Abstract
The mechanism of solute accumulation in the renal inner medulla remains an unresolved issue. Experiments were carried out in hamsters to address the possibility that the peristaltic contractions of the renal pelvic wall surrounding the inner medulla play a role in the inner medullary concentrating process. The right renal pelvis was subjected to one of four manipulations (surgical removal of the pelvic wall, paralysis of the pelvic wall with xylocaine, inhibition of pelvic contractions by direct application of heat, or sham treatment) followed by analysis of the inner medullary solute concentrations in the right kidney versus the untouched left kidney. Removal of the pelvic wall resulted in a marked reduction in inner medullary osmolality, confirming prior observations. Paralysis of the pelvic wall with xylocaine produced a similar decrease in inner medullary osmolality, despite the fact that urine flow was maintained. In contrast, sham treatment (surgical exposure of the right renal pelvic wall without any further manipulation) did not decrease inner medullary osmolality. To test whether the decrease in urinary osmolality following xylocaine treatment could have been due to a side effect of the drug, pelvic peristaltic contractions were eliminated in another way, by direct application of heat to denature the smooth muscle of the pelvic wall. This procedure also significantly decreased inner medullary osmolality. We conclude that elimination of the contractions of the renal pelvic wall in hamster significantly impairs inner medullary concentrating ability.
Keywords: urinary concentrating mechanism, urea, osmolality
INTRODUCTION
The mechanism by which solutes are concentrated in the inner medulla of the kidney is a longstanding question, which still lacks a definitive answer (12). One theory put forth originally by Kokko and Rector (11) and Stephenson (21) proposed that NaCl is concentrated in the inner medullary interstitium through a passive process that is dependent on urea reabsorption from the inner medullary collecting duct. Direct evidence contrary to this hypothesis has been provided by gene knockout studies in which deletion of collecting duct urea transporters did not diminish NaCl accumulation in the inner medulla of mice (6); (7). Another proposed theory states that the peristaltic contractions of the pelvic wall surrounding the inner medulla are somehow involved in the process that concentrates solutes in the inner medulla (16). It has recently been proposed that the energy from pelvic contractions is transduced by compressing the hyaluronic acid extracellular matrix present in the inner medulla (10). When the peristaltic compression is released, pressure gradients are believed to be generated that extract water from the descending limb of Henle’s loop and from the collecting duct, providing a single-effect mechanism. If this were true, one would predict not only that removal of the pelvic wall would reduce solute accumulation in the inner medulla as already demonstrated (20) (3), but also that procedures that abolish the pelvic contractions would also reduce solute accumulation in the inner medulla. In the present study, we tested this possibility in hamsters in which the pelvic contractions of one kidney were eliminated through use of a local anesthetic agent, xylocaine, or through exposure to high temperature, which locally damages the pelvic smooth muscle. The results support the theory that contractions of the renal pelvic wall play a role in generation of the inner medullary axial osmotic gradient.
METHODS
Male Syrian hamsters (body weight 80-100 g) were treated as described previously (18). Hamsters were housed, inventoried and cared for in compliance with the US Department of Agriculture (USDA) regulations, and their use was approved by the MDIBL animal care committee. Briefly, hamsters were anesthetized with Inactin (5-sec-butyl-5-ethyl-2-thiobarbituric acid, Byk Gulden, Konstanz, Germany, 15 mg per 100 gBW i.p.). Each anesthetized hamster was placed on a heated operating table, the jugular vein catheterized, the abdomen opened and the right kidney exposed. The abdominal cavity was filled with warm mineral oil to reduce evaporative cooling and increase visibility. The fat covering the pelvic wall was gently removed and a small fiberoptic light placed so that the papilla was transilluminated. A bolus of 0.1ml of 0.2% lissamine green in 0.9% saline was injected via the jugular vein. This allowed the experimenter to observe whether or not peristaltic contractions of the renal pelvis were occurring at any given time. Once steady contractions of the renal pelvis were documented, one of four treatments was initiated. These four types of experiments were: 1. Removal of the pelvic wall. In 12 hamsters, the right renal pelvis was removed by lifting the ureter with forceps just below the papilla and gently pushing up the papilla with a pair of scissors and then severing the pelvis wall as close to the rim of the cortex as possible. With this treatment, bolus flow in the collecting ducts was abolished giving instead a continuous flow of urine in the inner medullary collecting ducts. 2. Paralysis of the pelvic wall by xylocaine treatment. In 11 hamsters, a 2% xylocaine solution was applied to the right renal pelvic wall with a micropipette. The xylocaine solution would cling to and spread over the tissue to which it was applied due to the mineral oil in the pelvic cavity. The pelvic peristaltic contractions were completely inhibited by this treatment as evidenced by an uninterrupted flow of the green urine in the collecting ducts. The application of xylocaine did not affect the peristalsis in the ureter, which continued to send urine boluses toward the bladder, nor did it affect peristalsis in the contralateral pelvis or ureter. 3. Sham treatment. In 13 hamsters, the right pelvic wall was cleared of fat, but left untreated. Normal contractions continued. 4. Heat treatment. In 6 hamsters, heat was applied to the pelvic wall using a battery-operated Concept Accu-Temp Cautery device (Medtronic, Jacksonville, FL; Cat. No. 4200). Under microscopic observation, the tip of the device was manually moved along the tissue until contractions stopped. Care was taken to apply only enough heat to the pelvic wall to destroy just enough muscle tissue to inhibit pelvic peristalsis and to avoid damage to the papilla.
After a particular treatment had been implemented, the hamster was left to equilibrate for one hour. Saline was continuously infused i.v. at 10 ul per min. In the xylocaine experiments, it was necessary to repeat the application of xylocaine every 10-15 minutes in order to maintain complete inhibition of pelvic contractions. After 1 hour, the kidneys were removed. The inner medulla isolated and divided into segments for weighing, drying and analysis as previously described (19). Analysis was carried out in the middle third (IM2) and inner third (IM3) of the inner medulla. The following determinations were made on the tissue: osmolality, urea, Na, and K as previously described (19). Briefly, the tissues were excised rapidly from the animals, placed on pre-weighed foil envelopes and weighed on a Cahn electrobalance (model 21) to the nearest 0.001 mg. The envelopes were opened and dried over a desiccant at 60°C for 3-8 h. The envelopes were then cooled and reweighed to allow calculation of water content of the sample. The dried tissue samples were then suspended in 25 μl of deionized, distilled water in a microfuge tube (Centaur Sciences, MT 1005) and heated in a 100°C water bath for 3 min. Following a brief centrifugation (2-3 s) the tubes were stored at 4°C for 18-24 h prior to analysis. After recentrifugation, the supematant was analyzed for osmolality and urea, K and Na concentrations. Osmolality was determined with a Wescor vapor pressure osmometer (model 5100B) using 5 μl of the supernatant. Original osmolality was estimated from on the dilution factor as described (19). Sodium and potassium concentrations were determined in 5 μl samples diluted in 2 ml of 15 meq/liter lithium solution using an Instrumentation Laboratory flame spectrophotometer. Urea was determined by the microdiffusion method on duplicate 5 μl samples of the supernatant. The results of the tissue analysis were calculated as solute concentrations (mmol/l tissue water).
RESULTS
Effect of renal pelvic removal on inner medullary solute concentrations
Previous studies have established that removal of the renal pelvis causes a decline in medullary tissue osmolality (20); (3). To confirm this finding in hamsters, the right renal pelvis was removed and tissue solute concentrations were measured in the middle third and inner third of the inner medulla (“Mid-Inner Medulla” and “Papillary Tip”) of both the right (experimental) and left (control) kidneys. Figure 1 shows the difference in osmolality between the right and left kidneys in both inner medullary regions. Consistent with the previous observations, there was a statistically significant decline of osmolality in the inner medullas of kidneys from which the renal pelvis had been removed. The measured tissue osmolalities of the control inner medullas for these experiments were: mid-inner medulla, 1148 ± 150 mOsm; papillary tip, 1297 ± 160 mOsm. Table 1 shows the corresponding right versus left sodium, potassium and urea concentration differences in the inner medulla. As can be seen, the fall in osmolality is associated with a decrease in both sodium and urea concentration, but no change in potassium concentration.
Figure 1.
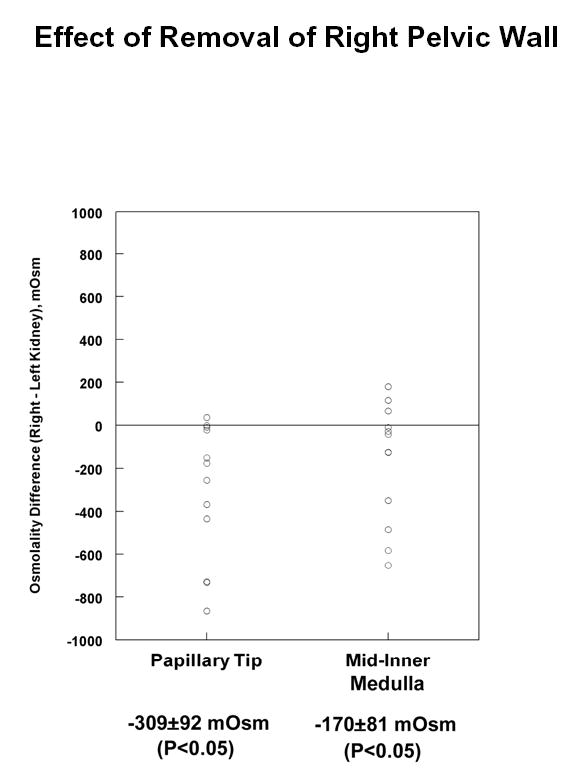
Difference in total solute concentration (osmolality) in experimental vs. control inner medullas of hamster kidneys after right renal pelvis removal. Kidneys were resected one hour after pelvic removal and analyzed as described in Methods. Total number of hamsters = 12.
Table 1.
Difference in sodium, potassium and urea concentration in experimental vs. control inner medullas of hamster kidneys after right renal pelvis removal. Total number of hamsters = 12.
| Papillary Tip | Mid-Inner Medulla | |
|---|---|---|
| Na (meq/L) | −86±32* | −56±21* |
| K (meq/L) | 3±6 | −8±4 |
| Urea (mM) | −97±26* | −49±37 |
P<0.05 vs 0.
Effect of xylocaine treatment on inner medullary solute concentrations
The fall in solute content with pelvic wall removal could be due to loss of pelvic peristalsis, which is hypothetically a source of energy for concentrating solutes in the inner medulla (10). To test this possibility, the pelvic peristalsis in the right renal pelvis was eliminated by direct application of xylocaine, and tissue solute concentrations were measured in the middle third and inner third of the inner medulla (“Mid-Inner Medulla” and “Papillary Tip”) of both the right (experimental) and left (control) kidneys. Figure 2 shows the difference in osmolality between the right and left kidneys in both inner medullary regions after treating the right kidney one hour. Osmolality was significantly decreased in both inner medullary regions after xylocaine treatment. The measured tissue osmolalities of the control inner medullas for these experiments were: mid-inner medulla, 1546 ± 130 mOsm; papillary tip, 1688 ± 167 mOsm. Table 2 shows the corresponding right versus left sodium, potassium and urea concentration differences in the inner medulla. As can be seen, the fall in osmolality is associated with a decrease in sodium in both inner medullary regions studied.
Figure 2.
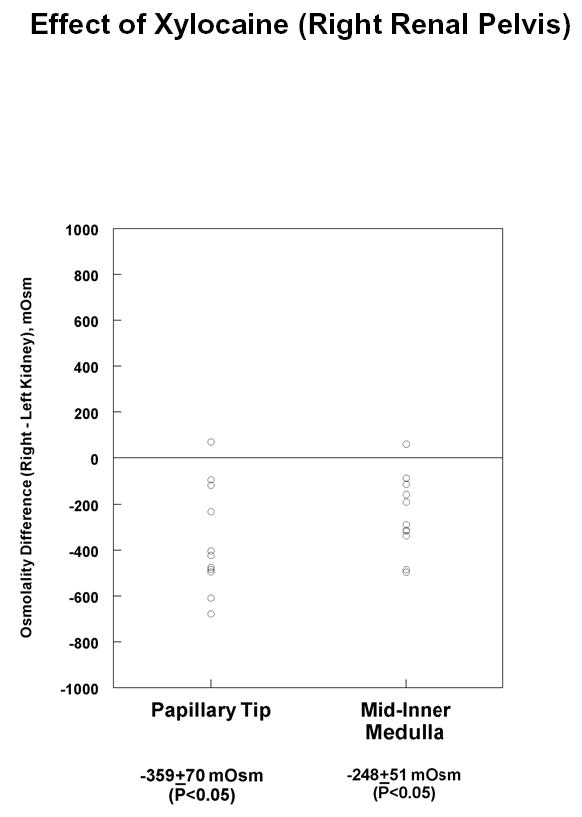
Difference in total solute concentration (osmolality) in experimental vs. control inner medullas of hamster kidneys after application of xylocaine to right renal pelvis. Kidneys were resected one hour after pelvic removal and analyzed as described in Methods. Total number of hamsters = 11.
Table 2.
Difference in sodium, potassium and urea concentration in experimental vs. control inner medullas of hamster kidneys after application of xylocaine. Total number of hamsters = 11.
| Papillary Tip | Mid-Inner Medulla | |
|---|---|---|
| Na (meq/L) | −97±27* | −58±13* |
| K (meq/L) | −2±3 | −6±2* |
| Urea (mM) | 4±5 | −50±48 |
P<0.05 vs 0.
Effect of sham surgery on inner medullary solute concentrations
The fall in solute content with pelvic wall removal and with inhibition of pelvic wall peristalsis could hypothetically be due to the surgical procedure involved with isolation of the pelvis rather than to the specific manipulations themselves. To test this possibility, we carried out sham experiments in which we isolated the pelvis of the right kidney and removed the fat covering the pelvic wall. After one hour, we measured tissue solute concentrations in the middle third and inner third of the inner medulla (“Mid-Inner Medulla” and “Papillary Tip”) of both the right (sham) and left (control) kidneys. The measured tissue osmolalities of the control inner medullas for these experiments were: mid-inner medulla, 1317 ± 100 mOsm; papillary tip, 1413 ± 140 mOsm. Figure 3 shows the corresponding right versus left osmolality differences in the inner medulla. Osmolality was not decreased, but rather was increased in the inner medulla of the sham-operated kidneys. Table 3 shows the corresponding data for sodium, potassium and urea in the inner medulla. The increase in total solute content appears to be largely due to increases in urea accumulation. Based on present evidence, we cannot assign a mechanism for this increase. Interestingly, evidence exists that accumulation of peripelvic fat may alter renal function and blood pressure (4), and we speculate that removal of fat as part of the sham surgery procedure could have contributed to the increase.
Figure 3.
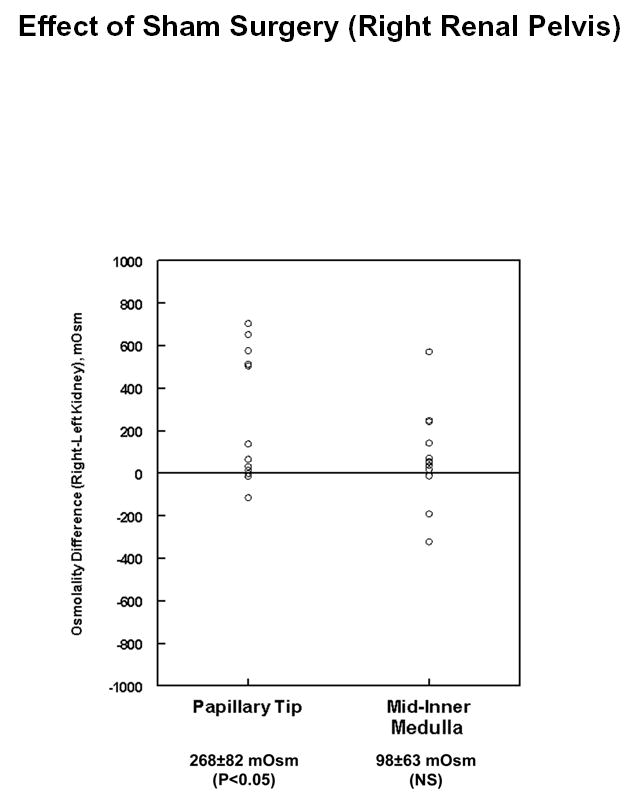
Difference in total solute concentration (osmolality) in sham (right) vs. control (left) inner medullas after sham surgery of right renal pelvis. Kidneys were resected one hour after sham surgery and analyzed as described in Methods. Total number of hamsters = 13.
Table 3.
Difference in sodium, potassium and urea concentration in experimental vs. control inner medullas of hamster kidneys after sham procedure. Total number of hamsters = 13.
| Papillary Tip | Mid-Inner Medulla | |
|---|---|---|
| Na (meq/L) | 38±23 | 11±93 |
| K (meq/L) | 0±2 | 3±4 |
| Urea (mM) | 61±35 | 75±33 |
P<0.05 vs 0.
Effect of heat treatment on inner medullary solute concentrations
To examine another means of inhibition of pelvic peristalsis, heat was applied to the outer surface of the pelvic wall as described in Methods and tissue solute concentrations were measured in the middle third and inner third of the inner medulla (“Mid-Inner Medulla” and “Papillary Tip”) of both the right (experimental) and left (control) kidneys. Figure 4 shows the difference in osmolality between the right and left kidneys in both inner medullary regions after heat treatment and one hour equilibration. Osmolality was significantly decreased in both inner medullary regions after heat treatment. The measured tissue osmolalities of the control inner medullas for these experiments were: mid-inner medulla, 1291 ± 196 mOsm; papillary tip, 1439 ± 210 mOsm.
Figure 4.
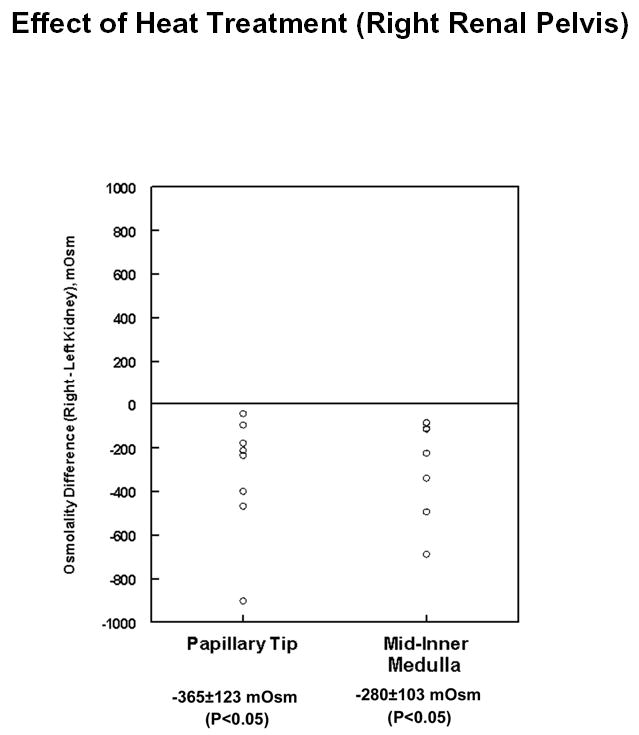
Difference in total solute concentration (osmolality) in experimental (right) vs. control (left) inner medullas after heat treatment of right renal pelvis. Kidneys were resected one hour after heat treatment and analyzed as described in Methods. Total number of hamsters = 6.
DISCUSSION
The renal pelvic wall surrounding the papillary portion of the inner medulla shows regular peristaltic contractions that strongly compress the medullary tissue, resulting in intermittent flow in the collecting ducts and loops of Henle (14) ; (19). In general, mathematical models of the urinary concentrating process in the inner medulla have assumed steady-state function, essentially ignoring the potential role of the periodic pelvic contractions (5); (10). At the same time, the mechanism whereby an axial solute gradient is generated in the renal inner medulla remains incompletely understood (15) ; (12) ; (10). It appears possible that the pelvic contractions significantly contribute to solute gradients in the inner medulla by providing energy for the concentrating process (17) ;(5) ; (10). To address this possibility, three different methods of abolishing pelvic contractions were used in one kidney and the inner medullary solute concentrations in the experimentally manipulated vs the opposite (control) kidney of hamsters were compared. The results showed that not only did removal of the renal pelvic wall attenuate tissue osmolality in the renal inner medulla (Figure 1) as previously demonstrated (20) (3), but that interruption of the pelvic contractions by application of a local anesthetic to the pelvic wall (Figure 2) or heat damage to the pelvic smooth muscle (Figure 4) also attenuated the osmolality of the inner medullary tissue. These latter procedures left the flow of urine intact and did not interrupt contractions of the ureter downstream from the pelvis. At the same time, sham manipulation of the right kidney (without pelvic wall removal, local anesthetic application or heat treatment) did not decrease inner medullary osmolality, but instead left the expected normal solute gradient intact. The manipulations were applied for only 1 hour in the current study, a period that is much shorter than the characteristic time required for the inner medulla to go from one steady state to another after various perturbationswhich has been reported to be 5-8 hours (1; 2; 8). Conceivably, longer treatment periods could have resulted in more profound effects on inner medullary osmolality.
In contrast to the conclusions of the present study, an earlier study in young rats (13) demonstrated that paralysis of the ureter with a calcium-channel blocker, (verapamil) downstream from the renal pelvis had little effect on urinary osmolality although peristalsis appeared to cease. The difference in conclusions between the two studies may have arisen from anatomical differences between the papillas of hamsters versus rats. In the former, the papilla protrudes much further beyond the edge of the cortex, allowing a greater portion of it to be affected by the manipulations applied to external portions of the pelvis (or ureter). In contrast, the two studies agree with regard to the effect of severing the pelvis/ureter, consistent with the idea that shunting the hydrostatic pressure changes normally associated with pelvic peristalsis interferes with the urinary concentrating process in the inner medulla.
Studies in mice in which the collecting duct urea transporters, UT-A1 and UT-A3, were knocked out (6) (7) demonstrated that NaCl accumulation in the inner medulla is not dependent on urea efflux from the inner medullary collecting duct, contrary to the predictions of the Kokko-Rector-Stephenson model (11); (21). Thus, alternative models need to be explored. One such model is a steady-state model that uses lactate, produced in the inner medulla from glucose, as a substitute for urea in the Kokko-Rector-Stephenson model (9). This model has not yet been tested experimentally. Another alternative is a model that uses the mechanical energy of the pelvic contractions which is transduced into chemical energy for concentrating solutes in the inner medulla (using the hyaluronic acid matrix of the inner medullary interstitium as an intermediary) has been recently proposed (10). This model lacks experimental verification at this point, but could potentially be verified through use of transgenic technology in mice. Nonetheless, the results of the present study support the theory that the inner medullary solute gradient might be generated in part through utilization of energy from the contractions of the pelvic wall, at least in rodents.
Acknowledgments
Supported by an extramural NIH Grant (AM-15972) and the intramural budget of the National Heart, Lung, and Blood Institute (Z01-HL-01282-KE).
Footnotes
{Footnote: Note that in this and all other experiments in this paper, there was a substantial osmotic gap between measured osmolality and the sum [urea] + 2([Na] + [K]). As a result, the difference in osmolality may not be explained fully by the differences in urea, Na, and K concentrations. As discussed previously (7), this gap is thought to be attributable to unmeasured solutes such as lactic acid, sorbitol, taurine, inositol, glycerophosphoryl choline, betaine, and ammonium. }
References
- 1.Atherton JC, Green R, Thomas S. Influence of lysine-vasopressin dosage on the time course of changes in renal tissue and urinary composition in the conscious rat. Journal of Physiology (London) 1971;213:291–309. doi: 10.1113/jphysiol.1971.sp009383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton JC, Hai MA, Thomas S. The time course of changes in renal tissue composition during water diuresis in the rat. Journal of Physiology (London) 1968;197:429–443. doi: 10.1113/jphysiol.1968.sp008568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang EL, Reineck JH, Osgood RW, Kunau RT. Studies on the mechanism of reduced urinary osmolality after the exposure of the renal papilla. Journal of Clinical Investigation. 1978;61:633–639. doi: 10.1172/JCI108974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dwyer TM, Mizelle HL, Cockrell K, Buhner P. Renal sinus lipomatosis and body composition in hypertensive, obese rabbits. Int J Obes Relat Metab Disord. 1995;19:869–874. [PubMed] [Google Scholar]
- 5.Dwyer TM, Schmidt-Nielsen B. The renal pelvis: machinery that concentrates urine in the papilla. News Physiol Sci. 2003;18:1–6. doi: 10.1152/nips.1416.2002. [DOI] [PubMed] [Google Scholar]
- 6.Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci U S A. 2004;101:7469–7474. doi: 10.1073/pnas.0401704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA. Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol. 2005;16:1583–1592. doi: 10.1681/ASN.2005010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hai MA, Thomas S. The time-course of changes in renal composition during lysine vasopressin infusion in the rat. Pfluegers Arch. 1969;310:297–319. doi: 10.1007/BF00587241. [DOI] [PubMed] [Google Scholar]
- 9.Hervy S, Thomas SR. Inner medullary lactate production and urine-concentrating mechanism: a flat medullary model. Am J Physiol Renal Physiol. 2003;284:F65–F81. doi: 10.1152/ajprenal.00045.2002. [DOI] [PubMed] [Google Scholar]
- 10.Knepper MA, Saidel GM, Hascall VC, Dwyer T. Concentration of solutes in the renal inner medulla: interstitial hyaluronan as a mechano-osmotic transducer. American Journal of Physiology: Renal Physiology. 2003;284:F433–F446. doi: 10.1152/ajprenal.00067.2002. [DOI] [PubMed] [Google Scholar]
- 11.Kokko JP, Rector FC., Jr Countercurrent multiplication system without active transport in inner medulla. Kidney International. 1972;2:214–223. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- 12.Masilamani S, Knepper MA and Burg MB Urine concentration and dilution. In: The Kidney, edited by Brenner BM. Philadelphia: Saunders, 2000, p. 595–636.
- 13.Oliver RE, Roy DR, Jamison RL. Urinary concentration in the papillary collecting duct of the rat. Role of the ureter. Journal of Clinical Investigation. 1982;69:157–164. doi: 10.1172/JCI110426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinking LN, Schmidt-Nielsen B. Peristaltic flow of urine in the renal papillary collecting ducts of hamsters. Kidney International. 1981;20:55060. doi: 10.1038/ki.1981.104. [DOI] [PubMed] [Google Scholar]
- 15.Sands JM, Kokko JP. Current concepts of the countercurrent multiplication system. Kidney International. 1996;57:S93–S99. [PubMed] [Google Scholar]
- 16.Schmidt-Nielsen B. The renal pelvis. Kidney International. 1987;31:621–628. doi: 10.1038/ki.1987.43. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Nielsen B. The renal concentrating mechanism in insects and mammals: a new hypothesis involving hydrostatic pressures. American Journal of Physiology. 1995;268:R1087–R1100. doi: 10.1152/ajpregu.1995.268.5.R1087. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Nielsen B, Churchill M, Reinking LN. Occurrence of renal pelvic refluxes during rising urine flow rate in rats and hamsters. Kidney International. 1980;18:419–431. doi: 10.1038/ki.1980.155. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Nielsen B, Graves B, Roth J. Water removal and solute additions determining increases in renal medullary osmolality. American Journal of Physiology. 1983;244:F472–F482. doi: 10.1152/ajprenal.1983.244.5.F472. [DOI] [PubMed] [Google Scholar]
- 20.Schuetz W, Schnermann J. Pelvic urine composition as a determinant of inner medullary solute concentration and urine osmolality. Pfluegers Archiv. 1972;334:154–166. doi: 10.1007/BF00586788. [DOI] [PubMed] [Google Scholar]
- 21.Stephenson JL. Concentration of urine in a central core model of the renal counterflow system. Kidney International. 1972;2:85–94. doi: 10.1038/ki.1972.75. [DOI] [PubMed] [Google Scholar]


