Abstract
‘Metal-responsive transcription factor-1’ (MTF-1), a zinc finger protein, is conserved from mammals to insects. In the mouse, it activates metallothionein genes and other target genes in response to several cell stress conditions, notably heavy metal load. The knockout of MTF-1 in the mouse has an embryonic lethal phenotype accompanied by liver degeneration. Here we describe the targeted disruption of the MTF-1 gene in Drosophila by homologous recombination. Unlike the situation in the mouse, knockout of MTF-1 in Drosophila is not lethal. Flies survive well under laboratory conditions but are sensitive to elevated concentrations of copper, cadmium and zinc. Basal and metal-induced expression of Drosophila metallothionein genes MtnA (Mtn) and MtnB (Mto), and of two new metallothionein genes described here, MtnC and MtnD, is abolished in MTF-1 mutants. Unexpectedly, MTF-1 mutant larvae are sensitive not only to copper load but also to copper depletion. In MTF-1 mutants, copper depletion prevents metamorphosis and dramatically extends larval development/lifespan from normally 4–5 days to as many as 32 days, possibly reflecting the effects of impaired oxygen metabolism. These findings expand the roles of MTF-1 in the control of heavy metal homeostasis.
Keywords: gene targeting/heavy metal/metallothionein/MRE/MTF-1
Introduction
Every organism must cope with environmental fluctuation of heavy metal concentrations. Non-essential, toxic heavy metals have to be exported or sequestered intracellularly, while the uptake, storage and distribution of essential heavy metals, such as zinc and copper, have to be ensured, with the additional problem that even these metals are toxic if present in excess.
Important components of the heavy metal homeostasis and detoxification system are the membrane-based heavy metal transporters (reviewed in Nelson, 1999; Puig and Thiele, 2002), intracellular metal chaperones for efficient distribution of scarce essential metals (reviewed in Harrison et al., 2000), and the metallothioneins, a group of small, cysteine-rich proteins that have the ability to bind and thereby sequester heavy metals (reviewed in Kägi, 1991; Palmiter, 1998; Andrews, 2000; Simpkins, 2000). There are >10 functional metallothionein genes in humans and four in the mouse (West et al., 1990; Quaife et al., 1994); in Drosophila, two genes were characterized before, designated Mtn/MtnA and Mto/MtnB (Lastowskiperry et al., 1985; Mokdad et al., 1987) (see also below). Trans cription of metallothionein genes is strongly induced by heavy metal load. This induction is mediated via conserved DNA sequence motifs, so-called metal response elements (MREs) of consensus TGCRCNC (R = A or G, and N = any nucleotide) that are present in the promoters of all metallothionein genes from insects to mammals (Stuart et al., 1985).
Previously, our laboratory isolated and characterized a zinc finger transcription factor that binds to MRE sequences. This protein was referred to as metal response element-binding transcription factor-1 (MTF-1, also designated metal-responsive transcription factor-1 or metal transcription factor-1) (Westin and Schaffner, 1988; Radtke et al., 1993; Brugnera et al., 1994; Auf der Maur et al., 1999; reviewed in Andrews, 2001; Giedroc et al., 2001; Lichtlen and Schaffner, 2001). In the mouse, MTF-1 plays an essential role in liver development; targeted deletion of the MTF-1 gene results in embryonic death due to liver degeneration (Günes et al., 1998). A search for MTF-1 target genes in the mouse has revealed metallothionein genes and a number of other genes, several of which contain MREs in the promoter and/or are involved in coping with cell stress (Andrews et al., 2001; Lichtlen et al., 2001). MTF-1 plays a role not only in heavy metal stress but also in other cell stress conditions such as oxidative stress, hypoxia and amino acid starvation (Murphy et al., 1999; Dalton et al., 2000; Adilakshmi and Laine, 2002; reviewed in Lichtlen and Schaffner, 2001).
Recently, we have characterized the MTF-1 homolog from Drosophila and demonstrated that it can activate metallothionein gene promoters in cell transfection experiments (Zhang et al., 2001). Here we show that inactivating the Drosophila MTF-1 gene using the technique of targeted gene disruption by homologous recombination (Rong et al., 2002) yields viable flies that are sensitive not only to high concentrations of copper, cadmium and zinc but, unexpectedly, also to copper depletion. We also describe two novel metallothionein genes MtnC and MtnD and show them to be, like the known metallothionein genes MtnA and MtnB, targets of MTF-1.
Results
Generation of MTF-1 mutant alleles
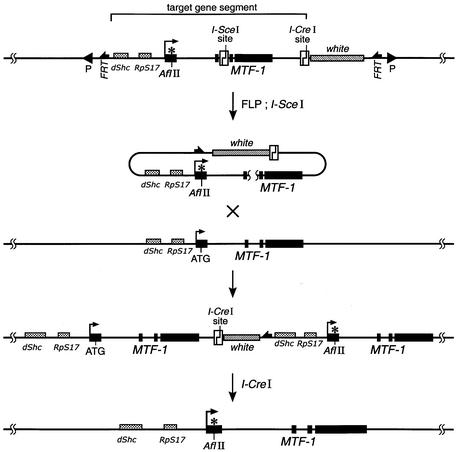
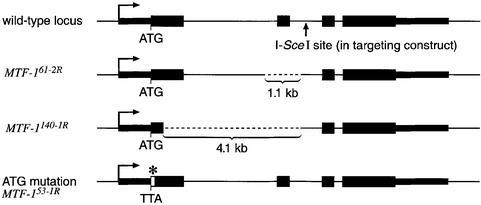
We decided to mutate the gene for MTF-1 in Drosophila by homologous recombination, since there are no P-elements close by in the corresponding 67B region of chromosome 3. For gene targeting, a 17 kb genomic fragment was modified by replacing the initiator ATG with TTA and inserting an I-SceI recognition site into the second intron of MTF-1. Targeting of the MTF-1 locus was achieved by a procedure essentially corresponding to that described by Rong and Golic (2000, 2001) and Rong et al. (2002) (Figure 1). By screening a total of 40 000 flies, we recorded 23 independent targeting events (frequency of one event in 1700 flies). Because of the design of an ‘ends-in’ recombination, the gene targeting led to a tandem duplication of the locus, resulting in a mutant and a wild-type gene copy. In a final step, one of the two gene copies could be removed by cleavage with subsequent recombin ation (Figure 1). Unexpectedly, in five independent lines, homologous integration of the targeting DNA into the MTF-1 locus resulted in additional sequence alterations, namely large deletions of 1–4 kb in the supposedly wild-type copy of MTF-1 (Figure 2). Similar deletions as side products of homologous gene targeting apparently were also observed by others (Rong and Golic, 2000; Seum et al., 2002). The origin of such deletions is enigmatic at present. In any case, they were a welcome addition to the intended ATG site mutation and allowed for an independent test of the mutant phenotype.
Fig. 1. Schematic view of the MTF-1 locus and targeting strategy. A transgene containing a mutant MTF-1 and the marker gene ‘white’ (w+) is circularized by FLP recombinase and linearized by the rare-cutter restriction enzyme I-SceI in germ cells. Alignment of the targeting DNA and resident MTF-1 locus by ‘ends-in’ recombination results in a duplication of MTF-1, with concomitant integration of the w+ gene into the MTF-1 locus. After identification of successful targeting events, the duplication is reduced to a single copy by means of homologous recombination, after inducing a double strand break in the genomic DNA with the other rare-cutter (I-CreI). The w+ marker gene is thereby lost. *, mutation of start colon.
Fig. 2. Overview of wild-type and mutant MTF-1 alleles. The gene structure of the wild-type MTF-1 allele is shown on top; below, two examples of spontaneous deletions that had occurred upon integration of the targeting DNA by homologous recombination are depicted. These deletions are missing 1.1 kb including exon 2 (allele MTF-161-2 and MTF-161-2R), or 4.1 kb including a substantial fraction of exon 1, together with the entire first intron and exon 2 (allele MTF-1140-1 and MTF-1140-1R). The targeting mutation in the initiator codon ATG→TTA in the original targeting construct is shown at the bottom (alleles MTF-1140-1, MTF-161-2 and MTF-153-1R). Alleles MTF-161-2 and MTF-1140-1 are duplications of the locus (as indicated in Figure 1) containing a ATG→TTA mutation in one copy and a deletion in the other copy of MTF-1. MTF-1140-1R, MTF-161-2R and MTF-153-1R are alleles reduced to a single copy.
Isogenic DNA does not increase targeting efficiency at MTF-1
In the mouse, the best targeting efficiency is observed if targeting and recipient DNA are isogenic, i.e. do not display DNA sequence polymorphisms (te Riele et al., 1992). In our case, the targeting construct used was not isogenic to recipient DNA, but differed by no less than 215 single nucleotide substitutions and 25 insertions/deletions of up to 12 bp within a segment of 17 kb. To test whether an isogenic background would increase the targeting efficiency, we performed the same experiment in a fly strain that does not contain any polymorphisms relative to the targeting construct (a gift of R.Jiao and M.Noll). Surprisingly, in our hands, isogenicity did not improve the frequency of targeting by homologous recombination: among 15 000 flies, merely two events were recorded. It might be that as the length of homology increases, there is less dependence on isogenic DNA.
MTF-1 mutant flies are sensitive to heavy metals
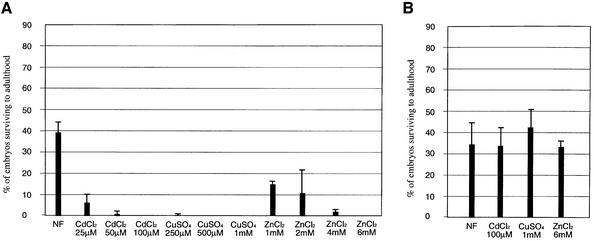
Disruption of the MTF-1 gene in the mouse results in embryonic lethality (Günes et al., 1998). Unexpectedly, this was not the case in Drosophila. We observed viable and fertile flies with single or tandemly arranged mutant genes, irrespective of whether they were kept over the large deficiency Df(3L)AC1 that also removes the MTF-1 locus, or were bred to homozygosity. In the mouse, MTF-1-deficient cells can be taken into culture before the deadly crisis of the embryo; such cells are sensitive to heavy metal load (Günes et al., 1998). We thus wanted to test whether the mutant flies are sensitive to elevated concentrations of cadmium, copper or zinc. Cadmium is a non-essential, highly toxic metal; in contrast, copper and zinc are essential trace metals whose homeostasis is regulated carefully (Palmiter and Findley, 1995; reviewed in Eide, 1998; Nelson, 1999). Indeed, as seen in Figure 3, MTF-1 mutant flies are more sensitive to cadmium toxicity than wild-type flies. Even though generally higher concentrations of copper and zinc were required to achieve toxic effects, the mutant flies were again considerably more sensitive to these trace metals. At the concentrations shown here, wild-type flies are not affected (Figure 3B). Severe toxicity for MTF-1 mutant flies (tested with allele MTF-1140-1R) is seen with 50 µM CdCl2, 250 µM CuSO4 and 4 mM ZnCl2, whereas the wild-type is able to grow on food supplemented with 300 µM CdCl2, 3 mM CuSO4 and 8 mM ZnCl2, respectively. The metal sensitivity phenotype of the mutant could be completely rescued by a P-element containing a genomic MTF-1 transgene (not shown), confirming that it was indeed lack of MTF-1 that caused the phenotype.
Fig. 3. Relative viability of MTF-1 mutant versus wild-type at different metal concentrations. The bar diagrams depict the percentage survival of mutant and wild-type embryos to adulthood. MTF-1–/– mutant (allele MTF-1140–1R) (A) or y w control flies (B) were allowed to deposit 150–300 eggs on food containing the indicated concentrations of metals, and eclosing adults were counted. NF = normal food. Error bars represent standard deviations calculated from the number of flies in at least three different tubes.
Metallothionein gene transcription depends on MTF-1
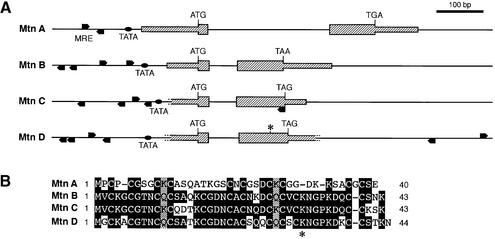
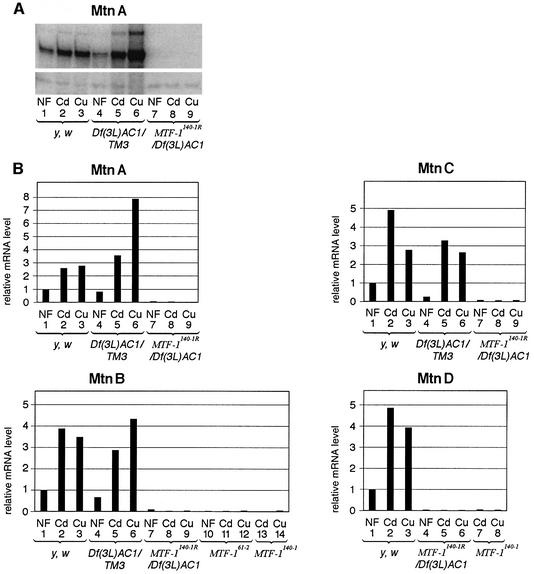
The question arose as to what target genes are most obviously affected by the lack of MTF-1. In mammals, the best characterized targets are the metallothionein genes and, also in cultured Drosophila cells, transcription of metallothionein genes MtnA and MtnB (formerly Mtn and Mto, respectively) is activated by MTF-1 via the typical MREs in their promoters (Zhang et al., 2001). When we searched the database for metallothioneins using the known sequences of MtnA and MtnB, two new metallothionein genes were identified that were accordingly named MtnC and MtnD (Figure 4A). Initially, the significance of MtnD was in doubt because of a premature stop codon (TAG) replacing AAG (lysine) at amino acid position 32. Otherwise, MtnD has all the attributes of a functional metallothionein gene, including MRE sequences in the promoter, which prompted us to determine the gene’s sequence in other fly strains as well. Interestingly, we found that this stop codon, most probably resulting in a non-functional pseudogene, is confined to the OregonR Drosophila strain, while the CantonS strain, as well as a wild catch in the village of Küsnacht, Switzerland, contain a lysine codon instead. This identifies MtnD as a novel metallothionein closely related to MtnB and MtnC (Figure 4B). We tested the effect of heavy metal load on the expression of these metallothionein genes and found that all four of them displayed greatly elevated transcript levels upon exposure to cadmium or copper (Figure 5A). Zinc is a relatively poor inducer of Drosophila metallothioneins (Zhang et al., 2001) and was therefore not tested in this experiment. Mutation of MTF-1 had a striking effect on the expression of all four metallothionein genes. As seen in Figure 5B, the heavy metal response was abrogated and even the basal expression level was strongly reduced.
Fig. 4. Drosophila metallothionein genes and proteins. (A) Overview of the four metallothionein genes; genomic location on 3R (MtnA at 85E9, MtnB at 92E12, MtnC at 92E4, MtnD at 92F1). In addition to the previously known MtnA and MtnB, two new metallothionein genes are present in the region of MtnB, designated MtnC and MtnD. (B) Comparison of metallothionein protein sequences. All four metallothioneins have a similar pattern of cysteines that are involved in metal complexation. The amino acid sequences of MtnB, C and D are closely related, indicating that these genes arose by duplication. However, several genes between them are not duplicated. OregonR contains a premature TAG stop codon at position 32 of Mtn D, indicated by an asterisk in the gene structure and in the amino acid sequence. In the CantonS strain and a Swiss wild catch, a conserved lysine is present instead of this premature stop codon.
Fig. 5. All four Drosophila metallothionein genes respond to heavy metal load and are dependent on MTF-1. Transcripts of metallothionein genes in Drosophila third instar larvae were determined by S1 nuclease protection (Weaver and Weissmann, 1979). Several MTF-1 mutant genotypes were used for this analysis, as indicated in the figure. NF = normal food; Cd = 50 µM CdCl2; Cu = 500 µM CuSO4. (A) Quantification of metallothionein (MtnA) transcripts. The lower part of the figure shows the signal from α-tubulin84B (loading control). (B) Compilation of the expression values of all four metallothionein genes (MtnA–D). Due to different specific activities of probes and hybridization optima, absolute transcript levels could not be compared easily between metallothionein genes. Therefore, the wild-type expression level in normal food was set arbitrarily to 1. Note that metallothionein expression in flies heterozygous for the MTF-1 locus is about the same as in y w flies wild-type for MTF-1 (compare lanes 1–3 of MtnA,B,C with lanes 4–6 of the same metallothioneins), while in MTF-1 mutants basal and metal-induced transcription activities of metallothionein genes are strongly reduced (compare lanes 1–3 of MtnA,B,C,D with lanes 7–9 of MtnA, lanes 7–14 of MtnB, lanes 7–9 of MtnC and lanes 4–8 of MtnD, respectively). MtnB expression is equally low with different mutant alleles: MTF-161-2, MTF-1140-1 and MTF-1140-1R (compare lanes 7–9 of MtnB with lanes 10–12, and with lanes 13 and 14).
MTF-1 mutants are also sensitive to copper depletion
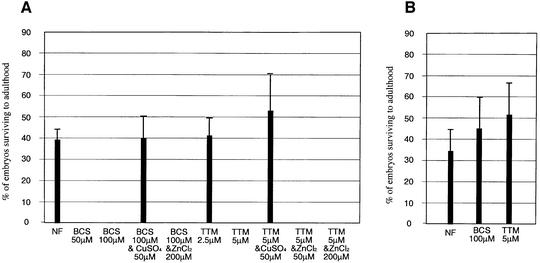
To see whether MTF-1 mutant flies were perhaps more resistant to a scarcity of an essential trace metal, flies were raised on food containing various concentrations of metal chelators. Three chelators were tested: bathocuproinedisulfonic acid (BCS), an extracellular copper chelator (Labbe et al., 1997); ammonium tetrathiomolybdate (TTM), a strong copper chelator also used in the treatment of Wilson’s disease patients who suffer from copper accumulation in the liver (Brewer et al., 1991; Jeannin et al., 1992); and bathophenanthrolinedisulfonic acid (BPS), an iron (II) chelator that also, however, has affinity for copper (Alcain et al., 1994; Georgatsou and Alexandraki, 1999). Unexpectedly, MTF-1 mutant larvae were exquisitely sensitive to the copper chelators TTM and BCS (Figure 6); they were also more sensitive to the iron (copper) chelator BPS, although >5-fold higher BPS concentrations were needed to achieve lethality as compared with the chemically very similar compound BCS (not shown).
Fig. 6. MTF-1 mutants are highly sensitive to copper chelators. The bar diagrams depict the percentage survival of mutant and wild-type embryos to adulthood. MTF-1–/– mutant (allele MTF-1140-1R) (A) or y w control flies (B) were allowed to deposit 150–300 eggs on food containing the indicated concentrations of chelator, and eclosing adults were counted. NF = normal food. Error bars represent standard deviations calculated from the number of flies in at least three different tubes.
Larvae died at first and second instar larval stages with 5 or 10 µM TTM, and at second or third instar and even the pupal stage in the case of 50 or 100 µM BCS, with a decreasing number of larvae escaping to the next stage. The effect of copper depletion was aggravated by breeding mutant flies for more than one generation: flies that had been raised under a sublethal BCS chelator condition for one generation failed to produce viable larvae if these were subjected to the same low copper conditions, whereas wild-type flies could be grown continuously in the same food. This suggests a maternal copper effect: if raised in normal food, both wild-type and mutant mothers might deposit copper in the egg. If raised in low copper food, however, the mutant mothers would be unable to provide the necessary copper for their offspring, resulting in early larval death. To see whether MTF-1 mutants were more sensitive to adverse nutrient conditions in general, we also raised them in a 10-fold dilution of the food in a constant amount of supporting agar. Even under such hardship which required extra activity of the larvae to collect enough nutrients, the mutant was not at a disadvantage: a few larvae from both wild-type or MTF-1 mutants survived and reached pupation and fertile adulthood, indicating that MTF-1 mutants do not suffer from a general weakness (not shown). The effect of the copper chelators is specific, because addition of copper but not zinc to food containing either BCS or TTM rescued the phenotype (Figure 6).
Another unexpected finding was a dramatically extended larval period of MTF-1 mutants upon copper depletion. Instead of the usual 4 days for passing through instar stages 1–3 to pupation at 25°C, some mutant larvae were roaming through the food cake for up to 32 days, apparently unable to continue on to pupation. While their size was approaching that of third instar larvae, other features, especially the tracheal system and mouth apparatus, corresponded to second instar larvae (Figure 7). These findings show that loss of MTF-1 makes animals hypersensitive to too high and too low concentrations of heavy metals, thus suggesting that MTF-1 plays a dual role in metal homeostasis.
Fig. 7. Treatment with the copper chelator TTM results in extreme prolongation of the larval period. Shown are an MTF-1 mutant larva (allele MTF-1140-1) at 22 days kept in 10 µM TTM and a wild-type (y w) control larva at the wandering stage. Note that in the mutant larva, although its size approaches that of a wandering third instar larva, several anatomical deviations, including tracheal system (arrowhead points to posterior spiracles) and mouth hooks (arrow) correspond to those of second instar larvae. Anterior spiracles, not visible here, also resemble second instar larvae. Note that the diameter of the tracheal tubes is smaller in the MTF-1 knockout than in y w controls. Such larvae cannot enter pupation and die between 20 and 32 days.
Discussion
To study the function of MTF-1 in Drosophila, we mutated the MTF-1 locus via homologous recombination. Drosophila had long been refractory to this kind of manipulation, even though many aspects of homologous recombination were described before (Engels et al., 1990; Gloor et al., 1991; Hagmann et al., 1998). Using an ‘ends-in’ recombination approach, we observed a targeting frequency of one targeting event in 1700 flies, a number that compares quite well with the best values obtained so far by Golic and co-workers at the yellow (y) gene locus (Rong and Golic, 2000). Our findings thus underline the usefulness of homologous gene targeting in Drosophila.
Unlike the MTF-1 mutant mice, which die from embryonic liver degeneration in utero, MTF-1 mutant flies are viable yet sensitive to heavy metals and copper depletion. This phenotype is very similar for combinations of a variety of different alleles including an initiator triplet mutation, a 1.1 kb deletion, a 4.1 kb deletion, and tandem or single mutant genes in combination with a chromosomal deficiency. Subtle differences exist amongst different allelic combinations in their sensitivity to either heavy metal load or copper chelators. The allele MTF-1140-1R carrying a 4.1 kb deletion of the coding region possesses the strongest phenotype and is most probably a null mutation.
In general, MTF-1 mutants are sensitive to distortions of heavy metal balance. One aspect, namely the sensitivity to high concentrations of cadmium but also to copper and zinc, is in agreement with earlier findings with cultured cells of MTF-1–/– mice (Günes et al., 1998) and Drosophila (Zhang et al., 2001). The failure of our MTF-1 mutants to induce metallothionein genes provides the most likely explanation for their sensitivity to heavy metal load.
Quite unexpected, however, is the exquisite sensitivity of MTF-1 mutants to copper depletion. This is particularly interesting because scarcity of trace heavy metals is probably encountered more often under natural conditions than heavy metal load. It is worth mentioning that another Drosophila mutant displays a similar phenotype: flies with a deletion of a copper transporter (Ctr1B) are also sensitive to both excess copper and copper depletion (D.J.Thiele, personal communication). The mechanism for this dual sensitivity may be a translocation of the protein from the outer membrane to vacuoles/lysosomes under limiting and excess copper concentrations, respectively (see also Petris et al., 1996; Schaefer et al., 1999; Borrelly et al., 2002). So far, it is unclear how MTF-1 enables a cell to cope with metal depletion. Our results suggest that MTF-1 regulates either import or efficient usage of copper, since wild-type flies may be grown continuously on copper chelator food, whereas MTF-1 mutants are able to do so under sublethal conditions for just a single generation. We speculate that MTF-1, upon copper depletion, activates a copper import pump and/or inactivates an export pump or, alternatively, regulates the expression of a copper chaperone. Drosophila metallothioneins themselves, which preferentially bind copper (Valls et al., 2000), may act not only as heavy metal scavengers upon heavy metal load, but also, under limiting copper concentrations, as copper chaperones similar to Cox17 and Atx1 (reviewed in Harrison et al., 1999). Alternatively, Drosophila copper-loaded metallothioneins may act as a storage pool for copper (see also Dalton et al., 1996). This scenario is compatible with our finding that MTF-1 is also required for the basal transcription of metallothionein genes, as seen in Figure 5. Thus, the lack of metallothioneins in MTF-1 mutants may also be responsible for their sensitivity to copper depletion.
Another enigma is the extreme extension of larval development in the presence of copper chelator in the food. It is not clear at present whether this prolonged larval period involves a genuine longevity effect. Several hypotheses can be envisaged to explain this phenomenon. (i) Extension of larval life could be due to a decrease in cytochrome c oxidase (COX) activity, a key copper-containing enzyme in the respiratory chain. In the fungus Podospora anserina, elimination of grisea, a copper-modulated transcription factor, affects the expression of a copper transporter and leads to impaired copper uptake. This correlates with a reduced activity of the COX complex and is associated with delayed growth and an extended lifespan. The same phenotype is produced by the direct elimination of COX5, a subunit of the cytochrome c oxidase (Begel et al., 1999; Dufour et al., 2000; Borghouts et al., 2002; reviewed in Osiewacz and Borghouts, 2000; see also Carr et al., 2002). Extension of lifespan in P.anserina can also be achieved by the mere addition of the copper chelator BCS to the medium (Borghouts et al., 2001). In our case, a decrease in cytochrome c oxidase activity due to insufficient copper supply could reduce ATP production, slow growth and, consequently, prolong the larval period. (ii) Copper on the one hand is an essential component of enzymes, including tyrosinase/phenol oxidase (Asada et al., 1999) and superoxide dismutase for radical scavenging, but on the other hand contributes directly to the formation of oxygen radicals in the Fenton reaction (Zhou et al., 2001); for the latter reason, copper depletion may result in less oxidative damage. This by itself would not explain stalled development, but rather why larvae survive that long. (iii) Mutant larvae raised with a copper chelator grow but retain features of second instar larvae including thinner tracheal ducts (Figure 7); thus insufficient oxygen supply could restrict growth and prolong the larval period, perhaps again in combination with less oxidative damage of tissues. (iv) Larvae raised in chelator-containing food also have smaller mouth hooks, which may prevent them from using the food efficiently. Thus extended larval life could be the result of a caloric restriction, which is known to delay growth and extend lifespan in a large variety of organisms from yeast to mammals (Lin et al., 2002; reviewed in Roth et al., 1995; Sohal and Weindruch, 1996; Masoro, 2000). We consider this unlikely, because starvation due to a 10-fold dilution of the food cake did not reveal any differences between wild-type and MTF-1 mutant larvae. (v) The gene for SHC adaptor protein (shc) involved in tyrosine kinase receptor signaling is located quite close to the MTF-1 transcription unit (two genes upstream, at 3.3 kb distance), and in the mouse, knockout of shc has been found to extend lifespan (Migliaccio et al., 1999). Thus the knockout of MTF-1 might adversely affect regulatory sequences of the shc gene. However, our simple mutation of three bases at the MTF-1 translation initia tion codon is unlikely to have such an effect. Further more, sequencing of the genomic region in the mutant flies revealed no difference from wild-type in the shc or Rps17 region, showing that the targeting process has not affected these two neighboring genes (data not shown).
Whatever the reason for this greatly expanded larval period, our results firmly establish for MTF-1 a central role in the heavy metal metabolism of the fly. The exquisite sensitivity of MTF-1 mutants to copper depletion points to a new role for this protein that waits to be explored also in mammals.
Materials and methods
DNA constructs
Targeting construct. The clone aj271817 containing the three genes shc, RpS17 and MTF-1 was cloned as a NotI fragment into pTV2 vector (Rong et al., 2002).
Changes were introduced by PCR with the following oligos (altered bases are underlined). The primers used to change the ATG→TTA were 5′-GCGAATAACAAATAATACGACTTAAGCGACCAAGAGAAAC AACACCAGC-3′ and 5′-GCTGGTGTTGTTTCTCTTGGTCGCTTAA GTCGTATTATTTGTTATTCGC-3′. Mutation of CATGAAC to CTTAAGC generates a new restriction site (AflII) for easy identification of mutant DNA.
Oligos used to introduce the I-SceI cleavage site were 5′-TATTCCTAGGGATAACAGGGTAATACGGATAACTCAAGCGCG GAG-3′ and 5′-TATTCCTAGGCTCGCTAAGGTATTCCTCCTCG-3′. The reason for mutating the ATG was that there are no other nearby in-frame ATGs further downstream that could give rise to a truncated, yet possibly functional protein. The next downstream in-frame initiation codon occurs at amino acid position 222 at the end of the fourth zinc finger; DNA binding of MTF-1 has been shown to be completely dependent on zinc fingers 1–4 (Chen et al., 1998, 1999).
Genomic rescue construct. The clone aj271817 containing the three genes shc, RpS17 and MTF-1 was cloned as a NotI fragment into a P-element vector and injected into y w flies.
Fly stocks and genetics
The stocks y w (v); P[ry+, 70FLP]4 P[v+, 70I-SceI]2B Sco/S2 CyO were provided by Y.Rong and K.Golic. Targeting was done from a single donor on the second chromosome. To maximize the efficiency of donor excision, three heat shocks (38°C, 60 min) were performed on days 2, 3 and 4 after egg laying. Heat-shocked virgins were crossed to y w, ey-Flp; D gl/TM3,y+ males, and only females were screened for the presence of the white+ (w+) gene. w+ flies represent homologous or non-homologous integrations, respectively, since the ey-Flp completely removes any unexcised donor. Successful targeting events into the resident MTF-1 locus on chromosome 3 were verified by Southern analysis (of 24 independent recombination events, just one was due to non-homologous insertion). Of the 23 targeted events, all except one retained the ATG→TTA mutation. A low frequency of gene conversion was to be expected, since the I-SceI recognition site is located 4.3 kb away from the TTA mutation in the targeting construct and it is known that gene conversion frequencies decrease with the distance from the double strand break (Gloor et al., 1991; Rong et al., 2002). For the reduction to a single copy allele by homologous recombination, the targeted alleles MTF-1140-1 and MTF-161-2 were chosen, both of which carry a deletion in one copy and an ATG point mutation in the other. Reduction by I-CreI was performed by crossing the targeted alleles (class III event; Rong and Golic, 2000) to y w; P[I-CreI]/TM3,Sb Ser, a P-element insertion kindly provided by Y.Rong and K.Golic. The offspring were given a single heat shock (36°C, 60 min) at the third larval stage. Males were re-crossed to y w; P[I-CreI]/TM3,Sb Ser females to select for w– flies. These were crossed individually to y w; TM3,y+/D gl to make stocks which were tested for the presence of either the introduced mutation or the spontaneous deletion. Since I-CreI cutting may result in elimination of a functional w+ gene either by homologous recombination or by exonuclease resection and DNA end joining, the correct removal of the duplication was checked by PCR and by Southern blotting (see below). The new single copy alleles were named as follows: MTF-1140-1R for the 4.1 kb deletion, MTF-161-2R for the 1.1 kb deletion and MTF-153-1R for the ATG point mutation. These alleles are identified by the PCR primers listed below. The reduction process by I-CreI worked with ∼10% efficiency even with our very long duplication, where >17 kb of DNA of the MTF-1 locus plus the w+ marker gene had to be removed, a total of 26 kb.
Molecular characterization of the targeted events
Southern blots were performed with a probe derived from a PCR product using genomic DNA with the primers 5′-CCTAATAGCATTGTT ATACTCG-3′ and 5′-AGGGAGCACATACGAATCAG-3′. Allele-specific PCR was used to check for the presence of the mutations: the two primers 5′-AAAGCGAATTGCGCGACGAG-3′ and 5′-GAATAACA AATAATACGACTTAAG-3′ amplify a 158 bp fragment only from the mutant allele with the ATG→TTA mutation.
The two primers 5′-CGTTCCGCCCATGGTCACACTGGTTC-3′ and 5′-TGCCGGGTGTAACCGTAGACAGCCAG-3′ amplify a 545 bp fragment from the MTF-1140-1 and MTF-1140-1R deletion allele and a 4.7 kb fragment from wild-type, and the primer pair 5′-CGCAAAAGCC CGCTGCCACAAGGAGC-3′ and 5′-GAGCCCTCCAGGAAACGGC TG-3′ amplifies a 0.9 kb fragment from the MTF-161-2 and MTF-161-2R deletion allele instead of a 1.6 kb fragment in wild-type.
Cloning of MtnC and MtnD
The two primer pairs 5′-ACTGGCAAACACAGTATTCAG-3′/5′-ACAGGGGCTATCATTTATTGG-3′ and 5′-GTATTTTATTTCGTTG TCATG-3′/5′-TTTTAACACAAAATGGGTTGC-3′ were used to amplify Mtn C and Mtn D by RT–PCR from total RNA, and cDNAs were cloned into pBluescript. MtnC is annotated at FlyBase (http://flybase.bio.indiana.edu/) as CG5097.
The fly strains used for sequencing the MtnD gene were OregonR, CantonS and a wild catch from Küsnacht, Switzerland, tentatively designated KantonZ. This sequence has been submitted to the DDBJ/EMBL/GenBank database under accession No. AF546903.
Fly food
Flies were raised on standard cornmeal molasses-based food supplemented with either CdCl2, CuSO4, ZnCl2, TTM (Sigma-Aldrich 32,344-6), BCS disodium salt hydrate (Sigma-Aldrich 14,662-5) or BPS disodium salt hydrate (Sigma-Aldrich 14,661-7). The concentrations of trace metals, based on the content of the individual ingredients, are ∼5 µM for copper and 150 µM for zinc.
RNA extraction and S1 nuclease protection assay
Third instar larvae were transferred for 6 h to food containing 50 µM CdCl2, 500 µM CuSO4, or to non-supplemented food. Total RNA was extracted using the TRIzol reagent (Life Technologies). Nuclease S1 mapping of transcripts with 50 µg of total RNA was performed as described previously (Weaver and Weissmann, 1979). The gels were developed using PhosphorImager (Molecular Dynamics) and bands were quantified.
Database searches and computer analysis of the sequences
Blast searches were performed using the BDGP BLAST service http://www.fruitfly.org/blast/. MREs were mapped using Sequencer™ 4.1 software. Sequence alignments were performed using the CLUSTAL_W and Boxshade programs.
Acknowledgments
Acknowledgements
We are grateful to Christoph Hugentobler for technical assistance, to Kent Golic and Yikang Rong for the generous gift of flies and plasmids for gene targeting, and to Renjie Jiao and Markus Noll for providing the fly strain isogenic to our targeting construct. We also thank Franz Wittwer, George Hausmann, Rolf Nöthiger and three anonymous reviewers for comments on the manuscript, Konrad Basler, Knud Nairz and Maria Jasin for valuable discussions, and Fritz Ochsenbein for the preparation of figures. D.E. is grateful to Inspiration. This work was supported by the Kanton Zürich and by the Swiss National Science Foundation.
References
- Adilakshmi T. and Laine,R.O. (2002) Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival or death. J. Biol. Chem., 277, 4147–4151. [DOI] [PubMed] [Google Scholar]
- Alcain F.J., Low,H., Crane,F.L. and Navas,P. (1994) Iron chelators hydroxyurea and bathophenanthroline disulfonate inhibit DNA-synthesis by different pathways. Biochem. Mol. Biol. Int., 34, 273–279. [PubMed] [Google Scholar]
- Andrews G.K. (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol., 59, 95–104. [DOI] [PubMed] [Google Scholar]
- Andrews G.K. (2001) Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals, 14, 223–237. [DOI] [PubMed] [Google Scholar]
- Andrews G.K., Lee,D.K., Ravindra,R., Lichtlen,P., Sirito,M., Sawadogo,M. and Schaffner,W. (2001) The transcription factors MTF-1 and USF1 cooperate to regulate mouse metallothionein-1 expression in response to the essential metal zinc in visceral endoderm cells during early development. EMBO J., 20, 1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada N., Kawamoto,N. and Sezaki,H. (1999) Deleterious effect of null phenoloxidase mutation on the survival rate in Drosophila melanogaster. Dev. Comp. Immunol., 23, 535–543. [DOI] [PubMed] [Google Scholar]
- Auf der Maur A., Belser,T., Elgar,G., Georgiev,O. and Schaffner,W. (1999). Characterization of the transcription factor MTF-1 from the Japanese pufferfish (Fugu rubripes) reveals evolutionary conservation of heavy metal stress response. Biol. Chem., 380, 175–185. [DOI] [PubMed] [Google Scholar]
- Begel O., Boulay,J., Albert,B., Dufour,E. and Sainsard-Chanet,A. (1999) Mitochondrial group II introns, cytochrome c oxidase and senescence in Podospora anserina. Mol. Cell. Biol., 19, 4093–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghouts C., Werner,A., Elthon,T. and Osiewacz,H.D. (2001) Copper-modulated gene expression and senescence in the filamentous fungus Podospora anserina. Mol. Cell. Biol., 21, 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghouts C., Scheckhuber,C., Stephan,O. and Osiewacz,H.D. (2002) Copper homeostasis and aging in the fungal model system Podospora anserina: differential expression of PaCtr3 encoding a copper transporter. Int. J. Biochem. Cell Biol., 34, 1355. [DOI] [PubMed] [Google Scholar]
- Borrelly G.P., Harrison,M.D., Robinson,A.K., Cox,S.G., Robinson,N.J. and Whitehall,S.K. (2002) Surplus zinc is handled by Zym1 metallothionein and Zhf endoplasmic reticulum transporter in Schizosaccharomyces pombe. J. Biol. Chem., 277, 30394–30400. [DOI] [PubMed] [Google Scholar]
- Brewer G.J., Dick,R.D., Yuzbasiyan-Gurkin,V., Tankanow,R., Young, A.B. and Kluin,K.J. (1991) Initial therapy of patients with Wilson’s disease with tetrathiomolybdate. Arch. Neurol., 48, 42–47. [DOI] [PubMed] [Google Scholar]
- Brugnera E., Georgiev,O., Radtke,F., Heuchel,R., Baker,E., Sutherland,G.R. and Schaffner,W. (1994) Cloning, chromosomal mapping and characterization of the human metal-regulatory transcription factor MTF-1. Nucleic Acids Res., 22, 3167–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr H.S., George,G.N., Winge,D.R. (2002) Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I)-binding protein. J. Biol. Chem., 277, 31237–31242. [DOI] [PubMed] [Google Scholar]
- Chen X.H., Agarwal,A. and Giedroc,D.P. (1998) Structural and functional heterogeneity among the zinc fingers of human MRE-binding transcription factor-1. Biochemistry, 37, 11152–11161. [DOI] [PubMed] [Google Scholar]
- Chen X.H., Chu,M.H. and Giedroc,D.P. (1999) MRE-binding transcription factor-1: weak zinc-binding finger domains 5 and 6 modulate the structure, affinity and specificity of the metal-response element complex. Biochemistry, 38, 12915–12925. [DOI] [PubMed] [Google Scholar]
- Dalton T., Fu,K., Palmiter,R.D. and Andrews,G.K. (1996) Transgenic mice that overexpress metallothionein-I resist dietary zinc deficiency. J. Nutr., 126, 825–833. [DOI] [PubMed] [Google Scholar]
- Dalton T.P., Solis,W.A., Nebert,D.W. and Carvan,M.J. (2000) Characterization of the MTF-1 transcription factor from zebrafish and trout cells. Comp. Biochem. Physiol., B, 126, 325–335. [DOI] [PubMed] [Google Scholar]
- Dufour E., Boulay,J., Rincheval,V. and Sainsard-Chanet,A. (2000) A causal link between respiration and senescence in Podospora anserina. Proc. Natl Acad. Sci. USA, 97, 4138–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D.J. (1998) The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu. Rev. Nutr., 18, 441–469. [DOI] [PubMed] [Google Scholar]
- Engels W.R., Johnsonschlitz,D.M., Eggleston,W.B. and Sved,J. (1990) High-frequency P element loss in Drosophila is homolog dependent. Cell, 62, 515–525. [DOI] [PubMed] [Google Scholar]
- Georgatsou E. and Alexandraki,D. (1999) Regulated expression of the Saccharomyces cerevisiae Fre1p/Fre2p Fe/Cu reductase related genes. Yeast, 15, 573–584. [DOI] [PubMed] [Google Scholar]
- Giedroc D.P., Chen,X. and Apuy,J.L. (2001) Metal response element (MRE)-binding transcription factor-1 (MTF-1): structure, function and regulation. Antioxidants Redox Signal., 3, 577–596. [DOI] [PubMed] [Google Scholar]
- Gloor G.B., Nassif,N.A., Johnsonschlitz,D.M., Preston,C.R. and Engels,W.R. (1991) Targeted gene replacement in Drosophila via P-element-induced gap repair. Science, 253, 1110–1117. [DOI] [PubMed] [Google Scholar]
- Günes C., Heuchel,R., Georgiev,O., Muller,K.H., Lichtlen,P., Bluthmann,H., Marino,S., Aguzzi,A. and Schaffner,W. (1998) Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J., 17, 2846–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann M., Bruggmann,R., Xue,L., Georgiev,O., Schaffner,W., Rungger,D., Spaniol,P. and Gerster,T. (1998) Homologous recombination and DNA-end joining reactions in zygotes and early embryos of zebrafish (Danio rerio) and Drosophila melanogaster. Biol. Chem., 379, 673–681. [DOI] [PubMed] [Google Scholar]
- Harrison M.D., Jones,C.E. and Dameron,C.T. (1999) Copper chaperones: function, structure and copper-binding properties. J. Biol. Inorg. Chem., 4, 145–153. [DOI] [PubMed] [Google Scholar]
- Harrison M.D., Jones,C.E., Solioz,M. and Dameron,C.T. (2000) Intracellular copper routing: the role of copper chaperones. Trends Biochem. Sci., 25, 29–32. [DOI] [PubMed] [Google Scholar]
- Jeannin Y., Secheresse,F., Bernes,S. and Robert,F. (1992) Molecular architecture of copper(I) thiometallate complexes—example of a cubane with an extra face, (Npr4)3 Ms4cu4cl5 (M = Mo, W). Inorg. Chim. Acta, 200, 493–505. [Google Scholar]
- Kägi J.H.R. (1991) Overview of metallothionein. Methods Enzymol., 205, 613–626. [DOI] [PubMed] [Google Scholar]
- Labbe S., Zhu,Z.W. and Thiele,D.J. (1997) Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J. Biol. Chem., 272, 15951–15958. [DOI] [PubMed] [Google Scholar]
- Lastowskiperry D., Otto,E. and Maroni,G. (1985) Nucleotide sequence and expression of a Drosophila metallothionein. J. Biol. Chem., 260, 1527–1530. [PubMed] [Google Scholar]
- Lichtlen P. and Schaffner,W. (2001) Putting its fingers on stressful situations: the heavy metal-regulatory transcription factor MTF-1. BioEssays, 23, 1010–1017. [DOI] [PubMed] [Google Scholar]
- Lichtlen P., Wang,Y., Belser,T., Georgiev,O., Certa,U., Sack,R. and Schaffner,W. (2001) Target gene search for the metal-responsive transcription factor MTF-1. Nucleic Acids Res., 29, 1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.J., Kaeberlein,M., Andalis,A.A., Sturtz,L.A., Defossez,P.A., Culotta,V.C., Fink,G.R. and Guarente,L. (2002) Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature, 418, 344–348. [DOI] [PubMed] [Google Scholar]
- Masoro E.J. (2000) Caloric restriction and aging: an update. Exp. Gerontol., 35, 299–305. [DOI] [PubMed] [Google Scholar]
- Migliaccio E., Giorgio,M., Mele,S., Pelicci,G., Reboidl,P., Pandolfi,P.P., Lanfrancone,L. and Pelicci,P.G. (1999) The p66(shc) adaptor protein controls oxidative stress response and life span in mammals. Nature, 402, 309–313. [DOI] [PubMed] [Google Scholar]
- Mokdad R., Debec,A. and Wegnez,M. (1987) Metallothionein genes in Drosophila melanogaster constitute a dual system. Proc. Natl Acad. Sci. USA, 84, 2658–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B.J. et al. (1999) Activation of metallothionein gene expression by hypoxia involves metal response elements and metal transcription factor-1. Cancer Res., 59, 1315–1322. [PubMed] [Google Scholar]
- Nelson N. (1999) Metal ion transporters and homeostasis. EMBO J., 18, 4361–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiewacz H.D. and Borghouts,C. (2000) Cellular copper homeostasis, mitochondrial DNA instabilities and lifespan control in the filamentous fungus Podospora anserina. Exp. Gerontol., 35, 677–686. [DOI] [PubMed] [Google Scholar]
- Palmiter R.D. (1998) The elusive function of metallothioneins. Proc. Natl Acad. Sci. USA, 95, 8428–8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R.D. and Findley,S.D. (1995) Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J., 14, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petris M.J., Mercer,J.F.B., Culvenor,J.G., Lockhart,P., Gleeson,P.A. and Camakaris,J. (1996) Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J., 15, 6084–6095. [PMC free article] [PubMed] [Google Scholar]
- Puig S. and Thiele,D.J. (2002) Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol., 6, 171–180. [DOI] [PubMed] [Google Scholar]
- Quaife C.J., Findley,S.D., Erickson,J.C., Froelick,G.J., Kelly,E.J., Zambrowicz,B.P. and Palmiter,R.D. (1994) Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry, 33, 7250–7259. [DOI] [PubMed] [Google Scholar]
- Radtke F., Heuchel,R., Georgiev,O., Hergersberg,M., Gariglio,M., Dembic,Z. and Schaffner,W. (1993) Cloned transcription factor MTF-1 activates the mouse metallothionein-I promoter. EMBO J., 12, 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y.K.S. et al. (2002) Targeted mutagenesis by homologous recombination in D.melanogaster. Genes Dev., 16, 1568–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y.S. and Golic,K.G. (2000) Gene targeting by homologous recombination in Drosophila. Science, 288, 2013–2018. [DOI] [PubMed] [Google Scholar]
- Rong Y.S. and Golic,K.G. (2001) A targeted gene knockout in Drosophila. Genetics, 157, 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G.S., Ingram,D.K. and Lane,M.A. (1995) Slowing aging by caloric restriction. Nat. Med., 1, 414–415. [DOI] [PubMed] [Google Scholar]
- Schaefer M., Hopkins,R.G., Failla,M.L. and Gitlin,J.D. (1999) Hepatocyte-specific localization and copper-dependent trafficking of the Wilson’s disease protein in the liver. Am. J. Physiol., 276, G639–G646. [DOI] [PubMed] [Google Scholar]
- Seum C., Pauli,D., Delattre,M., Jaquet,Y., Spierer,A. and Spierer,P. (2002) Isolation of Su(var)3–7 mutations by homologous recombination in Drosophila melanogaster. Genetics, 161, 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins C.O. (2000) Metallothionein in human disease. Cell. Mol. Biol., 46, 465–488. [PubMed] [Google Scholar]
- Sohal R.S. and Weindruch,R. (1996) Oxidative stress, caloric restriction and aging. Science, 273, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G.W., Searle,P.F. and Palmiter,R.D. (1985) Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. Nature, 317, 828–831. [DOI] [PubMed] [Google Scholar]
- te Riele H., Maandag,E.R. and Berns,A. (1992) Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc. Natl Acad. Sci. USA, 89, 5128–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls M., Bofill,R., Romero-Isart,N., Gonzalez-Duarte,R., Abian,J., Carrascal,M., Gonzalez-Duarte,P., Capdevila,M. and Atrian,S. (2000) Drosophila MTN: a metazoan copper-thionein related to fungal forms. FEBS Lett., 467, 189–194. [DOI] [PubMed] [Google Scholar]
- Weaver R.F. and Weissmann,C. (1979) Mapping of RNA by a modification of the Berk–Sharp procedure—5′ termini of 15-S β-globin messenger-RNA precursor and mature 10-S β-globin messenger-RNA have identical map coordinates. Nucleic Acids Res., 7, 1175–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A.K., Stallings,R., Hildebrand,C.E., Chiu,R., Karin,M. and Richards,R.I. (1990) Human metallothionein genes—structure of the functional locus at 16q13. Genomics, 8, 513–518. [DOI] [PubMed] [Google Scholar]
- Westin G. and Schaffner,W. (1988) A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J., 7, 3763–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Egli,D., Georgiev,O. and Schaffner,W. (2001) The Drosophila homolog of mammalian zinc finger factor MTF-1 activates transcription in response to heavy metals. Mol. Cell. Biol., 21, 4505–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G.D., Randerath,E. and Randerath,K. (2001) Effects of dietary transition metals on oxidative DNA lesions in neonatal rats. Mutat. Res., 479, 71–79. [DOI] [PubMed] [Google Scholar]