Abstract
Heat shock proteins (Hsps) can be found in two forms, intracellular and extracellular. The intracellular Hsps are induced as a result of stress and have been found to be cytoprotective in many instances due to their chaperone functions in protein folding and in protein degradation. The origin and role of extracellular Hsps is less clear. Although they were suspected originally to be released from damaged cells (necrosis), their presence in most normal individuals rather suggests that they have regulatory functions in circulation. As immunodominant molecules, Hsps can stimulate the immune system, leading to the production of autoantibodies recognizing epitopes shared by microbial and human Hsps. Thus, extracellular Hsps can influence the inflammatory response as evidenced by the production of inflammatory cytokines. Antibodies to Hsps have been found under normal conditions but seem to be increased in certain stresses and diseases. Such antibodies could regulate the inflammatory response positively or negatively. Here, we review the literature on the findings of antibodies to Hsps in situations of environmental or occupational stress and in a number of diseases and discuss their possible significance for the diagnosis, prognosis, or pathogenesis of these diseases.
INTRODUCTION
Heat shock proteins (Hsps) are highly conserved proteins found in prokaryotes and eukaryotes (Lindquist and Craig 1988; Morimoto et al 1994). The heat shock response was initially described over 40 years ago as the appearance of puffs in fly chromosomes induced by heat or treatment with respiration uncouplers (Ritossa 1962, 1964) and characterized by the rapid induction of a limited subset of proteins (Tissières et al 1974). This response and Hsps produced during this response continue to fascinate many scientists from both basic and applied fields because Hsps are important in the buildup of tolerance and cytoprotection against many stresses such as ischemia, hypoxia, and exposure to numerous xenobiotics. It has also been suggested that Hsps play a role in the pathogenesis, prognosis, and treatment of many diseases, although the exact mechanisms of how Hsps operate in these processes remain elusive in most cases (Welch 1992; Kauffmann and Schooel 1994; Minowada and Welch 1995; Favatier et al 1997; Frostegard et al 1997; Xu 2002; Todryk et al 2003; Xiao et al 2003; Jin et al 2004a; Mandal et al 2004; Ciocca and Calderwood 2005). In the mid-1990s, the presence of autoantibodies against Hsps was observed in humans and in animal models of various diseases. In many cases, these antibodies were associated with the pathogenesis, prognosis, and/or severity of disease especially in the heart and brain fields. Here, we review the present data on the presence of antibodies against Hsps in environmental stress-associated diseases and discuss their possible roles.
INDUCTION OF Hsps AND THEIR POSSIBLE ROLES
Most Hsps are expressed at a basal level under normal physiological conditions. However, their amount rapidly goes up when cells are submitted to a wide variety of stresses such as exposure to heat, xenobiotics, or drugs; to pathological stimuli such as viral, bacterial, or parasitic infections, fever, inflammation, malignancy, or autoimmunity; and to physiological stimuli such as growth factors, cell differentiation, or hormonal stimulation. Although many environmental xenobiotics induce the synthesis of Hsps, some like benzo(a)pyrene, a ubiquitous environmental pollutant and a potent procarcinogen and mutagen that can elicit tumors, inhibits their synthesis. This points to the special toxicity of these xenobiotics and at potential mechanisms of diseases caused by this chemical (Bartosiewicz et al 2001; Gao et al 2004).
Many Hsps act as molecular chaperones both in vitro and in vivo. This is important as they provide cells with a mechanism to prevent damage caused by misfolded, damaged, aggregated, or insoluble proteins resulting in the formation of toxic inclusion bodies and aggresomes (Hightower 1991). These structures have been associated with many neurodegenerative diseases and are thought to be cytotoxic (Muchovski et al 2000; Barral et al 2004; Muchovski and Walker 2005). Thus, misfolded or damaged proteins must either be properly refolded by chaperones or degraded through proteolytic pathways like the proteasome. This chaperone function of Hsps is also thought to be at the basis of cell protection at the organismic level; thus overexpression of Hsps has been linked to protection against ischemia-induced damages in brain, heart, and kidneys in mammals including humans (Currie et al 1993; Marber et al 1995; Plumier et al 1997) (reviewed in Benjamin and McMillan 1998; Beck et al 2000; Delogu et al 2001; Lachman 2001; Christians et al 2002; Mehta et al 2005). The cytoprotective property of Hsps, although beneficial in some cases, may be detrimental in others as in tumor progression or in conferring resistance to chemotherapy and apoptotic removal of tumor cells (reviewed in Ciocca and Calderwood 2005).
Hsps have also been shown to modulate immunological processes by acting at the level of antigen presentation and in transport of peptides to the major histocompatibility complexes (MHC) (Basu and Srivastava 2000; Wells and Malkovsky 2000).
ASSOCIATION OF ANTI-Hsps WITH ENVIRONMENTAL STRESSES
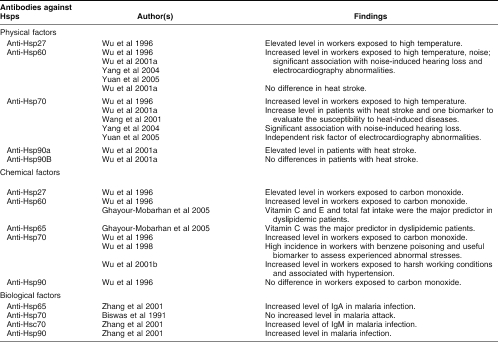
There are 3 main types of environmental factors: (1) physical factors such as exposure to high temperature, noise, ultraviolet light, radiation; (2) chemical factors with thousands of industrial xenobiotics including carbon monoxide, heavy metal, and dust; and (3) biological factors such as infection by viruses, bacteria, parasites, and fungi. Exposure to these stresses not only contributes to the induction of Hsps but may also result in production of autoantibodies against Hsps. Many factors can contribute to the production of such autoantibodies: genetic factors, infection, the denaturation and release of Hsps as a result of cell damage (necrosis), and the presence of antigen-specific lymphocytes related to environmental stress exposure. There are many observations suggesting that long-term exposure to environmental stresses may result in the production of antibodies against Hsps and such antibodies can be associated with abnormal changes of the body and with some diseases (Table 1). We first reported the presence of antibodies to Hsp27, Hsp60, Hsp70, and Hsp90 in plasma of workers in the steel industry who had long-term exposure to high temperature, carbon monoxide, and other chemicals in coke ovens (Wu et al 1996). It was suggested that the presence of such antibodies might potentially constitute useful biomarkers to assess whether workers were experiencing abnormal stress within their working and/or living environments (Wu et al 1996). We subsequently observed that there was a significantly increased frequency of antibodies against Hsp70 (anti-Hsp70) in workers exposed to benzene, dust, heat, and noise, and that these stresses seemed to contribute to the production of an antibody against Hsp70. Interestingly, the presence of such antibodies was associated with benzene-poisoning, hypertension, noise-induced hearing loss, and abnormal changes of electrocardiography (Wu et al 1998, 2001b; Yang et al 2004b; Yuan et al 2005). Equally interesting was the observation that antibodies against Hsp70 occurred in higher titers in individuals who were more susceptible to heat-induced illness (Wang et al 2001; Wu et al 2001a). Jin et al (2003) also reported that elevated levels of antibodies against metallothionein and Hsp70 were associated with metal allergy in atopic dermatitis patients. However, how environmental stresses cause the production of antibodies against Hsps and how such anti-Hsp antibodies are involved in the development of many environmentally related diseases remains unknown.
Table 1.
Antibodies against Hsps in environmental stresses and related diseases
ASSOCIATION OF ANTI-Hsps WITH DISEASES
The involvement of Hsps and their antibodies and their association with some diseases does not come as a surprise because 3 genes encoding for members of the Hsp70 family are located within the MHC in humans and genes coding for human leukocyte antigens (HLA) are associated with most diseases (Gunther 1991). Second, Hsps have been suggested to play a central role in the response to environmental stimuli, and possibly in autoimmune responses (Cohen and Young 1991). Third, many Hsps function as molecular chaperones and are particularly important in conformation diseases including most neurodegenerative diseases (Barral et al 2004). Here, we focus on data reporting the presence and the possible roles of antibodies against Hsps in different types of diseases.
IMMUNE AND AUTOIMMUNE DISEASES
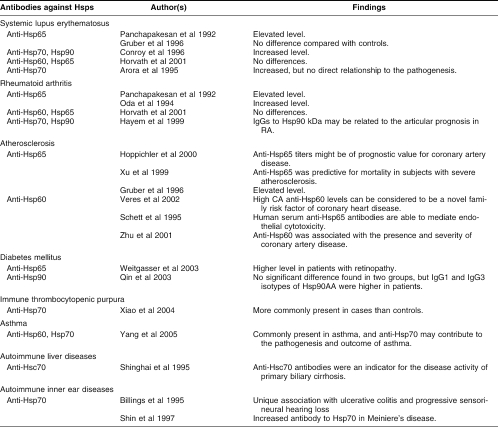
A role of Hsps in autoimmunity has been implicated from many studies and a role for Hsps as facilitators of immune response to proteins and peptides has been widely documented both in vivo and in vitro (Yang and Feige 1992; Kauffmann and Schooel 1994; Basu and Srivastava 2000; Srivastava 2000; Prohaszka et al 2002). Recent evidence from studies in animal models and in patients with autoimmune diseases has clearly suggested the involvement of Hsps in both the pathogenesis and the immunoregulation of these diseases; this may have important therapeutic implications for the treatment of human autoimmune diseases (Yang and Feige 1992). A list of autoimmune diseases presenting antibodies against Hsps is presented in Table 2.
Table 2.
Antibodies against Hsps and autoimmune diseases
The first report of antibodies against Hsps in a human disease is that of Jarjour et al (1991), who suggested that the difference in the levels of anti-Hsps antibodies seen in sera of patients with various rheumatoid and other inflammatory diseases compared to normal controls, could merely reflect disease-associated polyclonal B cell activation. Subsequent investigations have suggested that such antibodies or specific T cells against Hsps were associated with immune and autoimmune diseases. For example, Panchapakesan et al (1992) reported on the elevated levels of antimycobacterial Hsp65 in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Gruber et al (1996) did not observe any significant difference in the concentration of anti-Hsp65 between patients with SLE and controls, but antibodies were higher in patients with atherosclerosis and vasculitis. Conroy et al (1996) found that there was a similar increase of anti-Hsp70 and anti-Hsp90 antibodies in children with SLE as in adult patients and suggested that SLE in children and adults might share a similar pathological mechanism of disease. Horvath et al (2001) investigated the presence of antibodies against both human Hsp60 and mycobacterial Hsp65 in patients with systemic lupus erythematosus, systemic sclerosis, undifferentiated connective tissue diseases (UCTD), primary Raynaud syndrome, rheumatoid arthritis, polymyositis; they found that the levels of anti-Hsp60 and anti-Hsp65 antibodies were significantly higher only in the UCTD patients. Shinghai et al (1995) reported the presence of antibodies to Hsps in patients with autoimmune liver diseases and suggested that the presence of anti-Hsc70 antibodies was an indicator for the disease activity of primary biliary cirrhosis. Xiao et al (2004) found that antibodies against Hsp70 are more common in immune thrombocytopenic purpura (ITP) patients (15 of 29) than in control children (5 of 30). The prevalence of antibodies against Hsp70 (51.7%) in ITP-affected children is as high as antibodies against platelet membrane glycoproteins (58.3%). Recently, Yang et al (2005) reported that antibodies to Hsp60 and Hsp70 are commonly present in asthma patients and more so in subjects with the most severe symptoms, suggesting that such antibodies and especially anti-Hsp70 may play a role in the pathogenesis and outcome of asthma.
The presence of anti-Hsps in patients with multiple sclerosis, Grave's diseases, sarcoidosis, or insulin-dependent diabetes mellitus (IDDM) was also observed (Table 2). Antibodies to Hsp65 were suspected to be involved in the pathophysiological mechanisms of β-cell destruction in diabetes (Jones et al 1990). Tun et al (1994) found that serological reaction to mycobacterial Hsp65 occurred in type 1 diabetes, but was neither a characteristic nor a specific feature of the disease. Child et al (1995) suggested that the low levels of anti-Hsp65 found in patients with established type 1 and type 2 diabetes were probably a manifestation of impaired immunity induced by the diabetic disease. Mackay et al (1996) found that there was lack of autoimmune serological reactions in rodent models of IDDM. Horvath et al (2002) found that antibody levels to a specific peptide found in Mycobacteria bovis Hsp65 and human Hsp60 were significantly higher in a group of 83 children with type 1 diabetes than in healthy children. Antibodies to two epitope regions of Hsp60 were also detected in the diabetic children, and it was suggested that the presence of antibodies to these specific peptides might play a possible role in the autoimmune diabetogenic process of the early diabetes. In a study on 138 patients with type 1 diabetes, Weitgasser et al (2003) found that the Hsp65 antibody titer was positively related to the patient age but not to glycemic control and that patients with retinopathy had higher antibody titers than those without retinopathy. Sims et al (2002) found that median titers to Hsp70 were significantly higher in patients with type 1 diabetes/periodontitis than in nondiabetic controls and suggested that pretreatment profiles of serum antibody titers to Hsp65, Hsp70, and Hsp90 might be useful to predicting which patients with diabetes/periodontitis would have a poor response to nonsurgical periodontal therapy. Recent results from Qin et al (2003) suggested that there was a higher level of IgG1 and IgG3 isotypes of Hsp90 in patients with type 1 diabetes and first-degree relatives of these patients than in controls, and that autoimmunity leading to type 1 diabetes significantly altered anti-Hsp90 autoantibody isotype to autoantigen.
In summary, antibodies against Hsps have been detected in various autoimmune diseases, but they were neither a characteristic nor a specific feature of these immune diseases. Whether antibodies against Hsps are directly involved in the pathogenesis or can regulate the course of these autoimmune diseases remains to be clarified.
ANTI-Hsps AND INFECTIOUS DISEASES
Another type of environmental aggression is exposure to biological factors such as viruses, bacteria, parasites, and fungi. The immune system has a bias toward recognition of microbial antigens for protecting the host from infection at birth. Much data suggest that an important initial line of defense in this regard involves autologous heat shock proteins, especially highly conserved Hsp60s (Zugel and Kauffmann 1999). Given the high degree of amino acid sequence homology between Hsps of different species, the presence of antibodies against Hsps may, on the one hand, be helpful to protect the host from recurring infection; on the other hand, presence of such antibodies may contribute to autoimmunity through cross-reactivity between Hsps and tissue-specific proteins containing similar epitope motives. Therefore, it is no surprise that Hsps and/or their antibodies can be involved in the development of many autoimmune and/or inflammatory diseases such as SLE, IDDM, rheumatoid arthritis, multiple sclerosis, Hashimoto's thyroiditis, glomerulonephritis, scleroderma, pemphigoid, Addison's disease, chronic active hepatitis, primary biliary cirrhosis, and atherosclerosis (see Table 2). For example, Colebrook and Lightowlers (1997) reported that antibodies to Echinococcus granulosus Hsp70, but not to human Hsp70, were more frequent in sera of patients with hydatid diseases than in matched controls. Al-Shamma et al (1997) showed that there were higher mean titers of serum anti-human Hsp27 and Hsp90 IgG in patients with cystic fibrosis than in controls and higher anti-Hsp27 antibodies in cystic fibrosis patients with arthritis than patients without arthritis. They suggested that arthritis-associated cystic fibrosis was associated with more severe lung disease and with a greater inflammatory response to Hsps. In contrast, Lopatin et al (1999) reported that periodontal patients with higher anti-Hsps (Hsp90, Dnak, and GroEL) antibody levels tended to have significantly healthier periodontal tissues, reflecting the protective effects of anti-Hsp antibodies. Prohaszka et al (1999) reported that there was an increase of anti-hHsp60 and mycobacterial Hsp65 and a strong positive correlation between the levels of autoantibodies against C1q and antibodies to these Hsps in the sera of HIV-infected patients. There have been reports of an increase in serum Hsp70 and antibodies to Hsp70 in HIV-infected patients (Kocsis et al 2003). Antibodies to the Hsp70 cochaperone HspB1 have also been found in HIV-infected subjects but also in uninfected controls consistent with the absence of any relationship between the autoantibodies and clinical parameters (Papp et al 2005). Yunoki et al (2000) suggested that measurement of antibodies to Helicobacter pylori Hsp60 in serum of patients with H pylori–infected peptic ulcers was useful for early monitoring of effectiveness of eradication therapy. Kalabay et al (2002) reported that infection with the Helicobacter pylori bacterium in connective tissue disorders was associated with increased concentrations of antimycobacterial Hsp65. Zhang et al (2001) found that the antibodies IgG, IgM, and IgA to Hsp90, IgM to Hsp70, IgA to Hsp65 were significantly increased in patients with malaria, suggesting that the antigenic potential of Hsp90 was higher than those of Hsp70 and Hsp65 in malaria. Although many studies show that Hsp70 purified from virally infected cells can transfer and deliver antigenic peptides to antigen-presenting cells to elicit peptide-specific immunity and the administration of recombinant Hsp70 can attenuate experimental autoimmune disease, the exact role(s) and mechanisms of action of anti-Hsps antibodies in autoimmune and infectious diseases remain to be examined in more detail.
CARDIOVASCULAR DISEASES AND CEREBRAL INJURY
Cardiovascular and cerebral diseases are a leading cause of morbidity and death. Several studies in animal models have documented the cytoprotective activity of Hsps against ischemia and reperfusion-associated damage in the heart, and the brain. Excellent reviews have been written on the roles for Hsps and anti-Hsp60/Hsp65 antibodies in cardiovascular diseases (Frostegard et al 1997; Wick and Xu 1999; Latchman 2001; Pockley 2002; Xu 2002; Mandal et al 2004; Mehta et al 2005). Interestingly, data from many laboratories have demonstrated that serum antibodies against human Hsp60 or mycobacterial Hsp65 are associated with the pathogenesis, diagnosis, prognosis, and even possible treatment of cardiovascular diseases such as coronary heart diseases, hypertension, carotid atherosclerosis, and vascular diseases. However, no association between antibodies to Hsp70 and cardiovascular diseases was observed in investigations from distinct laboratories including our own (Portig et al 1997; Yang et al 2004a), although Hsp70 is known to play a key role in cytoprotection of heart from ischemia and other harmful stimuli. Recently, antibodies to Hsp70 were found in patients with severe angina, but not chronic angina, and interestingly, the former patients had an improved outcome following coronary artery bypass grafting (Vogt et al 2004).
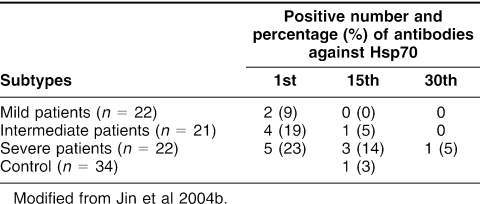
Rea et al (2001) observed that Hsp60, Hsp70, anti-Hsp60, anti-Hsp65, and anti-Hsp70 were detectable in 60 healthy individuals aged from 20 to 96 years. There was a progressive decline in Hsp60 and Hsp70 levels and an increased trend in Hsp70 antibody levels with age. Chan et al (1999) showed that there was a significant correlation between anti-Hsp70 antibody and different vascular diseases such as lower limb claudication and critical ischemia, abdominal aortic aneurysms, which suggested that Hsp70 and anti-Hsp70 might be involved in the pathogenesis and propagation of atherosclerosis. Gromadzka et al (2001) noted that humoral immunity to Hsp70 was common in patients (in the first 48 hours after stroke onset) with ischemic stroke mainly caused by the development of vascular lesions and that elevated levels of anti-Hsp70 antibody could be triggering factors for stroke. Recently, Jin et al (2004b) found that anti-Hsp70 antibodies in patients with cerebral infarction were significantly higher in the first 24 hours after stroke onset and then decreased and even disappeared after a recovery for 30 days (Table 3). Yuan et al (2005) found that there was a significant dose-response increase of plasma anti-Hsp70 in workers exposed to noise (without any treatment) and that the increase of anti-Hsp70 was associated with the independent risk of increased electrocardiography abnormality types, including sinus arrhythmia, chronic myocardial ischemia, or ectopic rhythm (odds ratio, 2.67; 95%confidence interval, 1.54–4.62; P = 0.000); this suggested that enhanced anti-Hsp70 might be involved in progression of abnormal electrocardiography changes and possibly cardiovascular diseases.
Table 3.
Dynamic changes of antibodies against Hsp70 in patients with mild, intermediate, and severe cerebral infarction on different days after infarction and in controls
In summary, the results of anti-Hsp70 antibody measurements in cardiovascular and cerebral diseases are still controversial. The frequency of this antibody varies in different studies and this may be dependent on different methods of detection and classification of disease states including stages, duration, and treatment of these diseases. So far, there have been no attempt to standardize these methods, and therefore the significance of these data should be cautiously interpreted for the time being.
ORGAN TRANSPLANTATION
During transplantation, the allograft undergoes a stress response, which results in an increased expression of Hsps and the recruitment and activation of Hsp-reactive lymphocytes. In early studies on animal models of cardiac allografts, a higher activation and expression of Hsps was accompanied by a higher rate of rejection of allografts (Moliterno et al 1995; Qian et al 1995). Lee et al (1995) reported the presence of antibodies to both inducible Hsp72 and constitutive Hsp73 in 3 of 14 allogeneic bone marrow transplant recipients, but not in autologous peripheral blood stem cell transplant recipients. Goral et al (2002) reported a significant increase of IgM anti-Hsp70 and/or anti-Hsp90, but not anti-Hsp60 in patients with graft-vs-host disease (GVHD) early (30–90 days) after transplantation, and this increase preceded or accompanied chronic GVHD and returned to normal with the next 400 days in the majority of these patients; this suggested that monitoring levels of anti-Hsp70 and anti-Hsp90 antibodies in stem cell transplant recipients might serve as a diagnostic tool and help to predict the onset of GVHD. Recently, Morgun et al (2004) found that there was significantly higher anti-myosin and anti-Hsps IgG antibodies in adult pretransplant cardiac allograft recipients than in controls. However, there was no significant difference of anti-Hsps during acute rejection than during the rejection-free period, suggesting a more pathogenic role for anti-myosin antibodies in cardiac transplant rejection than for anti-Hsps antibodies. In a mouse knockout model of Hsp70.1, Oh et al (2004) noted that the survival of skin grafts was longer when the donor had a disrupted Hsp70.1 gene suggesting that this gene product up-regulated graft rejection. Antibodies to Hsp70 were not measured in this study.
Although a number of investigations suggest that there is an association of Hsps and anti-Hsp reactivity with allograft rejection, the balance between protective and damaging effects and the precise influence of the heat shock response and the generation of autoantibodies to Hsps on graft outcome remains unclear (for a recent review, see Pockley and Muthana 2005).
CANCERS
Cancer prediction and outcome is an important facet of environmental and occupational stresses. Hsps are involved in the acquisition of tolerance to heat, chemotherapeutic drugs, oxidative stress, toxins, and radiation, and protect cells against injury including DNA damage and apoptosis caused by these stimuli (Buzzard 1998; Jäättelä et al 1998; Xiao et al 2002). Therefore, investigating the roles of Hsps in development, prognosis, and treatment of cancers is an important issue (Cornford et al 2000; Todryk et al 2003). Ciocca and Calderwood (2005) recently surveyed the literature for the implications of various Hsps in cancer: in general Hsp levels are not informative at the diagnostic level but can be useful markers of properties like aggressiveness and response to chemotherapy in some tissues. There are a few investigations on the presence of anti-Hsps antibodies in the tumor process. Conroy et al (1998a, 1998b) showed that antibodies to Hsp27, Hsp70, and Hsp90 were detectable in patients with breast cancer and that anti-Hsp27 and anti-Hsp90 antibodies were detectable only in patients, not in controls; they suggested that the increased levels of antibodies to Hsp27 were correlated with an improved survival, whereas high levels of antibodies to Hsp90 were correlated with the reduced survival of these patients. Korneeva et al (2000) also reported that there was a common prevalence of anti-Hsp27 IgA (but not IgG) in the genital tract of women with endometrial and with ovarian cancer before and after treatment, but not in 25 women with benign diagnoses nor in 46 healthy women; moreover, in the same study, IgA antibodies to Hsp70 were not cancer-specific, but IgA to Hsp90 were present in one-third of patients with ovarian cancer. Thus, cervical IgA antibodies to Hsp27 may be indicators of gynecologic malignancy. These data seem to be related with properties of distribution and possible functions of Hsp27 and Hsp90 in hormonal response.
ANTI-Hsps IN OTHER DISEASES
Hsps are present in almost all types of cells and tissues, and thus anti-Hsp antibodies may be associated with many types of diseases. Qureishi et al (1995) reported a significant increase of antibodies to Hsp70 in patients with thermal burns, implicating the presence of this antibody and its possible influence on the immune system. Freidank et al (1995) showed by immunoblot that there was significantly higher anti-Chlamydia trachomatis Hsp antibody rate in infertile female patients with complete tubal occlusion than in such patients with normal fallopian tubes. An increased presence of anti-Hsp70 and anti-Hsp90 was observed in patients with schizophrenia by Schwarz et al (1999) and Kim et al (2001), suggesting that these two antibodies, especially anti-Hsp70 antibody, might be involved in the pathogenesis of schizophrenia.
Antibodies to Hsp70 were also noted in sera of patients with idiopathic, progressive, bilateral sensorineural hearing loss (Bloch et al 1995). The presence of anti-Hsp70 antibody is significantly higher in patients with Meniere's diseases than in controls, but the association of this antibody with clinical features or course of Meniere's diseases is limited because of the high prevalence of the antibody found in healthy controls of this study (Rauch et al 2000). Moreover, another study of 27 patients with sudden deafness showed that there was no significant increase of anti-Hsp70 antibody in patients compared with controls, suggesting there was no clinical utility for diagnostic screening in this disease (Samuelsson et al 2003). Recently, Yang et al (2004b) examined the prevalence of antibodies to Hsp70 and Hsp60 in workers with noise-induced hearing loss: antibodies to both proteins were more prevalent in such workers, but curiously anti-Hsp70 was associated with high-frequency hearing loss, whereas anti-Hsp60 was associated with moderate low-frequency loss.
Finally, De Smet and Ramadan (2001) reported that levels of antibodies to human inducible Hsp70 were significantly higher in Behcet's disease, sarcoidosis, and pars planitis than in controls, suggesting that the circulating levels of this antibody might reflect the extent of disease involvement with the eye.
CONCLUSION AND PERSPECTIVES
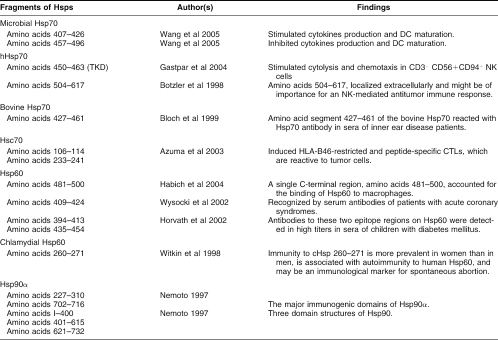
Antibodies against Hsps are found under normal physiological conditions, after exposure to environmental stresses, and in many diseases. The data accumulated so far suggest that the presence of such antibodies may be more foe than friend in humans. However, many of these data are still contradictory and the frequency or levels of anti-Hsps antibodies vary in different studies; one possible reason for this variance may be the use of different methods of detection. Most of the present data has been obtained using Western blot–based assays, a technique that lacks sensitivity and is rather imprecise for quantification. Because antibodies to Hsps are present in normal individuals, it will be important to establish antibody titers cutoff lines and variance between “normality” and disease states using more sensitive and quantifiable techniques. This would be facilitated by methods that can be automated to assess and titrate antibodies levels in numerous samples. Such methods should be accessible in terms of costs for routine work especially in developing countries. Another important aspect is the nature and specificity of the antigens recognized by these antibodies. Thus, many studies refer to Hsp70 without clearly specifying whether they are referring to the inducible member Hsp71 or to the constitutively expressed member, Hsc73. Because there are at least 8 members in the family of Hsp70 in humans, knowledge on the epitopes recognized by the anti-Hsp70 antibodies in different diseases is important for deciphering their functions. This is particularly important because the different members of the Hsp70 family seem to have distinct functions in processes such as cancer cell growth (Rohde et al 2005). Because Hsps are immunodominant molecules, identification of epitopes in some bacterial and human Hsps has begun and regions of Hsp70 involved in the modulation of cytokine production and in the natural killer (NK) immune response have been identified (Botzler et al 1998; Gastpar et al 2004; Wang et al 2005). Table 4 lists some of the epitopes identified in Hsp70, Hsp60, and Hsp90. For example, the C terminus of Hsp70 seems particularly important in the immune response, but it is also notable that very few Hsp70 epitopes have been defined in diseases with the exception of patients with inner ear and Menière's disease (Bloch et al 1999). This is slightly better defined in the case of Hsp60 where acute coronary syndrome patients and children with diabetes have been examined (Table 4). Thus, identifying the epitopes in specific diseases remains a priority, because it will aid in the understanding of immunological events leading to the production of such autoantibodies and the identification of their functions and fate in the disease process. Whether antibodies to Hsp70, for example, in different patients with different diseases recognize the same epitopes is presently unknown. Epitope mapping would also lead to substantial progress in the development of simple specific and sensitive assays. The peptide array field is rapidly expanding and could provide such precious information (Maercker 2005).
Table 4.
Epitopes of different Hsps and possible functions
Another reason for the variability of results from different laboratories is that diseases are very complex, especially for chronic and multifactorial diseases, suggesting that it is essential to consider disease states such as stage, duration, and extent of treatment, and to investigate more critically the risk factors related to the corresponding disease. From the present data, it is not always possible to assess these variables in different reports.
There is a reactivity or/and cross-reactivity among anti-Hsp antibodies, exogenous Hsps from infecting microorganism, and host endogenous Hsps including circulating Hsps in plasma, Hsps on the surface of cells, Hsps within cells, and denatured Hsps, suggesting that it will also be very important to detect different types and different characteristics of both Hsps and anti-Hsp antibodies in future research. Thus, it was recently reported that increased titers of antibodies against human Hsp60 indicated an adverse prognosis in patients with acute chest pain, whereas antibodies against mycobacterial Hsp65, a protein highly homologous to human Hsp60, were not predictive (Birnie et al 2005; and see editorial comment by Pockley and Frostegard 2005). Finally, do antibodies against Hsps neutralize circulating Hsps, activate complement, or target surface antigens on specific cells? Hsp70 in particular has been reported to be exposed at the surface of tumor cells where it can exert immunomodulatory functions (Multhoff et al 1995; Gastpar et al 2004). Specific members of the Hsp70 family may also present at the surface in different cells; thus hspA8 is specifically expressed at the surface of human embryonic stem cells and disappears during differentiation (Son et al 2005). Although a few investigations have preliminarily shown that antibodies against Hsps can down-modulate immune response via a self-Hsp-reactive, Th2-type mechanism, the unknown effects of this cross-reactivity on healthy individual and in patients with different diseases, and their precise mechanisms of action should continue to fascinate scientists from numerous disciplines.
Acknowledgments
This work was supported by research funds from the National Key Basic Research and Development Program (2002CB512905), the National Natural Science Foundation of China (NNSFC: 30200227 and 30430590) (to T.W.). T.W. and R.M.T. also acknowledge financial support from the NNSFC of China and the Canadian Institute of Health Research of Canada (CIHR) for a research exchange program and an operating CIHR grant (to R.M.T.). We thank Dr. Meian He for careful editing of the tables.
REFERENCES
- Al-Shamma MR, McSharry C, McLeod K, McCruden EA, Stack BH. Role of heat shock proteins in the pathogenesis of cystic fibrosis. Thorax. 1997;52:1056–1059. doi: 10.1136/thx.52.12.1056.0040-6376(1997)052[1056:ROHSPI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora SK, Singh G, Sehgal S. Comparative evaluation of anti-heat shock protein antibodies in SLE and healthy controls. Scand J Rheumatol. 1995;24:160–163. doi: 10.3109/03009749509099306.0300-9742(1995)024[0160:CEOASP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Azuma K, Shichijo S, Takedatsu H, Komatsu N, Sawamizu H, Itoh K. Heat shock cognate protein 70 encodes antigenic epitopes recognised by HLA-B4601-restricted cytotoxic T lymphocytes from cancer patients. Br J Cancer. 2003;89:1079–1085. doi: 10.1038/sj.bjc.6601203.0007-0920(2003)089[1079:HSCPEA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral JM, Broadley SA, Shaffar G, Hartl FU. Roles of molecular chaperones in protein misfolding diseases. Semin Cell Dev Biol. 2004;15:17–29. doi: 10.1016/j.semcdb.2003.12.010.1084-9521(2004)015[0017:ROMCIP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bartosiewicz M, Peen S, Buckpitt A. Application of gene arrays in environmental toxicology: fingerprints of gene regulation associated with cadmium chloride, benzo(a)pyrene, and trichloroethylene. Environ Health Perspect. 2001;109:71–74. doi: 10.1289/ehp.0110971.0091-6765(2001)109[0071:AOGAIE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Srivastava PK. Heat shock proteins: the fountain-head of innate and adaptive immune response. Cell Stress Chaperones. 2000;5:443–451. doi: 10.1379/1466-1268(2000)005<0443:hsptfo>2.0.co;2.1466-1268(2000)005[0443:HSPTFO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck FX, Neuhofer W, Müller E. Molecular chaperones in the kidney: Distribution, putative roles, and regulation. Am J Physiol Renal Physiol. 2000;279:F203–F215. doi: 10.1152/ajprenal.2000.279.2.F203.0363-6127(2000)279[F203:MCITKD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117.0009-7330(1998)083[0117:SHSPMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Billings PB, Keithley EM, Harris JP. Evidence linking the 68 kilodalton antigen identified in progressive sensorineural hearing loss patient sera with heat shock protein 70. Ann Otol Rhinol Laryngol. 1995;104:181–188. doi: 10.1177/000348949510400302.0003-4894(1995)104[0181:ELTKAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Birnie DH, Vickers LE, Hillis WS, Norrie J, Cobbe SM. Increased titres of anti-human heat shock protein 60 predict an adverse one year prognosis in patients with acute cardiac chest pain. Heart. 2005;91:1148–1153. doi: 10.1136/hrt.2004.040485.1355-6037(2005)091[1148:ITOAHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Sharma YD. Lack of correlation between red cell invasion by merozoites and anti-heat shock protein-70 antibody levels in malaria patients' sera. Int J Parasitol. 1991;21:213–217. doi: 10.1016/0020-7519(91)90011-u.0020-7519(1991)021[0213:LOCBRC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bloch DB, Gutierrez JA, Guerriero V Jr, Rauch SD, Bloch KJ. Recognition of a dominant epitope in bovine heat-shock protein 70 in inner ear disease. Laryngoscope. 1999;109:621–625. doi: 10.1097/00005537-199904000-00019.0023-852X(1999)109[0621:ROADEI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bloch DB, San Martin JE, Rauch SD, Moscicki RA, Bloch KJ. Serum antibodies to heat shock protein 70 in sensorineural hearing loss. Arch Otolaryngol Head Neck Surg. 1995;121:1167–1171. doi: 10.1001/archotol.1995.01890100075013.0886-4470(1995)121[1167:SATHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Botzler C, Li G, Issels RD, Multhoff G. Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones. 1998;3:6–11. doi: 10.1379/1466-1268(1998)003<0006:doeleo>2.3.co;2.1466-1268(1998)003[0006:DOELEO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–17153. doi: 10.1074/jbc.273.27.17147.0021-9258(1998)273[17147:HSPMPO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chan YC, Shukla N, and Abdus-samee M. et al. 1999 Anti-heat-shock protein 70 kDa antibodies in vascular patients. Eur J Vasc Endovasc Surg. 18:381–385. [DOI] [PubMed] [Google Scholar]
- Child DF, Williams CP, Jones RP, Hudson PR, Jones M, Smith CJ. Heat shock protein studies in type 1 and type 2 diabetes and human islet cell culture. Diabet Med. 1995;12:595–599. doi: 10.1111/j.1464-5491.1995.tb00548.x.0742-3071(1995)012[0595:HSPSIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Christians ES, Yan LJ, Benjamin IJ. Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit Care Med. 2002;30:S43–S50.0090-3493(2002)030[S43:HSFAHS]2.0.CO;2 [PubMed] [Google Scholar]
- Ciocca DR, Calderwood S. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1.1466-1268(2005)010[0086:HSPICD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IR, Young DB. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991;12:105–110. doi: 10.1016/0167-5699(91)90093-9.0167-5699(1991)012[0105:AMIATI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Colebrook AL, Lightowers MW. Serological reactivity to heat shock protein 70 in patient with hydatid disease. Parasite Immunol. 1997;19:41–46. doi: 10.1046/j.1365-3024.1997.d01-141.x.0141-9838(1997)019[0041:SRTHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Conroy SE, Sasieni PD, and Amin V. et al. 1998a Antibodies to heat-shock protein 27 are associated with improved survival in patients with breast cancer. Br J Cancer. 77:1875–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SE, Sasieni PD, Fentiman I, Latchman DS. Autoantibodies to the 90kDa heat shock protein and poor survival in breast cancer patients. Eur J Cancer. 1998b;34:942–943.0959-8049(1998)034[0942:ATTKHS]2.0.CO;2 [PubMed] [Google Scholar]
- Conroy SE, Tucker L, Latchman DS, Isenberg DA. Incidence of anti Hsp90 and 70 antibodies in children with SLE, juvenile dermatomyositis and juvenile chronic arthritis. Clin Exp Rheumatol. 1996;14:99–104.0392-856X(1996)014[0099:IOAHAA]2.0.CO;2 [PubMed] [Google Scholar]
- Cornford PA, Dodson AR, and Parsons KF. et al. 2000 Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 60:7099–7105. [PubMed] [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG. Heat-response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963.0009-7322(1993)087[0963:HALOTN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- De Smet MD, Ramadan A. Circulating antibodies to inducible heat shock protein 70 in patients with uveitis. Ocul Immunol Inflamm. 2001;9:85–92. doi: 10.1076/ocii.9.2.85.3973.0927-3948(2001)009[0085:CATIHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Favatier F, Bornman L, Hightower LE, Gunther E, Polla BS. Variation in hsp gene expression and Hsp polymorphism: do they contribute to differential disease susceptibility and stress tolerance? Cell Stress Chaperones. 1997;2:141–155. doi: 10.1379/1466-1268(1997)002<0141:vihgea>2.3.co;2.1466-1268(1997)002[0141:VIHGEA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidank HM, Clad A, Herr AS, Wiedmann-Al-Ahmad M, Jung B. Immune response to Chlamydia trachomatis heat-shock protein in infertile female patients and influence of Chlamydia pneumoniae antibodies. Eur J Clin Microbiol Infect Dis. 1995;14:1063–1069. doi: 10.1007/BF01590940.0934-9723(1995)014[1063:IRTCTH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Frostegard J, Lemmne C, Andersson B, Zee RVD, Liessling R, Faire U. Association of serum antibodies to heat shock protein 65 with borderline hypertension. Hypertension. 1997;29:40–44. doi: 10.1161/01.hyp.29.1.40.0194-911X(1997)029[0040:AOSATH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gao Y, Xiao C, and Chen S. et al. 2004 In vitro study on the role of Hsp70 expression in DNA damage in human embryonic lung cells exposed to benzo(a)pyrene. Biomed Environ Sci. 17:144–152. [PubMed] [Google Scholar]
- Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G. The cell surface localized heat shock protein 70 epitope TKD induces migration and cytolitic activity selectively in human NK cells. J Immunol. 2004;172:972–980. doi: 10.4049/jimmunol.172.2.972.0022-1767(2004)172[0972:TCSLHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ghayour-Mobarhan M, New SA, and Lamb DJ. et al. 2005 Dietary antioxidants and fat are associated with plasma antibody titers to heat shock proteins 60, 65, and 70 in subjects with dyslipidemia. Am J Clin Nutr. 81:998–1004. [DOI] [PubMed] [Google Scholar]
- Goral J, Shenoy S, Mohanakumar T, Clancy JJ. Antibodies to 70 kD and 90 kD heat shock proteins are associated with graft-versus-host disease in peripheral blood stem cell transplant recipients. Clin Exp Immunol. 2002;127:553–559. doi: 10.1046/j.1365-2249.2002.01770.x.0009-9104(2002)127[0553:ATKAKH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromadzka G, Zielinska J, Ryglewicz D, Fiszer U, Czlonkowska A. Elevated levels of anti-heat shock protein antibodies in patients with cerebral ischemia. Cerebrovasc Dis. 2001;12:235–239. doi: 10.1159/000047709.1015-9770(2001)012[0235:ELOASP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gruber R, Lederer S, Bechtel U, Lob S, Riethmuller G, Feucht HE. Increased antibody titers against mycobacterial heat-shock protein 65 in patients with vasculitis and arteriosclerosis. Int Arch Allergy Immunol. 1996;110:95–98. doi: 10.1159/000237318.1018-2438(1996)110[0095:IATAMH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gunther F. Heat shock protein genes and the major histocompatibility complex. Curr Top Microbiol Immunol. 1991;167:57–68. doi: 10.1007/978-3-642-75875-1_3.0070-217X(1991)167[0057:HSPGAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Habich C, Kempe K, Burkart V, Van Der Zee R, Lillicrap M, Gaston H, Kolb H. Identification of the heat shock protein 60 epitope involved in receptor binding on macrophages. FEBS Lett. 2004;568:65–69. doi: 10.1016/j.febslet.2004.05.010.0014-5793(2004)568[0065:IOTHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hayem G, De Bandt M, and Palazzo E. et al. 1999 Anti-heat shock protein 70 kDa and 90 kDa antibodies in serum of patients with rheumatoid arthritis. Ann Rheum Dis. 58:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress protein, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2.0092-8674(1991)066[0191:HSSPCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hoppichler F, Koch T, Dzien A, Gschwandtner G, Lechleitner M. Prognostic value of antibody titre to heat-shock protein 65 on cardiovascular events. Cardiology. 2000;94:220–223. doi: 10.1159/000047320.0008-6312(2000)094[0220:PVOATT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Horvath L, Cervenak L, and Oroszlan M. et al. 2002 Antibodies against different epitopes of heat-shock protein 60 in children with type 1 diabetes mellitus. Immunol Lett. 80:155–162. [DOI] [PubMed] [Google Scholar]
- Horvath L, Czirjak L, and Fekete B. et al. 2001 Levels of antibodies against C1q and 60 kDa family of heat shock proteins in the sera of patients with various autoimmune diseases. Immunol Lett. 75:103–109. [DOI] [PubMed] [Google Scholar]
- Jäättelä M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like protease. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124.1460-2075(1998)017[6124:HEIAFD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour WN, Jeffries BD, Davis JS, Welch WJ, Mimura T, Winfield JD. Autoantibodies to human stress proteins. Arthritis Rheum. 1991;34:1133–1138. doi: 10.1002/art.1780340909.0004-3591(1991)034[1133:ATHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jin GB, Nakayama H, and Shmyhlo M. et al. 2003 High positive frequency of antibodies to metallothionein and heat shock protein 70 in sera of patients with metal allergy. Clin Exp Immunol. 131:275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Wang R, and Xiao C. et al. 2004a Serum and lymphocyte levels of Hsp7l in aging: a study in the normal Chinese population. Cell Stress Chaperones. 9:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Xiao C, and Tanguay RM. et al. 2003 Dynamic changes of antibody against Hsp70 in elder patients with cerebral infarction. First International Congress on Stress Response in Biology and Medicine, Quebec, Canada, 10–14 September. P134. [Google Scholar]
- Jin X, Xiao C, and Tanguay RM. et al. 2004b Correlation of lymphocyte Hsp71 levels with neurological deficits in elder patients with cerebral infarction. Am J Med. 117:406–411. [DOI] [PubMed] [Google Scholar]
- Jones DB, Hunter NR, Duff GW. Heat-shock protein 65 as a beta cell antigen of insulin dependent diabetes. Lancet. 1990;336:583–585. doi: 10.1016/0140-6736(90)93390-b.0140-6736(1990)336[0583:HPAABC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kalabay L, Fekete B, and Czirjak L. et al. 2002 Helicobacter pylori infection in connective tissue disorders is associated with high levels of antibodies to mycobacterial hsp65 but not to human hsp60. Helicobacter. 7:250–256. [DOI] [PubMed] [Google Scholar]
- Kauffmann SHE, Schooel B 1994 Heat shock proteins as antigens in immunity against infection and self. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissières A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 495–531. [Google Scholar]
- Kim JJ, Lee SJ, Toh KY, Lee CU, Lee C, Paik IH. Identification of antibodies to heat shock proteins 90 kDa and 70 kDa in patients with schizophrenia. Schizophr Res. 2001;52:127–135. doi: 10.1016/s0920-9964(00)00091-8.0920-9964(2001)052[0127:IOATHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kocsis J, Prohaszka Z, Biro A, Fust G, Banhegyi D. Elevated levels of antibodies against 70 kDa heat shock proteins in the sera of patients with HIV infection. J Med Virol. 2003;71:480–482. doi: 10.1002/jmv.10507.0146-6615(2003)071[0480:ELOAAK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Korneeva I, Bongiovanni AM, Girotra M, Caputo TA, Witkin SS. IgA antibodies to the 27-kDa heat-shock protein in the genital tracts of women with gynecologic cancers. Int J Cancer. 2000;87:824–828. doi: 10.1002/1097-0215(20000915)87:6<824::aid-ijc11>3.0.co;2-k.0020-7136(2000)087[0824:IATTKH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res. 2001;51:637–646. doi: 10.1016/s0008-6363(01)00354-6.0008-6363(2001)051[0637:HSPACP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lee M, Hirokawa M, and Kuroki J. et al. 1995 Autoantibodies against 70-kDa heat shock proteins (HSP70) in allogeneic bone marrow transplant recipients. Bone Marrow Transplant. 16:583–588. [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Ann Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215.0066-4197(1988)022[0631:THSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lopatin DE, Sheburne CE, Van Poperin N, Kowalski CJ, Bagramian RA. Humoral immunity to stress proteins and periodontal diseases. J Periodontol. 1999;70:1185–1193. doi: 10.1902/jop.1999.70.10.1185.0022-3492(1999)070[1185:HITSPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mackay IR, Bone A, and Tuomi T. et al. 1996 Lack of autoimmune serological reactions in rodent models of insulin dependent diabetes mellitus. J Autoimmun. 9:705–711. [DOI] [PubMed] [Google Scholar]
- Maercker C. Protein arrays in functional genome research. Biosci Rep. 2005;25:57–70. doi: 10.1007/s10540-005-2848-y.0144-8463(2005)025[0057:PAIFGR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mandal K, Jahangiri M, Xu Q. Autoimmunity to heat shock proteins in atherosclerosis. Autoimmun Rev. 2004;3:31–37. doi: 10.1016/S1568-9972(03)00088-0. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi S, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;96:1446–1456. doi: 10.1172/JCI117815.0021-9738(1995)096[1446:OOTRIK]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metha TA, Greeman J, Ettelaie C, Venkatasubramaniam A, Chetter IC, McCollum PT. Heat shock proteins in vascular disease—a review. Eur J Endovasc Surg. 2005;29:395–402. doi: 10.1016/j.ejvs.2005.01.005.1078-5884(2005)029[0395:HSPIVD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest. 1995;95:3–12. doi: 10.1172/JCI117655.0021-9738(1995)095[0003:CIOTSR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliterno R, Valdivia L, Pan F, Duquesnoy RJ. Heat shock protein reactivity of lymphocytes isolated from heterotopic rat cardiac allografts. Transplantation. 1995;59:598–604.0041-1337(1995)059[0598:HSPROL]2.0.CO;2 [PubMed] [Google Scholar]
- Morgun A, Shulzhenko N, and Unterkircher CS. et al. 2004 Pre- and post-transplant anti-myosin and anti-heat shock protein antibodies and cardiac transplant outcome. J Heart Lung Transplant. 23:204–209. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Tissières A, and Georgopoulos C. ed. 1994 Progress and perspectives in the biology of heat shock proteins and molecular chaperones. In: The Biology of Heat Shock Proteins and Molecular Chaperones, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1–30. [Google Scholar]
- Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci USA. 2000;97:7841–7846. doi: 10.1073/pnas.140202897.0027-8424(2000)097[7841:HAHCCI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Walker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587.1471-0048(2005)006[0011:MONBMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, and Wiesnet M. et al. 1995 A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 61:272–279. [DOI] [PubMed] [Google Scholar]
- Nemoto T, Sato N, Iwanari H, Yamashita H, Takagi T. Domain structures and immunogenic regions of the 90-kDa heat-shock protein (HSP90): probing with a library of anti-HSP90 monoclonal antibodies and limited proteolysis. J Biol Chem. 1997;272:26179–26187. doi: 10.1074/jbc.272.42.26179.0021-9258(1997)272[26179:DSAIRO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Oda A, Miyata M, and Kodama E. et al. 1994 Antibodies to 65Kd heat-shock protein were elevated in rheumatoid arthritis. Clin Rheumatol. 13:261–264. [DOI] [PubMed] [Google Scholar]
- Oh KH, Kim JY, and Kim D. et al. 2004 Targeted gene disruption of the heat shock protein 72 gene (hsp70.1) in the donor tissue is associated with a prolonged rejection-free survival in the murine skin allograph model. Transplant Immunol. 13:273–281. [DOI] [PubMed] [Google Scholar]
- Panchapakesan J, Daglis M, Gatenby P. Antibodies to 65 kDa and 70 kDa heat shock proteins in rheumatoid arthritis and systemic lupus erythematosus. Immunol Cell Biol. 1992;70:295–300. doi: 10.1038/icb.1992.37.0818-9641(1992)070[0295:ATKAKH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Papp D, Prohaszka Z, and Kocsis J. et al. 2005 Development of a sensitive assay for the measurement of antibodies against heat shock protein binding protein 1 (HspBP1): increased levels of anti-HspBP1 IgG are prevalent in HIV infected subjects. J Med Virol. 76:464–469. [DOI] [PubMed] [Google Scholar]
- Plumier C, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2.1466-1268(1997)002[0162:TMETHI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins, inflammation, and cardiovascular disease. Circulation. 2002;105:1012–1017. doi: 10.1161/hc0802.103729.0009-7322(2002)105[1012:HSPIAC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pockley AG, Frostegard J. Heat shock proteins in cardiovascular disease and the progostic value of heat shock protein related measurements. Heart. 2005;91:1124–1126. doi: 10.1136/hrt.2004.059220.1355-6037(2005)091[1124:HSPICD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley A, Muthana M 2005 Heat shock proteins and allograft rejection. In Cellular Stress Responses in Renal Diseases, ed Razzaque MS, Taguchi T. Karger, Basel, 122–134. [DOI] [PubMed] [Google Scholar]
- Portig I, Pankuweit S, Maisch B. Antibodies against stress proteins in sera with dilated cardiopathy. J Mol Cell Cardiol. 1997;29:2245–2251. doi: 10.1006/jmcc.1997.0463.0022-2828(1997)029[2245:AASPIS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Daha MR, and Susal C. et al. 1999 C1q autoantibodies in HIV infection: correlation to elevated levels of autoantibodies against 60-kDa heat-shock proteins. Clin Immunol. 90:247–255. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Singh M, and Nagy K. et al. 2002 Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 7:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Moliterno R, and Donovan-Peluso MA. et al. 1995 Expression of stress proteins and lymphocyte reactivity in heterotopic cardiac allografts undergoing cellular rejection. Transpl Immunol. 3:114–123. [DOI] [PubMed] [Google Scholar]
- Qin HY, Mahon JL, Atkinson MA, Chaturvedi P, Lee-Chan E, Singh B. Type 1 diabetes alters anti-hsp90 autoantibody isotype. J Autoimmun. 2003;20:237–245. doi: 10.1016/s0896-8411(03)00035-0.0896-8411(2003)020[0237:TDAAAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Querishi T, Nagarwalla N, Sarela A, Ahmed AR. Antibodies to the 70-kDa heat-shock protein in patients with thermal burns. Clin Immunol Immunopathol. 1995;75:94–98. doi: 10.1006/clin.1995.1057.0090-1229(1995)075[0094:ATTKHP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rauch SD, Zurakowski D, Bloch DB, Bloch KJ. Anti-heat shock protein 70 antibodies in Meniere's disease. Laryngoscope. 2000;110:1516–1521. doi: 10.1097/00005537-200009000-00020.0023-852X(2000)110[1516:ASPAIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001;36:341–352. doi: 10.1016/s0531-5565(00)00215-1.0531-5565(2001)036[0341:SHSPAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rohde M, Daugaard M, Jensen MH, Helin K, Nylandsted J, Jäättelä M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19:570–582. doi: 10.1101/gad.305405.0890-9369(2005)019[0570:MOTHPF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573.0014-4754(1962)018[0571:ANPPIB]2.0.CO;2 [Google Scholar]
- Ritossa FM. Experimental activation of specific loci in polytene chromosomes of Drosophila. Exp Cell Res. 1964;35:601–607. doi: 10.1016/0014-4827(64)90147-8.0014-4827(1964)035[0601:EAOSLI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Samuelsson AK, Hyden D, Roberg M, Skogh T. Evaluation of anti-hsp70 antibody screening in sudden deafness. Ear Hear. 2003;24:233–235. doi: 10.1097/01.AUD.0000069230.36940.AC.0196-0202(2003)024[0233:EOAASI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schett G, Xu Q, and Amberger A. et al. 1995 Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 96:2569–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz MJ, Riedel M, Gruber R, Ackenheil M, Muller N. Antibodies to heat shock protein in schizophrenic patients: implications for the mechanism of the disease. Am J Psychiatry. 1999;156:1103–1104. doi: 10.1176/ajp.156.7.1103.0002-953X(1999)156[1103:ATHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shin SO, Billing PB, Keithley EM, Harris JP. Comparison of anti-heat shock protein (anti-Hsp70) and anti-68-kDa inner ear protein in the sera of patients with Meiniere's disease. Laryngoscope. 1997;107:222–227. doi: 10.1097/00005537-199702000-00015.0023-852X(1997)107[0222:COASPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shingai R, Maeda T, Onishi S, Yamamoto Y. Autoantibody against 70 kD heat-shock protein in patients with autoimmune liver diseases. J Hepatol. 1995;23:382–390. doi: 10.1016/0168-8278(95)80195-2.0168-8278(1995)023[0382:AAKHPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sims TJ, Lernmark A, Mancl LA, Schifferle RE, Page RC, Persson GR. Serum IgG to heat shock proteins and Porphyromonas gingivalis antigens in diabetic patients with periodontitis. J Clin Periodontol. 2002;29:551–562. doi: 10.1034/j.1600-051x.2002.290612.x.0303-6979(2002)029[0551:SITHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Son YS, Park JH, and Kang YK. et al. 2005 Heat shock 70-kD 8 protein isoform 1 (HSPA8) is expressed on the surface of human embryonic stem cells and down-regulated upon differentiation. Stem Cells. 23:1502–1513. [DOI] [PubMed] [Google Scholar]
- Srivastava PK. Immunotherapy of human cancer: lessons from mice. Nat Immun. 2000;1:363–366. doi: 10.1038/808795.1018-8916(2000)001[0363:IOHCLF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tissières A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1.0022-2836(1974)084[0389:PSISGO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology. 2003;110:1–9. doi: 10.1046/j.1365-2567.2003.01725.x.0019-2805(2003)110[0001:FOHSPS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun RY, Smith MD, Lo SS, Rook GA, Lydyard P, Leslie RD. Antibodies to heat shock protein 65kD in type 1 diabetes mellitus. Diabet Med. 1994;11:66–70. doi: 10.1111/j.1464-5491.1994.tb00232.x.0742-3071(1994)011[0066:ATHSPK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Veres A, Szamosi T, and Abloczy M. et al. 2002 Complement activating antibodies against the human 60 kDa heat shock protein as a new independent family risk factor of coronary heart disease. Eur J Clin Invest. 32:405–410. [DOI] [PubMed] [Google Scholar]
- Vogt S, Portigggg I, and Kusch B. et al. 2004 Detection of anti-hsp70 immunoglobulin G antibodies indicates better outcome in coronary artery bypass grafting patients suffering from severe preoperative angina. Ann Thorac Surg. 78:883–889. [DOI] [PubMed] [Google Scholar]
- Wang Y, Whittall T, McGowan E, Younson J, Kelly C, Bergmeier LA, Singh M, Lehner T. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol. 2005;172:3306–3316. doi: 10.4049/jimmunol.174.6.3306.0022-1767(2005)172[3306:IOSAIE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Wang CL, Wu T, Pan HN, Wang SK, Jiang JD. Autoantibody response to heat shock protein 70 in patients with heatstroke. Am J Med. 2001;111:654–657. doi: 10.1016/s0002-9343(01)00974-3.0002-9343(2001)111[0654:ARTHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Weitgasser R, Lechleitner M, and Koch T. et al. 2003 Antibodies to heat-shock protein 65 and neopterin levels in patients with type 1 diabetes mellitus. Exp Clin Endocrinol Diabetes. 111:127–131. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063.0031-9333(1992)072[1063:MSRCPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wells AD, Malkovsky M. Heat shock proteins, tumor immunogenicity and antigen presentation: an integrated view. Immunol Today. 2000;21:129–132. doi: 10.1016/s0167-5699(99)01558-3.0167-5699(2000)021[0129:HSPTIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wick G, Xu Q. Atherosclerosis—an autoimmune disease. Exp Gerontol. 1999;34:559–566.0531-5565(1999)034[0559:AAD]2.0.CO;2 [PubMed] [Google Scholar]
- Witkin SS, Askienazy-Elbhar M, Henry-Suchet J, Belaisch-Allart J, Tort-Grumbach J, Sarjdine K. Circulating antibodies to a conserved epitope of the Chlamydia trachomatis 60 kDa heat shock protein (hsp60) in infertile couples and its relationship to antibodies to C. trachomatis surface antigens and the Escherichia coli and human HSP60. Hum Reprod. 1998;13:1175–1179. doi: 10.1093/humrep/13.5.1175.0268-1161(1998)013[1175:CATACE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wu T, Chen S, and Sun Y. et al. 2001a Presence of antibody against the inducible Hsp71 in patients with acute heat-induced illness. Cell Stress Chaperones. 6:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ma J, and Chen S. et al. 2001b Association of plasma antibodies against the inducible Hsp70 with hypertension and harsh working conditions. Cell Stress Chaperones. 6:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Tanguay RM, and Wu Y. et al. 1996 Presence of antibodies to heat stress proteins and its possible significance in workers exposed to high temperature and carbon monoxide. Biomed Environ Sci. 9:370–379. [PubMed] [Google Scholar]
- Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM. Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones. 1998;3:161–167. doi: 10.1379/1466-1268(1998)003<0161:poaths>2.3.co;2.1466-1268(1998)003[0161:POATHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki J, Karawajczyk B, Gorski J, Korzeniowski A, Mackiewicz Z, Kupryszewski G, Glosnicka R. Human heat shock protein 60 (409–424) fragment is recognized by serum antibodies of patients with acute coronary syndromes. Cardiovasc Pathol. 2002;11:238–243. doi: 10.1016/s1054-8807(02)00109-6.1054-8807(2002)011[0238:HHSPFI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xiao C, Chen S, and Li J. et al. 2002 Association of HSP70 and genotoxic damage in lymphocytes of workers exposed to coke-oven emission. Cell Stress Chaperones. 7:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Chen S, and Yuan M. et al. 2004 Expression of Hsp60 and Hsp70 and presence of antibodies against Hsp70 in child patients with immune thrombocytopenic purpura. BMC Blood Disorders. 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Wu T, and Ren A. et al. 2003 Basal and inducible levels of Hsp70 in patients with acute heat-induced illness induced during training. Cell Stress Chaperones. 8:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–59. doi: 10.1161/01.atv.0000029720.59649.50.1079-5642(2002)022[1547:ROHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xu Q, Kiechl S, and Mayr M. et al. 1999 Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation. 100:1169–1174. [DOI] [PubMed] [Google Scholar]
- Yang M, Zheng J, and Tan H. et al. 2004b Frequency-specific association of antibodies against heat shock protein 60 and 70 with noise-induced hearing loss in workers. Cell Stress Chaperones. 9:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Tan H, and Chen Y. et al. 2004a Presence of antibodies against heat shock protein 70 in plasma of patients with coronary heart disease. Environ Occup Med. 21:139–140.(In Chinese). [Google Scholar]
- Yang M, Wu T, Cheng L, Wang F, Wei Q, Tanguay RM. Plasma antibodies against heat shock protein 70 correlate with the incidence and severity of asthma in a Chinese population. Resp Res. 2005;6:18. doi: 10.1186/1465-9921-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Feige U. Heat shock proteins in autoimmune disease: from causative antigen to specific therapy. Experientia. 1992;48:650–656. doi: 10.1007/BF02118311.0014-4754(1992)048[0650:HSPIAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yuan J, Yang M, and Yao H. et al. 2005 Plasma antibodies to heat shock protein 60 and heat shock protein 70 are associated with increased risk of electrocardiograph abnormalities in automobile workers exposed to noise. Cell Stress Chaperones. 10:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunoki N, Yokota K, and Mizuno M. et al. 2000 Antibody to heat shock protein can be used for early serological monitoring of Helicobacter pylori eradication treatment. Clin Diagn Lab Immunol. 7:574–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Hisaeda H, and Kano S. et al. 2001 Antibodies specific for heat shock proteins in human and murine malaria. Microbes Infect. 3:363–367. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, and Rott D. et al. 2001 Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation. 103:1071–1075. [DOI] [PubMed] [Google Scholar]
- Zugel U, Kaufmann SHE. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Micro Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19.1098-6618(1999)012[0019:ROHSPI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]