Abstract
It has been suggested that induction of the heat shock response in the mammalian embryo during the critical period of organogenesis can result in anatomical malformation. We measured serum heat shock protein 70 (Hsp70), anti-Hsp70, and anti-Hsp60 in samples taken from expectant mothers at 16 weeks gestation. Samples from women whose babies were born with a birth defect (n = 30) were compared with controls who gave birth to healthy babies (n = 46). Anti-Hsp70 levels were significantly elevated in patients who later gave birth to babies with cleft lip or palate or neurological abnormalities (n = 10): 260 (223–406) μg/mL compared to 150 (88–207) μg/mL in controls (P < 0.001). No significant differences were found in serum Hsp70 and anti-Hsp60 levels between cases and controls. This finding of increased maternal anti-Hsp70 in patients who later gave birth to babies with these abnormalities suggests a previous stressful event may have contributed to the pathogenesis. Further work is required to determine whether Hsp70 has a direct or indirect role in this pathogenesis or whether anti-Hsp70 is simply a marker of a prior increase in Hsp70 due to a physiological stress that itself resulted in the damage. This work is consistent with previous studies showing a buffering role for Hsps in evolution.
INTRODUCTION
It was suggested in the mid-1980s that induction of the heat shock response in the mammalian embryo during the critical period of organogenesis can alter the established program of activation and inactivation of genetic loci essential for normal interuterine development, the result being anatomical malformation (German 1984). More recently, heat shock protein 70 (Hsp70) has been shown to play an important role in mammalian embryonic development and cellular differentiation (Luft and Dix 1999), and in vitro studies have shown that antibodies to Hsp60 and Hsp70 have a direct adverse effect upon fertilization and early embryo development (Neuer et al 1998; Matwee et al 2001).
Cytosolic molecular chaperones (including heat shock proteins Hsp70 and Hsp90) are induced in response to environmental stresses, including hyperthermia, hypoxia, ischemia, inflammation, and oxidative stress (Benjamin and McMillan 1998). During a stressful event, hsp genes are switched on, resulting in increased intracellular Hsp protein levels. In vitro physiological stress has also recently been shown to increase levels of extracellular Hsp70 (Guzhova et al 2001; Hunter-Lavin et al 2004). It has been suggested that extracellular exposure of Hsps will lead to the generation of anti-Hsp antibodies, providing a recent historical record of a stressful event (Thomas and Cooper 2002). In addition to their role in binding misfolded proteins, both Hsp70 and Hsp90 have a role in modulating the apoptotic response (Dai et al 2003), and, in the unstressed cell, Hsp90 is known to interact and form complexes with transcription factors and signal transducing proteins (Yahara 1999).
Stress has also been proposed to be a driving force in evolution (McLaren 1999). Hsp90 can act as an evolutionary capacitor, hiding genotypic variation. Under conditions of environmental stress, its buffering capacity is overcome, allowing mutations to be expressed phenotypically (Rutherford and Lindquist 1998; Queitsch et al 2002). A similar role may also exist for other Hsps. Maternal physiological stress may redirect these Hsps from their normal chaperoning duties to protect cells under stress, resulting in damage or alterations to normal developmental processes. Antibodies to Hsps provide a historical record of Hsp exposure. We therefore hypothesized that elevated maternal antibodies to Hsp60 and Hsp70 would correlate to birth defects.
MATERIALS AND METHODS
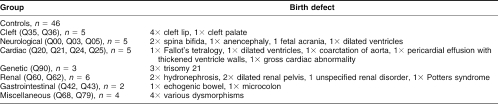
Ethics Committee permission was obtained for this case-control retrospective study. During a 12-month period, 2205 serum samples were collected from expectant mothers at approximately 16 weeks gestation. The samples were stored at −20°C. Women who gave birth to babies with congenital abnormalities were identified using the Wales Congenital Anomaly Register and Information Service Database (CARYS: Level 3, West Wing, Singleton Hospital, Swansea, SA2 8QA, UK). Thirty pregnancies resulting in a birth defect were identified during this time. Serum samples for these subjects were retrieved from storage, along with 46 samples selected randomly from subjects whose pregnancies ended in a normal delivery. The medical records of cases and controls were examined for details of smoking history, folate supplementation, gestational age, and blood pressure at the time of blood collection. The 30 affected births were classified as having a cardiac, genetic, renal, clefting, neurological, gastrointestinal, or miscellaneous disorder (Table 1).
Table 1.
Categorization of patients by ICD10 code
Serum Hsp70 was quantified in undiluted serum using an Hsp70 ELISA Kit (EKS-700; Stressgen Biotechnologies, Victoria, BC, Canada). Antibodies to Hsps were quantified in 1/1000 dilutions of serum using anti-human Hsp70 ELISA Kit and anti-human Hsp60 ELISA Kit (EKS-750 and EKS-650, respectively; StressGen Biotechnologies).
Median values are presented with interquartile (IQ) ranges. The Wilcoxon-Mann-Whitney test was used to compare median values, Fisher's exact test was used to compare proportions, and the Spearman correlation coefficient was used to assess the relationship between variables (StatXact version 4.0.1; Cytel Software Corporation, Cambridge, MA, USA). Logistic regression models were used in the case of binary dependent variables (LogXact version 4.0.2; Cytel Software Corporation) and conventional regression models in the case of continuous dependent variables (Statistica version 6.0; Statsoft Inc, Tulsa, OK, USA). In both cases, regression parameters are presented with their standard errors and associated P values.
RESULTS
There was no difference in median age between cases (27 years, 22–31) and controls (29 years, 24–33), nor between median gestational age at the time of blood sampling between cases (16 weeks, 16–16) and controls (16 weeks, 16– 17). A greater proportion of patients were taking folic acid supplements in the control group (60.0%), compared to the cases (48.3%), and there were fewer smokers in the control group (15.2% compared to 27.6%). In neither case, however, were the proportions significantly different (Fisher's exact test: P = 0.35 and P = 0.24, respectively).
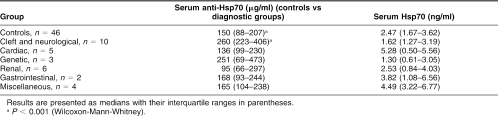
There was a significant difference in Hsp70 antibody levels between cases and controls: 223 (99–298) μg/mL compared to 150 (88–207) μg/mL (Wilcoxon-Mann-Whitney test, P = 0.05) (Table 2). No significant difference in Hsp70 protein levels or anti-Hsp60 levels was found between cases and controls (Table 2). There was no difference in systolic blood pressure (SBP) or diastolic blood pressure (DBP) between the 2 groups (Table 2). There was no correlation between gestational age and anti-Hsp70, anti-Hsp60, Hsp70, SBP or DBP. There was a correlation between anti-Hsp70 and both SBP and DBP (Spearman n: 0.37 and 0.36, respectively; P = 0.013 and 0.014). These correlations were confined to the cases (SBP and DBP: Spearman n: 0.49 and 0.53, respectively; P = 0.013 and P = 0.007) rather than the controls (SBP and DBP: Spearman n: 0.18 and 0.08, respectively; P = 0.5 and P = 0.7).
Table 2.
Analysis of maternal serum at 16 weeks gestation for serum Hsp70, anti-Hsp70, anti-Hsp60, systolic blood pressure (SBP), and diastolic blood pressure (DBP)
The effect of diagnostic category upon anti-Hsp70 concentration was assessed by stepwise forward multiple regression with dummy independent variables, one for each category. An F-value of 1.0 was used as the criteria to enter variables into the model. Only the variables ‘Control,’ ‘Cleft,’ and ‘Neurological’ were entered into the model, contributing 8.4%, 2.5%, and 2.8% to the multiple n2 statistic (F(3,72) = 3.82, P < 0.013), so further analysis was confined to the control group and the combined ‘Cleft’ and ‘Neurological’ group (‘C&N’). There was a highly significant difference between the anti-Hsp70 concentration in the C&N group and the controls: 260 (223– 406) μg/mL compared to 150 (88–207) μg/mL (P < 0.001) (Table 3). Additionally, there was also one single particularly high value in a patient whose pregnancy resulted in a baby with bilateral hydronephrosis in the ‘renal’ group (785 μg/mL).
Table 3.
Comparison of maternal anti-Hsp70 and Hsp70 levels at 16 weeks gestation between mothers who gave birth to babies with abnormalities within the diagnostic groups stated and those whose pregnancies resulted in a healthy baby (controls)
Increased Hsp70 antibodies have been associated with established hypertension (Pockley et al 2002). To confirm that the difference in anti-Hsp70 levels between the C&N group and the control group was independent of blood pressure, smoking status and folic acid supplementation, 2 logistic regression models were used, with case or control as the binary dependent variable, anti-Hsp70, DBP or SBP as continuous independent variables, and folic acid supplementation and smoking status as binary categorical variables. In the model with DBP, anti-Hsp70 concentration had a significant effect on the difference between cases and controls (regression coefficient: 0.012, SE 0.005, P = 0.012), with smoking status only having a weak effect (regression coefficient: 2.16, SE 1.20, P = 0.07). In the model with SBP, however, anti-Hsp70 concentration had the greatest effect (regression coefficient: 0.015, SE 0.006, P = 0.014), with significant effects of smoking (regression coefficient: 3.32, SE 1.62, P = 0.04) and folic acid supplementation (regression coefficient: −2.45, SE 1.26, P = 0.05). Differences in anti-Hsp70 between cases and controls were therefore independent of blood pressure, smoker status, and folic acid supplements.
DISCUSSION
Embryonic organogenesis occurs in the first trimester of pregnancy. This study suggests that, in the case of some birth defects, there is evidence of increased levels of a stress marker, namely anti-Hsp70, in blood collected several weeks later at 16 weeks gestation. Maternal physiological stress in early pregnancy may therefore be associated with increased cellular export, or cell surface presentation, of Hsp70, with a resulting increase in anti-Hsp70. This physiological stress, either directly by diverting Hsps from their normal chaperone functions or indirectly through transplacental transfer of anti-Hsp70 may be associated with cleft lip, palate, and neural tube defect.
Despite the correlations between anti-Hsp70 concentration and blood pressure in cases, there was no evidence that difference in anti-Hsp70 between C&N cases and controls was associated with differences in blood pressure. Although the protective effect of folic acid supplementation upon the incidence of neural tube defects is well established (Daly et al 1995), the effect of folic acid supplementation in protecting against cleft lip and palate may be more complex. A combination of environmental and genetic factors may contribute to this developmental disorder, some of these genes being involved in folate metabolism (Prescott and Malcolm 2002), including methyltetrahydrofolate reductase (van Rooij et al 2003). Oxidative stress may play a part in the development of neural tube defects (Chang et al 2003) and cleft palate (Knott et al 2003). The oxidative stress and apoptosis associated with folic acid deficiency, mediated by homocysteine-dependent overproduction of hydrogen peroxide and enhanced activation of NF-κB (Chern et al 2001), may therefore play a part in the development of these disorders.
It is interesting that, by the time of sampling (16 weeks gestation), serum Hsp70 levels showed no significant differences between cases and controls. There are 2 likely reasons for this: first, elevation of Hsp70 levels following increased physiological stress is transient (Walsh et al 2001; Campisi et al 2003). It is also possible that serum Hsp70 did not increase earlier in gestation, and that the increased anti-Hsp70 levels are a result of increased cell surface expression of Hsp70 and not release into the serum.
Another important observation is that there was no significant difference in anti-Hsp60 levels between cases and controls. This may suggest a pathological mechanism specific to Hsp70 and not due to a general induction of the heat shock response. It may also indicate that the stressful event is itself responsible for the damage, and that that stress specifically induces Hsp70 rather than Hsp60. In this case, anti-Hsp70 antibodies may simply be serving as markers that a stressful event has occurred with no involvement of Hsp70 in the pathogenesis. Oxidative stress is one example of a stress that induces Hsp70 more readily than Hsp60 (Kemp et al 2003) and is implicated in the development of neural tube defects (Chang et al 2003) and cleft palate (Knott et al 2003). The source of physiological stress, however, was not determined in this study, and oxidative stress is only one possibility. Bacterial and viral infections also have important potential to affect Hsp and anti-Hsp levels. Because this was a retrospective study, no detailed information on infections occurring during the pregnancies was collected, and so it is not possible to determine whether these were the source of, or contributed to, the maternal physiological stress.
Further work is required to determine the source of the maternal physiological stress and mechanisms that result in the increased levels of Hsp70 antibodies in patients whose babies suffer cleft lip or palate or neurological abnormalities, and whether Hsp70 has a role in the pathogenesis of these abnormalities. In addition, further studies should collect more cases with nonclefting and nonneurological abnormalities to confer increased statistical power on the analysis of these subgroups. Finally, more work is needed to determine the time point where maternal Hsp70 levels increased, resulting in the subsequent increase in anti-Hsp70 levels. This time point may have crucial implications in the resulting fetal abnormalities. Our results suggest a possible buffering role for Hsp70 in evolution, similar to that of Hsp90. In this case, maternal physiological stress during the early stages of pregnancy may divert Hsp70 from essential chaperoning duties during fetal development, resulting in birth defects.
REFERENCES
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117.0009-7330(1998)083[0117:SHSPMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2.1466-1268(2003)008[0272:SEHIAF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TI, Horal M, Jain SK, Wang F, Patel R, Loeken MR. Oxidant regulation of gene expression and neural tube development: insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia. 2003;46:538–545. doi: 10.1007/s00125-003-1063-2.0012-186X(2003)046[0538:OROGEA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chern CL, Huang RF, Chen YH, Cheng JT, Liu TZ. Folate deficiency-induced oxidative stress and apoptosis are mediated via homocysteine-dependent overproduction of hydrogen peroxide and enhanced activation of NF-kappaB in human Hep G2 cells. Biomed Pharmacother. 2001;55:434–442. doi: 10.1016/s0753-3322(01)00095-6.0753-3322(2001)055[0434:FDOSAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dai Q, Zhang CL, and Wu YX. et al. 2003 CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J. 22:5446–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274:1698–1702. doi: 10.1001/jama.1995.03530210052030.0098-7484(1995)274[1698:FLANTD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- German J. Embryonic stress hypothesis of teratogenesis. Am J Med. 1984;76:293–301. doi: 10.1016/0002-9343(84)90788-5.0002-9343(1984)076[0293:ESHOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3.0006-8993(2001)914[0066:IVSSTH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MMFVG, Marshall MJ, Andrew SM, Williams JHH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075.0006-291X(2004)324[0511:HRFPBM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kemp TJ, Causton HC, Clerk A. Changes in gene expression induced by H2O2 in cardiac myocytes. Biochem Biophys Res Commun. 2003;307:416–421. doi: 10.1016/s0006-291x(03)01215-4.0006-291X(2003)307[0416:CIGEIB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Knott L, Hartridge T, Brown NL, Mansell JP, Sandy JR. Homocysteine oxidation and apoptosis: a potential cause of cleft palate. In Vitro Cell Dev Biol Anim. 2003;39:98–105. doi: 10.1290/1543-706x(2003)039<0098:hoaaap>2.0.co;2.1543-706X(2003)039[0098:HOAAAP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Luft JC, Dix DJ. Hsp70 expression and function during embryogenesis. Cell Stress Chaperones. 1999;4:162–170. doi: 10.1379/1466-1268(1999)004<0162:heafde>2.3.co;2.1466-1268(1999)004[0162:HEAFDE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod. 2001;7:829–837. doi: 10.1093/molehr/7.9.829.1360-9947(2001)007[0829:TEOATH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- McLaren A. Too late for the midwife toad: stress, variability and Hsp90. Trends Genet. 1999;15:169–171. doi: 10.1016/s0168-9525(99)01732-1.0168-9525(1999)015[0169:TLFTMT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Neuer A, Mele C, Liu HC, Rosenwaks Z, Witkin SS. Monoclonal antibodies to mammalian heat shock proteins impair mouse embryo development in vitro. Hum Reprod. 1998;13:987–990. doi: 10.1093/humrep/13.4.987.0268-1161(1998)013[0987:MATMHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20:1815–1820. doi: 10.1097/00004872-200209000-00027.0263-6352(2002)020[1815:CHSPAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prescott NJ, Malcolm S. Folate and the face: evaluating the evidence for the influence of folate genes on craniofacial development. Cleft Palate Craniofac J. 2002;39:327–331. doi: 10.1597/1545-1569_2002_039_0327_fatfet_2.0.co_2.1545-1569(2002)039[0327:FATFET]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749.0028-0836(2002)417[0618:HAACOP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550.0028-0836(1998)396[0336:HAACFM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thomas MC, Cooper ME. Turning up the heat: heat shock proteins, hypertension and cardiovascular risk. J Hypertens. 2002;20:1713–1714. doi: 10.1097/00004872-200209000-00010.0263-6352(2002)020[1713:TUTHHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Vermeij-Keers C, and Kluijtmans LA. et al. 2003 Does the interaction between maternal folate intake and the methylenetetrahydrofolate reductase polymorphisms affect the risk of cleft lip with or without cleft palate? Am J Epidemiol. 157:583–591. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2.1466-1268(2001)006[0386:EISHIH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara I. The role of HSP90 in evolution. Genes Cells. 1999;4:375–379. doi: 10.1046/j.1365-2443.1999.00271.x.1356-9597(1999)004[0375:TROHIE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]