Abstract
The Drosophila melanogaster family of small heat shock proteins (sHsps) is composed of 4 main members (Hsp22, Hsp23, Hsp26, and Hsp27) that display distinct intracellular localization and specific developmental patterns of expression in the absence of stress. In an attempt to determine their function, we have examined whether these 4 proteins have chaperone-like activity using various chaperone assays. Heat-induced aggregation of citrate synthase was decreased from 100 to 17 arbitrary units in the presence of Hsp22 and Hsp27 at a 1:1 molar ratio of sHsp to citrate synthase. A 5 M excess of Hsp23 and Hsp26 was required to obtain the same efficiency with either citrate synthase or luciferase as substrate. In an in vitro refolding assay with reticulocyte lysate, more than 50% of luciferase activity was recovered when heat denaturation was performed in the presence of Hsp22, 40% with Hsp27, and 30% with Hsp23 or Hsp26. These differences in luciferase reactivation efficiency seemed related to the ability of sHsps to bind their substrate at 42°C, as revealed by sedimentation analysis of sHsp and luciferase on sucrose gradients. Therefore, the 4 main sHsps of Drosophila share the ability to prevent heat-induced protein aggregation and are able to maintain proteins in a refoldable state, although with different efficiencies. The functional reasons for their distinctive cell-specific pattern of expression could reflect the existence of defined substrates for each sHsp within the different intracellular compartments.
INTRODUCTION
Heat shock proteins (Hsps) are conserved proteins involved in multiple cellular processes, including protein folding, targeting, and translocation across membranes (Neupert 1997; Hartl and Hayer-Hartl 2002). Hsps are also important for cell viability and can prevent intracellular damage induced by environmental stress during aging (Verbeke et al 2001; Söti and Csermely 2002; Morrow and Tanguay 2003b) and in neurodegenerative diseases (Fonte et al 2002; Muchowski 2002; Sakahira et al 2002). Most Hsps are up-regulated following stress when they can act as molecular chaperones preventing protein dysfunction by facilitating protein refolding or disposal of aggregated protein through proteolytic pathways (Walter and Buchner 2002).
Although less conserved than the Hsps of the other families (Hsp100, Hsp90, Hsp70, and Hsp60), the small Hsps (sHsps) of different organisms, with molecular weight ranging from 10 to 40 kDa, share properties such as the presence of a C-terminal α-crystallin domain and a native oligomeric structure (de Jong et al 1998). In vitro, many sHsps can act as molecular chaperones, inhibiting stress-induced aggregation of different protein substrates (Ehrnsperger et al 1997; Lee et al 1997; Haslbeck et al 1999; Fernando and Heikkila 2000). In vivo, overexpression of sHsps has been reported to confer thermotolerance (Landry et al 1989; Rollet et al 1992; Kitagawa et al 2002), protection against tumor necrosis factor–α-induced and caspase-dependent apoptosis (Mehlen et al 1996; Arrigo 1998; Samali et al 2001; Concannon et al 2003), and stabilization of cytoskeletal elements (Lavoie et al 1993; Wieske et al 2001; Mounier and Arrigo 2002).
The Drosophila melanogaster genome contains 12 open reading frames for proteins having the characteristic α-crystallin domain of sHsps (Michaud et al 2002). Four of these sHsps have been examined in detail: Hsp22, Hsp23, Hsp26, and Hsp27. These 4 sHsps share high sequence homology, are coordinately expressed following stresses, but have distinct developmental expression pattern and intracellular localization (Michaud et al 1997, 2002). In Drosophila cells, Hsp22 localizes in the mitochondrial matrix (Morrow et al 2000), Hsp23 and Hsp26 in the cytosol, and Hsp27 in the nucleus (Beaulieu et al 1989; Marin and Tanguay 1996). Hsp23 and Hsp26 seem to be localized in different parts of the cytosol because Hsp26 staining is granular compared with the more uniform staining pattern of Hsp23. The developmental expression profile of each of these sHsps is intriguing and does not always correspond to periods of physiological stress. Hsp23, Hsp26, and Hsp27 are expressed at different distinct stages of early development, especially in the brain and the gonads (Michaud et al 1997, 2002). During embryogenesis, Hsp23 is expressed in a stage-specific manner in a restricted number of neuronal and glial lineages of the central nervous system (Michaud and Tanguay 2003). In adult flies, hsp26 and hsp27 mRNA remain stable, whereas in aged flies hsp23 mRNA is up-regulated 5-fold in the thorax and hsp22 mRNA is up-regulated up to 60-fold in the head and 20-fold in the thorax (Wheeler et al 1995; King and Tower 1999).
Why D melanogaster has at least 4 distinct, albeit structurally similar, sHsps is unclear. Their coordinated pattern of expression after heat shock contrasts with their cell-specific pattern of expression during development (Michaud et al 1997, 2002; Morrow and Tanguay 2003a), suggesting a common and general role under stress conditions and more specific function(s) during development and differentiation. It has recently been shown that overexpression of sHsps is beneficial to flies by extending lifespan and stress resistance (Seong et al 2001; Morrow et al 2004b; Wang et al 2004), but their mode of action in these processes remains to be determined.
As a further step aimed at identifying the function(s) of the different sHsps of D melanogaster, we analyzed their chaperone-like activity using different in vitro assays. These sHsps were tested for their efficiency in preventing heat-induced aggregation of 2 substrates commonly used in chaperoning assays: citrate synthase (CS) and luciferase. Because many sHsps of bacteria, plants, and higher vertebrates have been reported to act as reservoirs of misfolded proteins (Ehrnsperger et al 1997; Lee et al 1997; Haslbeck et al 1999; Mogk et al 2003; Basha et al 2004b; Chowdary et al 2004), we also measured the activity of D melanogaster sHsps in luciferase refolding assays in vitro in the presence of rabbit reticulocyte lysate. The data show that the 4 main sHsps of D melanogaster can prevent protein aggregation with different efficiencies and that they appear to have different requirements to allow reactivation of their substrate.
MATERIAL AND METHODS
Cloning of sHsp genes in pET30 expression vector
Full-length cDNA encoding the 4 sHsps were cloned in the pET30(a) expression vector (Novagen, Madison, WI, USA) by PCR with custom forward and reverse primers, except for hsp27, for which the universal SP6 promoter was used as the reverse primer (Table 1; Genosys, Oakville, ON, Canada). The forward primers contained a BspHI restriction enzyme site in the case of hsp22 and hsp23 or a BamHI restriction enzyme site for hsp26 and hsp27. All custom reverse primers had a XhoI restriction enzyme site. Amplification of each shsp gene was performed on the corresponding pRcCMV-sHsp plasmid containing the respective full-length cDNA from D melanogaster. For cloning of hsp27, the XhoI restriction site of the plasmid was used because the SP6 promoter was the reverse primer. Amplification of hsp22 and hsp23 was performed by mixing 100 ng of plasmid template with 5 μL of 10× buffer #3 (50 mM Tris-HCl pH 9.2, 160 mM [NH4]2SO4, 22.5 mM MgCl2, 20% dimethyl sulfoxide, and 1% Tween20), 3 μL of 25 mM MgCl2, 2 μL of 5 mM dNTP, 200 ng of each corresponding primer, and 0.5 μL of Taq polymerase (Amersham Pharmacia Biotech, Laval, Quebec, Canada). The reaction mixtures were covered with mineral oil and incubated for 5 min at 95°C, followed by 35 cycles of 30 seconds at 95°C, 30 seconds at 50°C, 1 minute 15 seconds at 72°C, and a final elongation of 5 minutes at 72°C. For hsp26 and hsp27 amplifications, a 10× Taq polymerase buffer (Amersham Pharmacia Biotech) was used instead of 10× buffer #3, MgCl2 was omitted, and annealing was performed at 58°C. The DNA fragments obtained were digested by their respective enzymes and ligated with T4 DNA ligase (New England Biolabs, Pickering, ON, Canada) to pET-30(a)-digested NcoI/XhoI for hsp22 and hsp23 or BamHI/XhoI for hsp26 and hsp27. We used the pET-30(a) vector to take advantage of the histidine tag for the purification step. All constructions were verified by restriction enzyme analysis and DNA sequencing. The Hsp22 amino acid sequence correspond to GI:24661519 (accession NP_729478.1), that of Hsp23 to GI:123565 (accession P02516), and Hsp27 to GI:123570 (accession P02518). The Hsp26 sequence correspond to GI:123566 (accession P02517), except for glutamate 192, which is replaced by an aspartic acid residue and the addition of 3 amino acids (DGK) at position 199. However, this Hsp26 sequence is identical to the one obtained by amplifying the hsp26 gene directly from D melanogaster genomic DNA.
Table 1.
Primers for Drosophilus melanogaster shsps gene amplification. Primers are listed in the 5′ → 3′ direction. Restriction sites are underlined and ATG is in bold
Expression and purification of sHsps
Each pET-sHsp construct was transformed in Escherichia coli BL21DE3 pLysS grown at 37°C in Luria-Bertani medium and protein expression was induced with 0.5 mM isopropylthio-B-d-galactoside (Invitrogen Life Technologies, Burlington, ON, Canada) to give His-sHsp proteins. His-Hsp22 and His-Hsp27 were soluble under these conditions and were purified in native conditions by affinity chromatography through their histidine tag on nickel-nitrilotriacetic acid superflow columns (Qiagen, Mississauga, ON, Canada). His-Hsp23 and His-Hsp26 were first solubilized in 8 M urea and refolded to their native state by dialysis. Sequential dialysis was performed with 10% glycerol (ACP Chemicals, Montreal, Quebec, Canada) and decreasing urea concentrations (from 6 to 0 M). His-Hsp22 was also purified under denaturing conditions to assess the effect of the purification procedure on the chaperoning function. Once purified proteins were obtained, the histidine tag was removed with recombinant enterokinase, as suggested by the manufacturer (Novagen). Protein purity was assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on purified fractions as described in Morrow et al (2000).
CS and luciferase heat-induced aggregation assay
The heat-induced aggregation assay was conducted essentially as described in Fernando and Heikkila (2000). CS (0.1 μM; molecular weight [MW] 51,629; Sigma, Oakville, ON, Canada) or luciferase (0.1 μM; MW 61,000; Promega, Madison, WI, USA) were heat-denatured at 42°C in the absence or presence of either His-Hsp22, Hsp22, Hsp23, Hsp26, Hsp27 (0.05 μM, 0.1 μM, or 0.5 μM) or bovine albumin serum (BSA; 0.1 μM; ICN Biochemicals, Costa Mesa, CA, USA) as a control for 90 minutes for CS or 30 minutes for luciferase. Aggregation of CS and luciferase was determined by a light-scattering assay at 320 nm on a spectrophotometer with thermostated cells (Varian, Montreal, Quebec, Canada, Cary 100). Data are representative of 6 different assays and expressed as the mean ± standard deviation.
Luciferase refolding assay in reticulocyte lysate
This assay was adapted from Lee et al (1997). Firefly luciferase (0.2 μM) was heat-denatured at 42°C in the absence or presence of Hsp22, Hsp23, Hsp26, Hsp27 (1.0 μM) or BSA (1.0 μM, control) for 15 minutes. The refolding step was performed at 30°C for 90 minutes in untreated rabbit reticulocyte lysate (Promega) supplemented with 2 mM ATP (Amersham Pharmacia Biotech). Luciferase activity was measured on a luminometer (LKB Wallac, Turku, Finland, model 1250) according to standard protocol. Data are representative of 5 different assays, calculated as a percentage of luciferase activity after 15 minutes at 22°C and expressed as the mean ± standard deviation.
Sedimentation analysis on sucrose gradients
Sedimentation analysis of sHsps and luciferase were performed on 10–40% sucrose gradients as described previously (Morrow et al 2000). Each sHsp was incubated with luciferase at a molar ratio of 5 sHsps:1 luciferase to reproduce the luciferase refolding assay conditions for either 15 minutes at 4°C, 15 minutes at 42°C, or 15 minutes at 42°C followed by a 90-minute incubation at 30°C in the presence of rabbit reticulocyte lysate supplemented with ATP. Samples were then loaded on sucrose gradients and centrifuged for 22 hours at 39 600 rpm. Following this step, gradients were divided into 25 fractions of 10 drops each, and protein localization was assessed by SDS-PAGE (Morrow et al 2000) and Western blotting with the use of specific antibodies.
RESULTS
To perform the chaperone assays, the 4 sHsps of D melanogaster were overexpressed in E coli BL21DE3 pLySs with the use of the corresponding pET-sHsp construct and were purified through their histidine tag on a nickel-affinity column. The yield of His-sHsp (histidine-tagged sHsp) was of the order of 5 mg/L of bacteria.
The 4 small Hsps of D melanogaster prevent heat-induced aggregation of proteins
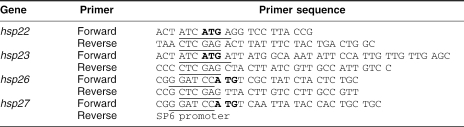
Because sHsps of other organisms have been shown to have chaperone-like activity in vitro, we tested whether each of the sHsps of D melanogaster could prevent protein denaturation and loss of function. Because Hsp23 and Hsp26 were insoluble when induced in bacteria, we first analyzed whether the purification conditions (native or urea) could influence the ability of His-sHsps to prevent heat-induced CS aggregation. CS was incubated at 42°C for 90 minutes either alone or in presence of the 2 His-Hsp22 preparations (purification under native conditions and with urea). As observed in Figure 1A, the urea purification procedure did not affect the ability of His-Hsp22 to prevent CS aggregation.
Fig 1.
Blocking the N-terminus of Hsp22 with a histidine tag reduces its efficiency to prevent citrate synthase (CS) heat-induced aggregation. CS (0.1 μM) was incubated at 42°C for 90 minutes either alone (open lozenge) or in the presence of 0.1 μM of His-Hsp22 purified under native conditions (A and B, open triangle) or purified with urea (A, open circle) or of untagged-Hsp22 purified under native conditions (B, closed triangle). Protein aggregation was determined by the light-scattering assay at 320 nm. Data are representative of 6 trials and are expressed as the mean ± standard deviation
Next, we determined whether the tag could influence the chaperone-like activity of D melanogaster His-sHsps. At a 1:1 (His-Hsp22:CS) molar ratio, the aggregation of CS was reduced from 100 arbitrary units to 47.8 ± 6.3 arbitrary units compared with 17.5 ± 3.2 units in the presence of Hsp22 (untagged; Fig 1B). Thus, untagged Hsp22 was at least 2 times more efficient in preventing heat-induced aggregation of CS than His-Hsp22 at the same molar ratio. The same difference in chaperone efficiency was observed with the other sHsps (data not shown) and at molar ratios of 2:1 and 5:1 (data not shown). Because these results suggested an influence of the histidine tag on the ability of D melanogaster sHsps to prevent protein aggregation, tags were removed from each His-sHsp protein with enterokinase before performing the chaperone assays.
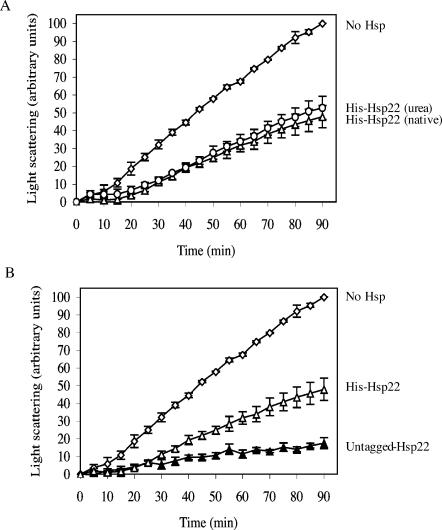
Incubation of CS with the different sHsps prevented its aggregation, and this protection was dependent on the Hsp/CS molar ratio used (Fig 2A–D). Thus, incubation of CS in the presence of an equal amount of sHsp (1:1 sHsp:CS) resulted in an aggregation decrease from 100 arbitrary units after 90 minutes to 17.5 ± 3.2 units in the presence of Hsp22 (Fig 2A), 64.3 ± 4.3 units in the presence of Hsp23 (Fig 2B), 40.3 ± 2.4 units in the presence of Hsp26 (Fig 2C), and 17.7 ± 5.8 units in the presence of Hsp27 (Fig 2D). A 5-fold molar excess of Hsp23 or Hsp26 (5:1 sHsp:CS) was needed to obtain the same protection efficiency as Hsp22 and Hsp27 in this assay (Fig 2B, C). Addition of a 5-fold excess of BSA (5:1 BSA:CS) had only a small effect on protein aggregation because 78.1 ± 6.4 arbitrary units of CS were still aggregated after 90 minutes. Hence, each of the 4 sHsps of D melanogaster can prevent the heat-induced aggregation of CS, albeit with different efficiencies.
Fig 2.
D melanogaster sHsps inhibits heat-induced aggregation of citrate synthase (CS). CS (0.1 μM) was incubated at 42°C for 90 min either alone (opened lozenge) or in presence of BSA (0.5 μM, opened square), Hsp22 (A), Hsp23 (B), Hsp26 (C) or Hsp27 (D) at 2 different concentrations (0.05 μM: closed square, 0.1 μM: closed circle or 0.5 μM: closed triangle). Protein aggregation was determined by the light-scattering assay at 320 nm. Data are representative of 6 trials and are expressed as the mean ± standard deviation
The ability to prevent aggregation depends on the nature of the substrate
To investigate the ability of Drosophila sHsps to bind different substrates, the heat-induced aggregation assay was also performed with luciferase instead of CS as a substrate. Because luciferase is particularly sensitive to heat, the aggregation assay was performed for 30 minutes. As in the CS assay, Hsp23 and Hsp26 were less effective than Hsp22 and Hsp27 in preventing heat-induced aggregation (Fig 3). In the presence of an equal ratio of Hsp22 or Hsp27 (1:1 sHsp:luciferase), luciferase aggregation was decreased from 100 arbitrary units to 12.3 ± 4.2 and 39.6 ± 7.8 arbitrary units, respectively (Fig 3A, D). Incubation of luciferase in the presence of Hsp23 or Hsp26 resulted in decreased luciferase aggregation to 65.9 ± 6.9 and 71.0 ± 5.3 arbitrary units (Fig 3B, C). A 5-fold excess of these 2 sHsps (5:1 sHsp:luciferase) was necessary to prevent luciferase aggregation as well as the 1:1 ratio for Hsp22.
Fig 3.
D melanogaster sHsps inhibit heat-induced aggregation of luciferase. Luciferase (0.1 μM) was incubated at 42°C for 30 minutes either alone (opened lozenge) or in the presence of BSA (0.5 μM, open square), Hsp22 (A), Hsp23 (B), Hsp26 (C), or Hsp27 (D) at 2 different concentrations (0.1 μM: closed circle, 0.5 μM: closed triangle). Protein aggregation was determined by the light-scattering assay at 320 nm. Data are representative of 6 trials and are expressed as the mean ± standard deviation
Together, these results indicate that Hsp22 and Hsp27 are more efficient in preventing heat-induced protein aggregation than Hsp23 and Hsp26 (Figs 2 and 3). However, unlike Hsp26 and Hsp27, Hsp22 and Hsp23 chaperone-like activity does not seem to be dependent on the nature of the substrate because an equivalent molar ratio prevented CS and luciferase aggregation with similar efficiency (Figs 2 and 3).
Drosophila sHsps can maintain heat-denatured protein in a refoldable state
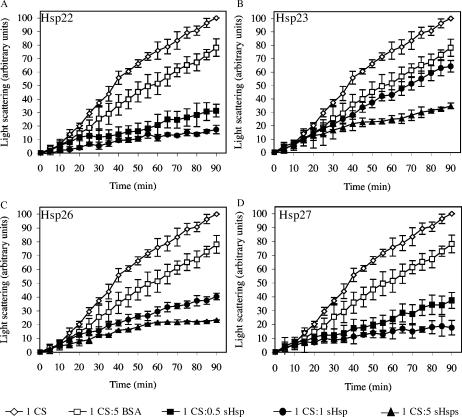
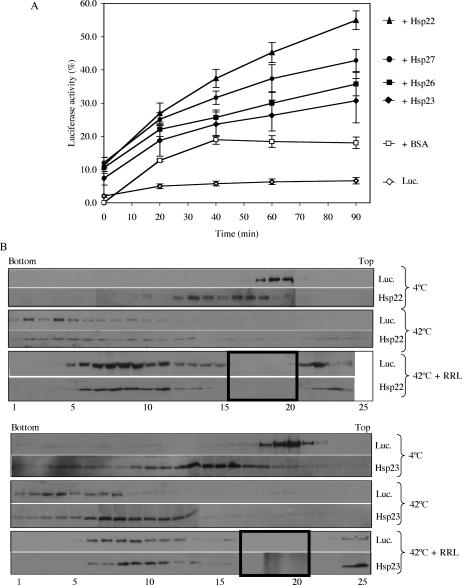
To ascertain that the proteins trapped by the sHsps were in a refoldable state and that a relationship between the sHsp-substrate complex and ATP-dependent chaperones was possible, an in vitro luciferase refolding assay was next performed. In this assay, luciferase was heat-treated in the absence or presence of sHsp, and the mixture was then supplemented with the other members of the chaperone machinery present in rabbit reticulocyte lysate and ATP. When luciferase was kept at room temperature, its activity remained stable for the entire refolding period, whereas a preincubation of 15 minutes at 42°C resulted in a drastic decrease in its activity (2.0% ± 0.4%), with no more than 6.7% ± 1.0% activity recovered after the refolding period in the absence of Hsps (Fig 4A), probably because of endogenous chaperones present in the reticulocyte lysate. After 90 minutes, 54.9% ± 2.8% of the activity was recovered when luciferase was heat-denatured at 42°C in the presence of Hsp22. Heat treatment in the presence of Hsp27 resulted in a 42.8% ± 3.3% luciferase activity, whereas recovery of luciferase activity was 30.7% ± 6.7% and 35.7% ± 3.5% in the presence of Hsp23 and Hsp26, respectively.
Fig 4.
The sHsps of D melanogaster maintain heat-denatured luciferase in a refoldable state in vitro. (A) Luciferase (0.2 μM) was incubated at 42°C for 15 minutes either alone (open lozenge) or with 1 μM BSA (open square), Hsp22 (closed triangle), Hsp23 (closed lozenge), Hsp26 (closed square), or Hsp27 (closed circle). The refolding step was performed at 30°C for 90 minutes in reticulocyte lysate supplemented with ATP, and luciferase activity was determined at different time points. Data are representative of 3–5 trials and are presented as a percentage of luciferase activity after 15 minutes of incubation at 22°C. Data are expressed as mean ± standard deviation. (B) Luciferase was incubated with Hsp22 or Hsp23 for 15 minutes at 4°C, 15 minutes at 42°C, or 15 minutes at 42°C and 90 minutes at 30°C in the presence of reticulocyte lysate and ATP. Samples were applied on 10–40% sucrose gradients and were analyzed by SDS-PAGE. Small Hsps and luciferase were localized by Western blotting. 1–25: sucrose gradients fractions (1 being at the bottom of the gradient and 25 at the top); bold boxes: fractions in which protein precipitation was likely disturbed by the hemoglobin present in the reticulocyte lysate
To understand the differences in chaperone-like activity of the 4 sHsps at the molecular level, we performed sucrose gradient analysis following incubation of sHsps and luciferase at 4°C, 42°C, and after a 90-minute incubation with rabbit reticulocyte lysate. As can be seen in Figure 4B, at 4°C, Hsp22 and Hsp23 are mainly localized in fractions 10 to 17, whereas luciferase is localized in fractions 18 to 21. A 15-minute incubation at 42°C resulted in a shift of luciferase, mainly in fractions 1 to 10, whereas sHsps were shifted to fractions 5 to 14. After incubation in the reticulocyte lysate, luciferase and Hsp22 were found in fractions 5 to 24 (protein precipitation was likely disturbed by the huge amount of hemoglobin from the reticulocyte lysate in fractions 16 to 20). In the case of Hsp23, incubation in the reticulocyte lysate resulted in a distribution of Hsp23 similar to that obtained after the 15-minute heat shock (fractions 8 to 13), whereas luciferase was observable in fractions 6 to 12. These results suggest that, after heat shock, less Hsp23 binds luciferase compared with Hsp22. Moreover, for an unknown reason, after the 90-minute incubation in the reticulocyte lysate, luciferase seemed to be trapped with Hsp23 and less accessible to the ATP-dependent chaperones. Experiments with Hsp26 gave results similar to those with Hsp23, whereas the Hsp27 were similar to the Hsp22 experiments (data not shown).
Altogether, these results demonstrate that Hsp22, Hsp23, Hsp26, and Hsp27 can maintain heat-treated luciferase in a refoldable state, from which it can be refolded by other chaperones into an active enzyme. Differences in luciferase reactivation efficiency obtained in the reticulocyte lysate further suggest that each of these sHsps binds heat-denatured luciferase differently and has special requirements to accomplish its function.
DISCUSSION
Despite reports that many sHsps from yeast, plants, and mammals present chaperone-like activity in in vitro aggregation assays, their function in vivo remain unknown. Overexpression of sHsps, including the Hsp27 of D melanogaster (Rollet et al 1992; Mehlen et al 1993), has been shown to confer resistance to supraoptimal temperatures. Mammalian Hsp27 has also been shown to confer resistance to heat shock and to various drugs (Huot et al 1991; Lavoie et al 1993; Van de Ijssel et al 1994; Mehlen et al 1996; Fortin et al 2000). However, the different cellular mechanisms suggested for the resistance remain controversial. In D melanogaster, the different sHsps show a cell- and stage-specific pattern of expression at developmental periods that do not necessarily coincide with peaks of physiological stress (Michaud et al 1997, 2002). In addition, the 4 main sHsps are localized in different cell compartments, with Hsp22 in the mitochondria, Hsp23 and Hsp26 in the cytosol, and Hsp27 in the nucleus. Thus, the sHsps could have different intracellular targets for their activity. Interestingly, overexpression of Hsp22 (Morrow et al 2004b), Hsp23 (Morrow et al. in preparation), Hsp26 (Seong et al 2001; Wang et al 2004), and Hsp27 (Wang et al 2004) has been shown to increase lifespan and resistance to stress to different extents. Flies that do not express mitochondrial Hsp22 show a decreased lifespan and are sensitized to mild stress (Morrow et al 2004a).
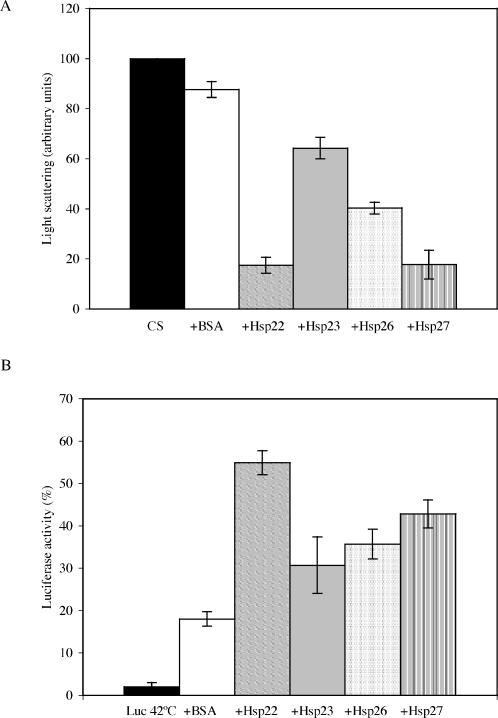
To further understand the function of sHsps in vivo, we have examined their ability to act as molecular chaperones in vitro. In such assays, each of the 4 distinct sHsps of D melanogaster showed chaperone-like activity, and all 4 sHsps were able to inhibit heat-induced aggregation of substrates such as CS and luciferase. Furthermore, Hsp22, Hsp23, Hsp26, and Hsp27 can maintain heat-denatured luciferase in a folding competent state such that it can be refolded in the presence of the chaperones (Hsp60 and Hsp70) present in the reticulocyte lysate. Figure 5 summarizes these data in graphic format and displays the different efficiencies of sHsps to act as molecular chaperones. Thus, the 4 main sHsps of D melanogaster can perform functions similar to sHsps of other organisms in in vitro chaperone assays (Horwitz 1992; Ehrnsperger et al 1997; Lee et al 1997; Haslbeck et al 1999; Fernando and Heikkila 2000; Lindner et al 2000; Abdulle et al 2002; Panensenko et al 2002; Van Montfort et al 2002).
Fig 5.
Summary of chaperone activity of D melanogaster sHsps. (A) Prevention of citrate synthase (CS) heat-induced aggregation at a 1:1 (sHsp:CS) molar ratio after 90 minutes at 42°C. See the legend of Figure 2 for details. (B) Reactivation of luciferase at a 5:1 (sHsp: luciferase) molar ratio after a 15-minute heat shock (42°C) and 90 minutes of recovery at 30°C in the presence of reticulocyte lysate. See the legend of Figure 4A for details
Although the 4 Drosophila sHsps share a high degree of homology, they display differences in their capacity to prevent aggregation of a common substrate, suggesting differences in their mode of action. Hsp22 and Hsp27 were more efficient in preventing CS heat-induced aggregation than Hsp26 and Hsp23. This difference is not caused by their mode of purification, as demonstrated with His-Hsp22 purified under native conditions and with urea. Small Hsps of other organisms have also been reported to show differences in their chaperone activity; among others, this is the case for Xenopus laevis Hsp30C and Hsp30D (Abdulle et al 2002), for mammalian αA-crystallin and αB-crystallin (Datta and Rao 1999; Van Boekel et al 1999; Reddy et al 2000) and recently for mammalian Hsp22 (Chowdary et al 2004) and S. cerevisiae Hsp42 and Hsp26 (Haslbeck et al 2004). For example, despite the high degree of sequence homology and structural similarity of αA- and αB-crystallin, αA-crystallin is more efficient in preventing thermal aggregation of proteins whereas αB-crystallin performs better against reduction-induced aggregation of proteins (Datta and Rao 1999).
As demonstrated in the CS and luciferase heat-induced aggregation assay, the ability of D melanogaster sHsps to prevent denaturation varies according to the nature of the substrate. This has also been observed in the case of other sHsps, such as pea Hsp18.1 (Lee et al 1997) and murine Hsp25 and yeast Hsp26 (Stromer et al 2003). Lee et al (1997) have suggested that substrate binding was determined by 3 major parameters: substrate protein susceptibility to heat denaturation, exposure of structural elements recognized by sHsps, and steric considerations. In addition, Stromer et al (2003) recently showed that the morphology of the sHsp-substrate complex, as seen by electron microscopy, is dependent on the nature of the substrate and that the first substrate bound determines the morphology of the final complex. Thus, a specific complex morphology could permit the binding of more substrate proteins.
As in the prevention of heat-induced aggregation assay, the 4 sHsps of D melanogaster displayed differences in their ability to maintain heat-treated luciferase in a refoldable state. This is not surprising because these sHsps have differences in their amino acid sequences and are localized in different cellular compartments, suggesting an adaptation for precise functions. For example, the overall good chaperone ability of Hsp22 could be an advantage because mitochondria are particularly sensitive to stress (Marcillat et al 1989; Zhang et al 1990; Li et al 2002) and it is possible that mitochondrial proteins are more prone to damage. Moreover, this could explain the beneficial effect that we have obtained by overexpressing this sHsp in the fruit fly (Morrow et al 2004a, 2004b).
One reason that could account for the differences in luciferase reactivation efficiency of D melanogaster sHsps is their ability to bind heat-denatured luciferase and interact directly or indirectly with ATP-dependent chaperones to promote luciferase refolding. Indeed, luciferase is reactivated to a lesser extent in the presence of Hsp23 than in that of Hsp22. As can be seen following sedimentation on sucrose gradients (Fig 4B), fewer Hsp23 colocalize with luciferase than Hsp22 after a 15-minute heat shock, and luciferase seems to be trapped with Hsp23 in higher molecular mass complexes after 90 minutes of incubation with reticulocyte lysate. This argues for special requirements by each sHsp to interact with ATP-dependent chaperones to accomplish their chaperone-like function.
The 4 main sHsps of D melanogaster can prevent protein aggregation in vitro and maintain unfolded proteins in a refoldable state, albeit with different efficiencies. Refolding can then be performed by ATP-dependent chaperones such as Hsp70, as demonstrated in vitro for pea Hsp18.1 (Lee and Vierling 2000) and Xenopus Hsp30C (Abdulle et al 2002). Thus, D melanogaster sHsps might act as a general molecular chaperones after stress to prevent protein aggregation within their respective cell compartments. This role of general molecular chaperone has recently been shown in vivo for Synechocystis Hsp16.6 (Basha et al 2004a) and S cerevisiae Hsp26 and Hsp42 (Haslbeck et al 2004), which interact with proteins involved in cellular processes as different as transcription, translation, and signalization after heat shock. The functional reason behind the distinctive cell-specific developmental expression pattern of D melanogaster sHsps and their differences in chaperone-like activity remain unknown, but our results suggest the existence of different substrates and requirements for each sHsp to accomplish their function.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) to R.M.T., CIHR and FRSQ-FCAR studentships to G.M., and a grant from the National Sciences and Engineering Research Council of Canada to J.J.H. J.J.H. is a recipient of a Canada Research Chair in Stress Protein Gene Research. We thank Marie-Claire Goulet and Geneviève Robert for technical help.
REFERENCES
- Abdulle R, Mohindra A, Fernando P, Heikkila JJ. Xenopus small heat shock proteins, Hsp30C and Hsp30D, maintain heat- and chemically denatured luciferase in a folding-competent state. Cell Stress Chaperones. 2002;7:6–16. doi: 10.1379/1466-1268(2002)007<0006:xshsph>2.0.co;2.1466-1268(2002)007[0006:XSHSPH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26.1431-6730(1998)379[0019:SSPCTA]2.0.CO;2 [PubMed] [Google Scholar]
- Basha E, Lee GJ, Breci LA, Hausrath AC, Buan NR, Giese KC, Vierling E. The identity of proteins associated with small heat shock protein during heat stress in vivo indicates that these chaperones protect a wide range of cellular functions. J Biol Chem. 2004a;279:7566–7575. doi: 10.1074/jbc.M310684200.0021-9258(2004)279[7566:TIOPAW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Basha E, Lee GJ, Demeler B, Vierling E. Chaperone activity of cytosolic small heat shock proteins from wheat. Eur J Biochem. 2004b;271:1426–1436. doi: 10.1111/j.1432-1033.2004.04033.x.0014-2956(2004)271[1426:CAOCSH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Beaulieu JF, Arrigo AP, Tanguay RM. Interaction of Drosophila 27,000 Mr heat-shock protein with the nucleus of heat-shocked and ecdysone-stimulated culture cells. J Cell Sci. 1989;92:29–36. doi: 10.1242/jcs.92.1.29.0021-9533(1989)092[0029:IODMHP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chowdary TK, Raman B, Ramakrishna T, Rao CM. Mammalian Hsp22 is a heat-inducible small heat-shock protein with chaperone-like activity. Biochem J. 2004;381:379–387. doi: 10.1042/BJ20031958.0264-6021(2004)381[0379:MHIAHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/a:1021601103096.1360-8185(2003)008[0061:OTROHI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Datta SA, Rao CM. Differential temperature-dependent chaperone-like activity of αA- and αB-crystallin homoaggregates. J Biol Chem. 1999;274:34773–34778. doi: 10.1074/jbc.274.49.34773.0021-9258(1999)274[34773:DTCAOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- de Jong WW, Caspers GJ, Leunissen JA. Genealogy of the alpha-crystallin-small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0.0141-8130(1998)022[0151:GOTAHP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221.1460-2075(1997)016[0221:BONPTH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando P, Heikkila JJ. Functional characterization of Xenopus small heat shock protein, Hsp30C: the carboxyl end is required for stability and chaperone activity. Cell Stress Chaperones. 2000;5:48–59. doi: 10.1379/1466-1268(2000)005<0148:fcoxsh>2.0.co;2.1466-1268(2000)005[0048:FCOXSH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonte V, Kapulkin V, Taft A, Fluet A, Frieman D, Link CD. Interaction of intracellular beta amyloid peptide with chaperone proteins. Proc Natl Acad Sci U S A. 2002;99:9439–9444. doi: 10.1073/pnas.152313999.0027-8424(2002)099[9439:IOIBAP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin A, Rayboud-Diogene H, Tetu B, Deschènes R, Huot J, Landry J. Overexpression of the 27 KDa heat shock protein is associated with thermoresistance and chemoresistance but not with radioresistance. Int J Rad Oncol Biol Phys. 2000;46:1259–1266. doi: 10.1016/s0360-3016(99)00410-1.0360-3016(2000)046[1259:OOTKHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408.0193-4511(2002)295[1852:MCITCF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Braun N, Stromer T, Richter B, Model N, Weinkauf S, Buchner J. Hsp42 is the general small heat shock protein in the cytosol of Saccharomyces cerevisiae. EMBO J. 2004;23:638–649. doi: 10.1038/sj.emboj.7600080.1460-2075(2004)023[0638:HITGSH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen S, Saibil HR, Buchner J. Hsp26: a temperature-regulated chaperone. EMBO J. 1999;18:6744–6751. doi: 10.1093/emboj/18.23.6744.1460-2075(1999)018[6744:HATC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449.0027-8424(1992)089[10449:ACFAAM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot J, Roy G, Lambert H, Chrétien P, Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991;51:5245–5252.0008-5472(1991)051[5245:ISATWA]2.0.CO;2 [PubMed] [Google Scholar]
- King V, Tower J. Aging-specific expression of Drosophila hsp22. Dev Biol. 1999;207:107–118. doi: 10.1006/dbio.1998.9147.0012-1606(1999)207[0107:AEODH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Miyakawa M, Matsumura Y, Tsuchido T. Escherichia coli small heat shock proteins, IbpA and IbpB, protect enzymes from inactivation by heat and oxidants. Eur J Biochem. 2002;269:2907–2917. doi: 10.1046/j.1432-1033.2002.02958.x.0014-2956(2002)269[2907:ECSHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Landry J, Chrétien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7.0021-9525(1989)109[0007:HSRCBE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429.0021-9258(1993)268[3420:IOCHHG]2.0.CO;2 [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659.1460-2075(1997)016[0659:ASHSPS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Vierling E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 2000;122:189–197. doi: 10.1104/pp.122.1.189.0032-0889(2000)122[0189:ASHSPC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Mao HP, Ruchalski KL, Wang YH, Choy W, Schwartz JH, Borkan SC. Heat stress prevents mitochondrial injury in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol. 2002;283:C917–C926. doi: 10.1152/ajpcell.00517.2001.0363-6143(2002)283[C917:HSPMII]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lindner RA, Carver JA, and Ehrnsperger M. et al. 2000 Mouse Hsp25, a small shock protein. The role of its C-terminal extension in oligomerization and chaperone action. Eur J Biochem. 267:1923–1932. [DOI] [PubMed] [Google Scholar]
- Marcillat O, Zhang Y, Davies KJA. Oxidative and non-oxidative mechanisms in the inactivation of cardiac mitochondrial electron transport chain components by doxorubicin. Biochem J. 1989;259:181–189. doi: 10.1042/bj2590181.0264-6021(1989)259[0181:OANMIT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin R, Tanguay RM. Stage-specific localization of the small heat shock protein Hsp27 during oogenesis in Drosophila melanogaster. Chromosoma. 1996;105:142–149. doi: 10.1007/BF02509495.0009-5915(1996)105[0142:SLOTSH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Briolay J, Smith L, Diaz-Latoud C, Fabre N, Pauli D, Arrigo AP. Analysis of the resistance to heat and hydrogen peroxide stresses in COS cells transiently expressing wild type or deletion mutants of the Drosophila 27-kDa heat-shock protein. Eur J Biochem. 1993;215:277–284. doi: 10.1111/j.1432-1033.1993.tb18032.x.0014-2956(1993)215[0277:AOTRTH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human Hsp27, Drosophila Hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J. 1996;15:2695–2706.1460-2075(1996)015[2695:HHDHAH]2.0.CO;2 [PMC free article] [PubMed] [Google Scholar]
- Michaud S, Marin R, Tanguay RM. Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol Life Sci. 1997;53:104–113. doi: 10.1007/PL00000572.1420-682X(1997)053[0104:ROHSGI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud S, Morrow G, Marchand J, Tanguay RM. Drosophila small heat shock proteins: cell and organelle-specific chaperones? Prog Mol Subcell Biol. 2002;28:79–101. doi: 10.1007/978-3-642-56348-5_5.0079-6484(2002)028[0079:DSHSPC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Michaud S, Tanguay RM. Expression of the Hsp23 chaperone during Drosophila embryogenesis: association to distinct neural and glial lineages. BMC Dev Biol. 2003;3:9. doi: 10.1186/1471-213X-3-9.1471-213X(2003)003[0009:EOTHCD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Deuerling E, Vorderwulbecke S, Vierling E, Bukau B. Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol Microbiol. 2003;50:585–595. doi: 10.1046/j.1365-2958.2003.03710.x.0950-382X(2003)050[0585:SHSPCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morrow G, Battistini S, Zhang P, Tanguay RM. Decreased lifespan in absence of expression of the mitochondrial small heat shock protein Hsp22 in Drosophila. J Biol Chem. 2004a;279:43382–43385. doi: 10.1074/jbc.C400357200.0021-9258(2004)279[43382:DLIAOE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morrow G, Inaguma Y, Kato K, Tanguay RM. The small heat shock protein Hsp22 of Drosophila melanogaster is a mitochondrial protein displaying oligomeric organization. J Biol Chem. 2000;275:31204–31210. doi: 10.1074/jbc.M002960200.0021-9258(2000)275[31204:TSHSPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, Tanguay RM. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J. 2004b;18:598–599. doi: 10.1096/fj.03-0860fje.0892-6638(2004)018[0598:OOTSMH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morrow G, Tanguay RM. Heat shock proteins and aging in Drosophila melanogaster. Semin Cell Dev Biol. 2003a;14:291–299. doi: 10.1016/j.semcdb.2003.09.023.1084-9521(2003)014[0291:HSPAAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morrow G, Tanguay RM 2003b Molecular chaperones and cellular aging. In: Aging of Cells In and Outside the Body, ed Kaul S, Wadhwa R. Kluwer Academic Press, Lancaster, UK, 207–223. [Google Scholar]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2.1466-1268(2002)007[0167:ACASHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron. 2002;35:9–12. doi: 10.1016/s0896-6273(02)00761-4.0896-6273(2002)035[0009:PMAFAN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863.0066-4154(1997)066[0863:PIIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Panensenko OO, Seit Nebi A, Bukach OV, Marston SB, Gusev NB. Structure and properties of avian small heat shock protein with molecular weight 25 kDa. Biochim Biophys Acta. 2002;1601:64–74. doi: 10.1016/s1570-9639(02)00430-2.0006-3002(2002)1601[0064:SAPOAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Reddy GB, Das KP, Petrash JM, Surewics WK. Temperature-dependent chaperone activity and structural properties of human alphaA- and alphaB-crystallins. J Biol Chem. 2000;275:4565–4570. doi: 10.1074/jbc.275.7.4565.0021-9258(2000)275[4565:TCAASP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rollet E, Lavoie JN, Landry J, Tanguay RM. Expression of Drosophila's 27 kDa heat shock protein into rodent cells confers thermal resistance. Biochem Biophys Res Commun. 1992;185:116–120. doi: 10.1016/s0006-291x(05)80963-5.0006-291X(1992)185[0116:EODKHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sakahira H, Breuer P, Hayer-Hartl MK, Hartl FU. Molecular chaperones as modulators of polyglutamine protein aggregation and toxicity. Proc Natl Acad Sci U S A. 2002;99:16412–16418. doi: 10.1073/pnas.182426899.0027-8424(2002)099[16412:MCAMOP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Robertson JD, and Peterson E. et al. 2001 Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 6:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong K-H, Ogashiwa T, Matsuo T, Fuyama Y, Aigaki T. Application of the gene search system to screen for longevity genes in Drosophila. Biogerontology. 2001;2:209–217. doi: 10.1023/a:1011517325711.1389-5729(2001)002[0209:AOTGSS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Söti C, Csermely P. Chaperones come of age. Cell Stress and Chaperones. 2002;7:186–190. doi: 10.1379/1466-1268(2002)007<0186:ccoa>2.0.co;2.1466-1268(2002)007[0186:CCOA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromer T, Ehrnsperger M, Gaestel M, Buchner J. Analysis of the interaction of small heat shock proteins with unfolding proteins. J Biol Chem. 2003;278:18015–18021. doi: 10.1074/jbc.M301640200.0021-9258(2003)278[18015:AOTIOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Van Boekel MA, de Lange F, de Grip WJ, de Jong WW. Eye lens alphaA- and alphaB-crystallin: complex stability versus chaperone-like activity. Biochim Biophys Acta. 1999;1434:114–123. doi: 10.1016/s0167-4838(99)00178-8.0006-3002(1999)1434[0114:ELAAAC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Van de Ijssel PR, Overkamp P, Knauf U, Gaestel M, de Jong WW. Alpha A-crystallin confers cellular thermoresistance. FEBS Lett. 1994;355:54–56. doi: 10.1016/0014-5793(94)01175-3.0014-5793(1994)355[0054:AACCT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Van Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem. 2002;59:105–156. doi: 10.1016/s0065-3233(01)59004-x.0065-3233(2002)059[0105:SAFOTS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Verbeke P, Fonager J, Clark BFC, Rattan SIS. Heat-shock response and ageing: mechanisms and applications. Cell Biol Int. 2001;25:845–857. doi: 10.1006/cbir.2001.0789.1065-6995(2001)025[0845:HRAAMA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Walter S, Buchner J. Molecular chaperones—cellular machines for protein folding. Angew Chem Int Ed Engl. 2002;41:1098–1113. doi: 10.1002/1521-3773(20020402)41:7<1098::aid-anie1098>3.0.co;2-9.0570-0833(2002)041[1098:MCMFPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang HD, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc Natl Acad Sci U S A. 2004;101:12610–12615. doi: 10.1073/pnas.0404648101.0027-8424(2004)101[12610:MAFIOD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JC, Bieschke ET, Tower J. Muscle-specific expression of Drosophila Hsp70 in response to aging and oxidative stress. Proc Natl Acad Sci U S A. 1995;92:10408–10412. doi: 10.1073/pnas.92.22.10408.0027-8424(1995)092[10408:MEODHI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieske M, Benndorf R, Behlke J, Dölling R, Grelle G, Bielka H, Lutsch G. Defined sequence segments of the small heat shock proteins HSP25 and αB-crystallin inhibit actin polymerization. Eur J Biochem. 2001;268:2083–2090. doi: 10.1046/j.1432-1327.2001.02082.x.0014-2956(2001)268[2083:DSSOTS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 1990;265:16330–16336.0021-9258(1990)265[16330:TOIOME]2.0.CO;2 [PubMed] [Google Scholar]