Abstract
p26, an abundantly expressed small heat shock protein, is thought to establish stress resistance in oviparously developing embryos of the crustacean Artemia franciscana by preventing irreversible protein denaturation, but it might also promote survival by inhibiting apoptosis. To test this possibility, stably transfected mammalian cells producing p26 were generated and their ability to resist apoptosis induction determined. Examination of immunofluorescently stained transfected 293H cells by confocal microscopy demonstrated p26 is diffusely distributed in the cytoplasm with a minor amount of the protein in nuclei. As shown by immunoprobing of Western blots, p26 constituted approximately 0.6% of soluble cell protein. p26 localization and quantity were unchanged during prolonged culture, and the protein had no apparent ill effects on transfected cells. Molecular sieve chromatography in Sepharose 6B revealed p26 oligomers of about 20 monomers, with a second fraction occurring as larger aggregates. A similar pattern was observed in sucrose gradients, but overall oligomer size was smaller. Mammalian cells containing p26 were more thermotolerant than cells transfected with the expression vector only, and as measured by annexin V labeling, Hoescht 33342 nuclear staining and procaspase-3 activation, transfected cells effectively resisted apoptosis induction by heat and staurosporine. The ability to confer thermotolerance and limit heat-induced apoptosis is important because Artemia embryos are frequently exposed to high temperature in their natural habitat. p26 also blocked apoptosis in transfected cells during drying and rehydration, findings with direct relevance to Artemia life history characteristics because desiccation terminates cyst diapause. Thus, in addition to functioning as a molecular chaperone, p26 inhibits apoptosis, an activity shared by other small heat shock proteins and with the potential to play an important role during Artemia embryo development.
INTRODUCTION
Oviparously developing embryos of the crustacean Artemia franciscana possess an abundant small heat shock protein (sHSP) termed p26, which is thought to have an important role in stress resistance (Clegg et al 1995; Jackson and Clegg 1996; Liang et al 1997a, 1997b; Liang and MacRae 1999; Viner and Clegg 2001; Crack et al 2002; Day et al 2003; MacRae 2003; Sun et al 2004). As examples of stress tolerance, encysted Artemia embryos (cysts) survive diapause, a genetically predetermined process in which development and metabolism are arrested (Clegg et al 1996; Jackson and Clegg 1996; Clegg and Jackson 1998; MacRae 2001, 2003, 2005). The embryos are indifferent to desiccation, which normally kills most organisms but is probably required for diapause termination in Artemia (Drinkwater and Crowe 1987), and they endure anoxia-imposed quiescence lasting several years (Clegg 1997; Clegg and Jackson 1998; Clegg et al 2000; Warner and Clegg 2001). Like sHSPs from other species (MacRae 2000; Haslbeck 2002; Narberhaus 2002; Laksanalamai and Robb 2004; Sun and MacRae 2005a, 2005b), p26 forms oligomers and functions as a molecular chaperone in vitro, preserving citrate synthase, insulin, and tubulin against denaturation and aggregation (Viner and Clegg 2001; Day et al 2003; Sun et al 2004, 2005; Sun and MacRae 2005c). p26 confers thermotolerance on transformed bacteria (Liang and MacRae 1999; Crack et al 2002, Sun et al 2004, 2005; Sun and MacRae 2005c), and with the reducing sugar trehalose, it protects transfected mammalian cells during drying and rehydration (Ma et al 2005).
p26 represents approximately 10% of the soluble protein in Artemia cysts, an amount sufficient to prevent irreversible denaturation of key proteins required for embryo growth on termination of diapause and quiescence. However, sHSPs function in other ways that might occur in Artemia. They bind membranes in Mycobacterium tuberculosis (Zhang et al 2005), Synechocystis sp (Török et al 2001; Tsvetkova et al 2002), and the mammalian lens (Cobb and Petrash 2000) by influencing fluidity. sHSPs interact with cytoskeletal elements such as actin (Mounier and Arrigo 2002; Panasenko et al 2003), tubulin (Day et al 2003), and intermediate filaments (Wang et al 2003; Duverger et al 2004), shielding against insult, modulating polymerization, and affecting cell motility. Intact cytoskeletal elements are essential to mitosis and cell division, both of which are delayed during Artemia postdiapause development until larvae emerge from the cyst shell (Nakanishi et al 1962; Olson and Clegg 1978). p26 might bind tubulin in encysted Artemia embryos and prevent microtubule formation until the sHSP is sufficiently depleted during postdiapause development (Day et al 2003). sHSPs, including p26, reversibly enter nuclei, in which they associate with splicing-associated speckles, nucleoli, and lamins with uncertain cellular consequences (Liang and MacRae 1999; Willsie and Clegg 2002; Adhikari et al 2004; den Engelsman et al 2004). Another important process influenced by sHSPs is apoptosis (Arrigo et al 2005; Fan et al 2005; Kamradt et al 2005). In this paper, the crustacean small heat shock protein p26 is shown to confer thermotolerance on stably transfected mammalian cells and inhibit apoptosis induction on exposure to heat shock, drying/rehydration, and staurosporine. By extrapolation, p26 suppresses apoptosis in Artemia, increasing the protective capacity of the sHSP during diapause and on exposure to other physiological stresses experienced by encysted embryos.
MATERIALS AND METHODS
Eukaryotic cell transfection
Purified p26 cDNA excised from pRSETC-p26-3-6-3 (Liang et al 1997b) with BamHI and XhoI was inserted into linearized pSecTag2A (Invitrogen, Burlington, ON, Canada) and used to transform Escherichia coli DH5α (Invitrogen). The identity of the cDNA insert was confirmed by sequencing (DNA Sequencing Facility, Centre for Applied Genomics, Hospital for Sick Children, Toronto, ON, Canada). The p26 cDNA-containing construct was used to transfect 293H human kidney cells maintained at 37°C under 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) containing 10% fetal bovine serum (FBS; Invitrogen) and 1% antibiotic-antimycotic (Invitrogen) as reported previously (Ma et al 2005). Transfection mixtures containing 250 μL of DMEM lacking FBS and antibiotic; 1 μg of pSecTag2A plasmid, either with or without p26 cDNA; and 3 μL of Lipofectamine 2000 (Invitrogen) were mixed, incubated at room temperature for 20 minutes, then added to each well of a 6-well plate containing 293H cells grown to 80–85% confluence before incubation at 37°C for 24 hours. The transfection mixtures were replaced with fresh DMEM containing FBS, and incubation continued 24 hours. The cells were trypsinized; collected by centrifugation at 80 × g for 5 minutes; resuspended in DMEM supplemented with FBS, antibiotic-antimycotic, and 200 μg/mL Zeocin™ (Invitrogen); and incubated at 37°C for 2 weeks in 100-mm tissue culture plates. Individual colonies, transferred to 96-well plates and maintained in selection medium containing Zeocin, were expanded and screened for p26.
Immunodetection of p26
Cells grown to 60% confluence on coverslips for immunofluorescent staining were rinsed twice with phosphate-buffered saline (PBS; 140 mM NaCl, 2.7 mM KCl, 8.0 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4), fixed with 4% paraformaldehyde for 20 minutes at room temperature, rinsed twice with PBS, and permeabilized with PBS containing 0.2% Triton X-100 (Sigma, Oakville, ON, Canada) for 8 minutes. The cells were rinsed twice with PBS containing 0.2% Triton X-100 and incubated for 20 minutes with anti-p26 antibody (Liang et al 1997a) diluted in PBS containing 0.2% Triton X-100. Three washes of 3 minutes each with PBS containing 0.2% Triton X-100 preceded incubation for 20 minutes in AlexaFlour®488-labeled, anti-rabbit IgG secondary antibody (Molecular Probes, Eugene, OR, USA) diluted in PBS containing 0.2% Triton X-100. The cells were washed 3 times with PBS containing 0.2% Triton X-100, incubated with 5 mg/mL RNaseA (Amersham Biosciences, Baie d'Urfé, Quebec, Canada) for 10 minutes, washed twice in PBS, and incubated for 2 minutes with 0.40 μg/μL propidium iodide (Sigma). The coverslips were rinsed with PBS, dipped in H2O, inverted onto 12 μL of mounting medium (0.2 M 1,4-diazabicyclo[2.2.2]octane [DABCO] in 80% glycerol), sealed with clear nail polish, and viewed with a Zeiss confocal laser scanning microscope.
For immunoprobing of Western blots, transfected mammalian cells grown to 100% confluence in 100-mm tissue culture plates were rinsed with PBS; suspended in 100 μL of PBS containing 0.04 mg/mL each of soybean trypsin inhibitor (Sigma), leupeptin (Sigma), and pepstatin A (Sigma) and 0.08 mg/mL phenylmethylsufonyl fluoride (PMSF; Sigma); and sheared with a 25-gauge needle before centrifugation at 12 000 × g for 10 minutes at 4°C. Supernatant protein concentrations were determined with the Bio-Rad protein assay (BioRad, Mississauga, ON, Canada). Protein samples, including Artemia cyst extracts (Sun et al 2004), were electrophoresed in 12.5% sodium dodecyl sulfate (SDS) polyacrylamide gels and either stained with Coomassie blue or blotted to nitrocellulose. Western blots were blocked with 5% low-fat Carnation milk powder in TBS-Tween (10 mM Tris-HCl, 140 mM NaCl, 0.1% Tween 20, pH 7.4) for 30 minutes and incubated for 15 minutes in anti-p26 antibody (Liang et al 1997a) diluted in TBS-Tween. The blots were washed 5 times for 3 minutes with TBS-Tween, incubated for 15 minutes with HRP-conjugated goat anti-rabbit IgG antibody (Jackson Immunologicals) diluted in HST (10 mM Tris-HCl, 1 M NaCl, 0.5% Tween 20, pH 7.4), and washed 5 times for 3 minutes in TBS-Tween, followed by 1 wash for 5 minutes in TBS (10 mM Tris-HCl, 140 mM NaCl, pH 7.4). p26 was detected with the Western Lighting Chemiluminescence Reagent Plus (Perkin Elmer Life Sciences, Boston, MA, USA) and Super RX FujiFilm.
p26 was quantified in protein extracts prepared from transfected mammalian cells by electrophoresing concurrently in 12.5% SDS polyacrylamide gels with protein extracts from nontransfected cells to which known amounts of bacterially produced p26 had been added (Sun et al 2004). Proteins were transferred to nitrocellulose membranes and probed with antibody, and p26 band density was measured at 400 dpi with a UMAX Astra 1200S scanner (UMAX Technologies, Inc, Dallas, TX, USA) for comparison to a standard curve generated each time p26 was measured. Standard curves were produced with the Trendline function of Microsoft Excel by plotting p26 band pixel densities obtained with the AnalystPro300 Soft Imaging System GmbH program (Lakewood, CO, USA) against known amounts of bacterially produced p26.
p26 oligomerization
p26 oligomer mass was determined by chromatography of cell-free protein extracts in 0.1 M Tris-glycine buffer, pH 7.4, at a flow rate of 5–10 mL/h in a Sepharose 6B (Sigma) column (48 cm × 1.0 cm) with collection of 1-mL samples. Protein extracts from transfected mammalian cells were also centrifuged at 288,000 × g for 15 hours at 4°C in 10 mL, 10–50% continuous sucrose gradients in 0.1 M Tris-glycine buffer, pH 7.4, with a Beckman SW41 Ti rotor and Beckman Ultra-Clear™ tubes (14 × 89 mm) (Beckman Coulter Canada Inc, Ville Saint-Laurent, Quebec, Canada). The tubes were bottom-punctured with an 18-gauge needle, and 1-mL samples were collected. Pellets were recovered in the first sample. Samples from gradients and columns were electrophoresed in 12.5% SDS polyacrylamide gels, blotted to nitrocellulose, and probed with anti-p26 antibody.
Thermotolerance of transfected cells
Stably transfected mammalian cells were seeded at 5 × 105 cells/mL in 30-mm dishes and incubated at 37°C for 24 hours. The dishes were sealed with Parafilm, heated in a VWR programmable water bath (VWR International, Mississauga, ON, Canada) at 46°C for up to 1 hour and then incubated for 24 hours at 37°C. Nonattached and trypsinized cells were pooled, centrifuged at 80 × g for 5 minutes and suspended in 2 mL of cold PBS. To ascertain cell viability, 0.5 mL of the PBS cell suspension was added to 24 mL of DMEM supplemented with FBS, antibiotic-antimycotic, and Zeocin in a T75 vented tissue culture flask and incubated 2 weeks. The cells were rinsed in PBS, stained with crystal violet (0.5% crystal violet [Sigma], 50% methanol) for 15 minutes, rinsed gently with PBS, air dried, and scanned with a UMAX Astra 1200S scanner at 400 dpi. Scan density, in arbitrary units, was determined with the AnalystPro300 Soft Imaging System GmbH program. Percentages were calculated by comparing the scan density of each flask with the flask density after heating for 1 hour, which killed all cells. Experiments were done in triplicate.
Apoptosis in heat-stressed cells
Heat-stressed mammalian cells, prepared as described, were examined for entry into apoptosis by suspension in 100 μL of annexin V binding buffer (10 mM HEPES, 140 mM NaCl, 25 mM CaCl2, pH 7.4), followed by addition of 25 μL of annexin V-Alexa Fluor®488 conjugate (Molecular Probes) and 2 μL of 10 mg/mL propidium iodide (Molecular Probes). The cells were placed on poly-l-lysine–coated coverslips, incubated for 15 minutes in the dark at room temperature, rinsed with annexin V binding buffer, fixed in cold 4% paraformaldehyde for 30 seconds, rinsed with PBS, and inverted on 12 μL of mounting medium. Alternatively, 0.5 mL of cell suspension was applied to poly-l-lysine–coated coverslips for 15 minutes, rinsed in PBS, fixed 30 seconds in cold 4% paraformaldehyde, and rinsed with PBS before staining. The cells were also stained with 10 μg/mL Hoescht 33342 (Molecular Probes). All coverslips were sealed with clear nail polish and viewed with a Leitz Aristoplan fluorescence microscope. Experiments were performed at least in triplicate, and 500 or more cells were counted each time.
Apoptosis in transfected cells during drying and rehydration
Stably transfected mammalian cells, vacuum dried in the presence of trehalose at room temperature in 50-μL round droplets to 0.35 g H2O per gram dry weight, were rehydrated with DMEM supplemented with 10% bovine calf serum, 1% penicillin/streptomycin, 1% MEM nonessential amino acids solution, 0.05% Zeocin, and 1% HEPES buffer solution (Ma et al 2005). Cells were then incubated in either the presence or absence of 30 μM OPH-109, a pan-caspase inhibitor (MP Biomedicals, Irvine, CA, USA) at 37°C for 24 hours in medium containing 100 mM trehalose. To quantitate apoptosis and viability, cells were stained with FITC-DEVD-FMK, a fluorescently labeled inhibitor of activated caspase-3 (Oncogene Research Products, San Diego, CA, USA), and propidium iodide before analysis in a Beckman Coulter Cytomics FC 500 flow cytometry system.
Apoptosis in staurosporine-exposed cells
Transfected mammalian cells seeded at 5 × 105 cells/mL in 30-mm dishes were incubated at 37°C for 24 hours. Growth medium was removed, fresh medium containing 0.2 μM staurosporine (Sigma) was added, and cells were incubated at 37°C for up to 30 hours. Nonattached and trypsinized cells were pooled, centrifuged at 80 × g for 5 minutes, suspended in 2 mL of cold PBS, and stained with either annexin V-Alexa Fluor®488 conjugate and 2 μL of 10 mg/mL propidium iodide or 10 μg/mL Hoescht 33342.
Inhibition of procaspase-3 activation
Nonattached and trypsinized cells, obtained 6 hours after either heat shock or staurosporine exposure, were pooled, centrifuged at 80 × g for 5 minutes, rinsed with cold PBS, resuspended in 2× treatment buffer (0.25 M Tris, 5.5 M glycerol, 3 M 2-mercaptoethanol, 8% SDS), and heated in a boiling water bath for 3 minutes. The samples were electrophoresed in 12.5% SDS–polyacrylamide gels, blotted to nitrocellulose, and probed with a mouse monoclonal antibody to caspase-3 (IMGENEX, San Diego, CA, USA) followed by HRP-conjugated goat anti-mouse IgG antibody.
RESULTS
p26 in stably transfected mammalian cells
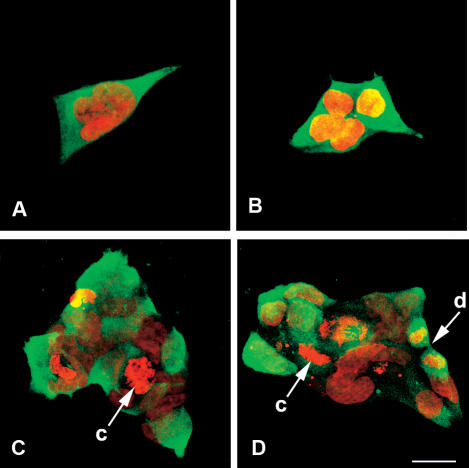
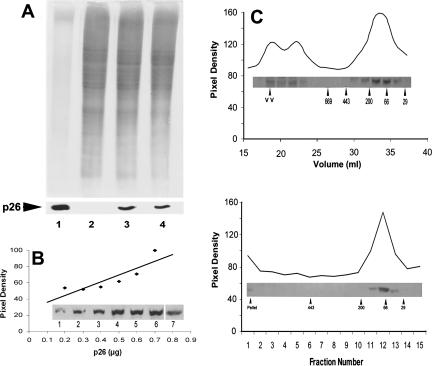
Transfection of 293H cells with the p26 cDNA–containing eukaryotic expression vector pSecTag2A, followed by selection in Zeocin, yielded several stably transfected cell lines that varied in staining intensity on exposure to anti-p26 antibody. The clone designated E11‘L, shown previously to possess enhanced dehydration tolerance in the presence of trehalose (Ma et al 2005), was selected for further examination because it reacted strongly with anti-p26 antibody and fluorescence was relatively constant from cell-to-cell, although a few cells stained weakly or failed to react with antibody. Nontransfected cells and those transfected with vector only lacked immunofluorescent staining with antibody to p26. The E11‘L line contained single cells, some with morphologically complex nuclei (Fig 1A), small clumps of cells (Fig 1B), and larger clumps of varying size (Fig 1C, D), as well as cells in monolayer sheets that looked very much like nontransfected lines (see Fig 3C). p26 was mainly cytoplasmic with minor quantities in nuclei. As revealed by propidium iodide, some transfected cells contained chromosomes, an observation indicative of growth and division (Fig 1C, D). Immunoprobing of Western blots containing E11‘L protein extract resolved in SDS polyacrylamide gels revealed a reactive band of the size expected for p26, confirming synthesis of the protein (Fig 2A). p26 remained at 0.6% ± 0.2% of total soluble protein during extended culture of the E11‘L line (Fig 2B), and the protein caused no apparent detrimental effects on the cells. p26 oligomers in E11‘L cell extracts reached approximately 400 kDa in mass (20 subunits) as determined by Sepharose 6B chromatography, but only 150 kDa (6 subunits) if measured by sucrose density gradient centrifugation (Fig 2C). Most p26 oligomers contained 4 to 8 subunits, with large aggregates eluting near the void volume of Sepharose 6B columns and forming pellets on centrifugation. pSecTag2A, one of many vectors tested during this work for p26 production in mammalian cells, is a secretion vector, but p26 was not detected in medium used for culture of E11‘L to confluence, even when medium was concentrated 5-fold (Ma et al 2005). Additionally, E11‘L cell cytoplasm lacked discrete staining foci indicating p26 was not in membrane-bound cytoplasmic organelles.
Fig 1.
Immunostaining of E11′L cells. E11′L cells immunostained with antibody to p26 and Alexa Fluor®488-labeled anti-rabbit IgG secondary antibody (green) were incubated with propidium iodide to reveal nuclei (red) and examined by confocal microscopy. c, chromosomes; d, dividing cell. Bar = 25 μm (D). All figures are the same magnification
Fig 3.
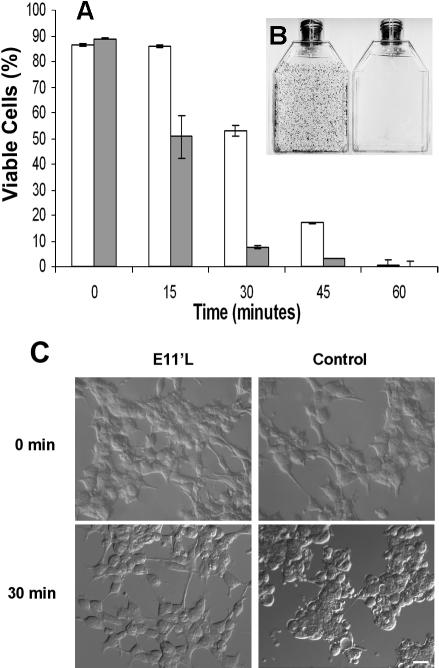
p26-dependent thermotolerance in transfected mammalian cells. (A) E11′L cells and cells transfected with vector only were heat shocked for 0, 15, 30, 45, and 60 min at 46°C; incubated in T75 vented tissue culture flasks for 2 weeks; and stained with crystal violet. Nonshaded bar, E11′L; shaded bar, cells lacking p26. The data are the mean ± standard error of 3 independent experiments. (B) Crystal violet–stained flasks containing E11′L (left side) and control (right side) cells heat shocked for 30 min before incubation for 2 weeks. (C) Nomarski images of cells either containing (E11′L) or lacking (control) p26 after heat shock for 30 minutes. The bar in the lower right panel represents 50 μm, and all pictures are the same magnification
Fig 2.
p26 from E11′L cells. (A) Protein extracts from E11′L cells were electrophoresed in 12.5% SDS polyacrylamide gels and either stained with Coomassie blue (upper panel) or blotted to nitrocellulose and immunostained with antibody to p26 (lower panel). Lane 1: 10.0 and 5.0 μg of Artemia embryo protein for gel and blot, respectively; lane 2: 30 μg of protein extract from 293H cells transfected with vector only; lane 3: 30 μg of protein extract from E11′L cells early in culture; lane 4: 30 μg of protein extract from E11′L cells after 50 days in culture. (B) E11′L protein extract and purified p26 produced in bacteria were electrophoresed concurrently in 12.5% gels, blotted to nitrocellulose, probed with anti-p26 antibody, and scanned with the UMAX Astra 1200S scanner. The amount of p26 was determined by comparing the p26 band pixel density in the E11′L lane to the standard curve derived with bacterially produced p26. Insert lane 1: 0.2 μg; lane 2: 0.3 μg; lane 3: 0.4 μg; lane 4: 0.5 μg; lane 5: 0.6 μg; lane 6: 0.7 μg of protein. Lane 7 contained 40 μg of E11′L cell protein. (C) Protein extracts from E11′L cells were either chromatographed in Sepharose 6B columns (upper panel) or centrifuged in 10–50% continuous sucrose gradients (lower panel). Collected fractions were electrophoresed in 12.5% SDS polyacrylamide gels, blotted to nitrocellulose, and immunostained with antibody to p26 (inserts). Labeled arrowheads, molecular mass markers × 10−3
Enhanced thermotolerance of E11′L cells
E11‘L cells were significantly more resistant to heating than cells transfected with vector only (Fig 3). Almost 100% of E11‘L cells were viable after 15 minutes at 46°C compared with 58% of controls transfected with vector only. Enhanced survival of E11‘L cells occurred at other time points, with the most pronounced disparity at 30 minutes, but there was no difference after 1 hour at 46°C. Variation in survival was readily apparent on visual examination of culture flasks stained with crystal violet (Fig 3B), and cells lacking p26 were more rounded and granular after heating than were those possessing the protein (Fig 3C).
p26 reduces apoptosis in heat-stressed cells
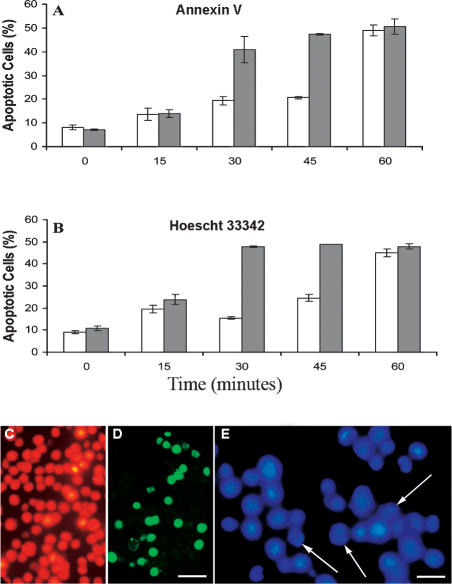
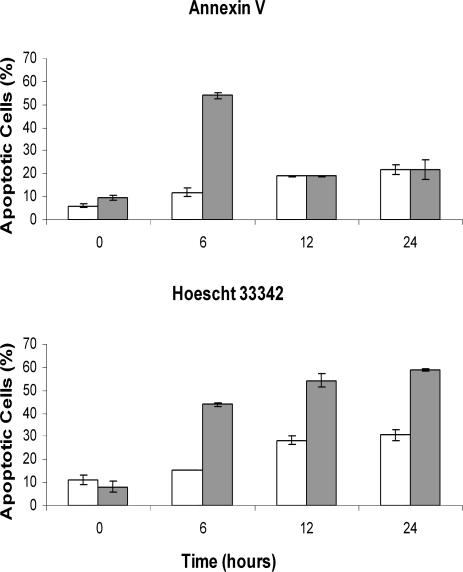
Less than 20% of E11‘L cells exposed to a 30-minute heat shock and then incubated for 24 hours showed annexin V staining, whereas under the same conditions, over 40% of cells transfected with vector only were apoptotic (Fig 4A, C, D). Similar results were seen after 45 minutes of heat shock, but after 60 minutes, apoptosis was almost identical in both cell types. The findings were comparable to those obtained by staining with Hoescht 33342 (Fig 4B, E), confirming that p26 inhibited heat-induced apoptosis.
Fig 4.
p26 reduces heat-induced apoptosis. E11′L and cells transfected with vector only were heat shocked at 46°C for the times indicated, incubated at 37°C for 24 hours, and stained with either annexin V-Alexa Fluor®488 conjugate (A) or Hoescht 33342 (B). Nonshaded bar, E11′L cells; shaded bar, cells lacking p26. The data are the mean ± standard error of 3 independent experiments. Cells heat shocked for 30 minutes were either double-stained with propidium iodide (red) and annexin V-Alexa Fluor®488 conjugate (green) (C, D) or stained with Hoescht 33342 (E). Arrows in panel E indicate apoptotic cell nuclei. Bar = 40 μm (D), which has the same magnification as (C). Bar = 15 μm (E)
p26 limits apoptosis induction by dehydration and rehydration
On drying and rehydration, apoptosis was significantly reduced and viability increased in E11‘L cells compared with cells transfected with vector only (Fig 5). Approximately 15% of trehalose-containing E11‘L cells were apoptotic after drying and rehydration, as shown by staining with FITC-DEVD-FMK and propidium iodide, in contrast to approximately 45% of cells lacking p26 (Fig 5A, C, E). OPH-109 had little effect on E11‘L cells, presumably because p26 suppressed caspase activation (Fig 5C–E), whereas exposure to this pan-caspase inhibitor tended to decrease apoptosis and increase viability in cells lacking p26 (Fig 5A, B, E).
Fig 5.
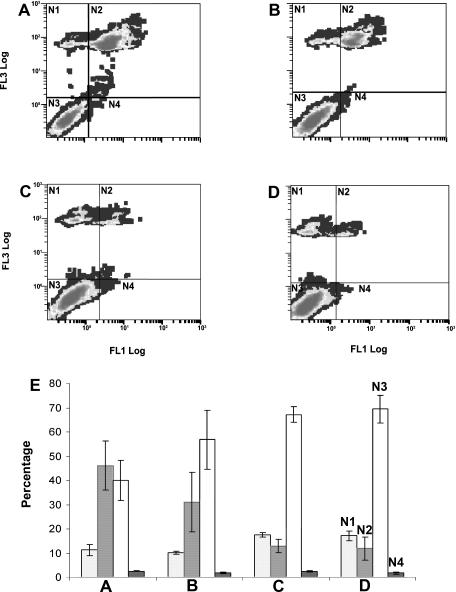
p26 blocks apoptosis on cell drying and rehydration. E11′L cells and cells transfected with vector only were vacuum dried to 0.35 g H2O per gram dry weight, rehydrated, incubated in either the presence or absence of the pan-caspase inhibitor OPH-109, then stained with FITC-DEVD-FMK, a fluorescently labeled inhibitor of activated caspase-3, and propidium iodide before analysis by flow cytometry. (A) 293H cells transfected with vector only; (B) 293H cells transfected with vector only and incubated with OPH-109; (C) E11′L cells; (D) E11′L cells incubated with OPH-109. (E) Experiments in panels A–D were done in triplicate; the results were averaged and plotted with standard error. N1, dead cells; N2, apoptotic and dead cells; N3, live cells; N4, apoptotic cells
p26 decreases apoptosis in cells exposed to staurosporine
E11‘L cells were more resistant to staurosporine exposure, a commonly used apoptosis inducer, than were cells lacking p26 (Fig 6). After a 6-hour exposure to staurosporine, approximately 12% of E11‘L cells exhibited annexin staining, whereas 55% of 293H cells transfected with vector only were apoptotic (Fig 6A). This difference was transient and not observed following 12 hours or more of staurosporine treatment, reflecting increased difficulty in detecting annexin-stained cells because of membrane fragmentation. Staining with Hoescht 33342 verified that p26 inhibited staurosporine-induced apoptosis with approximately 2 to 3 times more apoptotic cells after 6 hours or more of staurosporine exposure in cells lacking p26 compared with those with the protein (Fig 6B).
Fig 6.
p26 reduces staurosporine-induced apoptosis. E11′L cells and cells transfected with vector only were treated with 0.2 M staurosporine for the times indicated and then stained with either annexin V-Alexa Fluor®488 conjugate (A) or Hoescht 33342 (B). Nonshaded bar, E11′L cells; shaded bar, cells lacking p26
Inhibition of procaspase-3 activation by p26
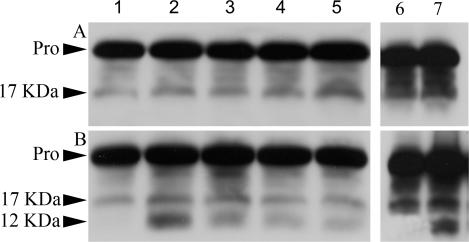
Reduction in apoptosis suggested that procaspase-3 activation is inhibited. This possibility was investigated by probing cell-free protein extracts for procaspase-3 fragmentation, which is indicative of enzyme activation. Heat shock and staurosporine exposure generated a 12-kDa caspase fragment in 293H cells lacking p26, but not in E11‘L cells, demonstrating that lower levels of apoptosis were accompanied by reduced caspase-3 activation (Fig 7). The 17-kDa fragment increased moderately in heated E11‘L cells, but the 12-kDa fragment was not observed even after 1 hour at 46°C. In contrast, procaspase-3 fragmentation reached its maximum in cells lacking p26 after 15 minutes of heat treatment and then declined. Procaspase-3 fragmentation was also different after exposure to staurosporine for 6 hours, the only time tested.
Fig 7.
Inhibition of procaspase-3 activation by p26. E11′L cells (A) and cells transfected with vector only (B) were either heat shocked at 46°C or exposed to staurosporine for 6 hours, as described in Materials and Methods. Protein extracts were prepared from these cells, electrophoresed in 12.5% SDS polyacrylamide gels, blotted to nitrocellulose, and probed with anti–caspase-3 antibody. Lanes 1– 5: protein extracts from cells heat shocked for 0, 15, 30, 45, and 60 minutes, respectively. Lanes 6, 7: extracts from cells exposed to 0.0 and 0.2 μm, respectively, of staurosporine for 6 hours. Approximately equal amounts of protein were applied to each lane. Pro, procaspase-3; 17 kDa, 17-kDa fragment from procaspase-3; 12 kDa, 12-kDa fragment from procaspase-3
DISCUSSION
p26 is thought to function as a molecular chaperone, preventing irreversible protein denaturation in oviparously developing Artemia embryos (Liang et al 1997a; Liang and MacRae 1999; Viner and Clegg 2001; Crack et al 2002; Day et al 2003; Sun et al 2004; Sun and MacRae 2005c). Protection is important because encysted Artemia embryos must survive greatly reduced metabolic activity associated with diapause, as well as high temperature, anoxia, and desiccation (Clegg et al 1996; Jackson and Clegg 1996; Clegg 1997; Clegg and Jackson 1998; Clegg et al 2000; Warner and Clegg 2001; MacRae 2003). In vitro experiments indicate protection is due to chaperoning; however, p26 might function in other ways, including insertion into membranes and inhibition of apoptosis, properties not previously investigated for this sHSP. The purpose of this study was to determine whether p26 possesses anti-apoptotic activity, thereby indicating a second way the protein shields oviparous Artemia embryos while contributing to the general appreciation of sHSP function.
Several vectors were employed in conjunction with immunofluorescent staining to obtain stably transfected mammalian cells synthesizing p26, and unexpectedly, the pSecTag2A vector designed for secretion of exogenous protein proved most successful. The E11‘L line chosen for experiments described in this paper exhibited diffuse p26 distribution within the cytoplasm. Moreover, the protein was not detected extracellularly by immunoprobing of Western blots with the enhanced chemiluminescence procedure, even though cell confluence was reached in culture dishes and growth medium used in experiments was concentrated 5-fold before electrophoreses and blotting (Ma et al 2005). Why p26 failed to exit transfected cells is unknown but might reflect the general properties of sHSPs, such as oligomerization and interaction with protein substrates, or a characteristic peculiar to p26. Approximately 0.6% of the soluble protein from E11‘L cells was p26 compared with 0.05–0.22% for sHSPs, including murine fibrosarcoma L929 cell Hsp27 (Paul et al 2002), human αA- and αB-crystallins in human lens epithelial cells (Mao et al 2004), and mouse lens epithelial cell αB-crystallin (Andley et al 2000). p26 production was maintained during long-term culture with no apparent effects on cells, which is of interest because p26 binds tubulin and could disrupt cell activities by inhibiting microtubule-dependent processes (Day et al 2003). However, because transfected cells grow and divide, interaction with p26 appears to protect tubulin in stressed cells but not provide a mechanism to modulate cell growth and division per se. Oligomerization is important for sHSP function (Sun and MacRae 2005b), and the predominant size for p26 in E11‘L cells is 4–8 subunits, although as indicated by Sepharose 6B chromatography, some oligomers consisted of 20 subunits. p26 maximum oligomer size was smaller when determined by sucrose density gradient centrifugation compared with Sepharose 6B chromatography, reflecting differences in the methods. Nonetheless, p26 oligomers smaller on average than those in encysted Artemia embryos (Liang et al 1997a) prevent death and apoptosis in heat-stressed mammalian cells. Previous analysis demonstrated that other HSPs did not increase in response to p26 in the E11‘L line (Ma et al 2005).
p26 is shown for the first time to confer thermotolerance on eukaryotic cells and, in concert with trehalose, to protect cells during dehydration (Ma et al 2005). Additionally, staining with annexin V-Alexa Fluor®488 conjugate, which binds to the plasma membrane of cells early in apoptosis, and Hoescht 33342, used to detect fragmented or condensed nuclei, demonstrated that p26 reduced apoptosis induction by heating more than 50%. Similar results were obtained when cells were exposed to staurosporine. Procaspase-3 fragmentation, indicative of enzyme activation, was suppressed by p26 in cells on heat shock or exposure to staurosporine, indicating a direct association between levels of caspase-3 activity and apoptosis. Dehydration/rehydration-induced apoptosis was repressed substantially by p26 and, as shown by indifference to the pan-caspase inhibitor OPH-109, caspase was inhibited in rehydrated cells containing p26.
Examination of other sHSPs suggests how p26 modulates apoptosis, a process that might vary depending on how apoptosis is induced. As one example, heat-induced release of cytochrome c from L929 cell mitochondria, an early apoptotic event, is decreased by Hsp27, repressing caspase activation and cell death (Samali et al 2001; Paul et al 2002). Hsp27 suppresses apoptosis in U937 human leukemic cells on exposure to ectoposide, a topoisomerase II–reactive chemical, by interacting with cytochrome c, thus interfering with ATP-dependent activation of procaspase-9 and subsequently procaspase-3 by apoptotic protease activating factor (Garrido et al 1999; Bruey et al 2000). Hsp27 interferes with cytochrome c release caused by the protein kinase inhibitor staurosporine, perhaps by intracellular signaling related to microfilament damage, as is thought to occur in transfected murine fibrosarcoma L929 cell lines (Paul et al 2002). The ability of human Hsp27 to prevent apoptosis initiation by staurosporine in murine L929 cells is shared by Drosophila DHsp27 and human αA- and αB-crystallins, suggesting that inhibition depends on the conserved α-crystallin domain (Mehlen et al 1996; Arrigo 1998; Andley et al 2000; Paul et al 2002). Hsp27 also slows apoptosis by interacting directly with procaspase-3 (Pandey et al 2000; Concannon et al 2003).
Human αB-crystallin hinders apoptosis in lens epithelial cells exposed to UVA by suppressing the Raf/MEK/ ERK signaling pathway, whereas αA-crystallin prevents apoptosis by activating AKT kinase (Liu et al 2004). As another mechanism, human αA- and αB-crystallins interact with the apoptosis-promoting Bcl-2 family members, Bax and Bcl-Xs, preventing movement from cytosol into mitochondria and inhibiting staurosporine-induced apoptosis by preserving mitochondrial integrity and limiting cytochrome c release (Mao et al 2004). αB-crystallin protects cancer cells against caspase-3 activation and apoptosis on exposure to tumor necrosis factor–related apoptosis-inducing ligand by a mechanism that might depend on αB-crystallin oligomerization (Kamradt et al 2005). αB-crystallin averts apoptosis by H2O2 by interacting with procaspase-3 and partially processed procaspase-3 in rabbit lens epithelial cells N/N1003A (Mao et al 2001) and human MDA-MB-231 cells (Kamradt et al 2001), thereby antagonizing caspase-3 processing. The proteolytic activation of caspase-3 caused by growth factor deprivation of cultured murine myogenic C2C12 cells is repressed by αB-crystallin (Kamradt et al 2002).
Relationships between sHSPs, development, and cell differentiation are of interest in the context of p26 function during Artemia diapause, but studies on these relationships are limited. Early transient expression of Hsp27 counters apoptosis that occurs normally during dopamine-dependent differentiation of rat olfactory neuroblasts, suggesting that Hsp27 influences the decision between death and differentiation (Mehlen et al 1999). Constitutive overexpression of Hsp27 enhances the early phase of CGR8 murine embryonic stem cell differentiation accompanied by reduced cell proliferation, whereas decreasing Hsp27 leads to greater cell death (Mehlen et al 1997). In addition, growth factor deprivation in myoblasts causes either differentiation into multinucleated myoblasts or apoptosis. Myogenic precursors must acquire resistance to apoptosis if they are to develop, which might depend on caspase-3 inhibition by αB-crystallin but not Hsp27 early in differentiation (Kamradt et al 2002).
The sHSPs modulate apoptosis by reacting with cytochrome c, signaling pathway molecules, mitochondrial interactive proteins, and procaspase-3, clearly regulating both mitochondrial and death receptor apoptotic pathways. Determining how p26 inhibits apoptosis in transfected mammalian cells will provide an interesting perspective on sHSP function and indicate how p26 protects encysting Artemia embryos from apoptosis, a capability of the protein shown for the first time in this report.
Acknowledgments
This work was supported by a Nova Scotia Health Research Foundation/Canadian Institutes of Health Regional Partnership Plan Grant, a Heart and Stroke Foundation of Nova Scotia Grant, DARPA contract N00173-01-1-G016, DARPA grant N66001-02-C-8055, and a NSHRF Student Fellowship to Y.S.
REFERENCES
- Adhikari AS, Rao KS, Rangaraj N, Parnaik VK, Rao ChM. Heat stress-induced localization of small heat shock proteins in mouse myoblasts: intranuclear lamin A/C speckles as target for αB-crystallin and Hsp25. Exp Cell Res. 2004;299:393–403. doi: 10.1016/j.yexcr.2004.05.032.0014-4827(2004)299[0393:HSLOSH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of αA- and αB-crystallin in lens epithelial cells. J Biol Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200.0021-9258(2000)275[36823:DPAOAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Arrigo A-P. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26.1431-6730(1998)379[0019:SSPCTA]2.0.CO;2 [PubMed] [Google Scholar]
- Arrigo A-P, Firdaus WJJ, Mellier G, Moulin M, Paul C, Diaz-latoud C, Kretz-remy C. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. Methods. 2005;35:126–138. doi: 10.1016/j.ymeth.2004.08.003.1046-2023(2005)035[0126:CEIBOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bruey J-M, Ducasse C, and Bonniaud P. et al. 2000 Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2:645–652. [DOI] [PubMed] [Google Scholar]
- Clegg JS. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J Exp Biol. 1997;200:467–475. doi: 10.1242/jeb.200.3.467.0022-0949(1997)200[0467:EOAFSF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Clegg JS, Drinkwater LE, Sorgeloos P. The metabolic status of diapause embryos of Artemia franciscana (SFB) Physiol Zool. 1996;69:49–66.0031-935X(1996)069[0049:TMSODE]2.0.CO;2 [Google Scholar]
- Clegg JS, Jackson SA. The metabolic status of quiescent and diapause embryos of Artemia franciscana (Kellogg) Arch Hydrobiol Spec Issues Adv Limnol. 1998;52:425–439. [Google Scholar]
- Clegg JS, Jackson SA, Liang P, MacRae TH. Nuclear-cytoplasmic translocations of protein p26 during aerobic-anoxic transitions in embryos of Artemia franciscana. Exp Cell Res. 1995;219:1–7. doi: 10.1006/excr.1995.1197.0014-4827(1995)219[0001:NTOPPD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Clegg JS, Jackson SA, Popov VI. Long-term anoxia in encysted embryos of the crustacean, Artemia franciscana: viability, ultrastructure, and stress proteins. Cell Tiss Res. 2000;301:433–446. doi: 10.1007/s004410000249.0302-766X(2000)301[0433:LAIEEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cobb BA, Petrash JM. Characterization of α-crystallin-plasma membrane binding. J Biol Chem. 2000;275:6664–6672. doi: 10.1074/jbc.275.9.6664.0021-9258(2000)275[6664:COCMB]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/a:1021601103096.1360-8185(2003)008[0061:OTROHI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Crack JA, Mansour M, Sun Y, MacRae TH. Functional analysis of a small heat shock/α-crystallin protein from Artemia franciscana: oligomerization and thermotolerance. Eur J Biochem. 2002;269:933–942. doi: 10.1046/j.0014-2956.2001.02726.x.0014-2956(2002)269[0933:FAOASH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Day RM, Gupta JS, MacRae TH. A small heat shock/α-crystallin protein from encysted Artemia embryos suppresses tubulin denaturation. Cell Stress Chaperones. 2003;8:183–193. doi: 10.1379/1466-1268(2003)008<0183:ashcpf>2.0.co;2.1466-1268(2003)008[0183:ASHCPF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Engelsman J, Bennink EJ, and Doerwald L. et al. 2004 Mimicking phosphorylation of the small heat-shock protein αB-crystallin recruits the F-box protein FBX4 to nuclear SC35 speckles. Eur J Biochem. 271:4195–4203. [DOI] [PubMed] [Google Scholar]
- Drinkwater LE, Crowe JH. Regulation of embryonic diapause in Artemia: environmental and physiological signals. J Exp Zool. 1987;241:297–307.0022-104X(1987)241[0297:ROEDIA]2.0.CO;2 [Google Scholar]
- Duverger O, Paslaru L, Morange M. HSP25 is involved in two steps of the differentiation of PAM212 keratinocytes. J Biol Chem. 2004;279:10252–10260. doi: 10.1074/jbc.M309906200.0021-9258(2004)279[10252:HIIITS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fan G-C, Ren X, and Qian J. et al. 2005 Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 111:1792–1799. [DOI] [PubMed] [Google Scholar]
- Garrido C, Bruey J-M, Fromentin A, Hammann A, Arrigo A-P, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061.0892-6638(1999)013[2061:HICCAO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Haslbeck M. sHsps and their role in the chaperone network. Cell Mol Life Sci. 2002;59:1649–1657. doi: 10.1007/PL00012492.1420-682X(2002)059[1649:SATRIT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SA, Clegg JS. Ontogeny of low molecular weight stress protein p26 during early development of the brine shrimp, Artemia franciscana. Dev Growth Differ. 1996;38:153–160. doi: 10.1046/j.1440-169X.1996.t01-1-00004.x.0012-1592(1996)038[0153:OOLMWS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Cryns VL. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200.0021-9258(2001)276[16059:TSHSPB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein αB-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–38736. doi: 10.1074/jbc.M201770200.0021-9258(2002)277[38731:TSHSPB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Lu M, and Werner ME. et al. 2005 The small heat shock protein αB-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 280:11059–11066. [DOI] [PubMed] [Google Scholar]
- Laksanalamai P, Robb FT. Small heat shock proteins from extremophiles: a review. Extremophiles. 2004;8:1–11. doi: 10.1007/s00792-003-0362-3.1431-0651(2004)008[0001:SHSPFE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liang P, Amons R, MacRae TH, Clegg JS. Purification, structure and in vitro molecular-chaperone activity of Artemia p26, a small heat-shock/α-crystallin protein. Eur J Biochem. 1997a;243:225–232. doi: 10.1111/j.1432-1033.1997.0225a.x.0014-2956(1997)243[0225:PSAIVM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liang P, Amons R, Clegg JS, MacRae TH. Molecular characterization of a small heat shock/α-crystallin protein in encysted Artemia embryos. J Biol Chem. 1997b;272:19051–19058. doi: 10.1074/jbc.272.30.19051.0021-9258(1997)272[19051:MCOASH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. The synthesis of a small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138.0012-1606(1999)207[0445:TSOASH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liu J-P, Schlosser R, and Ma W-Y. et al. 2004 Human αA- and αB-crystallins prevent UVA-induced apoptosis through regulation of PKCα, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 79:393–403. [DOI] [PubMed] [Google Scholar]
- Ma X, Jamil K, and MacRae TH. et al. 2005 A small stress protein acts synergistically with trehalose to confer desiccation tolerance on mammalian cells. Cryobiology. 51:15–28. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/α-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733.1420-682X(2000)057[0899:SAFOSH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH 2001 Do stress proteins protect embryos during metabolic arrest and diapause? In: Molecular Mechanisms of Metabolic Arrest: Life in Limbo, ed Storey KB. BIOS Scientific Publishers, Oxford, 169–186. [Google Scholar]
- MacRae TH. Molecular chaperones, stress resistance and development in Artemia franciscana. Sem Cell Dev Biol. 2003;14:251–258. doi: 10.1016/j.semcdb.2003.09.019.1084-9521(2003)014[0251:MCSRAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- MacRae TH. Diapause: diverse states of developmental and metabolic arrest. J Biol Res. 2005;3:3–14. [Google Scholar]
- Mao Y-W, Liu J-P, Xiang H, Li DW-C. Human αA- and αB-crystallins bind to Bax and Bcl-Xs to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384.1350-9047(2004)011[0512:HAABBT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mao Y-W, Xiang H, Wang J, Korsmeyer S, Reddan J, Li DW-C. Human bcl-2 gene attenuates the ability of rabbit lens epithelial cells against H2O2-induced apoptosis through down-regulation of the αB-crystallin gene. J Biol Chem. 2001;276:43435–43445. doi: 10.1074/jbc.M102195200.0021-9258(2001)276[43435:HBGATA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Coronas V, Ljubic-Thibal V, Ducasse C, Granger L, Jourdan F, Arrigo A-P. Small stress protein Hsp27 accumulation during dopamine-mediated differentiation of rat olfactory neurons counteracts apoptosis. Cell Death Differ. 1999;6:227–233. doi: 10.1038/sj.cdd.4400483.1350-9047(1999)006[0227:SSPHAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mehlen A, Godet J, Arrigo A-P. hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem. 1997;272:31657–31665. doi: 10.1074/jbc.272.50.31657.0021-9258(1997)272[31657:HAASBD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo A-P. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks FAS/APO-1–and staurosporine-induced cell death. J Biol Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510.0021-9258(1996)271[16510:SSPANR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mounier N, Arrigo A-P. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2.1466-1268(2002)007[0167:ACASHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi YH, Iwasaki T, Okigaki T, Kato H. Cytological studies of Artemia salina. 1. Embryonic development without cell multiplication after the blastula stage in encysted dry eggs. Annot Zool Jpn. 1962;35:223–228.0003-5092(1962)035[0223:CSOASE]2.0.CO;2 [Google Scholar]
- Narberhaus F. α-Crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Micro Mol Biol Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002.1092-2172(2002)066[0064:CHSPSM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CS, Clegg JS. Cell division during the development of Artemia salina. Roux's Arch Dev Biol. 1978;184:1–13. doi: 10.1007/BF00848665.0340-0794(1978)184[0001:CDDTDO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Panasenko OO, Kim MV, Marston SB, Gusev NB. Interaction of the small heat shock protein with molecular mass 25 kDa (hsp25) with actin. Eur J Biochem. 2003;270:892–901. doi: 10.1046/j.1432-1033.2003.03449.x.0014-2956(2003)270[0892:IOTSHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pandey P, Farber R, and Nakazawa A. et al. 2000 Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 19:1975–1981. [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo A-P. Hsp27 as a negative regulator of cytochrome c release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002.0270-7306(2002)022[0816:HAANRO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Robertson JD, and Peterson E. et al. 2001 Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 6:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005a;62:2460–2476. doi: 10.1007/s00018-005-5190-4.1420-682X(2005)062[2460:SHSPMS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. The small heat shock proteins and their role in human disease. FEBS J. 2005b;272:2613–2627. doi: 10.1111/j.1742-4658.2005.04708.x.1742-464X(2005)272[2613:TSHSPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. Characterization of novel sequence motifs within amino- and carboxy-terminal extensions of p26, a small heat shock protein from Artemia franciscana. FEBS J. 2005c;272:5230–5243. doi: 10.1111/j.1742-4658.2005.04920.x.1742-464X(2005)272[5230:CONSMW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sun Y, Mansour M, Crack JA, Gass GL, MacRae TH. Oligomerization, chaperone activity, and nuclear localization of p26, a small heat shock protein from Artemia franciscana. J Biol Chem. 2004;279:39999–40006. doi: 10.1074/jbc.M406999200.0021-9258(2004)279[39999:OCAANL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Török Z, Goloubinoff P, and Horváth I. et al. 2001 Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc Natl Acad Sci U S A. 98:3098–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova NM, Horváth I, and Török Z. et al. 2002 Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A. 99:13504–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner RI, Clegg JS. Influence of trehalose on the molecular chaperone activity of p26, a small heat shock/α-crystallin protein. Cell Stress Chaperones. 2001;6:126–135. doi: 10.1379/1466-1268(2001)006<0126:iototm>2.0.co;2.1466-1268(2001)006[0126:IOTOTM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Klevitsky R, Huang W, Glasford J, Li F, Robbins J. αB-crystallin modulates protein aggregation of abnormal desmin. Circ Res. 2003;93:998–1005. doi: 10.1161/01.RES.0000102401.77712.ED.0009-7330(2003)093[0998:BMPAOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Warner AH, Clegg JS. Diguanosine nucleotide metabolism and the survival of artemia embryos during years of continuous anoxia. Eur J Biochem. 2001;268:1568–1576.0014-2956(2001)268[1568:DNMATS]2.0.CO;2 [PubMed] [Google Scholar]
- Willsie JK, Clegg JS. Small heat shock protein p26 associates with nuclear lamins and HSP70 in nuclei and nuclear matrix fractions from stressed cells. J Cell Biochem. 2002;84:601–614.0730-2312(2002)084[0601:SHSPPA]2.0.CO;2 [PubMed] [Google Scholar]
- Zhang H, Fu X, Jiao W, Zhang X, Liu C, Chang Z. The association of small heat shock protein 16.3 with the plasma membrane of Mycobacterium tuberculosis: dissociation of oligomers is a prerequisite. Biochem Biophys Res Commun. 2005;330:1055–1061. doi: 10.1016/j.bbrc.2005.03.092.0006-291X(2005)330[1055:TAOSHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]