Abstract
DNA polymerases specifically insert the hydrophobic pyrene deoxynucleotide (P) opposite tetrahydrofuran (F), an stable abasic site analog, and DNA duplexes containing this non-hydrogen-bonded pair possess a high degree of thermodynamic stability. These observations support the hypothesis that steric complementarity and stacking interactions may be sufficient for maintaining stability of DNA structure and specificity of DNA replication, even in the absence of hydrogen bonds across the base pair. Here we report the NMR characterization and structure determination of two DNA molecules containing pyrene residues. The first is a 13mer duplex with a pyrene·tetrahydrofuran pair (P·F pair) at the ninth position and the second mimics a replication intermediate right after incorporation of a pyrene nucleoside opposite an abasic site. Our data indicate that both molecules adopt right-handed helical conformations with Watson– Crick alignments for all canonical base pairs. The pyrene ring stays inside the helix close to its baseless partner in both molecules. The single-stranded region of the replication intermediate folds back over the opposing strand, sheltering the hydrophobic pyrene moiety from water exposure. The results support the idea that the stability and replication of a P·F pair is due to its ability to mimic Watson–Crick structure.
INTRODUCTION
Of all the non-covalent forces contributing to the structural integrity of nucleic acids and the fidelity of DNA replication, hydrogen bonds have in the past been generally regarded as the most essential component. However, recent studies have demonstrated that DNA replication can be achieved in the absence of hydrogen bonds across the newly formed base pair, a fact that led to the proposal of a steric matching mechanism as a driving force for DNA polymerase activity (1–4). Some exocyclic base lesions, such as 3,N4-ethenocytosine and 1,N2-propanoguanosine, miscode for dA incorporation in vitro and in vivo without forming hydrogen bonds (5–9). The structures of these mutagenic intermediates show stable right-handed duplexes with non-coplanar lesion-containing base pairs, which are stabilized mainly by hydrophobic stacking interactions (10–11). Furthermore, the Klenow fragment of DNA polymerase I specifically incorporates a non-polar shape mimic of thymine opposite a non-polar adenine analog and vice versa (12). Although the structure displays a coplanar alignment for the non-polar thymidine mimic/A pair, the duplex has reduced thermodynamic stability, possibly due to the missing hydrogen bond contribution or to disruption of local solvation (13,14).
Search for the steric match of abasic sites led to the discovery that DNA duplexes containing a pyrene·tetrahydrofuran pair (P·F pair) have melting temperatures close to that of A·T containing control samples. Furthermore, the free energy of duplex formation was consistently more favorable in (P·F)-containing duplexes than in duplexes having the pyrene or abasic site residues paired to any of the four natural nucleosides or in the same duplex with the P·F pair removed altogether (1). Primer extension studies revealed that the Klenow fragment of DNA polymerase I incorporates pyrene residues opposite abasic sites two orders of magnitude more efficiently than to any of the natural bases. Remarkably, the steady-state efficiency (Vmax / Km) for the formation of a P·F pair was only 3.7 times lower than that of a hydrogen-bonded T·A pair. Even in the presence of a 4-fold dATP excess, the Klenow fragment inserts pyrene opposite the abasic site 100 times more efficiently than adenine, an observation that has led to the proposition that pyrene can act as a unique agent in sequencing DNA containing abasic sites (2).
The shape exclusion model of polymerase fidelity suggests that, to achieve high replication efficiency, base pairs should fit within the double helical structure without distortions. Molecular modeling demonstrates that a pyrene deoxyriboside spans nearly the entire distance between the sugar-phosphate backbones of a DNA duplex and, with a surface of 220 A2, covers an area close to that of a regular base pair (270 A2). Thus, a pyrene moiety provides an optimal steric match to pair to an abasic site and, additionally, has an undisrupted π-electron system capable of effective stacking with flanking base pairs. Abasic sites in DNA have the potential to cause significant structural distortions to the helix depending upon the sequence context and partner base (15,16). Therefore, it is important to evaluate whether P·F pairs may form stable replication intermediates or not. Here we communicate the solution structure of two DNA molecules containing pyrene residues. One molecule is a 13mer duplex having a P·F pair located at the ninth position and the other molecule represents a replication intermediate right after incorporation of pyrene opposite an abasic site. The chemical structure of pyrene and tetrahydrofuran abasic site residues and the DNA sequences used in our studies are shown in Figure 1.
Figure 1.
Chemical structure of pyrene and tetrahydrofuran residues. Sequence and numbering scheme of the (P·F)-duplex and the (P·F)-intermediate.
MATERIALS AND METHODS
Sample preparation
The synthesis of modified precursors for solid-phase DNA synthesis and their incorporation into duplex DNA has been described (1–2,17). Purification of oligodeoxynucleotides followed standard procedure (18). Briefly, 5′-dimethoxytritylated sequences were isolated by treatment of crude synthesis products with concentrated aqueous ammonia for 46 h at room temperature. Purification of the sequences was accomplished by reverse-phase HPLC on a preparative Dynamax (300 × 25 mm) C4 column. The mobile phase consisted of solvent A (0.1 M triethylamine acetic acid buffer, pH 6.8) and solvent B (acetonitrile). Using a linear gradient of 0–50% of B in 50 min, the main fraction containing the desired compound eluted at 31 min. The terminal 5′-dimethoxytrityl group was cleaved by subsequent acidic treatment (80% acetic acid for 30 min) and this solution was extracted with ether three times before a second round of purification by HPLC. Desalting on a Sephadex G-25 column yielded pure deoxyoligonucleotide sequences that were converted to the sodium salt by percolation through a Dowex 50W cation exchange resin column. A 1:1 strand stoichiometry in the samples was achieved by monitoring the intensity of isolated NMR signals during the gradual addition of the tetrahydrofuran-containing strand to the pyrene-containing strand. NMR samples consisted of 260 and 280 OD260 for the (P·F)-intermediate and (P·F)-duplex, respectively, dissolved in 0.6 ml of 50 mM phosphate buffer, pH 6.8, 0.1 M NaCl in either 99.96% D2O or 90% H2O–10% D2O (v/v). Samples were vacuum-degassed before NMR data collection.
NMR experiments
One- and two-dimensional spectra were collected using Varian Inova spectrometers working at 11.75 and 14.1 T field strength. Proton chemical shifts were referenced relative to sodium 3-(trimethylsilyl)-propionate-2,2,3,3,-d4 (TSP). Phase-sensitive (19) NOESY (50, 100, 150, 200 and 300 ms mixing time), TOCSY (70 and 130 ms mixing time), COSY, DQF-COSY and COSY45 spectra in 100% D2O buffer were recorded at 35°C for the (P·F)-duplex and at 25°C for the (P·F)-intermediate. In these experiments, the residual water signal was suppressed by pre-saturation during the 1.5 s of relaxation delay. Phase-sensitive proton NOESY (150 ms mixing time) spectra in 90% H2O buffer were recorded at 0°C for both samples, using a jump and return reading pulse (20). Time-domain data sets consisted of 2048 × 300 complex data points in the t2 and t1 dimensions, respectively. A COSY45 spectrum of each duplex was acquired with a double number of complex points in both dimensions. Phase-sensitive [1H-31P]-HETCOR spectra in 100% D2O buffer were recorded with 2048 × 128 complex data points in the t2 and t1 dimensions, respectively, using the indirect detection mode (21). Phosphorus chemical shifts were referenced to TMP. NMR data were processed and analyzed using the Felix program (Molecular Simulations Inc.) running on Silicon Graphics computers. Time-domain data were multiplied by 90° shifted sinebell window functions prior to Fourier transformation. No base line corrections were applied to the transformed spectra.
Molecular dynamics
Interproton distance calculation and molecular dynamics were carried out using XPLOR3.1 on Silicon Graphics workstations (22). Approximately 300 experimental distances were calculated for each molecule by directly inputting NOE cross-peak intensities obtained from all NOESY spectra into a relaxation protocol (23). Only a relaxation energy term related to the difference between the back-calculated and experimental NOE intensities was included during minimization. A grid search was performed to find the best-fit isotropic correlation time of 2.25 ns. Reflecting the quality of the data, distance bounds were ±0.5 or ±0.7 Å of the calculated distances. As indicated by the NMR data, sugar pseudorotation angles were kept within 130–190° and backbone dihedrals angles enforced in a range encompassing A- and B-form DNA. Molecular dynamics simulations were performed in vacuo using a CHARMM-derived force field (24). Dynamics simulations run for 160–180 ps followed protocols described elsewhere (18). Twenty refined structures were calculated for each molecule by using four different values of starting temperatures and five different time-periods at the high temperature step. The last coordinate frame of the simulation was energy minimized yielding the structures presented here. Six refined structures of the (P·F)-duplex with pair-wise RMSD smaller than 1.5 Å with respect to their average and eight refined structures of the (P·F)-intermediate with pair-wise RMSD smaller than 1.5 Å with respect to their average composed the final ensemble of structures. Visualization and analysis of the structures was done with MidasPlus (25) and structural parameters computed with Curves 5.1 (26).
RESULTS
NMR spectra
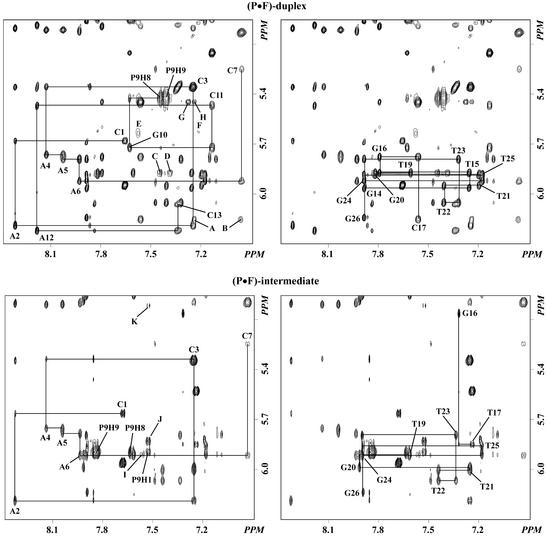
The assignment of exchangeable and non-exchangeable proton signals resulted from the analysis of NOESY, COSY and TOCSY spectra following standard methods (27,28). Figure 2 shows contour plots of the fingerprint region of NOESY (300 ms mixing time) spectra recorded at 35°C for the (P·F)-duplex and at 25°C for the (P·F)-intermediate. Each base proton (purine-H8/pyrimidine-H6) exhibits NOE cross-peaks to the H1′ proton of the same and the 5′-flanking residue. Similarly, other regions of these spectra reveal sequential NOE interactions between base protons and the sugar H2′, H2″ and H3′ protons of the 5′-attached residue. This directionality of the sequential NOE interactions implies that both samples adopt stable right-handed helical structures in solution. The specific assignment of pyrene protons followed the analysis of NOESY and DQF-COSY spectra recorded in 100% D2O buffer. In the symmetrical base–base region of DQF-COSY spectra, cross-peaks link all vicinal proton pairs within the aromatic moiety and, in addition, identify the H6 proton because is the only signal having two COSY peaks in this region of the spectra. The analysis of NOESY data confirmed these assignments and correlated neighboring groups of vicinal proton pairs via the observation of H2–H3, H4–H5 and H7–H8 cross-peaks, resulting in the identification of all aromatic pyrene protons in the samples (Fig. S1, Supplementary Material). Proton chemical shifts of the (P·F)-duplex and the (P·F)-intermediate are listed in Tables S1 and S2, respectively.
Figure 2.
Contour plots showing the ‘fingerprint’ region of 600 MHz NOESY (300 ms mixing time) spectra recorded in 100% D2O buffer at 35°C for the (P·F)-duplex (top) and at 25°C for the (P·F)-intermediate (bottom). Contour plots are drawn in duplicate showing the assignment of the pyrene-containing strand in the left and the tetrahydrofuran-containing strand on the right side in each panel. Solid lines link intra residue base to H1′ connectivities (labeled in the plot) with the sequential base to H1′ cross-peaks. Labeled peaks in the (P·F)-duplex are assigned as follows: A, P9H5–C17H1′; B, P9H6–C17H1′; C, P9H8–T19H1′; D, P9H7–T19H1′; E, P9H1–A8H1′; F, P9H2–A8H1′; G, P9H4–C17H5; H, P9H5–C17H5. Labeled peaks in the (P·F)-intermediate are assigned as follows: I, P9H7–T19H1′; J, P9H1–A8H1′; K, P9H1–A8H3′.
Figure 3 shows the imino region of exchangeable proton spectra of the samples dissolved in 10% D2O buffer collected at low temperature. In the case of the (P·F)-duplex, each thymine imino proton exhibits an NOE peak to the adenine H2 proton across the A·T base pair (Fig. 3, peaks A–E, H). Similarly, each guanine imino proton display NOE cross-peaks to the hydrogen-bonded and exposed amino protons of their partner cytosine within G·C base pairs (Fig. 3, peaks I/I′–M/M′). In the case of the (P·F)-intermediate, analogous peaks are present for all A·T base pairs (Fig. 3, peaks A, C–E, H) and G·C base pairs (Fig. 3, peaks J/J′, L/L′ and S/S′) of the sample. These observations establish the formation of Watson–Crick base pair alignments throughout the (P·F)-duplex and for the double-stranded region of the (P·F)-intermediate. In addition, strong NOE cross-peaks are observed in the symmetrical imino proton region of these NOESY spectra indicating proper base pair stacking in both samples (Fig. S4). The chemical shift of exchangeable protons is also listed in Tables S1 and S2.
Figure 3.
(Top) Imino proton region of one-dimensional proton spectra recorded in the 10% D2O buffer at 0°C at 500 MHz for the (P·F)-duplex (left) and at 600 MHz (P·F)-intermediate (right). The assignment of imino protons is given in the spectra. In the (P·F)-duplex, (*) points to a minor T19 conformation. In the (P·F)-intermediate, (#) points to signals of the unpaired imino protons of the single-stranded region of the sample (G14N1H, T15N3H and G16N1H protons). (Bottom) Contour plots of regions of NOESY (150 ms mixing time) spectra recorded in 10% D2O buffer at 0°C at 500 MHz for the (P·F)-duplex (left) and at 600 MHz (P·F)-intermediate (right). Labeled peaks are assigned as follows: A, T25N3H–A2H2; B, T15N3H–A12H2; C, T21N3H–A6H2; D, T22N3H–A5H2; E, T23N3H–A4H2; F, T22N3H–A6H2; G, T21N3H–A5H2; H, T19N3H–A8H2; I, G14N1H–C13N4Hhb; I′, G14N1H–C13N4Hex; J, G24N1H–C3N4Hhb; J′, G24N1H–C3N4Hex; K, G16N1H–C11N4Hhb; K′, G16N1H–C11N4Hex; L, G20N1H–C7N4Hhb; L′, G20N1H–C7N4Hex; M, G10N1H–C17N4Hhb; M′, G10N1H–C17N4Hex; O, G16N1H–A12H2; P, G24N1H–A2H2; Q, G24N1H–A4H2; R, G20N1H–A6H2; S, G26N1H–C1N4Hhb; S′, G26N1H–C1N4Hex.
Within the temperature range used for the characterization of the samples (–5 to 45°C), proton signals of the A8 residue positioned at the 5′ site of the pyrene residue are always broad. In the NOESY spectra shown in Figure 2, cross-peaks involving the A8H8 and A8H2 are absent and connectivities of the sugar protons of this residue are very weak. In contrast, proton signals of G10, flanking the pyrene residue at the 3′ site in the (P·F)-duplex, are sharp and NOE interactions for this residue are readily identified in all regions on the NOESY spectrum (Fig. 2). These observations indicate the existence of an exchange process localized at the 5′ side of the pyrene residue. Further evidence of conformational flexibility is found on the symmetrical imino proton region of a NOESY spectrum where T19H3 displays an exchange cross-peak in the (P·F)-duplex (Fig. S4). Moreover, as a result of the exchange process at the 5′ side of the pyrene moiety, we note that the imino proton signal of the C7·G20 base pair is as broad as that of terminal base pairs in both samples (Fig. 3). While the nature of this exchange remains under investigation, the fast disappearance of the minor T19H3 signal (labeled with an asterisk in Fig. 3) upon temperature increase suggest the lack of hydrogen bonding in that conformation.
Pyrene conformation
Several spectroscopic properties establish the intrahelical conformation of the P·F pair in both samples. P9H6 and P9H7 show NOE cross-peaks to the T19H1′, C17H1′ (Fig. 2, peaks B, D and I), F18H1′, F18H1″, F18H2′ and F18H2″ protons (Fig. 4, peaks A–H) located in the sugar-phosphate backbone of the tetrahydrofuran-containing strand. Moreover, the chemical shift of T19 and G10 imino protons in the (P·F)-duplex and of T19 imino proton in the (P·F)-intermediate are significantly up-field from the expected region of the spectra indicating shielding of these protons by induced currents of the pyrene ring (Fig. 3). Altogether, these observations position the aromatic pyrene moiety inside the helix pointing its H5–H7 edge towards the tetrahydrofuran-containing strand in both samples. Furthermore, the NOESY spectra of both samples show very strong P9H9–P9H1′ and P9H8–P9H1′ cross-peaks (Fig. 2) establishing a short distance between these pairs of protons and, thus, positioning the H8–H9 pyrene edge in the minor groove of the double helix. Corroborating evidence for this conformation is the observation of P9H4–C17H5, P9H5–C17H5 (Fig. 2, peaks G and H), P9H6–T19CH3 and P9H7–T19CH3 (Fig. 4, peaks I and J) NOE peaks that position the H4–H5 pyrene edge in the major groove of the helix. Pyrene protons chemical shifts are listed in Table S3.
Figure 4.
Contour plots of regions of 600 MHz NOESY (300 ms mixing time) spectra, recorded in 100% D2O buffer at 35°C for the (P·F)-duplex (left) and at 25°C for the (P·F)-intermediate (right), showing NOE interactions across the P·F pair. Labeled peaks are assigned as follows: A, P9H7–F18H1′; B, P9H6–F18H1′; C, P9H7–F18H1″; D, P9H6–F18H1″; E, P9H7–F18H2″; F, P9H6–F18H2″; G, P9H7–F18H2′; H, P9H6–F18H2′; I, P9H7–T19CH3; J, P9H6–T19CH3.
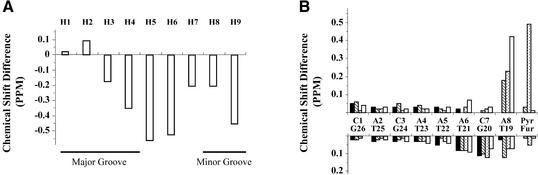
Pyrene proton signals are sharper in the spectra of the (P·F)-intermediate than in the (P·F)-duplex (Fig. 4). This feature reveals increased mobility and solvent exposure of the terminal pyrene ring in the (P·F)-intermediate. However, the observation of several NOE cross-peaks between protons of the aromatic moiety and of G16 and T15 residues indicates that the single-stranded segment of the (P·F)-intermediate turns toward the complementary strand protecting the hydrophobic moiety from water exposure. The chemical shift of pyrene protons shows that, except for the case of the H2 signal, they resonate at high frequencies (down-field) in the (P·F)-intermediate (Fig. 5A), suggesting incomplete shielding from water. In contrast, the comparison of proton chemical shifts in the (P·F)-duplex and the double-stranded segment of the (P·F)-intermediate reveals small differences for protons of the first six base pairs of the samples (Fig. 5B). Furthermore, chemical shift differences among the exchangeable protons are small and phosphorous chemical shifts in both samples are very similar (Tables S1 and S2). Taken together, these observations suggest that the solution structure of both DNA molecules is very similar for the first six base pairs of the samples and differs at the P·F and flanking base pairs.
Figure 5.
Proton chemical shift differences between the (P·F)-duplex and (P·F)-intermediate samples. (A) Real value differences among the aromatic pyrene protons [negative values correspond to a downfield position in the (P·F)-intermediate], and (B) absolute value differences of the major groove base and H2′ protons (black- and line-filled bars, respectively) and minor groove H1′ and H2″ protons (dot- and white-filled bars, respectively).
Three-dimensional structures
Overlap views of refined models for the (P·F)-duplex and (P·F)-intermediate obtained through restrained molecular dynamics are shown in Figures S2 and S3, respectively [coordinates of the refined structures have been deposited in the Protein Data Bank, accession nos 1FZL for the (P·F)-duplex and 1FZS for the (P·F)-intermediate]. Pair-wise RMSD between converging (P·F)-duplex or between converging (P·F)-intermediate structures are smaller than 1.5 Å and a similar value is measured between the double-stranded region of the (P·F)-intermediate and the corresponding segment of the (P·F)-duplex. The structures of the (P·F)-duplex display appropriate convergence throughout the molecule that diminishes at the A8 residue and at the ends of the duplex. In the case of the (P·F)-intermediate, convergence is good only for the double-stranded segment but the structures are disordered in the single-stranded region of the molecule.
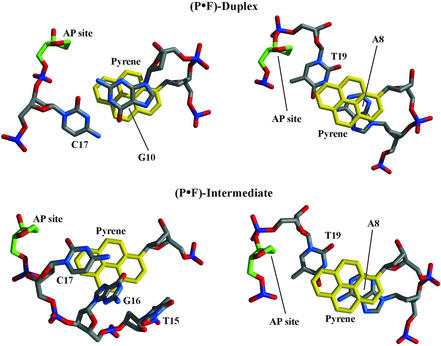
Figure 6 shows stereo views with the major groove prominent of the minimized average structure of the two duplexes and Table 1 lists the main structural parameters. Refined models are right-handed helices with all residues having glycosidic torsion angles in anti and sugar conformations in the south range. Helical twist and rise values are generally close to those observed in canonical B-form DNA, with slight deviations at and near the P·F pair (Table 1). Both structures have the pyrene residue inside the helix, with its H8–H9 edge in the minor groove and the deoxyribose sugar in the C2′-endo conformation. The abasic site residue is also intrahelical, adopts a C2′-endo conformation, and positions C1′ and C2′ close to the H5–H6 edge of its aromatic partner. The single-strand segment of the (P·F)-intermediate turns back towards the major groove of the molecule shielding the hydrophobic pyrene moiety from water exposure (Figs 6 and 7).
Figure 6.
Cross-eye stereo view of the (P·F)-duplex and (P·F)-intermediate structures. The picture shows the major groove prominent with atoms colored by type, the pyrene residue in yellow and the abasic site in green. Three base pairs at one end and the terminal base pair at the other end are omitted from the picture.
Table 1. Structural parametersa.
| Helical parametersb | ||
| Twist A8/P9 (°) | 36–44 | 29–36 |
| Twist P9/G10 (°) | 29–33 | – |
| Rise A8/P9 (Å) | 3.0–3.3 | 2.8–3.9 |
| Rise P9/G10 (Å) | 3.1–3.4 | – |
| Sugar conformation | ||
| Pyrene | C2′-endo | C2′-endo |
| Abasic site | C2′-endo | C2′-endo |
| Solvent accessible surfacec | ||
| Initial model (%) | 13 | 31 |
| Refined model (%) | 7 | 14 |
| NOE restraintsd | ||
| Non-exchangeable | 307 (1) | 260 (3) |
| Hydrogen-bonding | 28 (0) | 17 (0) |
aComputed with Curves5.1. The first set of values correspond to the (P·F)-duplex and the second to the (P·F)-intermediate.
bB-form values are twist 36°, rise 3.4 Å.
cPyrene area accessible to water.
dNumber of distance restraints (violations > 0.1 Å).
Figure 7.
Helical axis view of the (P·F)-duplex and (P·F)-intermediate structures depicting stacking interactions of pyrene and flanking residues. The G10- pyrene step is shown on the left and the pyrene-A8 step on the right with the pyrene residue in yellow, the abasic site in green, and other atoms colored by type.
Figure 7 details the modified pair structure on both samples as viewed from the helical axis of the molecules. In the (P·F)-duplex structure, the pyrene ring stacks extensively with the flanking A8 and G10 residues and partially with T19 in the tetrahydrofuran-containing strand. Similarly, the pyrene moiety also stacks extensively with the A8·T19 base pair in the structure of the (P·F)-intermediate. These observations are in direct agreement with the NMR data that established up-field chemical shifts for protons of the A8, G10 and T19 residues in both molecules (Tables S1 and S2).
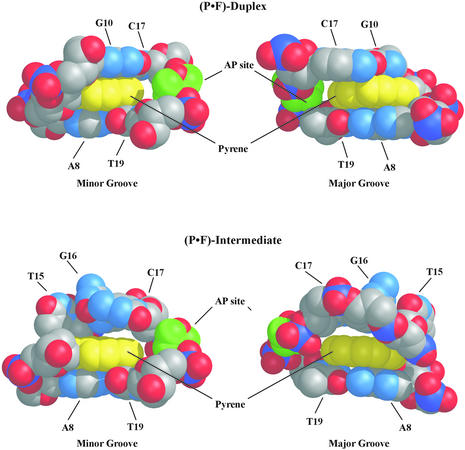
The space-filling models displayed in Figure 8 reveal that the size of the pyrene ring is insufficient to establish tight Van der Waals contacts with the abasic site residue in the opposing strand. In the case of the (P·F)-duplex, there is a gap within the P·F pair visible from both grooves of the duplex, which can potentially accommodate a water molecule after a minor conformational adjustment of the structure. An even larger hole is present in the (P·F)-intermediate structure where the distance between the abasic site and the H5–H6 edge of the pyrene is longer than in the (P·F)-duplex. As a result, 14% of the pyrene surface is accessible to solvent in the (P·F)-intermediate structure, very close to a 13% measured on a canonical B-form DNA duplex containing a P·F pair but still larger than the value observed in the refined (P·F)-duplex structures (7%).
Figure 8.
Space-filling representation of the P·F and flanking base pairs pair on the (P·F)-duplex and (P·F)-intermediate structures with the major groove (right) and minor groove (left) prominent. This picture display the pyrene residue in yellow, the abasic site in green, and other atoms colored by type.
DISCUSSION
The P·F pair represents the first reported example of a non-hydrogen-bonded base pair that stabilizes duplexes in which it is substituted. In addition, it is the first such pair (in which both nucleosides are non-natural) that was shown to be replicated by polymerase enzymes. Since both these phenomena are thought to be strongly dependent on geometry, studies of the structure of this pair and its effect on the local and global DNA structure seem justified.
On the whole, we find that the P·F pair causes little if any distortion of the duplex structure, but instead has observable effects on local base pair dynamics. The experimental spectroscopic evidence and the refined three-dimensional models indicate that the structure of DNA containing a P·F pair is a stable right-handed helix at room temperature (Figs 2 and 6). The abasic site and pyrene residues are both intrahelical and the aromatic moiety stacks properly with flanking base pairs (Figs 4 and 7). However, the presence of two inter-converting T19(N3H) signals in the (P·F)-duplex sample (Figs 3 and S4) and the observation of broad proton resonances for the A8 residue (Fig. 2) indicate that A8·T19, at the 5′ side of the pyrene residue, undergoes conformational exchange. In contrast, within the range of temperatures used in our study, the P·F pair in both molecules and the G10·C17 base pair, flanking the lesion at the in the 3′ side in the (P·F)-duplex, adopt a single conformation. The increased dynamics for the neighboring A·T base pair is unexpected since a P·F pair has essentially the same thermodynamic stability than that of an A·T base pair (1), and since the pyrene moiety stacks more strongly with DNA bases than natural bases do (29). It is worth noting that duplex DNA containing the hydrophobic 1,N2-propanoguanosine lesion opposite an abasic site reveals increased local mobility for the exocyclic adduct while the flanking base pairs remain stable (30). Furthermore, duplexes containing a tetrahydrofuran abasic site residue paired to purine bases have decreased thermodynamic stability but the lesion site and flanking base pairs show a single intrahelical conformation (31–34). Therefore, it appears that the presence of a highly hydrophobic moiety partially alters the dynamics of local stacking or pairing of the more polar DNA bases without decreasing overall thermodynamic stability of the duplex. It is possible that the localized bond dipoles present in the natural DNA base pairs may hinder two face-to-face bases from slipping past one another as easily as when one of the ‘bases’ has an unpolarized hydrophobic surface. Alternatively, as suggested by the three-dimensional models, a transient water molecule present in the gap across the P·F pair may contact the hydrophobic methyl group of T19 resulting in destabilization of the A8·T19 base pair (Fig. 8).
DNA polymerases preferentially incorporate pyrene opposite abasic sites but fail to extend primer DNA containing a P·F pair at the template/primer junction (2). One reason for this behavior may be the absence of a hydrogen bond acceptor in the minor groove of the crescent duplex, known to facilitate primer extension (35). However, the increased conformational dynamics observed for the P·F pair may contribute as well. Pyrene proton signals in the (P·F)-intermediate have significantly narrower line widths than the same protons in the (P·F)-duplex (Fig. 4). This difference may be attributed to a shorter local correlation time of the pyrene moiety in the (P·F)-intermediate where, due to absence of a 3′-attached nucleotide, it has increased conformational freedom (Figs 6 and 8). Furthermore, unlike canonical base pairs, the absence of tight contacts within the P·F pair increases even further the mobility of the pyrene residue at the 3′ end of a template/primer junction. Therefore, it seems possible that the relatively unrestrained bulky moiety may hinder a correct positioning of the ensuing nucleotide causing polymerase stalling, after insertion of a pyrene residue, and dissociation of the replication fork complex. Whether replacement of pyrene with a larger base would provide for tighter contact and allow extension of DNA beyond the non-polar pair yet remains to be seen.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Acknowledgments
ACKNOWLEDGEMENTS
E.T.K. acknowledges support from the NIH (GM52956) and from the US Army Research Office. C.D.S. acknowledges support from the NIH (CA77094 and CA47995).
PDB accession nos 1FZL, 1FZS
REFERENCES
- 1.Matray T.J. and Kool,E.T. (1998) Selective and stable DNA base pairing without hydrogen bonds. J. Am. Chem. Soc., 120, 6191–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matray T.J. and Kool,E.T. (1999) A specific partner for abasic damage in DNA. Nature, 399, 704–708. [DOI] [PubMed] [Google Scholar]
- 3.Kool E.T. (1998) Replication of non-hydrogen bonded bases by DNA polymerases: a mechanism for steric matching. Biopolymers, 48, 3–17. [DOI] [PubMed] [Google Scholar]
- 4.Kool E.T. (2002) Active site tightness and substrate fit in DNA replication. Annu. Rev. Biochem., 71, 191–219. [DOI] [PubMed] [Google Scholar]
- 5.Litinski V., Chenna,A., Sagi,J. and Singer,B. (1997) Sequence context is an important determinant in the mutagenic potential of 1,N6-ethenodeoxyadenosine (εA): formation of εA base pairs and elongation in defined templates. Carcinogenesis, 18, 1609–1615. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W., Johnson,F., Grollman,A.P. and Shibutani,S. (1995) Miscoding by the exocyclic and related DNA adducts 3,N4-etheno-2′-deoxycytidine, 3,N4-ethano-2′-deoxycytidine and 3-(2-hydroxyethyl)-2′-deoxyuridine. Chem. Res. Toxicol., 8, 157–163. [DOI] [PubMed] [Google Scholar]
- 7.Palejwala V.A., Rzepka,R.W. and Humayun,M.Z. (1993) UV irradiation of Escherichia coli modulates mutagenesis at a site-specific ethenocytosine residue on M13 DNA. Evidence for an inducible recA-independent effect. Biochemistry, 32, 4112–4120. [DOI] [PubMed] [Google Scholar]
- 8.Basu A.K., Wood,M.L., Niedernhofer,L.J., Ramos,L.A. and Essigmann,J.M. (1993) Mutagenic and genotoxic effects of three vinyl chloride-induced DNA lesions: 1,N6-ethenoadenine, 3,N4-ethenocytosine and 4-amino-5-(imidazol-2-yl)imidazole. Biochemistry, 32, 12793–12801. [DOI] [PubMed] [Google Scholar]
- 9.Moriya M., Zhang,W., Johnson,F. and Grollman,A.P. (1994) Mutagenic potency of exocyclic DNA adducts: marked differences between Escherichia coli and simian kidney cells. Proc. Natl Acad. Sci. USA, 91, 11899–11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouchakdjian M., Eisenberg,M., Live,D., Marinelli,E., Grollman,A.P. and Patel,D.J. (1990) NMR studies of an exocyclic 1,N2-propanodeoxyguanosine adduct (X) located opposite deoxyadenosine (A) in DNA duplexes at basic pH: simultaneous partial intercalation of X and A between stacked bases. Biochemistry, 29, 4456–4465. [DOI] [PubMed] [Google Scholar]
- 11.Korobka A., Cullinan,D., Cosman,M., Grollman,A.P., Patel,D.J., Eisenberg,M. and de los Santos,C. (1996) Solution structure of an oligodeoxynucleotide duplex containing the exocyclic lesion 3,N4-etheno-2′-deoxycytidine opposite 2′-deoxyadenosine determined by NMR spectroscopy and restrained molecular dynamics. Biochemistry, 35, 13310–13318. [DOI] [PubMed] [Google Scholar]
- 12.Morales J.C. and Kool,E.T. (1998) Efficient replication between non-hydrogen-bonded nucleoside shape analogs. Nature Struct. Biol., 5, 950–954. [DOI] [PubMed] [Google Scholar]
- 13.Guckian K.M., Krugh,T.R. and Kool,E.T. (1998) Solution structure of a DNA duplex containing a replicable difluorotoluene-adenine pair. Nature Struct. Biol., 5, 954–959. [DOI] [PubMed] [Google Scholar]
- 14.Cubero E., Laughton,C.A., Luque,F.J. and Orozco,M. (2000) Molecular dynamics study of oligonucleotides containing difluorotoluene J. Am. Chem. Soc., 122, 6891–6899. [Google Scholar]
- 15.Cline S.D., Jones,W.R., Stone,M.P. and Osheroff,N. (1999) DNA abasic lesions in a different light: solution structure of an endogenous topoisomerase II poison. Biochemistry, 38, 15500–15507. [DOI] [PubMed] [Google Scholar]
- 16.Singh M.P., Hill,G.C., Péoc’h,D., Rayner,B., Imbach,J.-L. and Lown,J.W. (1994) High-field NMR and restrained molecular modeling studies on a DNA heteroduplex containing a modified apurinic abasic site in the form of covalently linked 9-aminoellipticine. Biochemistry, 33, 10271–10285. [DOI] [PubMed] [Google Scholar]
- 17.Takeshita M., Chang,C.-N., Johnson,F., Will,S. and Grollman,A.P. (1987) Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J. Biol. Chem., 262, 10171–10179. [PubMed] [Google Scholar]
- 18.Smirnov S., Johnson,F., Marumoto,R. and de los Santos,C. (2000) Structure of an 11-mer DNA duplex containing the carbocyclic analog: 2′-deoxyaristeromycin. J. Biomol. Struct. Dynam., 17, 981–991. [DOI] [PubMed] [Google Scholar]
- 19.States D.J., Habekorn,R.A. and Ruben,D.J. (1982) A two-dimensional nuclear Overhauser experiment with pure absorption phase in four quadrants. J. Magn. Res., 48, 286–292. [Google Scholar]
- 20.Plateau P. and Gueron,M. (1982) Exchangeable proton NMR without base-line distortion, using new strong-pulse sequences. J. Am. Chem. Soc., 104, 7310–7311. [Google Scholar]
- 21.Sklenar V., Mishayiro,H., Zon,G., Miles,H.T. and Bax,A. (1986) Assignment of the 31P and 1H resonances in oligonucleotides by two-dimensional NMR spectroscopy. FEBS Lett., 208, 94–98. [DOI] [PubMed] [Google Scholar]
- 22.Brünger A. (1993) XPLOR Version 3.1. A System for X-Ray Crystallography and NMR. Yale University Press, New Haven, CT.
- 23.Yip P. and Case,D.A. (1989) A new method for refinement of macromolecular structures based on nuclear Overhauser effect spectra. J. Magn. Reson., 83, 643–648. [Google Scholar]
- 24.Brooks B.R., Buccoleri,R.E., Olafson,B.D., States,D.J., Swaminathan,S. and Karplus,M. (1983) CHARMM: a program for macromolecular energy, minimization and dynamics calculations. J. Comput. Chem., 4, 187–217. [Google Scholar]
- 25.MidasPlus (1994) Molecular Interactive Display and Simulation. Computer Graphics Laboratory. University of California at San Francisco, CA, USA.
- 26.Lavery R. and Sklenar,H. (1988) The definition of generalized helicoidal parameters and of axis curvature for irregular nucleic acids. J. Biomol. Struct. Dyn., 6, 655–667. [DOI] [PubMed] [Google Scholar]
- 27.van de Ven J.M. and Hilbers,C.W. (1988) Nucleic acids and nuclear magnetic resonance. Eur. J. Biochem., 178, 1–18. [DOI] [PubMed] [Google Scholar]
- 28.de los Santos C. (1999) Probing DNA structure by NMR spectroscopy. In Kool,E. (vol. ed.) and Barton,D., Nakanishi,K. and Meth-Cohn,O. (eds), Comprehensive Natural Products Chemistry, Volume 7. DNA and Aspects of Molecular Biology. Elsevier Science Ltd., Oxford, UK, pp. 55–80.
- 29.Guckian K.M., Ren,R.X., Chaudhuri,N.C., Tahmassebi,D.C. and Kool,E.T. (2000) Factors contributing to aromatic stacking in water: evaluation in the context of DNA. J. Am. Chem. Soc., 122, 2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouchakdjian M., Eisenberg,M., Johnson,F., Grollman,A.P. and Patel,D.J. (1991) Structural features of an exocyclic adduct positioned opposite an abasic site in a DNA duplex. Biochemistry, 30, 3262–3270. [DOI] [PubMed] [Google Scholar]
- 31.Cuniasse Ph., Sowers,L.C., Eritja,R., Kaplan,B., Goodman,N.F., Cognet,J.A.H., LeBret,M., Guschlbauer,W. and Fazakerly,G.V. (1987) An abasic site in DNA. Solution conformation determined by proton NMR and molecular mechanics calculations. Nucleic Acids Res., 15, 8003–8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalnick M.W., Chang,C.N., Grollman,A.P. and Patel,D.J. (1988) NMR studies of abasic sites in DNA duplexes: deoxyadenosine stacks into the helix opposite the cyclic analogue of 2-deoxyribose. Biochemistry, 27, 924–931. [DOI] [PubMed] [Google Scholar]
- 33.Kalnick M.W., Chang,C.N., Johnson,F., Grollman,A.P. and Patel,D.J. (1989) NMR studies of abasic sites in DNA duplexes: deoxyadenosine stacks into the helix opposite acyclic lesions. Biochemistry, 28, 3373–3383. [DOI] [PubMed] [Google Scholar]
- 34.Withka J.M., Wilde,J.A., Bolton,P.H., Mazumder,A. and Gerlt,J.A. (1991) Characterization of conformational features of DNA heteroduplexes containing aldehydic abasic sites. Biochemistry, 30, 9931–9940. [DOI] [PubMed] [Google Scholar]
- 35.Morales J.C. and Kool,E.T. (1999) Minor groove interactions between polymerase and DNA: more essential to replication than Watson–Crick hydrogen bonds? J. Am. Chem. Soc., 121, 2323–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.