Abstract
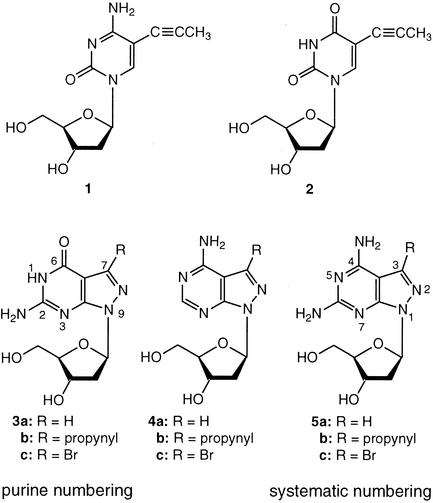
Oligonucleotides incorporating the 7-propynyl derivatives of 8-aza-7-deaza-2′-deoxyguanosine (3b) and 8-aza-7-deaza-2′-deoxyadenosine (4b) were synthesized and their duplex stability was compared with those containing the 5-propynyl derivatives of 2′-deoxycytidine (1) and 2′-deoxyuridine (2). For this purpose phosphoramidites of the 8-aza- 7-deazapurine (pyrazolo[3,4-d]pyrimidine) nucleosides were prepared and employed in solid-phase synthesis. All propynyl nucleosides exert a positive effect on the DNA duplex stability because of the increased polarizability of the nucleobase and the hydrophobic character of the propynyl group. The propynyl residues introduced into the 7-position of the 8-aza-7-deazapurines are generally more stabilizing than those at the 5-position of the pyrimidine bases. The duplex stabilization of the propynyl derivative 4b was higher than for the bromo nucleoside 4c. The extraordinary stability of duplexes containing the 7-propynyl derivative of 8-aza-7- deazapurin-2,6-diamine (5b) is attributed to the formation of a third hydrogen bond, which is apparently not present in the base pair of the purin-2,6-diamine 2′-deoxyribonucleoside with dT.
INTRODUCTION
The thermal stability of oligonucleotide duplexes depends on their length and their base pair composition (1). Efforts were undertaken to increase the duplex stability by chemical modification of the DNA constituents. The sugar moiety (2) or the nucleobase (1,3–7) of the DNA constituents as well as the oligonucleotide backbone (8) were structurally modified. These changes have broadened the application of oligonucleotides in the field of antisense technology (9–11) or in hybridization techniques used for diagnostic purposes (12). All approaches focused either on strengthening the hydrogen bonds between the bases and/or the increase of the stacking interactions among them. Our search for more stable base pairs led us to the pyrazolo[3,4-d]pyrimidine (8-aza-7-deazapurine) 2′-deoxyribonucleosides (13–16), which can be considered as ideal shape mimics of the parent purine nucleosides. Their 7-halogenated and alkynylated derivatives induce a positive effect on the base pair stability of duplex DNA (1,17,18).
Among the various groups introduced into oligonucleotides for duplex stabilization, the propynyl group found particular attention (19–21). This group was introduced in the 5-position of the pyrimidine nucleosides, e.g. in 2′-deoxycytidine (1) (19–23) and 2′-deoxyuridine (2) (24–26) or in the 7-position of pyrrolo[2,3-d]pyrimidine nucleosides (27). Also, 8-propynylated 2′-deoxyadenosine and 2′-deoxyguanosine derivatives were studied (26,28). However, 8-substituted purine derivatives destabilize DNA due to steric hindrance. As the 7-substituents of 8-aza-7-deazapurines have steric freedom within a DNA-duplex, propynyl residues were now introduced into the 7-position of oligonucleotides incorporating pyrazolo[3,4-d]pyrimidines (8-aza-7-deazapurines) (1,13,18). This study reports on the synthesis of 7-propynyl derivatives of the 8-aza-7-deaza-2′-deoxyguanosine (3b) and the corresponding 2′-deoxyadenosine derivative (4b). The work also pays attention to the extraordinary stability of duplexes incorporating the propynyl derivative of 8-aza-7-deazapurin-2,6-diamine 2′-deoxyribonucleoside (5b) (18) and compares the stabilities of ‘dA–dT’ versus ‘dG–dC’ base pairs replacing either one or two of the propynylated base analogs shown in Scheme 1.
Scheme 1.
MATERIALS AND METHODS
General
For general remarks see Seela and Becher (1). Thin-layer chromatography (TLC) was performed on TLC aluminium sheets coated with silica gel 60 F254 (0.2 mm; Merck, Darmstadt, Germany). Flash chromatography (FC) was performed on silica gel 60 H at 0.4 bar (Merck). Reverse-phase HPLC was carried out on a 4 × 250 mm RP-18 (10 µm) LiChrosorb column (Merck) with a Merck-Hitachi HPLC pump (model 655 A-12) connected with a variable wavelength monitor (model 655-A), a controller (model L-5000), and an integrator (model D-2000). UV spectra were recorded on a U-3200 spectrophotometer (Hitachi, Japan), λmax in nm, ε in dm3 mol–1 cm–1. NMR spectra were measured on an Avance DPX 250 and an AMX 500 spectrometer (Bruker, Germany); chemical shifts (δ) are in p.p.m. downfield from internal TMS (1H, 13C) or external 85% H3PO4 (31P). The J values are given in Hz. CD spectra of duplexes were measured on a Jasco J-600 CD spectropolarimeter (Jasco, Japan) in a thermostatted cell with 1 cm path length at 20°C. Microanalyses were performed by Mikroanalytisches Labor Beller (Göttingen, Germany). The solvents were purified and dried according to standard procedures.
The UV melting of oligonucleotides was performed on a Cary-1/1E UV/VIS spectrophotometer (Varian, Australia) equipped with a Cary thermoelectrical controller. The Tm values were obtained from the melting curves. Each melting curve was fit to a non-self-complementary two-state model, and the thermodynamic parameters were obtained with the Meltwin 3.0 software package (29); UV spectra: 150-20 spectrometer (Hitachi, Japan); MALDI-TOF mass spectra: Biflex III spectrometer (Bruker Saxonia, Leipzig, Germany), HPA (3-hydroxypicolinic acid) as matrix.
Oligonucleotides
The oligonucleotide syntheses were carried out on an ABI 392-08 DNA synthesizer (Applied Biosystems, Weiterstadt, Germany) in a 1 µmol scale (Applied Biosystems) following the synthesis protocol for 3′-cyanoethylphosphoramidites. After cleavage from the solid support, the oligonucleotides were deprotected in 25% aqueous ammonia solution for 12–16 h at 60°C; room temperature (r.t.) deprotection (3 h) was used in the case of the oligonucleotides incorporating compound 2.
Purification of the 5′-dimethoxytrityl oligomers was performed by reversed-phase HPLC (RP-18) with the following solvent gradient system [A: 0.1 M (Et3NH)OAc (pH 7.0)/MeCN 95:5; B: MeCN]: 0–3 min, 15% B in A with a flow rate of 1.0 ml/min, 3–15 min, 15–60% B in A with a flow rate of 1 ml/min. The solution was dried and treated with 2.5% CHCl2COOH/CH2Cl2 for 5 min at r.t. to remove the 4,4′-dimethoxytrityl residues. The detritylated oligomers were purified by reversed-phase HPLC with the gradient: 0–20 min, 0–20% B in A, with a flow rate of 1 ml/min. The oligomers were desalted and lyophilized on a Speed-Vac evaporator to yield colorless solids.
The enzymatic hydrolysis of the oligonucleotides was performed as described (1) using snake-venom phosphodiesterase (EC 3.1.15.1, Crotallus adamanteus) and alkaline phosphatase (EC 3.1.3.1, Escherichia coli) provided by Roche Diagnostics GmbH, Germany. The molecular masses of the oligonucleotides were determined by MALDI-TOF mass spectrometry (Table 1).
Table 1. Molecular masses [M+H]+ of selected oligonucleotides determined by MALDI-TOF mass spectrometry.
| Oligonucleotide | MH+ (calc.) | MH+ (found) |
|---|---|---|
| 3′-d(ATC CA3b TTA TGA) (18) | 3682 | 3682 |
| 3′-d(ATC CA3b TTA T3bA) (19) | 3720 | 3721 |
| 5′-d(TA3b 3bTC AAT ACT) (20) | 3720 | 3722 |
| 5′-d(TAG GT1 AAT ACT) (21) | 3682 | 3683 |
| 3′-d(AT1 1AG TTA TGA) (22) | 3720 | 3720 |
| 5′-d(TAG GT1 AAT A1T) (23) | 3720 | 3720 |
| 3′-d(ATC CA10c TTA TGA) (24) | 3697 | 3698 |
| 5′-d(TA10c 10cTC AAT ACT) (25) | 3750 | 3752 |
| 5′-d(1TAG GTC AAT ACT) (26) | 3979 | 3971 |
| 5′-d(TAG GTC 4bAT ACT) (27) | 3682 | 3682 |
| 3′-d(ATC C4bG TT4b TGA) (28) | 3720 | 3720 |
| 5′-d(TAG GTC 4b4bT ACT) (29) | 3720 | 3720 |
| 5′-d(T4bG GTC 4b4bT 4bCT) (30) | 3796 | 3795 |
| 5′-d(TAG G2C AA2 ACT) (33) | 3692 | 3694 |
| 5′-d(AGT A22 GAC CTA) (34) | 3692 | 3696 |
| 5′-d(TAG G2C AAT ACT) (35) | 3668 | 3668 |
| 5′-d(2TA GGT CAA TAC T) (36) | 3979 | 3979 |
| 5′-d(TAG GTC 5bAT ACT) (37) | 3697 | 3697 |
| 3′-d(ATC C5bG TT5b TGA) (38) | 3750 | 3750 |
| 5′-d(TAG GTC 5b5bT ACT) (39) | 3750 | 3749 |
| 5′-d(T5bG GTC 5b5bT 5bCT) (40) | 3858 | 3857 |
6-Amino-1-(2-deoxy-β-d-erythro-pentofuranosyl)-1,5-dihydro-3-propynyl-4H-pyrazolo[3,4-d]-pyrimidin-4-one (3b)
A mixture of 8-aza-7-deaza-7-iodo-2′-deoxyguanosine (6a) (3) (0.27 g, 0.69 mmol), tetrakis(triphenylphosphine)palladium(0) [(PPh3)4Pd(0)] (94 mg, 0.08 mmol), CuI (31.1 mg, 0.16 mmol), and triethylamine (0.24 ml, 1.72 mmol) in DMF (2.5 ml) was saturated with propyne at 0°C, sealed, and stirred at r.t. for 24 h. Afterwards, a second portion of the same amounts of reagents was added, and the solution was saturated with propyne again. After stirring for an additional 24 h, the reaction mixture was absorbed on silica gel (20 g) and subjected to FC (CH2Cl2/MeOH, 20:1) to obtain compound 3b as a colorless solid (0.15 g, 72%). TLC (CH2Cl2/MeOH, 9:1): Rf 0.49. UV (MeOH): λmax 216 (18 700), 242 (27 300). 1H-NMR [(D6)DMSO]: 2.06 (s, CH3); 2.15, 2.64 [m, H2-C(2′)]; 3.44 [m, H2-C(5′)]; 3.76 [m, H-C(4′)]; 4.35 [m, H-C(3′)]; 4.72 [t, J = 5.7, HO-C(5′)]; 5.23 [d, J = 4.3, HO-C(3′)]; 6.27 [‘t’, J = 6.5, H-C(1′)]; 6.72 (br, NH2); 10.72 (m, arom. H). Anal. calc. for C13H15N5O4 (305.3): C, 51.14; H, 4.95; N, 22.94; found: C, 51.18; H, 5.03; N, 22.10.
1-(2-Deoxy-β-d-erythro-pentofuranosyl)-1,5-dihydro-6-[(2-methylpropanoyl)amino]-3-propynyl-4H-pyrazolo [3,4-d]-pyrimidin-4-one (7)
Compound 6b (15) (0.75 g, 1.62 mmol) was reacted with tetrakis(triphenylphosphine)-palladium(0) [(PPh3)4Pd(0)] (94 mg, 0.08 mmol), CuI (65.5 mg, 0.34 mmol), triethylamine (0.24 ml, 1.72 mmol) and propyne in anhydrous DMF under Ar as described for 3b. The reaction mixture was subjected to FC (CH2Cl2/MeOH, 20:1). Compound 7 was obtained as a colorless solid (0.73 g, 90%). Rf (CH2Cl2/MeOH, 20:1): 0.66. UV (MeOH): λmax 202 (17 000), 250 (23 000), 270 (16 000). 1H-NMR [(D6)DMSO]: 1.12 [m, CH(CH3)2]; 2.10 (s, CH3); 2.19 [m, Hα-C(2′)]; 2.68 [m, Hβ-C(2′)]; 2.76 [m, CH(CH3)2]; 3.47 [m, H2-C(5′)]; 3.78 [m, H-C(4′)]; 4.39 [m, H-C(3′)]; 4.73 [t, J = 5.6, HO-C(5′)]; 5.27 [d, J = 4.1, HO-C(3′)]; 6.36 [‘t’, J = 6.3, H-C(1′)]; 11.79, 11.91 (2s, 2NH). Anal. calc. for C17H21N5O5 (375.4): C, 54.39; H, 5.64; N, 18.66; found: C, 54.10; H, 5.80; N, 18.47.
1-[2-Deoxy-5-(4,4′-dimethoxytrityl)-β-d-erythro-pentofuranosyl]-1,5-dihydro-6-[(2-methylpropanoyl)amino]-3-propynyl-4H-pyrazolo[3,4-d]-pyrimidin-4-one (8)
Compound 7 (0.3 g, 0.8 mmol) was co-evaporated three times with anhydrous pyridine before dissolving in anhydrous pyridine (1 ml). DMT-Cl (0.3 g, 0.89 mmol) was added in portions. After stirring for 3 h at r.t., the reaction was quenched with methanol (1 ml) and the solvents removed in vacuum. The residue was dissolved in CH2Cl2, washed with 5% NaHCO3, dried with Na2SO4, and the solvent was removed. The residue was applied to FC (CH2Cl2/MeOH, 20:1) to give a colorless foam of 8 (0.47 g, 87%). TLC (CH2Cl2/MeOH, 95:5): Rf 0.38. UV (MeOH): λmax 202 (83 000), 234 (36 000), 273 (18 000). 1H-NMR [(D6)DMSO]: 1.14 [m, 2(CH3)2CH]; 2.10 (s, CH3); 2.28 [m, Hα-C(2′)]; 2.77 [m, Hβ-C(2′), CH(CH3)2]; 3.02 [m, H2-C(5′)]; 3.71 (s, OCH3); 3.91 [m, H-C(4′)]; 4.51 [m, H-C(3′)]; 5.34 [d, J = 4.6, HO-C(3′)]; 6.38 [m, H-C(1′)]; 6.76–7.32 (m, arom. H); 11.88, 11.93 (s, NHCO). Anal. calc. for C38H39N5O7 (677.8): C, 67.34; H, 5.80; N, 10.33; found: C, 67.27; H, 5.70; N, 10.36.
1-[2-Deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pentofuranosyl]-1,5-dihydro-6-[(2-methylpropanoyl)amino]-3-propynyl-4H-pyrazolo[3,4-d]-pyrimidin-4-one 3′-[(2-cyanoethyl)-N,N-diisopropylphosphoramidite (9)
To the solution of compound 8 (0.4 g, 0.59 mmol) in anhydrous dichloromethane (25 ml) N,N-diisopropylethylamine (DIPEA) (0.2 ml, 1.15 mmol) and 2-cyanoethyl diisopropylphosphoramido chloridite (0.2 ml, 0.9 mmol) were added under an Ar atmosphere. The reaction mixture was stirred at r.t. for 1 h, diluted with dichloromethane (20 ml), was washed with 5% aq. NaHCO3, and dried over Na2SO4. Upon evaporation, the residue was submitted to FC (CH2Cl2/acetone, 9:1) yielding a colorless foam of 9 (0.45 g, 87%). TLC (CH2Cl2/acetone, 9:1): Rf 0.56, 0.65. 31P-NMR (CDCl3): 149.12. 1H-NMR [(D6)DMSO], 1.19 [m, CH(CH3)2]; 2.16 (s, CH3); 2.35–3.35 [m, H2-C(2′), CH(CH3)2, H2-C(5′)]; 3.45–3.94 (m, CH2CH2); 3.80 (s, CH3O); 4.23 [m, H-C(4′)]; 4.82 [m, H-C(3′)]; 6.45 [m, H-C(1′)]; 6.77–7.47 (m, arom. H); 9.65, 11.73 (br, NH).
6-Amino-1-(2-deoxy-β-d-erythro-pentofuranosyl)-1,5-dihydro-3-(3-phthaloylaminopropynyl)-4H-pyrazolo [3,4-d]-pyrimidin-4-one (10a)
Compound 6a (0.27g, 0.69 mmol) (3) was reacted with (PPh3)4Pd(0) (94 mg, 0.08 mmol), CuI (31.1 mg, 0.16 mmol), and phthaloylamidopropyne (1.5 g, 8.1 mmol) (30) in anhydrous DMF under Ar at r.t. as described for 3b. TLC indicated that the reaction was finished after 24 h, the second addition of the reagents was not necessary. The reaction mixture was subjected to FC (CH2Cl2/MeOH, 20:1); compound 3c was obtained as colorless solid (0.18 g, 58%). TLC (CH2Cl2/MeOH, 9:1): Rf 0.19. UV (MeOH): λmax 240 (36 900), 217 (51 080). 1H-NMR [(D6)DMSO], 2.13, 2.63 [m, 2H-C(2′)]; 3.32 [m, 2H-C(5′)]; 3.74 [m, H-C(4′)]; 4.32 [m, H-C(3′)]; 4.66 (s, CH2), 4.72 [t, J = 5.7, HO-C(5′)]; 5.23 [d, J = 4.3, HO-C(3′)]; 6.26 [‘t’, J = 6.5, H-C(1′)]; 6.77 (br, NH2); 7.93 (m, arom. H). Anal. calc. for C21H18N6O6 (450.4): C, 56.00; H, 4.03; N, 18.66; found: C, 55.84; H, 4.13; N, 18.27.
1-(2-Deoxy-β-d-erythro-pentofuranosyl)-1,5-dihydro-6-[(2-methylpropanoyl)amino]-3-(3-phthaloylaminopropynyl)-4H-pyrazolo[3,4-d]-pyrimidin-4-one (10b)
The cross-coupling reaction of compound 6b (15) (0.8 g, 1.73 mmol) with phthaloylamidopropyne (3.0 g, 16.2 mmol) was performed in anhydrous DMF (20 ml) under Ar in the presence of (PPh3)4Pd(0) (207 mg, 0.18 mmol), CuI (67.2 mg, 0.35 mmol) and triethylamine (0.5 ml, 3.59 mmol) at r.t. as described for 3b. After chromatographic purification (CH2Cl2/MeOH, 20:1) a colorless solid (0.89 g, quantitative yield) was obtained. TLC (CH2Cl2/CH3OH, 9:1): Rf 0.41. UV (MeOH): λmax 217 (51 400), 239 (30 000), 249 (26 000), 271 (16 300). 1H-NMR [(D6)DMSO], 1.11 (m, 2CH3); 2.25 [m, H-C(2′)]; 2.73 [m, H-C(2′), CH(CH3)2)]; 3.45 [m, 2H-C(5′)]; 3.77 [m, H-C(4′)]; 4.37 [m, H-C(3′)]; 4.70 (s, CH2); 4.73 [HO-C(5′)]. Anal. calc. for C25H24N6O7 (520.5): C, 57.69; H, 4.65; N, 16.15; found: C, 57.45; H, 4.56; N, 15.95.
1-[2-Deoxy-5-(4,4′-dimethoxytrityl)-β-d-erythro-pentofuranosyl]-1,5-dihydro-6-[(2-methylpropanoyl) amino]-3-(3-phthaloylaminopropynyl)-4H-pyrazolo [3,4-d]-pyrimidin-4-one (11)
1-[2-Deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pentofur anosyl]-1,5-dihydro-3-iodo-6-[(2-methylpropanoyl)amino]-4H-pyrazolo[3,4-d]pyrimidin-4-one (15) (130 mg, 0.17 mmol) was subjected to the cross-coupling reaction with phthaloylamidopropyne (351.5 mg, 1.9 mmol) under the same conditions as for 3b. CuI (7.3 mg, 0.04 mmol), (PPh3)4Pd(0) (22.5 mg, 0.02 mmol), and triethylamine (57 µl, 0.41 mmol) were used. The reaction mixture was subjected to FC (CH2Cl2/MeOH, 20:1) to furnish 11 as a colorless solid (0.1 g, 72%). TLC (CH2Cl2/MeOH 9:1): Rf 0.76. UV (MeOH): λmax 202 (110 000), 274 (19 000). 1H-NMR [(D6)DMSO], 1.13 (m, 2CH3); 2.26 [m, H-C(2′)]; 2.73 [m, H-C(2′)]; 3.01 [m, CH(CH3)2, 2H-C(5′)]; 3.68 (s, OCH3); 3.88 [m, H-C(4′)]; 4.42 [m, H-C(3′)]; 4.69 (s, CH2); 5.33 [d, J = 4.3, HO-C(3′)]; 6.39 [‘t’, J = 6.5, H-C(1′)]; 6.70–7.27 (m, arom. H); 11.90, 11.96 (s, 1-NH, NHCO). Anal. calc. for C46H42N6O9 (822.9): C, 67.14; H, 5.14; N, 10.21; found: C, 67.18; H, 5.09; N, 9.99.
1-[2-Deoxy-5-(4,4′-dimethoxytrityl)-β-d-erythro-pentofuranosyl]-1,5-dihydro-6-[(2-methylpropanoyl)amino]-3-(3-phthaloylaminopropynyl)-4H-pyrazolo-[3,4-d]-pyrimidin-4-one 3′-(2-cyanoethyl diisopropylphosphoramidite) (12)
The conversion from 11 (0.51 g, 0.62 mmol) was conducted as for 9, with DIPEA (0.25 ml, 1.44 mmol) and 2-cyanoethyl diisopropylphosphoramido chloridite (250 µl, 1.12 mmol) in anhydrous CH2Cl2 (20 ml) under Ar. The product was purified by FC (CH2Cl2/acetone, 85:15) to yield a colorless foam (0.51 g, 80%). TLC (CH2Cl2/acetone, 85:15): Rf 0.61, 0.67. 1H-NMR (CDCl3): 1.15 (m, 2CH3); 2.46 [m, H-C(2′), CH(CH3)2]; 3.12 [m, H-C(2′)], 3.69 (m, CH2CH2); 3.79 [m, 2H-C(5′)]; 3.79 (s, OCH3); 4.25 [m, H-C(4′)]; 4.76 [m, H-C(3′)]; 4.79 (s, CH2); 6.40 [‘t’, J = 6.5, H-C(1′)]; 6.74–8.26 (m, arom. H); 11.73 (s, NHCO). 31P-NMR: 149.04, 149.11.
1-(2-Deoxy-β-d-erythro-pentofuranosyl)-4-{[(dimethylamino)methylidene]amino}-3-propynyl-1H-pyrazolo[3,4-d]pyrimidine (13)
A solution of compound 4b (7) (250 mg, 0.87 mmol) in MeOH (30 ml) was stirred with N,N-dimethylformamide dimethyl acetal (3 ml, 21.4 mmol) for 30 min at r.t. After evaporation, the residue was applied to FC (CH2Cl2/MeOH, 95:5). Compound 13 was isolated as a colorless foam (270 mg, 91%). TLC (CH2Cl2/MeOH, 9:1): Rf 0.43. UV (MeOH) λmax: 321 nm (26 500), 223 (26 100). 1H-NMR [(D6)DMSO]: δ 2.27 [m, 1H, Hα-C(2′)]; 2.77 [m, 1H, Hβ-C(2′)]; 3.18, 3.21 (2s, 6H, Me2N); 3.72–3.81 [m, 2H, H-C(4′), H-C(5′)]; 4.42 [m, 1H, H-C(3′)]; 4.87 [m, J = 5.6, 1H, HO-C(5′)]; 5.40 [d, J = 4.5, 1H, HO-C(3′)]; 6.56 [t, J = 6.4, 1H, H-C(1′)]; 8.42 [s, 1H, H-C(6)]; 8.87 (s, 1H, N=CH). Anal. calc. for C16H20N6O3 (344.4): C, 55.80; H, 5.85; N, 24.40; found: C, 55.75; H, 5.95; N, 24.25.
1-(2-Deoxy-5-O-(4,4′-dimethoxytriphenylmethyl)-β-d-erythro-pentofuranosyl)-4-{[(di-methylamino) methylidene]amino}-3-propynyl-1H-pyrazolo [3,4-d]pyrimidine (14)
Compound 14 was obtained by the tritylation of compound 13 (0.2 g, 0.58 mmol) with DMT-Cl (0.24 g, 0.7 mmol) as described for 8 as a colorless foam purified by FC (CH2Cl2/MeOH, 20:1) (350 mg, 93%). Rf (CH2Cl2/MeOH, 9:1): 0.29. UV (MeOH) λmax: 224 nm (41 500), 320 (25 900). 1H-NMR [(D6)DMSO]: δ 2.33 [m, 1H, Hα-C(2′)]; 2.79 [m, 1H, Hβ-C(2′)]; 2.94, 3.04 [m, 2H, H-C(5′)]; 3.17, 3.21 (2s, 6H, Me2N); 3.68, 3.69 (2s, 6H, 2 MeO); 3.90 [m, 1H, H-C(4′)], 4.56 [m, 1H, H-C(3′)]; 5.44 [d, J = 5.0, 1H, HO-C(3′)]; 6.58 [m, 1H, H-C(1′)]; 6.69–7.29 (m, arom. H); 8.45 [s, 1H, H-C(6)]; 8.86 (s, 1H, N=CH). Anal. calc. for C37H38N6O5 (646.7): C, 68.71; H, 5.92; N, 12.99; found: C, 68.59; H, 6.00; N, 13.02.
1-(2-Deoxy-5-O-(4,4′-dimethoxytriphenylmethyl)-β-d-erythro-pentofuranosyl)-4-{[(di-methylamino) methylidene]amino}-3-propynyl-1H-pyrazolo [3,4-d]pyrimidine 3′-[(2-cyanoethyl)-N,N-diisopropylphosphoramidite] (15)
Compound 15 was synthesized from 14 (300 mg, 0.47 mmol) in anhydrous CH2Cl2 (5 ml) with anhydrous DIPEA (0.16 ml, 0.92 mmol) and 2-cyanoethyl diisopropylphosphoramido chloridite (0.21 ml, 0.94 mmol) under Ar. The residue was applied to FC (CH2Cl2/Me2CO, 10:1) to yield the title compound 15 as a colorless foam (290 mg, 74%). TLC (CH2Cl2/Me2CO, 8:1): Rf 0.18, 0.24. 1H-NMR [(D6)DMSO]: δ 1.16 [m, 6H, 2CHMe2]; 2.18 (s, 3H, Me); 2.48, 2.66 [m, 2H, H2-C(2′)]; 3.23, 3.30 [2s, 6H, 2CH3]; 3.25 [m, H2-C(5′)]; 3.73 (m, CH2CH2); 3.79 (2s, OCH3); 4.22 [m, 1H, H-C(4′)]; 4.81 [m, 1H, H-C(3′)]; 6.74–7.42 [m, H-C(1′), arom. H]; 8.54 [s, 1H, H-C(6)]; 8.84 (s, 1H, N=CH). 31P-NMR (CDCl3): 149.61, 149.71.
RESULTS AND DISCUSSION
Monomers
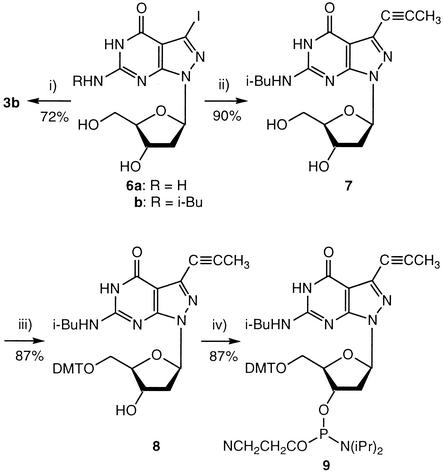
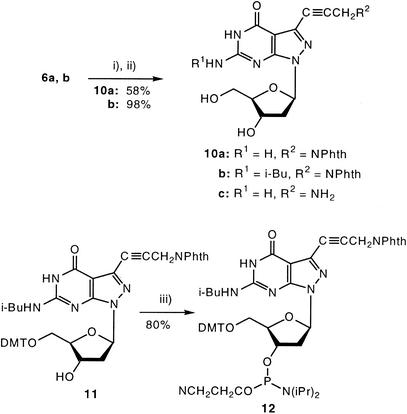
The propynyl nucleosides 1 (25), 2 (25), 4b (7) and 5b (18) were prepared according to the literature. The 7-propynyl and 7-(3-phthaloylamidopropynyl) substituted 8-aza-7-deaza-2′-deoxyguanosine derivatives 3b, 10a were synthesized from the 7-iodo nucleosides 6a (3) or 6b (15) by the Sonogashira cross-coupling reaction (16,31–33) (Schemes 2 and 3). The reaction was catalyzed by Pd(0)[(PPh)3]4/CuI and was performed in DMF in the presence of triethylamine. For 3b, DMF was saturated with propyne at 0°C. Its amino group was protected with an isobutyryl group by transient protection (34) (7). The protected 7 was converted into the DMT derivative 8 and further transformed to the phosphoramidites 9 under standard conditions. The phthaloylamino derivatives 10a,b and 11 were prepared from 6a,b, and the DMT derivative (15) employing phthaloylamidopropyne in the cross-coupling reaction. Compound 11 was converted into the phosphoramidite 12.
Scheme 2. (i) and (ii) Propyne, Pd(0)(PPh3)4, CuI, Et3N, Ar, r.t., 18 h. (iii) (MeO)2TrCl, pyridine, r.t.; 3 h. (iv) 2-Cyanoethyl diisopropylphosphoramido chloridite, CH2Cl2, r.t., 30 min.
Scheme 3. (i) and (ii) Phthaloylamidopropyne Pd(0)(PPh3)4, CuI, Et3N, Ar, r.t., 21 h. (iii) 2-Cyanoethyl diisopropylphosphoramido chloridite, CH2Cl2, r.t., 30 min.
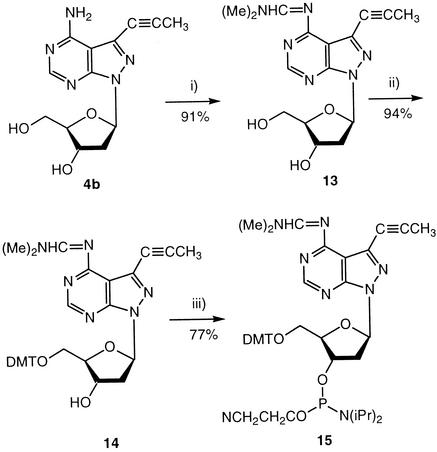
Finally, the phosphoramidite 15 was prepared (Scheme 4). For this purpose, compound 4b was protected at the amino group with the dimethylaminomethylidene residue resulting in 13. Then, the DMT residue was introduced to the 5′-hydroxyl group yielding compound 14, which was transformed into the phosphoramidite 15. All compounds were characterized by elemental analysis as well as by 1H- and 13C-NMR spectra (Table 2 and Materials and Methods).
Scheme 4. (i) N,N-Dimethylformamide dimethyl acetal in methanol, r.t. (ii) DMT-Cl, dry pyridine, 3 h at r.t. (iii) 2-Cyanoethyl diisopropylphosphoramido chloridite, diisopropylethylamine, CH2Cl2, 1 h, at r.t.
Table 2. 13C-NMR chemical shifts of pyrazolo[3,4-d]-pyrimidine 2′-deoxyribonucleosidesa.
| Compound | C(2)b,d C(6)c,d | C(4)b,d C(7a)c,d | C(5)b C(3a)c | C(6)b,d C(4)c,d | C(7)b C(3)c | C≡C | CH3/CH2 | N(CH3)2/C=N | C(1′) | C(2′) | C(3′) | C(4′) | C(5′) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3a (4) | 154.6 | 155.2 | 99.7 | 157.4 | 134.9 | 83.1 | 37.9 | 71.0 | 87.3 | 62.4 | |||
| 3b | 155.1 | 155.4 | 99.8 | 157.0 | 130.4 | 72.2, 89.4 | 3.9 | 83.0 | 37.6 | 70.9 | 87.5 | 62.4 | |
| 4a (7) | 156.9 | 154.5 | 101.3 | 158.9 | 133.9 | 84.9 | 38.8 | 72.0 | 88.4 | 63.3 | |||
| 4b | 156.5 | 153.5 | 100.8 | 157.6 | 127.4 | 71.1, 92.9 | 4.0 | 83.3 | 38.0 | 71.3 | 87.4 | 62.7 | |
| 5a | 156.9 | 158.3 | 95.5 | 162.7 | 133.3 | 83.0 | 37.6 | 71.0 | 87.3 | 62.5 | |||
| 5b | 156.7 | 157.8 | 95.6 | 162.7 | 127.3 | 72.0, 91.5 | 3.8 | 83.1 | 37.6 | 70.8 | 87.4 | 62.3 | |
| 6a (3) | 154.9 | 155.8 | 93.9 | 157.3 | 101.9 | 83.5 | 37.7 | 70.7 | 87.7 | 62.2 | |||
| 7 | 152.7 | 150.3 | 102.8 | 155.0 | 130.7 | 71.5, 90.6 | 4.18 | 83.7 | 37.8 | 70.2 | 87.7 | 63.9 | |
| 8 | 152.6 | 150.3 | 103.0 | 155.1 | 130.5 | 71.6, 90.4 | 4.0 | 87.3 | 37.6 | 70.9 | 83.1 | 62.4 | |
| 10a | 155.2 | 155.5 | 100.2 | 156.8 | 123.4 | 74.5, 86.5 | 27.4 | 83.8 | 37.8 | 70.9 | 87.6 | 62.3 | |
| 10b | 152.8 | 150.5 | 103.2 | 154.9 | 129.3 | 73.8, 87.5 | 27.4 | 84.5 | 37.6 | 70.6 | 87.5 | 62.1 | |
| 11 | 152.6 | 150.4 | 103.4 | 154.9 | 123.4 | 73.9, 85.0 | 27.4 | 87.3 | 37.8 | 70.2 | 83.9 | 63.9 | |
| 13 | 155.8 | 154.3 | 107.7 | 162.2 | 129.1 | 72.5, 90.7 | 3.8 | 34.6, 157.5 | 83.8 | 37.8 | 70.9 | 87.5 | 62.3 |
| 14 | 155.8 | 154.3 | 107.8 | 162.2 | 129.0 | 72.7, 90.4 | 3.9 | 34.5, 157.8 | 83.5 | e | 70.3 | 85.0 | 63.9 |
aMeasured in (D6)DMSO.
bPurine numbering.
cSystematic numbering.
dTentative.
eSuperimposed by DMSO.
Oligonucleotides
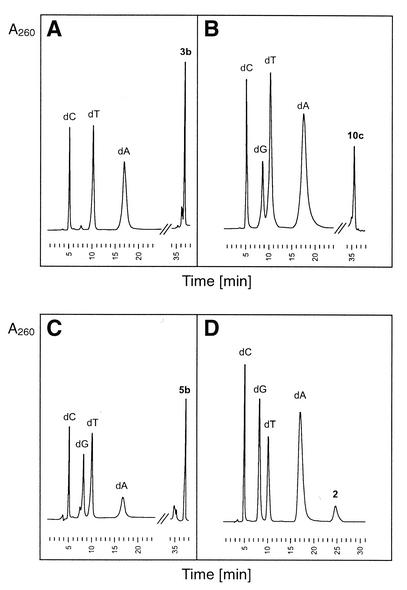
Synthesis and characterization. The oligonucleotides 16–47 were prepared in a 1 µmol scale on an ABI 392-08 synthesizer (trityl-on mode) employing phosphoramidite chemistry (35). Apart from the phosphoramidites 9, 12 and 15, standard phosphoramidites were used. In the case of the oligonucleotides containing 5-propynyl-2′-deoxyuridine (2), the tac [(4-(tert-butyl)phenoxyacetyl]-protected building blocks were used. The coupling efficiency was always higher than 95%. The deprotection of the oligonucleotides was performed in 25% aq. NH3 for 12–18 h at 60°C or at r.t. for 2–3 h for oligonucleotides containing compound 2. The oligonucleotides were purified before and after detritylation by reversed-phase HPLC (for conditions of purification see Materials and Methods). The composition analysis of oligomers was performed by enzymatic hydrolysis with snake-venom phosphodiesterase followed by alkaline phosphatase (Fig. 1). Also, MALDI-TOF mass spectrometry was performed confirming the constitution of the oligonucleotides.
Figure 1.
HPLC profile of enzymatic analysis of oligonucleotides 20 containing 3b (A), 24 containing 10c (B), 39 containing 5b (C), 34 containing 2 (D) by phosphodiesterase and alkaline phosphatase in 0.1 M Tris–HCl buffer (pH 8.3) at 37°C. Condition: reversed-phase HPLC at 260 nm on an RP-18 column (200 × 10 mm) with 100% B as the eluent, 0.7 ml/min (for composition of B, see Materials and Methods).
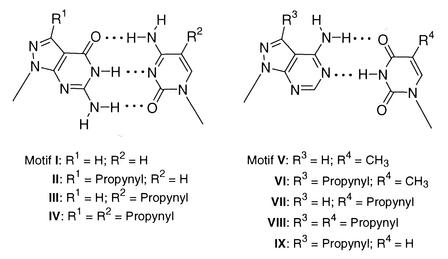
Duplex stability of oligonucleotides containing propynyl residues and related side chains. Earlier, it was reported that 8-aza-7-deazapurine nucleosides with bromo or iodo substituents and also longer alkynyl chains linked to the 7-position are well accommodated in the major groove of DNA, thereby increasing the thermal stability of duplexes (1,18,36). Moreover, it was found that alkynyl chains with three to six carbon atoms led to a significant stabilization of duplex DNA while longer chains are too hydrophobic to fulfill this objective, in particular when multiple incorporations were made. This study makes use of propynyl residues being part of the 8-aza-7-deazapurine nucleosides 3b, 4b and 5b. The already known pyrimidine nucleosides 1 and 2 were employed to study the role of the propynyl group on both sides of the ‘dG–dC’ or the ‘dA–dT’ base pair (motifs I–XVI, Schemes 5 and 6). As all four propynyl nucleosides related to the canonical DNA constituents were now accessible, a rather complete picture of the stabilizing effects of propynyl groups on Watson–Crick base pairs of oligonucleotide duplexes was developed.
Scheme 5.
Scheme 6.
The stability of the tridentate ‘dG–dC’ base pair modified by 8-aza-7-deaza-7-propynyl-2′-deoxyguanosine (3b) and/or 5-propynyl-2′-deoxycytidine (1). In a first series of experiments, the role of the propynyl group was studied with the duplex 5′-d(TAG GTC AAT ACT) (16) 3′-d(ATC CAG TTA TGA) (17) incorporating compound 3b in the place of dG. The Tm values of a series of oligonucleotides are shown in Table 3. According to Table 3, the 7-propynyl nucleoside 3b increases the stability of a ‘dG–dC’ base pair (Scheme 5, motif I) significantly. The incorporation of 3b (motif II) residue results in a Tm increase of 2.5–3°C per modification (data taken in the high salt buffer) depending on the position of substitution. The contribution of the 7-aminopropynyl residue (10c) is smaller (1–2°C), which is ∼1°C less than for 3b. In a similar way oligonucleotide duplexes were studied in which the dC residues of dG–dC base pairs were replaced by 5-propynyl-2′-deoxycytidine (1). The stability increase of such a modified base pair (motif III) was found to be between 2.0 and 3.0°C, which is slightly lower than that found for the 3b–dC pair. Also, both bases of the ‘dG–dC’ pair were substituted by the propynyl nucleosides 1 and 3b (motif IV) in which the Tm increase was 5.0–5.5°C for two propynyl modifications per base pair, which corresponds to ∼2.5–2.75°C for each single nucleoside substitution (Tables 3 and 4).
Table 3. Tm values and thermodynamic data of oligonucleotides containing 8-aza-7-deaza-7-propynyl-2′-deoxyguanosine 3b, 1, or the aminopropynyl derivative 10ca.
| Duplex | Tm (°C) | ΔTmb (°C) | ΔH° (kcal/mol) | ΔS° (cal/mol K) | ΔG°310 (kcal/mol) |
|---|---|---|---|---|---|
| 5′-d(TAG GTC AAT ACT) (16) | 50 | –90 | –252 | –11.8 | |
| 3′-d(ATC CAG TTA TGA) (17) | (47) | –89 | –253 | –10.9 | |
| 5′-d(TAG GTC AAT ACT) (16) | 53 | 3 | –96 | –270 | –12.5 |
| 3′-d(ATC CA3b TTA TGA) (18) | (49) | 2 | |||
| 5′-d(TAG GTC AAT ACT) (16) | 56 | 3 | –105 | –293 | –13.8 |
| 3′-d(ATC CA3bTTA T3bA) (19) | (53) | 3 | |||
| 5′-d(TA3b 3bTC AAT ACT) (20) | 56 | 3 | –105 | –294 | –14.5 |
| 3′-d(ATC CAG TTA TGA) (17) | (52) | 2.5 | |||
| 5′-d(TA3b 3bTC AAT ACT) (20) | 58 | 2.7 | –98 | –272 | –14.0 |
| 3′-d(ATC CA3b TTA TGA) (18) | (56) | 3 | |||
| 5′-d(TA3b 3bTC AAT ACT) (20) | 60 | 2.5 | –99 | –272 | –14.6 |
| 3′-d(ATC CA3b TTA T3bA) (19) | (58) | 2.8 | –93 | –256 | –13.7 |
| 5′-d(TAG GT1 AAT ACT) (21) | 55 | 2.5 | –89 | –245 | –12.8 |
| 3′-d(ATC CA3b TTA TGA) (18) | |||||
| 5′-d(TA3b 3bTC AAT ACT) (20) | 61 | 2.75 | –90 | –243 | –14.3 |
| 3′-d(AT1 1AG TTA TGA) (22) | (58) | 2.75 | |||
| 5′-d(TAG GT1 AAT A1T) (23) | 60 | 2.5 | –97 | –265 | –14.6 |
| 3′-d(ATC CA3b TTA T3bA) (19) | (57) | 2.5 | |||
| 5′-d(TAG GTC AAT ACT) (16) | 52 | 2.0 | –87 | –242 | –11.8 |
| 3′-d(ATC CA10c TTA TGA) (24) | (48) | 1.0 | |||
| 5′-d(TA10c 10cTC AAT ACT) (25) | 54 | 2.0 | –90 | –250 | –12.6 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TA10c 10cTC AAT ACT) (25) | 55 | 1.75 | –97 | –269 | –13.3 |
| 3′-d(ATC CA10c TTA TGA) (24) |
aMeasured in 1 M NaCl, 100 mM MgCl2 and 60 mM Na-cacodylate (pH 7.0) with 5 µM single-strand concentration. Data in parentheses were measured in 100 mM NaCl, 10 mM MgCl2 and 10 mM Na-cacodylate (pH 7.0) with 5 µM oligonucleotide.
bRefers to the contribution of the modified residues divided by the number of replacements.
Table 4. Tm values and thermodynamic data of oligonucleotides incorporating 5-propynyl-deoxycytidine 1 and 3ba.
| Duplex | Tm (°C) | ΔTmb (°C) | ΔH° (kcal/mol) | ΔS° (cal/mol K) | ΔG°310 (kcal/mol) |
|---|---|---|---|---|---|
| 5′-d(TAG GT1 AAT ACT) (21) | 53 | 3 | –90 | –250 | –12.4 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TAG GT1 AAT A1T) (23) | 54 | 2 | –93 | –259 | –12.8 |
| 3′-d(ATC CAG TTA TGA) (17) | (52) | 2.5 | |||
| 5′-d(TAG GTC AAT ACT) (16) | 55.5 | 2.75 | –87 | –239 | –12.8 |
| 3′-d(AT1 1AG TTA TGA) (22) | |||||
| 5′-d(1 TAG GTC AAT ACT) (26) | 52 | 2.0 | –96 | –271 | –12.2 |
| 3′-d(ATC CAG TTA TGA) (17) | (49) | 2.0 | |||
| 5′-d(TAG GT1 AAT ACT) (21) | 58.5 | 2.7 | –87 | –237 | –13.4 |
| 3′-d(AT1 1AG TTA TGA) (22) | |||||
| 5′-d(TAG GT1 AAT A1T) (23) | 60 | 2.5 | –91 | –248 | –14.0 |
| 3′-d(AT1 1AG TTA TGA) (22) | |||||
| 5′-d(1 TAG GTC AAT ACT) (26) | 57 | 2.3 | –90 | –247 | –13.1 |
| 3′-d(AT1 1AG TTA TGA) (22) | |||||
| 5′-d(1 TAGGTC AAT ACT) (26) | 54 | 2.0 | –89 | –247 | –12.6 |
| 3′-d(ATC CA3bTTA TGA) (18) | (51) | 2.0 | |||
| 5′-d(1 TAG GTC AATACT) (26) | 57 | 2.3 | –92 | –255 | –13.4 |
| 3′-d(ATC CA3bTTA T3bA) (19) |
aMeasured in 1 M NaCl, 100 mM MgCl2 and 60 mM Na-cacodylate (pH 7.0) with 5 µM single-strand concentration. Data in parentheses were measured in 100 mM NaCl, 10 mM MgCl2 and 10 mM Na-cacodylate (pH 7.0) with 5 µM oligonucleotides.
bSee Table 3.
From the Tm values shown above, it can be concluded that a 7-propynyl residue linked to the 7-position of an 8-aza-7-deaza-2′-deoxyguanosine residue (3b) causes a slightly better stabilization of a DNA duplex than that of the 5-propynyl residue of a 2′-deoxycytidine moiety (1). The modification at both sites of the ‘dG–dC’ base pair (motif IV) shows an addition of propynyl effects (Table 3). The 3-amino propynyl residues destabilize the duplex compared to the propynyl groups (37). The thermodynamic data taken from the shape analyses of the melting profiles have to be interpreted with care. An error of ∼15% has to be taken into account for the thermodynamic data while the accuracy of the Tm measurements is within ±0.5°C.
The bidentate ‘dA–dT’ base pair modified by 7-propynylated 8-aza-7-deazapurin-6-amine 2′-deoxyribonucleoside (4b) and/or 5-propynyl-2′-deoxyuridine (2). Similar experiments as performed for the ‘dG–dC’ base pair were undertaken on the ‘dA–dT’ pair (Scheme 5, motifs V–IX). Single and multiple substitutions of dA residues by the propynyl derivative 4b are shown in Table 5. A ΔTm of 2–3°C is observed for each modification of a dA residue by compound 4b when measured in low salt buffer (motif VI versus V) (18). These values are lower than for the propynylated dG derivative 3b. Even lower are the ΔTm values (0.75–1.5°C) for the modification of dT by the propynyl nucleoside 2 (motif VII). However, in these cases it has to be taken into account that the values refer to a dA–dT base pair containing already a methyl group at the dT residue. The propynyl group at both sides of the base pair 4b–2 (motif VIII) shows an additive effect on the Tm increase. The 5-propynyl group is more effective than the 5-methyl group (4b–2 versus 4b–dT, motif VIII versus VI). The base pair 4b–dU (motif IX) shows the lowest Tm value (Tables 5 and 6).
Table 5. Tm values and thermodynamic data of oligonucleotides containing 8-aza-7-deaza-7-propynyl-2′-deoxyadenosine 4ba.
| Duplex | Tm (°C) | ΔTmb (°C) | ΔH° (kcal/mol) | ΔS° (cal/mol K) | ΔG°310 (kcal/mol) |
|---|---|---|---|---|---|
| 5′-d(TAG GTC 4bAT ACT) (27) | 50 | 3 | –83 | –233 | –11.0 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TAG GTC AAT ACT) (16) | 53 | 3 | –94 | –263 | –12.6 |
| 3′-d(ATC C4bG TT4b TGA) (28) | |||||
| 5′-d(TAG GTC 4b4bT ACT) (29) | 52 | 2.5 | –85 | –234 | –11.8 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TAG GTC 4bAT ACT) (27) | 56 | 3 | –94 | –262 | –13.2 |
| 3′-d(ATC C4bG TT4b TGA) (28) | |||||
| 5′-d(TAG GTC 4b4bT ACT) (29) | 59 | 3 | –88 | –240 | –13.6 |
| 3′-d(ATC C4bG TT4b TGA) (28) | |||||
| 5′-d(T4bG GTC 4b4bT 4bCT) (30) | 56 | 2.5 | –94 | –261 | –13.2 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(T4bG GTC 4b4bT 4bCT) (30) | 63 | 2.7 | –99 | –269 | –15.7 |
| 3′-d(ATC C4bG TT4b TGA) (28) | |||||
| 5′-d(TAG GTC 4b4bT ACT) (29) | 51 | 2 | –93 | –263 | –11.8 |
| 3′-d(ATC CAG UUA TGA) (31) | |||||
| 5′-d(TAG GUC AAU ACT) (32) | 51 | 2 | –83 | –231 | –11.8 |
| 3′-d(ATC C4bG TT4b TGA) (28) |
aMeasured in 100 mM NaCl, 10 mM MgCl2 and 10 mM Na-cacodylate (pH 7.0) with 5 µM + 5 µM single-strand concentration.
bSee Table 3.
Table 6. Tm values and thermodynamic data of oligonucleotides containing 5-propynyl-2′-deoxyuridine (2) and 4ba.
| Duplex | Tm (°C) | ΔTmb (°C) | ΔH° (kcal/mol) | ΔS° (cal/mol K) | ΔG°310 (kcal/mol) |
|---|---|---|---|---|---|
| 5′-d(TAG G2C AA2 ACT) (33) | 53 | 1.5 | –98 | –274 | –12.6 |
| 3′-d(ATC CAG TTA TGA) (17) | (50) | 1.5 | |||
| 5′-d(TAG GTC AAT ACT) (16) | 52 | 1.0 | –93 | –262 | –12.2 |
| 3′-d(ATC CAG 22A TGA) (34) | (50) | 1.5 | |||
| 5′-d(TAG G2C AA2 ACT) (33) | 53 | 0.75 | –101 | –285 | –12.8 |
| 3′-d(ATC CAG 22A TGA) (34) | (53) | 1.5 | |||
| 5′-d(TAG G2C AAT ACT) (35) | 52 | 2.0 | –91 | –256 | –11.9 |
| 3′-d(ATC CAG TTA TGA) (17) | (50) | 3.0 | |||
| 5′-d(2 TAG GTC AAT ACT) (36) | 52 | 2.0 | –96 | –271 | –12.2 |
| 3′-d(ATC CAG TTA TGA) (17) | (48) | 1.0 | |||
| 5′-d(TAG G2C AAT ACT) (35) | 53 | 1.0 | –91 | –254 | –12.5 |
| 3′-d(ATC CAG 22A TGA) (34) | (51) | 1.3 | |||
| 5′-d(2 TAG GTC AAT ACT) (36) | 53 | 1.0 | –100 | –280 | –12.8 |
| 3′-d(ATC CAG 22A TGA) (34) | (51) | 1.3 | |||
| 5′-d(TAG GTC 4b4bT ACT) (29) | 56 | 1.5 | –100 | –278 | –13.6 |
| 3′-d(ATC CAG 22A TGA) (34) |
aMeasured in 1 M NaCl, 100 mM MgCl2 and 60 mM Na-cacodylate (pH 7.0) with 5 µM single-strand concentration. Data in parentheses were measured in 100 mM NaCl, 10 mM MgCl2 and 10 mM Na-cacodylate (pH 7.0) with 5 µM oligonucleotides.
bSee Table 3.
Comparison of the duplex stabilities of base pairs incorporating the 7-propynyl as well as the bromo derivatives of 8-aza-7-deaza-2′-deoxyguanosine (3b,c), 8-aza-7-deaza-2′-deoxyadenosines (4b,c) and the 8-aza-7-deazapurin-2,6-amine 2′-deoxyribonucleosides (5b,c). Earlier, it was already reported on the extraordinary stability of oligonucleotide duplexes incorporating the diamino nucleoside 5b (18). It forms a stable base pair with dT (Scheme 6, motif XI) as the tridentate dG–dC pair. This study was now extended to a series of oligonucleotide duplexes incorporating compound 5b at various positions of the oligonucleotide chain and containing the modified nucleoside in one, the other, or in both strands (Table 7). From the data shown in Table 7 (low salt buffer), it is apparent that the Tm increase amounts to 4.3–6°C for a single modification of compound 5b. As the corresponding non-propynylated nucleoside 5a and the parent purin-2,6-diamine 2′-deoxyribonucleoside have only a minor influence on the duplex stability (see Table 7), this stabilization is caused by the combination of two favorable effects: one caused by the propynyl group and the other by the 2-amino group, which forms the tridentate base pair with the 2-oxo group of the uracil base (motif XI). The propynyl effect of the nucleoside 5b is diluted by the rather low stabilizing propynylated pyrimidine nucleoside 2, as is seen by the comparison of four incorporations of compound 5b (duplex 38·39) with four incorporations (2 × compound 5b and 2 × compound 2) in the duplex 33·38 (motif XII). The former amounts to a ΔTm of 21°C compared to the unmodified duplex 16·17 and only to 13°C for the duplex 33·38. The propynyl effect of compound 2 is seen when comparing the duplexes 34·39 with 31·39. The propynyl group amounts only to 1°C per modification in comparison to the unsubstituted dU (motif XIII).
Table 7. Tm values and thermodynamic data of oligonucleotides incorporating the base pairs of the 8-aza-7-deazapurin-2,6-diamine 2′-deoxyribonucleosides 5b–dT and 2–5ba.
| Duplex | Tm (°C) | ΔTmb (°C) | ΔH° (kcal/mol) | ΔS° (cal/mol K) | ΔG°310 (kcal/mol) |
|---|---|---|---|---|---|
| 5′-d(TAG GTC 5a5aT ACT)c | 50 | 1.5 | –93 | –263 | –11.7 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TAG GT C 5a5aT ACT)c | 51 | 2.0 | –99 | –279 | –12.3 |
| 3′-d(ATC C5aG TT5a TGA)c | |||||
| 5′-d(TAG GTC 5bAT ACT) (37) | 53 | 6 | –88 | –244 | –11.9 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TAG GTC AAT ACT) (16) | 58 | 5.5 | –101 | –279 | –14.4 |
| 3′-d(ATC C5bG TT5bTGA) (38) | |||||
| 5′-d(TAG GTC 5b5bT ACT) (39) | 58 | 5.5 | –100 | –277 | –13.8 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TAG GTC 5bAT ACT) (37) | 64 | 6 | –104 | –284 | –16.0 |
| 3′-d(ATC C5bG TT5b TGA) (38) | |||||
| 5′-d(TAG GT C 5b5bT ACT) (39) | 68 | 5 | –111 | –300 | –18.0 |
| 3′-d(ATC C5bG TT5b TGA) (38) | |||||
| 5′-d(T5bG GTC 5b5bT 5bCT) (40) | 65 | 4.3 | –109 | –297 | –17.1 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(T5bG GTC 5b5bT 5bCT) (40) | 75 | 4.7 | –115 | –303 | –20.6 |
| 3′-d(ATC C5bG TT 5b TGA) (38) | |||||
| 5′-d(TAG G2C AA2 ACT) (33) | 60 | 3.25 | –96 | –264 | –14.3 |
| 3′-d(ATC C5bGTT5b TGA) (38) | |||||
| 5′-d(TAG GTC 5b5bT ACT) (39) | 59 | 3.00 | –91 | –249 | –14.0 |
| 3′-d(ATC CAG 22A TGA) (34) | |||||
| 5′-d(TAG GTC 5b5bT ACT) (39) | 56 | 2.25 | –104 | –289 | –13.8 |
| 3′-d(ATC CAG UUA TGA) (31) |
Next, the base pair stabilization of the 7-propynyl group versus the 7-bromo substituent was studied. Table 8 contains a series of the duplexes incorporating the propynylated 8-aza-7-deazapurine nucleosides 3b, 4b and 5b and the brominated compounds 3c, 4c and 5c for comparison, which are substituted at exactly the same positions. In oligonucleotide duplexes incorporating either 3b or 3c, four duplexes were available for comparison. In one pair the propynyl effect is greater than that of a bromo substituent, in the other case the Tm values are identical so it is not possible to make a clear cut conclusion in this series, incorporating the tridentate base pair related to the dG–dC pair (in high salt buffer). However, when we go to oligonucleotide duplexes incorporating either the propynylated 4b (motif XV) or the brominated 4c (motif XVI) the stabilization of the propynyl group is definitely superior to that of the halogen substituent. It seems to us that a bidentate base pair reacts more sensitively to this change than a tridentate pair. Only due to a hydrogen bond is this pair more flexible, and the various substituents directed into the major groove can distort the base pair to a much greater extent than that within a tridentate pair. This idea is supported by the data of duplexes incorporating the propynylated compound 5b (motif XI) in comparison to the brominated 5c (motif XIV). Here, an almost identical contribution for the two kinds of residues are observed, which amount to a ΔTm of +4.5 –6°C in both cases. Thus, the use of either 5b or 5c is equally favorable for the stabilization of oligonucleotide duplexes being used in antisense technology or biochips for diagnostic purposes employing hybridization either in solution or on a solid surface.
Table 8. Comparison of Tm values and thermodynamic data of oligonucleotide duplexes incorporating 7-propynyl versus 7-bromo nucleosides in tridentate and bidentate base pairsa.
| Duplex | Tm (°C) | ΔTmb (°C) | ΔH° (kcal/mol) | ΔS° (cal/mol K) | ΔG°310 (kcal/mol) |
|---|---|---|---|---|---|
| 5′-d(TA3b 3bTC AAT ACT) (20) | 58 | 2.75 | –95 | –262 | –13.8 |
| 3′-d(ATC CA3b TTA T3bA) (19) | |||||
| 5′-d(TA3c 3cTC AAT ACT) (41) | 55 | 2.00 | –78 | –219 | –14.3 |
| 3′-d(ATC CA3c TTA T3cA) (42) | |||||
| 5′-d(TA3b 3bTC AAT ACT) (20) | 52 | 2.5 | –94 | –264 | –12.1 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TA3c 3cTC AAT ACT) (41) | 52 | 2.5 | –105 | –301 | –12.4 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(T4bG GTC 4b4bT 4bCT) (30) | 63 | 2.7 | –99 | –269 | –15.7 |
| 3′-d(ATC C4bG TT4b TGA) (28) | |||||
| 5′-d(T4cG GTC 4c4cT 4cCT) (43) | 57 | 1.7 | –111 | –308 | –15.2 |
| 3′-d(ATC C4cG TT4c TGA) (44) | |||||
| 5′-d(T4bG GTC 4b4bT 4bCT) (30) | 56 | 2.25 | –94 | –261 | –13.2 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(T4cG GTC 4c4cT 4cCT) (43) | 52 | 1.25 | –87 | –242 | –12.3 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TAGGTC AAT ACT) (16) | 55 | 4.0 | –89 | –246 | –12.4 |
| 3′-d(ATC C4bG TT4b TGA) (28) | |||||
| 5′-d(TAGGTC AAT ACT) (16) | 51 | 2.0 | – | – | – |
| 3′-d(ATC C4cG TT4c TGA) (44) | |||||
| 5′-d(T5bG GTC 5b5bT 5bCT) (40) | 75 | 4.7 | –115 | –303 | –20.6 |
| 3′-d(ATC C5bG TT5b TGA) (38) | |||||
| 5′-d(T5cG GTC 5c5cT 5cCT) (45) | 75 | 4.7 | –107 | –283 | –19.5 |
| 3′-d(ATC C5cG TT5c TGA) (46) | |||||
| 5′-d(TAGGTC 5b5bT ACT) (39) | 68 | 5.25 | –111 | –300 | –18.0 |
| 3′-d(ATC C5bG TT5b TGA) (38) | |||||
| 5′-d(TAGGTC 5c5cT ACT) (47) | 67 | 5.0 | –105 | –285 | –17.0 |
| 3′-d(ATC C5cG TT5c TGA) (46) | |||||
| 5′-d(TAGGTC 5b5bT ACT) (39) | 58 | 5.5 | –100 | –277 | –13.8 |
| 3′-d(ATC CAG TTA TGA) (17) | |||||
| 5′-d(TAGGTC 5c5cT ACT) (47) | 56 | 4.5 | –91 | –251 | –13.4 |
| 3′-d(ATC CAG TTA TGA) (17) |
aMeasured in 100 mM NaCl, 10 mM MgCl2 and 10 mM Na-cacodylate (pH 7.0) with 5 µM + 5 µM oligonucleotide.
bSee Table 3.
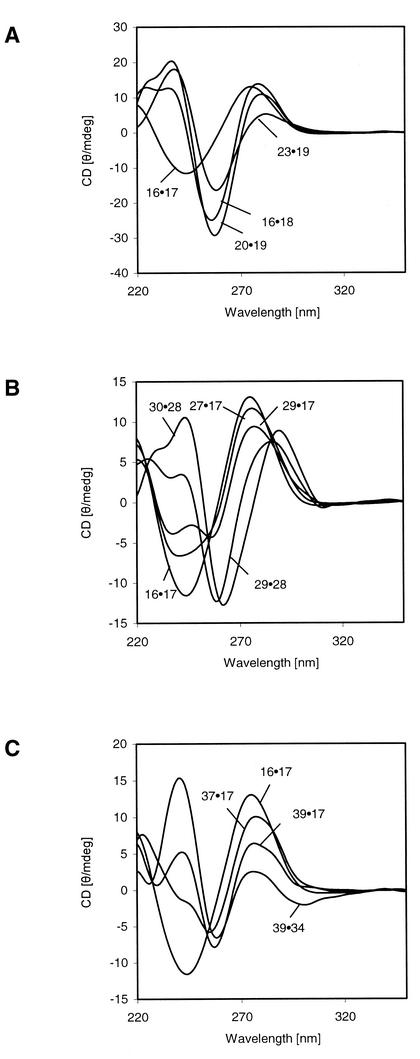
Oligonucleotide duplexes incorporating base pairs with propynyl residues show rather different CD spectra than their unmodified counterparts (Fig. 2A–C). In particular, in the region around 250 nm rather strong changes are observed. It is not clear whether these changes are due to the hydrophobic character of the propynyl residues or the altered polarizability of the nucleobases. Thus, water molecules being present in the major groove are expelled, which can effect base pairing and base stacking.
Figure 2.
The CD spectra of oligonucleotides containing 1 and 3b (A), 4b (B) and 5b (C). Measured at 10°C in buffers as indicated in Tables 3–8.
CONCLUSIONS
The synthesis of the phosphoramidites 12 and 15, together with the already known analogs of dC and dU, made oligonucleotides accessible, incorporating propynyl groups at the pyrimidine and/or the purine bases of the dA–dT and dG–dC base pairs. The propynyl group increases the stability of DNA no matter if this group is linked to the 5-position of a pyrimidine or to the 7-position of a ‘purine’ (8-aza-7-deazapurine) base. Because of its linear structure and coplanarity towards the heterocyclic base, it increases stacking interactions. It also tends to make the major groove hydrophobic and expels water molecules. The contribution of the propynyl group to the ‘dG–dC’ versus the ‘dA–dT’ base pairs shows differences. While the introduction of this group in the dG and dC analogs (3b and 1) causes a similar duplex stabilization (2–3°C for a 12mer duplex), it is less stabilizing in the case of dA and dT residues 4b and 2. The propynyl modification causes an extraordinary stabilization when the group is part of the pyrazolo[3,4-d]pyrimidine nucleoside 5b. Although the propynyl group, as well as the bromo substituent, increase the polarizibility of the various nucleobases, significant differences are observed regarding their stabilizing effects. The propynyl group is more stabilizing than a bromo substituent within the bidentate ‘4b,c–dT’ base pairs, while they show an almost identical behavior in the tridentate 5b,c–dT pairs.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Mr Yang He for the measurement of NMR spectra, Mrs Elisabeth Feiling and Mr Khalil Shaikh for the oligonucleotide synthesis and the MALDI-TOF mass spectra, and Mrs Monika Dubiel for the help in preparing the manuscript. Financial support from Roche Diagnostics GmbH is gratefully acknowledged.
REFERENCES
- 1.Seela F. and Becher,G. (2001) Pyrazolo[3,4-d]pyrimidine nucleic acids: adjustment of dA–dT to dG–dC base pair stability. Nucleic Acids Res., 29, 2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boudou V., Kerremans,L., De Bouvere,B., Lescrinier,E., Schepers,G., Busson,R., Van Aerschot,A. and Herdewijn,P. (1999) Base pairing of anhydrohexitol nucleosides with 2,6-diaminopurine, 5-methylcytosine and uracil as base moiety. Nucleic Acids Res., 27, 1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seela F. and Becher,G. (1998) Synthesis of 7-halogenated 8-aza-7-deaza-2′-deoxy-guanosines and related pyrazolo[3,4-d]pyrimidine 2′-deoxyribonucleosides. Synthesis, 207–214. [Google Scholar]
- 4.Seela F. and Steker,H. (1986) Synthesis of 2′-deoxyribofuranosides of 8-aza-7-deazaguanine and related pyrazolo[3,4-d]pyrimidines. Helv. Chim. Acta, 69, 1602–1613. [Google Scholar]
- 5.Seela F., Winkeler,H.-D., Driller,H. and Menkhoff,S. (1984) Synthesis of 7-deaza-2′-deoxyguanosine by phase-transfer glycosylation and preparation of suitable derivatives for oligonucleotide synthesis. Nucleic Acids Res., 14, 245–246. [Google Scholar]
- 6.Seela F. and Steker,H. (1985) Facile synthesis of 2′-deoxyribofuranosides of allopurinol and 4-amino-1H-pyrazolo [3,4-d]pyrimidine via phase-transfer glycosylation. Helv. Chim. Acta, 68, 563–570. [Google Scholar]
- 7.Seela F. and Zulauf,M. (1998) Synthesis of 7-alkynylated 8-aza-7-deaza-2′-deoxy-adenosines via the Pd-catalysed cross-coupling reaction. J. Chem. Soc. [Perkin 1], 3233–3239. [Google Scholar]
- 8.Haaima G., Hansen,H.F., Christensen,L., Dahl,O. and Nielsen,P.E. (1997) Increased DNA binding and sequence discrimination of PNA oligomers containing 2,6-diaminopurine. Nucleic Acids Res., 25, 4639–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanghvi Y.S., Hoke,G.D., Freier,S.M., Zounes,M.C., Gonzalez,C., Cummins,L., Sasmor,H. and Cook,P.D. (1993) Antisense oligodeoxynucleotides: synthesis, biophysical and biological evaluation of oligodeoxynucleotides containing modified pyrimidines. Nucleic Acids Res., 21, 3197–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhlmann E., Peyman,A., Ryte,A., Schmidt,A. and Buddecke,E. (2000) Use of minimally modified antisense oligonucleotides for specific inhibition of gene expression. Methods Enzymol., 313, 268–284. [DOI] [PubMed] [Google Scholar]
- 11.Lamm G.M., Blencowe,B.J., Sproat,B.S., Iribarren,A.M., Ryder,U. and Lamond,A.I. (1991) Antisense probes containing 2-aminoadenosine allow efficient depletion of U5 snRNP from HeLa splicing extracts. Nucleic Acids Res., 19, 3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailly C. and Waring,M.J. (1998) The use of diaminopurine to investigate structural properties of nucleic acids and molecular recognition between ligands and DNA. Nucleic Acids Res., 26, 4309–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seela F. and Zulauf,M. (1998) 7-Deazaadenine-DNA: bulky 7-iodo substituents or hydrophobic 7-hexynyl chains are well accommodated in the major groove of oligonucleotide duplexes. Chem. Eur. J., 4, 1781–1790. [Google Scholar]
- 14.Seela F. and Zulauf,M. (1999) Synthesis of oligonucleotides containing pyrazolo[3,4-d]pyrimidines: the influence of 7-substituted 8-aza-7-deazaadenines on the duplex structure and stability. J. Chem. Soc. [Perkin 1], 479–488. [Google Scholar]
- 15.Seela F. and Becher,G. (1999) Oligonucleotides containing pyrazolo [3,4-d]pyrimidines: the influence of 7-substituted 8-aza-7-deaza-2′-deoxyguanosines on the duplex structure and stability. Helv. Chim. Acta, 82, 1640–1655. [Google Scholar]
- 16.Seela F., Becher,G. and Zulauf,M. (1999) 8-Aza-7-deazapurine DNA: synthesis and duplex stability of oligonucleotides containing 7-substituted bases. Nucl. Nucl., 18, 1399–1400. [Google Scholar]
- 17.Becher G., He,J. and Seela,F. (2001) Major-groove-halogenated DNA: the effects of bromo and iodo substituents replacing H-C(7) of 8-aza-7-deazapurine-2,6-diamine or H-C(5) of uracil residues. Helv. Chim. Acta, 84, 1048–1065. [Google Scholar]
- 18.He J. and Seela,F. (2002) 8-Aza-7-deazapurine-pyrimidine base pairs: the contribution of 2- and 7-substituents to the stability of duplex DNA. Tetrahedron, 58, 4535–4542. [Google Scholar]
- 19.Sági J., Szemzö,A., Ébinger,K., Szabolcs,A., Sági,G., Ruff,É. and Ötvös,L. (1993) Base-modified oligodeoxynucleotides. I. Effect of 5-alkyl, 5-(1-alkenyl) and 5-(1-alkynyl) substitution of the pyrimidines on duplex stability and hydrophobicity. Tetrahedron Lett., 34, 2191–2194. [Google Scholar]
- 20.Barnes T.W. III and Turner,D.H. (2001) Long-range cooperativity in molecular recognition of RNA by oligodeoxynucleotides with multiple C5-(1-propynyl) pyrimidines. J. Am. Chem. Soc., 123, 4107–4118. [DOI] [PubMed] [Google Scholar]
- 21.Barnes T.W. III and Turner,D.H. (2001) C5-(1-propynyl)-2′-deoxy-pyrimidines enhance mismatch penalties of DNA:RNA duplex formation. Biochemistry, 40, 12738–12745. [DOI] [PubMed] [Google Scholar]
- 22.Wagner R.W., Matteucci,M.D., Lewis,J.G., Gutierrez,A.J., Moulds,C. and Froehler,B.C. (1993) Antisense gene inhibition by oligonucleotides containing C-5 propyne pyrimidines. Science, 260, 1510–1513. [DOI] [PubMed] [Google Scholar]
- 23.Froehler B.C., Wadwani,S., Terhorst,T.J. and Gerrard,S.R. (1992) Oligodeoxynucleotides containing C-5 propyne analogs of 2′-deoxyuridine and 2′-deoxycytidine. Tetrahedron Lett., 33, 5307–5310. [Google Scholar]
- 24.Gutierrez A.J., Matteucci,M.D., Grant,D., Matsumura,S., Wagner,R.W. and Froehler,B.C. (1997) Antisense gene inhibition by C-5-substituted deoxyuridine-containing oligodeoxynucleotides. Biochemistry, 36, 743–748. [DOI] [PubMed] [Google Scholar]
- 25.Ahmadian M., Zhang,P. and Bergstrom,D.E. (1998) A comparative study of the thermal stability of oligodeoxyribonucleotides containing 5-substituted 2′-deoxyuridines. Nucleic Acids Res., 26, 3127–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham D., Parkinson,J.A. and Brown,T. (1998) DNA duplexes stabilized by modified monomer residues: synthesis and stability. J. Chem. Soc. [Perkin 1], 1131–1138. [Google Scholar]
- 27.Buhr C.A., Wagner,R.W., Grant,D. and Froehler,B.C. (1996) Oligodeoxynucleotides containing C-7 propyne analogs of 7-deaza-2′-deoxyguanosine and 7-deaza-2′-deoxyadenosine. Nucleic Acids Res., 24, 2974–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catalanotti B., Galeone,A., Gomez-Paloma,L., Mayol,L. and Pepe,A. (2000) 2′-deoxy-8-(propyn-1-yl)adenosine-containing oligonucleotides: effects on stability of duplex and quadruplex structures. Bioorg. Med. Chem. Lett., 10, 2005–2009. [DOI] [PubMed] [Google Scholar]
- 29.McDowell J.A. and Turner,D.H. (1996) Investigation of the structural basis for thermodynamic stabilities of tandem GU mismatches: solution structure of (rGAGGUCUC)2 by two-dimensional NMR and simulated annealing. Biochemistry, 35, 14077–14089. [DOI] [PubMed] [Google Scholar]
- 30.Seela F., Ramzaeva,N., Leonard,P., Chen,Y., Debelak,H., Feiling,E., Kröschel,R., Zulauf,M., Wenzel,T., Fröhlich,T. and Kostrzewa,M. (2001) Phosphoramidites and oligonucleotides containing 7-deazapurines and pyrimidines carrying aminopropargyl side chains. Nucleosides Nucleotides Nucleic Acids, 20, 1421–1424. [DOI] [PubMed] [Google Scholar]
- 31.Hobbs F.W. (1989) Palladium-catalyzed synthesis of alkynylamino nucleosides. A universal linker for nucleic acids. J. Org. Chem., 54, 3420–3422. [Google Scholar]
- 32.Robins M.J., Vinayak,R.S. and Wood,S.G. (1990) Solvent, not palladium oxidation state, is the primary determinant for successful coupling of terminal alkynes with iodo-nucleosides. Tetrahedron Lett., 31, 3731–3734. [Google Scholar]
- 33.Korshun V.A., Manasova,E.V. and Berlin,Yu.A. (1997) Alkynylated nucleosides and their analogues. I. Methods of synthesis. Russian J. Bioorg. Chem., 23, 300–362. [Google Scholar]
- 34.Ti G.S., Gaffney,B.L. and Jones,R.A. (1982) Transient protection: efficient one-flask syntheses of protected deoxynucleosides. J. Am. Chem. Soc., 104, 1316–1319. [Google Scholar]
- 35.User Manual of the DNA Synthesizer. Applied Biosystems, Weiterstadt, Germany.
- 36.Seela F. and Zulauf,M. (1999) Oligonucleotides containing 7-deazaadenines: the influence of the 7-substituent chain length and charge on the duplex stability. Helv. Chim. Acta, 82, 1878–1898. [Google Scholar]
- 37.Ramzaeva N., Mittelbach,C. and Seela,F. (1997) 7-Deazaguanine DNA: oligonucleotides with hydrophobic or cationic side chains. Helv. Chim. Acta, 80, 1809–1822. [Google Scholar]