Abstract
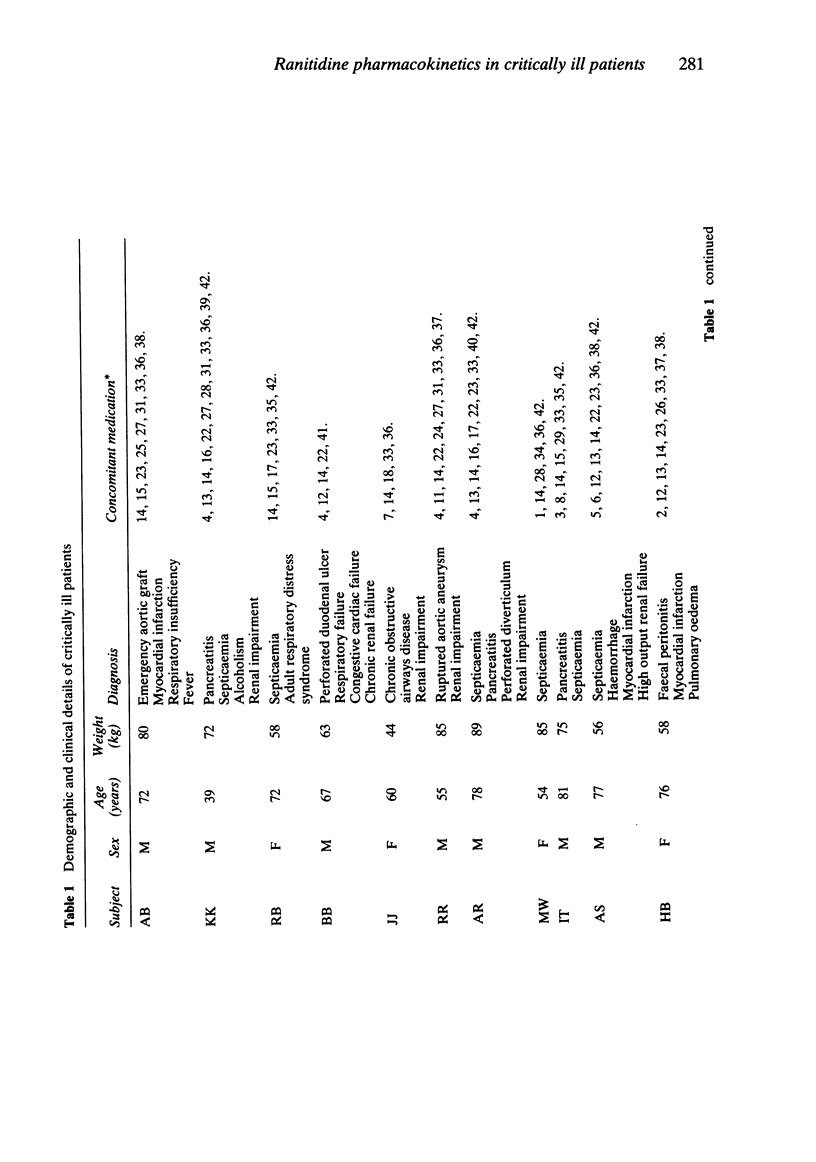
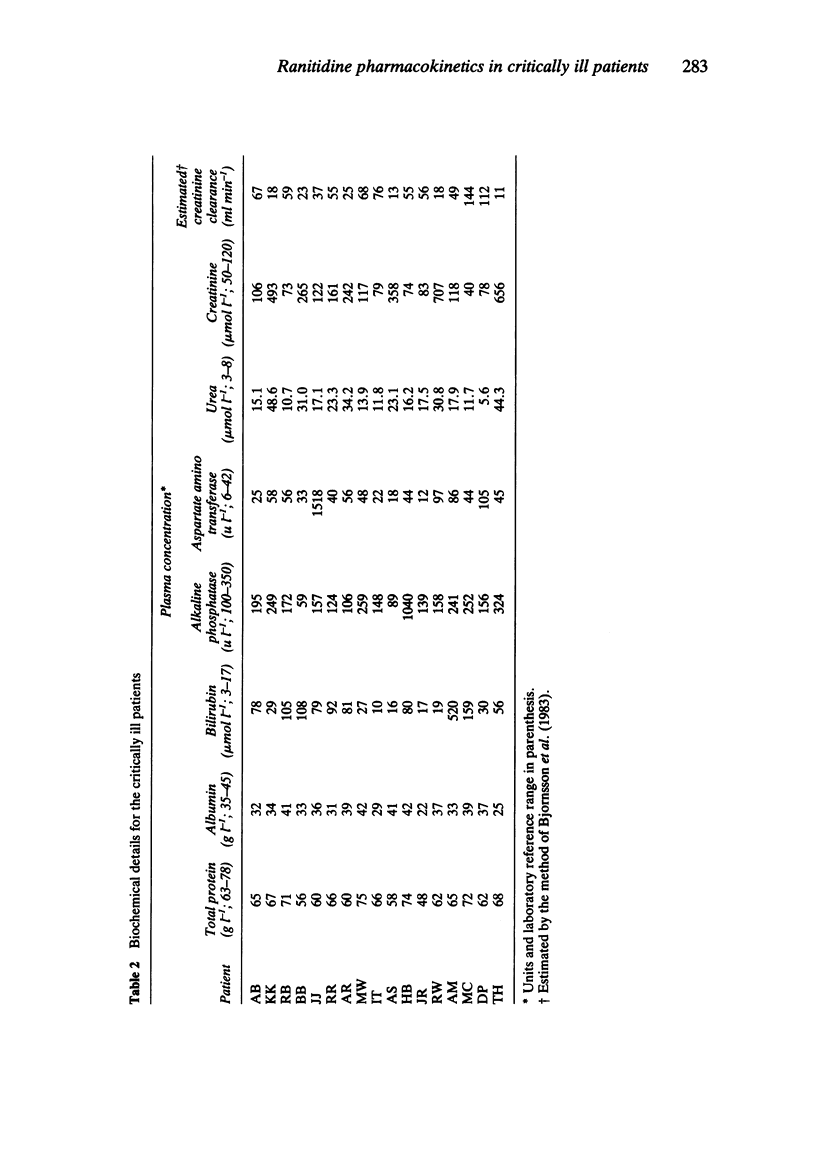
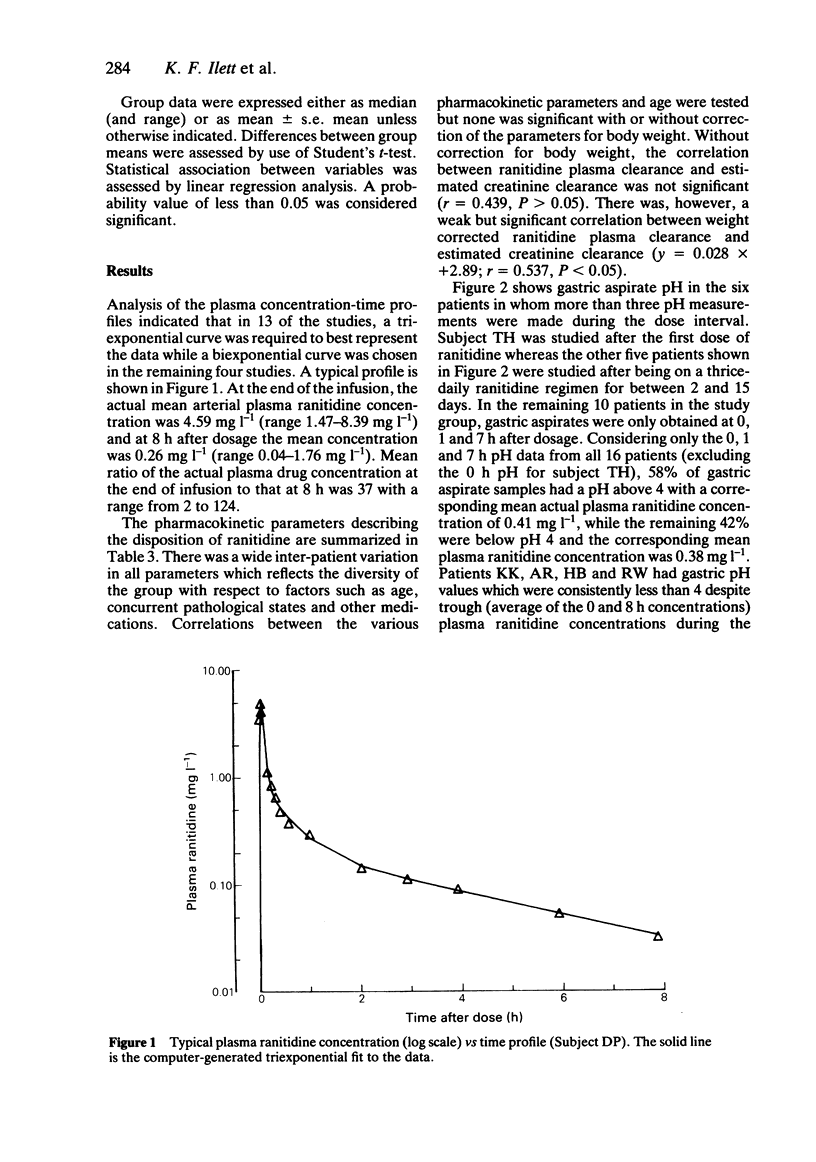
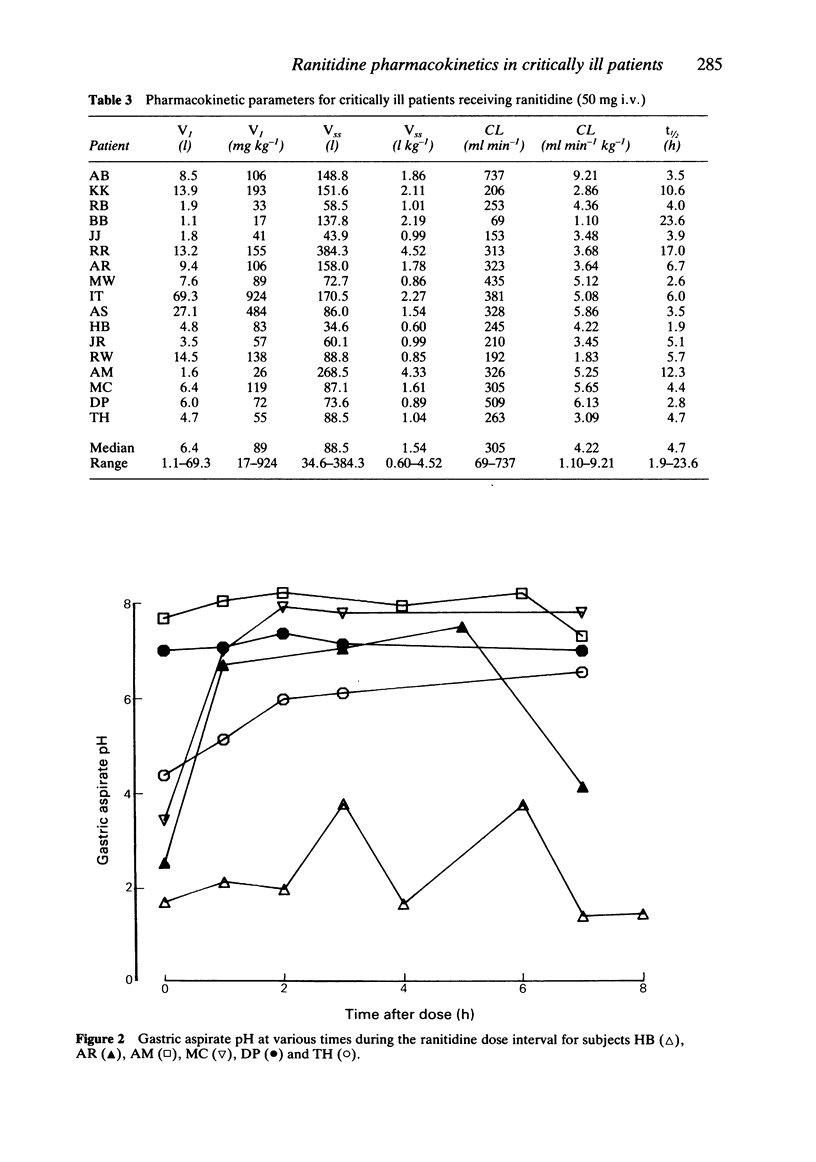
The plasma pharmacokinetics of ranitidine (50 mg i.v.) have been studied in 17 critically ill patients in an intensive care unit. Measurements of gastric aspirate pH were also made in 16 of these patients. Ranitidine therapy was part of the patients' normal drug regimen. Ranitidine plasma concentration was measured by high performance liquid chromatography and appropriate polyexponential equations were fitted to concentration-time data to enable calculation of relevant pharmacokinetic parameters. Values of the volume of the initial dilution space (median = 89 ml kg-1) and volume of distribution at steady state (median = 1.54 l kg-1) were about 60% of corresponding mean literature values for healthy controls. Plasma clearance (median = 4.22 ml min-1 kg-1) and terminal half-life (median = 4.7 h) were about 2-3 fold less and 2-3 fold greater, respectively, than values for healthy controls. There was wide interpatient variation in all the pharmacokinetic parameters. Renal impairment was considered to be largely responsible for the low plasma clearance. Gastric aspirate pH was measured at 0, 1 and 7 h after ranitidine administration and 58% of samples were found to be above pH 4. Four patients had gastric pH values which were consistently below pH 4 despite average trough plasma ranitidine concentrations equal to or greater than those required for a 50% suppression of gastric acid secretion in normal volunteers.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth H. O., Berg P., Brunner G., Dammann H. G., Friedl W., Franken F. H., Greiner L., Groitl J., Möckel W., Müller P. Ranitidin und Cimetidin in der Prophylaxe der Stressulcus-Blutung: Eine prospektive Multicenter-Vergleichsstudie. Langenbecks Arch Chir. 1984;362(2):131–138. doi: 10.1007/BF01254187. [DOI] [PubMed] [Google Scholar]
- Bjornsson T. D., Cocchetto D. M., McGowan F. X., Verghese C. P., Sedor F. Nomogram for estimating creatinine clearance. Clin Pharmacokinet. 1983 Jul-Aug;8(4):365–369. doi: 10.2165/00003088-198308040-00007. [DOI] [PubMed] [Google Scholar]
- Brogden R. N., Carmine A. A., Heel R. C., Speight T. M., Avery G. S. Ranitidine: a review of its pharmacology and therapeutic use in peptic ulcer disease and other allied diseases. Drugs. 1982 Oct;24(4):267–303. doi: 10.2165/00003495-198224040-00002. [DOI] [PubMed] [Google Scholar]
- Chau N. P., Zech P. Y., Pozet N., Hadj-Aissa A. Ranitidine kinetics in normal subjects. Clin Pharmacol Ther. 1982 Jun;31(6):770–774. doi: 10.1038/clpt.1982.109. [DOI] [PubMed] [Google Scholar]
- Greene W. L., Bollinger R. R. Cimetidine for stress-ulcer prophylaxis. Crit Care Med. 1984 Jul;12(7):571–575. doi: 10.1097/00003246-198407000-00005. [DOI] [PubMed] [Google Scholar]
- Hastings P. R., Skillman J. J., Bushnell L. S., Silen W. Antacid titration in the prevention of acute gastrointestinal bleeding: a controlled, randomized trial in 100 critically ill patients. N Engl J Med. 1978 May 11;298(19):1041–1045. doi: 10.1056/NEJM197805112981901. [DOI] [PubMed] [Google Scholar]
- Holloway R. H., Kuljian B., Eshelman F., McCallum R. W. Effects of ranitidine and of cimetidine on pentagastrin-stimulated gastric acid secretion. Clin Pharmacol Ther. 1984 Feb;35(2):203–207. doi: 10.1038/clpt.1984.27. [DOI] [PubMed] [Google Scholar]
- Lebert P. A., MacLeod S. M., Mahon W. A., Soldin S. J., Vandenberghe H. M. Ranitidine kinetics and dynamics. I. Oral dose studies. Clin Pharmacol Ther. 1981 Oct;30(4):539–544. doi: 10.1038/clpt.1981.200. [DOI] [PubMed] [Google Scholar]
- Lebert P. A., Mahon W. A., MacLeod S. M., Soldin S. J., Fenje P., Vandenberghe H. M. Ranitidine kinetics and dynamics. II. Intravenous dose studies and comparison with cimetidine. Clin Pharmacol Ther. 1981 Oct;30(4):545–550. doi: 10.1038/clpt.1981.201. [DOI] [PubMed] [Google Scholar]
- Macchi H., Fiasse R., Reynaert M., Desager J. P., Dive C. Effect of ranitidine (inhibitor of H2 receptors) after i.v. injection to patients treated in an intensive care unit: control of gastric acid secretion and estimation of drug plasma levels. Arch Int Pharmacodyn Ther. 1982 Apr;256(2):306–307. [PubMed] [Google Scholar]
- McFadyen M. L., Folb P. I., Miller R., Keeton G. R., Marks I. N. Pharmacokinetics of ranitidine in patients with chronic renal failure. Eur J Clin Pharmacol. 1983;25(3):347–351. doi: 10.1007/BF01037946. [DOI] [PubMed] [Google Scholar]
- McNeil J. J., Mihaly G. W., Anderson A., Marshall A. W., Smallwood R. A., Louis W. J. Pharmacokinetics of the H2- receptor antagonist ranitidine in man. Br J Clin Pharmacol. 1981 Sep;12(3):411–415. doi: 10.1111/j.1365-2125.1981.tb01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffin P. J., Grgurinovich N., Brooks P. M., Miners J. O., Cochran M., Stranks G. Ranitidine disposition in patients with renal impairment. Br J Clin Pharmacol. 1983 Dec;16(6):731–734. doi: 10.1111/j.1365-2125.1983.tb02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignon M., Chau N. P., Nguyen-Phuoc B. K., Sauvage M., Leguy F., Bonfils S. Ranitidine upon meal-induced gastric secretion: oral pharmacokinetics and plasma concentration effect relationships. Br J Clin Pharmacol. 1982 Aug;14(2):187–193. doi: 10.1111/j.1365-2125.1982.tb01960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe H. J., Skillman J. J., Bushnell L. S., Long P. C., Silen W. Antacid versus cimetidine in preventing acute gastrointestinal bleeding. A randomized trial in 75 critically ill patients. N Engl J Med. 1980 Feb 21;302(8):426–430. doi: 10.1056/NEJM198002213020802. [DOI] [PubMed] [Google Scholar]
- Smith I. L., Ziemniak J. A., Bernhard H., Eshelman F. N., Martin L. E., Schentag J. J. Ranitidine disposition and systemic availability in hepatic cirrhosis. Clin Pharmacol Ther. 1984 Apr;35(4):487–494. doi: 10.1038/clpt.1984.65. [DOI] [PubMed] [Google Scholar]
- Stothert J. C., Jr, Simonowitz D. A., Dellinger E. P., Farley M., Edwards W. A., Blair A. D., Cutler R., Carrico C. J. Randomized prospective evaluation of cimetidine and antacid control of gastric pH in the critically ill. Ann Surg. 1980 Aug;192(2):169–174. doi: 10.1097/00000658-198008000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. G. Linear pharmacokinetic equations allowing direct calculation of many needed pharmacokinetic parameters from the coefficients and exponents of polyexponential equations which have been fitted to the data. J Pharmacokinet Biopharm. 1976 Oct;4(5):443–467. doi: 10.1007/BF01062831. [DOI] [PubMed] [Google Scholar]
- Young C. J., Daneshmend T. K., Roberts C. J. Effects of cirrhosis and ageing on the elimination and bioavailability of ranitidine. Gut. 1982 Oct;23(10):819–823. doi: 10.1136/gut.23.10.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hecken A. M., Tjandramaga T. B., Mullie A., Verbesselt R., de Schepper P. J. Ranitidine: single dose pharmacokinetics and absolute bioavailability in man. Br J Clin Pharmacol. 1982 Aug;14(2):195–200. doi: 10.1111/j.1365-2125.1982.tb01961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


