Abstract
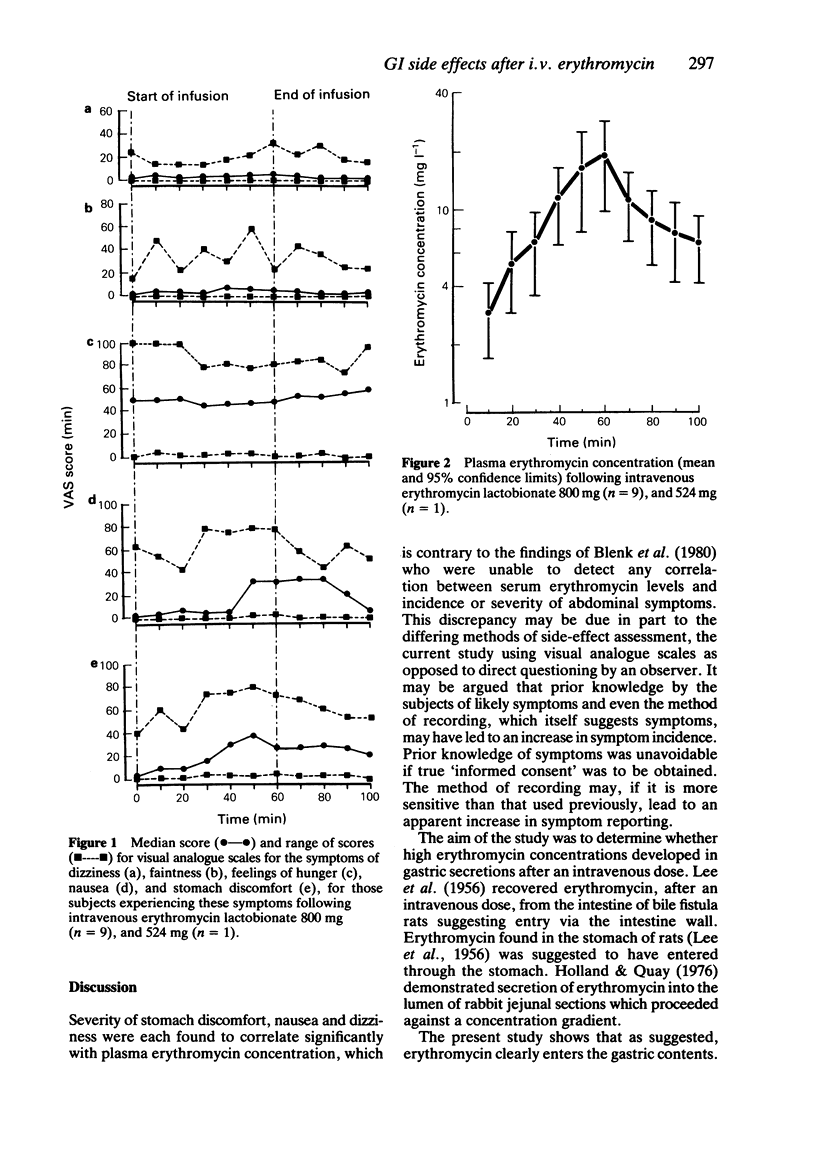
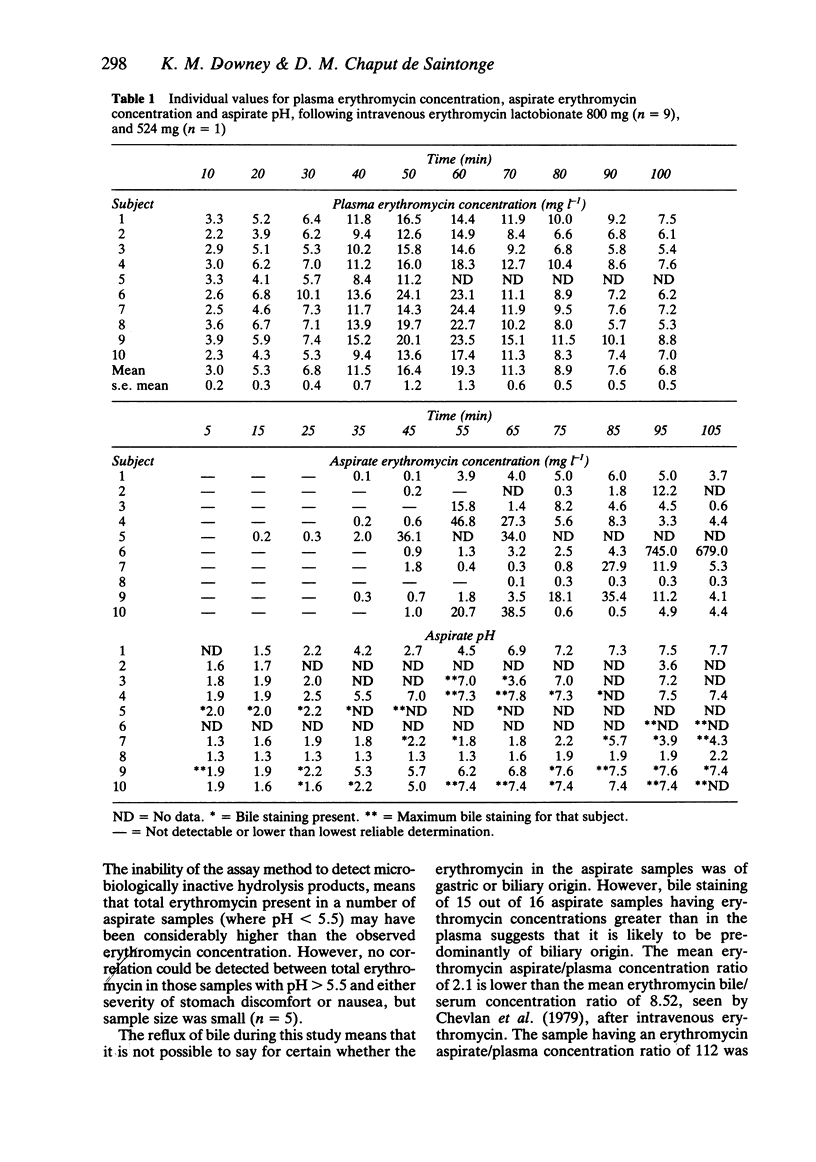
Ten healthy normal volunteers received an intravenous infusion of erythromycin lactobionate over 60 min to a total dose of 800 mg (n = 9), and 524 mg (n = 1). Blood samples were collected at 10 min intervals for 100 min and gastric contents aspirated, via a nasogastric tube, from pre-dose to 105 min after start of infusion. Incidence and severity of three gastrointestinal symptoms (nausea, stomach discomfort and feelings of hunger), two CNS symptoms (dizziness and faintness) and a 'control' symptom (back pain) were measured using 100 mm visual analogue scales. Rate of infusion and plasma erythromycin concentration correlated with nausea (P less than 0.001) and stomach discomfort (P less than 0.001); plasma erythromycin concentration was also correlated with dizziness (P less than 0.05). Concentrations of active erythromycin in the aspirate were pH dependent. In one subject the concentration of erythromycin in the aspirate exceeded that in the plasma by 100 fold. Bile staining of samples containing the highest levels of microbiologically active erythromycin makes the origin of the erythromycin in these samples uncertain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin K. L., Mather L. E., Philpot C. R., McDonald P. J. Intersubject and dose-related variability after intravenous administration of erythromycin. Br J Clin Pharmacol. 1980 Sep;10(3):273–279. doi: 10.1111/j.1365-2125.1980.tb01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenk H., Blenk B., Jahneke G., Simm K., von Lucke B., LaFranier D. Erythromycin infusions for treatment of infections in the ear, nose and throat region. J Int Med Res. 1980;8 (Suppl 2):41–46. [PubMed] [Google Scholar]
- Chelvan P., Hamilton-Miller J. M., Brumfitt W. Biliary excretion of erythromycin after parenteral administration. Br J Clin Pharmacol. 1979 Sep;8(3):233–235. doi: 10.1111/j.1365-2125.1979.tb01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D. R., Quay J. F. Intestinal secretion of erythromycin base. J Pharm Sci. 1976 Mar;65(3):417–419. doi: 10.1002/jps.2600650325. [DOI] [PubMed] [Google Scholar]
- Klika L. J., Goodman J. M. Gastrointestinal tract symptoms from intravenously administered erythromycin. JAMA. 1982 Sep 17;248(11):1309–1309. doi: 10.1001/jama.1982.03330110017010. [DOI] [PubMed] [Google Scholar]
- LEE C. C., ANDERSON R. C., CHEN K. K. Distribution and excretion of radioactivity in rats receiving N-methyl-C14-erythromycin. J Pharmacol Exp Ther. 1956 Jul;117(3):265–273. [PubMed] [Google Scholar]
- Marlin G. E., Thompson P. J., Jenkins C. R., Burgess K. R., LaFranier D. A. Study of serum levels, venous irritation and gastrointestinal side-effects with intravenous erythromycin lactobionate in patients with bronchopulmonary infection. Hum Toxicol. 1983 Oct;2(4):593–605. doi: 10.1177/096032718300200404. [DOI] [PubMed] [Google Scholar]
- Putzi R., Blaser J., Lüthy R., Wehrli R., Siegenthaler W. Side-effects due to the intravenous infusion of erythromycin lactobionate. Infection. 1983 May-Jun;11(3):161–163. doi: 10.1007/BF01641296. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Wagner J. G. Fluorometric determination of erythromycin and erythromycin propionate in whole blood or plasma and correlation of results with microbiological assay. Anal Chem. 1976 Feb;48(2):348–353. doi: 10.1021/ac60366a033. [DOI] [PubMed] [Google Scholar]


