Abstract
We describe the regulatory interactions that cause anterior extension of the mouse 5′ Hoxb expression domains from spinal cord levels to their definitive boundaries in the posterior hindbrain between embryonic day E10 and E11.5. This anterior expansion is retinoid dependent since it does not occur in mouse embryos deficient for the retinoic acid-synthesizing enzyme retinaldehyde dehydrogenase 2. A retinoic acid response element (RARE) was identified downstream of Hoxb5 and shown to be essential for expression of Hoxb5 and Hoxb8 reporter transgenes in the anterior neural tube. The spatio-temporal activity of this element overlaps with rostral extension of the expression domain of endogenous Hoxb5, Hoxb6 and Hoxb8 into the posterior hindbrain. The RARE and surrounding sequences are found at homologous positions in the human, mouse and zebrafish genome, which supports an evolutionarily conserved regulatory function.
Keywords: mouse Hox genes/posterior hindbrain patterning/retinoic acid response/transcriptional regulation
Introduction
In mammals, 39 Hox genes are arranged in four genomic clusters. Their sequential activation results in spatially and temporally restricted expression patterns along the antero–posterior (AP) axis of the embryo (reviewed by Krumlauf, 1994; Deschamps et al., 1999). Gain-of-function and loss-of-function studies have shown that Hox genes are involved in patterning structures along this main body axis (Krumlauf, 1994). The positioning of the definitive anterior expression boundaries is of decisive importance for proper patterning of axial and paraxial structures. Transient alteration of the early timing and level of expression of these genes also can lead to defects in AP patterning (van der Hoeven et al., 1996; Zákány et al., 1997; Greer et al., 2000). Therefore, tight spatio-temporal regulation of Hox genes is essential for correct patterning of target tissues. This regulation seems to depend on a hierarchy of molecular controls (van der Hoeven et al., 1996). At the highest level, the progressive accessibility of Hox genes for transcription is thought to be controlled by the release of a repression acting on the whole cluster during early development (Kondo and Duboule, 1999). Subsequently, differentially expressed transcriptional activators would bind to cis-acting elements to induce expression of individual genes.
Reporter transgenes containing proximal regulatory elements of 3′ Hoxb genes (Hoxb1–Hoxb4) recapitulate their correct anterior expression boundaries (Marshall et al., 1994; Gould et al., 1998). In contrast, expression patterns of the 5′ Hoxb genes such as Hoxb8 were not reproduced faithfully using only surrounding genomic sequences (Charité et al., 1995). The anterior extent of the expression domain of these genes in the neural tube was never recapitulated using reporter transgenes, despite extensive reporter scanning between Hoxb5 and Hoxb9 (Eid et al., 1993; Valarché et al., 1997; R.Vogels and J.Deschamps, unpublished data). At a location >30 kb 3′ from the Hoxb8 promoter, we identified a 550 bp element (called the distal element, DE) between Hoxb4 and Hoxb5, which is able to mediate Hoxb8 expression with the correct rostral expression boundary in the neural tube, when combined on a transgene with the Hoxb8 proximal regulatory sequences (Valarché et al., 1997). Interestingly, the Hoxb8 DE maps within the 3.5 kb sequence found by Sharpe et al. (1998) to drive the expression of Hoxb5 in the neural tube. This suggested that the same cis-acting element and transcriptional activator(s) may control some aspects of the expression of both Hoxb5 and Hoxb8.
Retinoid signalling has been shown to play a crucial role in setting the anterior boundary of 3′ Hox genes at the level of the first to the fifth inter-rhombomeric boundaries in the hindbrain (reviewed by Gavalas and Krumlauf, 2000). Retinoic acid (RA) response elements (RAREs) present in the 3′ part of the Hoxb cluster have been implicated in the regulation of Hoxb1, Hoxb2 and Hoxb4 in vivo (Studer et al., 1994; Gavalas et al., 1998; Gould et al., 1998; Huang et al., 1998; Gavalas and Krumlauf, 2000; see Manzanares et al., 2001). In contrast, no RARE identified so far has been found to control Hox genes more 5′ than paralogy group 4, in spite of the fact that RA was shown to induce sequential 3′ to 5′ activation of Hoxb genes in human embryonal carcinoma cells (Simeone et al., 1990). Moreover, experiments using chicken embryos suggested that Hoxb8 is a direct target of RA in limb bud tissues (Lu et al., 1997), but it had remained unclear which mechanism underlies this RA sensitivity.
In this study, we show that the late rostral extension of 5′ Hoxb gene expression in the neural tube depends on endogenous retinoids, and that it is abolished in Raldh2-null (Niederreither et al., 1999) mutant embryos. We identify a novel RARE, which provides transcriptional stimulatory activity to the DE regulatory element, and which is sufficient to induce rostral expression of Hoxb8/lacZ reporters in the neural tube. In addition, this RARE induces anterior neural expression of a Hoxb5/lacZ transgene. The spatio-temporal window of RA sensitivity of this element matches the windows of activation of the 5′ Hoxb genes in the most anterior part of their neural expression domains. This RARE has been well conserved between mammals and fish, which suggests that it may play an important role in mediating RA-dependent patterning of the caudal hindbrain through 5′ Hoxb genes.
Results
5′ genes of cluster Hoxb undergo a late anterior extension of their expression domain under control of a previously unknown mechanism
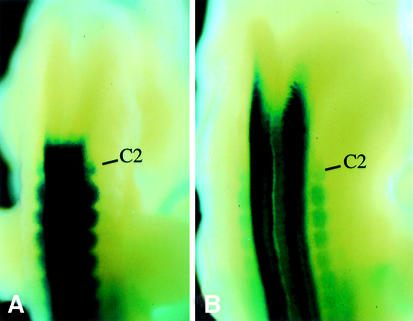
The domain of transcript accumulation of Hoxb8 in the neural tube undergoes a late phase of rostral extension between E10.5 and E11.5. The mechanism responsible for this change was not understood. The anterior expression boundary of Hoxb8 in the central nervous system (CNS) was at the level of the upper spinal cord (first dorsal root ganglion, level of somite 5–6 boundary) at E10.5, but shifted to an AP level located well within the hindbrain by E11.5 (Figure 1), whereas the boundaries in mesoderm remained unchanged. We postulated the existence of a regulatory interaction specifically responsible for the induction of this Hox gene between upper spinal cord and hindbrain levels. This assumption was based on the fact that none of the reporter transgenes containing Hoxb8 genomic sequences covering the interval between Hoxb9 and Hoxb5 reproduced this rostral extension of the transcription domain in the neural tube (Charité et al., 1995; Valarché et al., 1997). In addition, the expression domain of Hoxb6 and Hoxb5 also spread rostrally in the neural tube during these advanced developmental stages (T.Oosterveen, F.Meijlink and J.Deschamps, in preparation).
Fig. 1. Expression of Hoxb8 expands anteriorly into the posterior hindbrain between E10.5 and E11.5. Expression pattern of the Hoxb8lacZ knockin (van den Akker et al., 1999) in an E10.5 (A) and an E11.5 (B) embryo. C2 is the second spinal ganglion, the most anterior persistent dorsal root ganglion.
The rostral extension of the expression domain of 5′ Hoxb genes in the neural tube depends on retinoid signalling
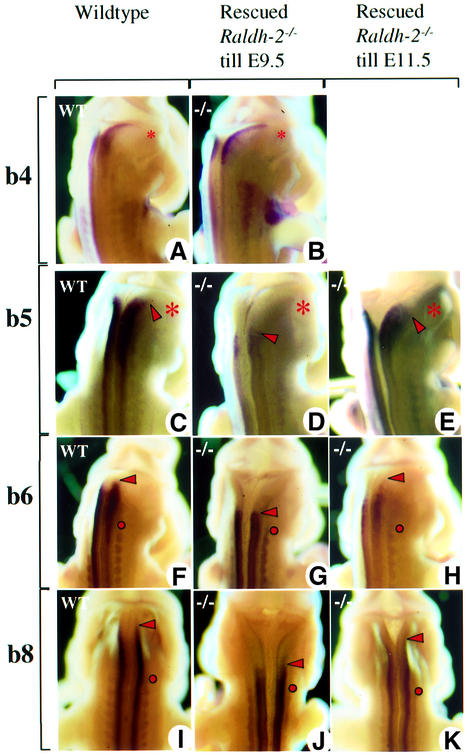
We analysed the evolution of the expression patterns of the 5′ Hoxb genes from E9.5 to 11.5 in mutants severely impaired in their biosynthesis of RA following inactivation of the enzyme retinaldehyde dehydrogenase 2 (Raldh2) (Niederreither et al., 2000). In order to overcome the early lethality of this mutation due to cardiac defects, subteratogenic doses of RA were administered from E7.5 to E9.0, i.e. prior to the window of Hox gene regulation under investigation. Such transiently RA-supplemented Raldh2 mutant and wild-type littermate embryos developed normally, as documented by marker gene expression (see Figure 2A and B; Materials and methods). The expression boundaries of Hoxb8, Hoxb6 and Hoxb5 were positioned correctly in the upper spinal cord at time points earlier than the anterior expansion of the expression domains described above for these genes (data not shown). At E11.5, the expected anterior expansion of neural expression had not occurred for any of these 5′ Hoxb genes in Raldh2 mutant embryos (Figure 2D, G and J), whereas it had occurred normally in wild-type controls (Figure 2C, F and I). In wild-type embryos, there was no difference between the Hox expression domains of early RA-supplemented and non-treated controls (data not shown). Furthermore, supplementation of Raldh2 mutant embryos with RA until E11.5 fully rescued the anterior expansion of 5′ Hoxb gene expression into the posterior hindbrain (Figure 2E, H and K).
Fig. 2. Alteration of 5′ Hoxb hindbrain expression in Raldh2–/– embryos transiently rescued from E7.5 to E9.5, or completely rescued up to E11.5. Hoxb4 (A and B) has already reached its rostralmost expression boundary at E9.5, and is therefore not affected in transiently rescued E11.5 Raldh2-null embryos. The neural expression boundary of Hoxb5 (C–E), Hoxb6 (F–H) and Hoxb8 (I–K) in transiently rescued E11.5 Raldh2-null embryos is more posterior than in the wild-types and completely rescued mutants. (A), (C), (F) and (I), untreated wild-type embryo; (B), (D), (G) and (J), Hox gene expression in early and transiently rescued Raldh2-null embryos; (E), (H) and (K), Hox gene expression in completely rescued Raldh2-null embryos, serving as controls. Asterisks give the position of the otic vesicle, the red dot the position of dorsal root ganglion C2, and the red arrowheads the anterior boundary of gene expression.
A conserved RARE confers expression of Hoxb8DE/lacZ and Hoxb5DE/lacZ transgenes in the posterior hindbrain
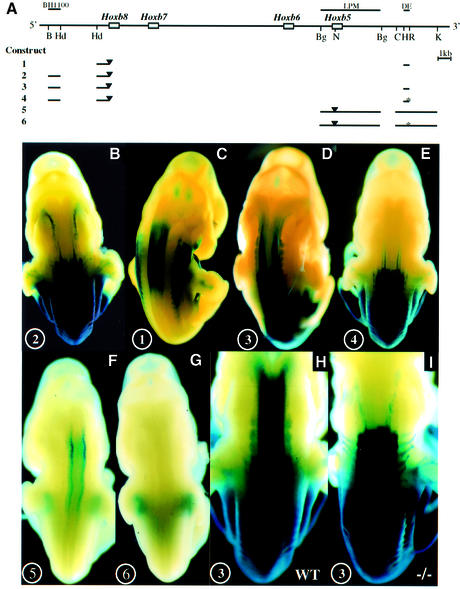
In a distal genomic region potentially participating in the regulation of Hoxb8 (called DE), we discovered a putative RARE element matching the consensus A/GGT/GTCA nnnnnA/GGT/GTCA (Figure 3A; Mader et al., 1993). This sequence is highly conserved between mouse, human and zebrafish (Figure 3A), as it was found in all three genomes at a corresponding position, between the paralogues of Hoxb4 and Hoxb5. Moreover, sequences flanking the consensus on both sides reveal a 74% conservation of identity over 160 nucleotides. The consensus behaved as a functional RARE by binding retinoic acid receptor (RAR)–retinoid X receptor (RXR) heterodimers in vitro (Figure 3B) and by activating a reporter gene in an RA-dependent manner in cultured cells (data not shown). In E11.5 transgenic embryos, the DE did provide a lacZ reporter driven by a minimum Hoxb8 promoter (Figure 4A, construct 1) with expression localized where RA signalling is known to act (Figure 4C; Shen et al., 1992). The DE was also inserted into a transgene controlled by the promoter and proximal regulatory sequences of Hoxb8 or Hoxb5, which normally is expressed in a posterior region at E11.5 (Figure 4A, constructs 3 and 5, respectively). The resulting constructs were expressed in the rostral neural tube up to the posterior hindbrain region (Figure 4D and F). The combination of the DE with the Hoxb5 promoter and this proximal regulatory element gave rise to a weaker lacZ activity than with the Hoxb8 promoter and proximal element. This was observed for three independent integration sites of both transgenes (data not shown), and may therefore result from the features of the respective promoter–enhancer combination. Both transgenes, however, depend on the DE RARE to be expressed in the rostral neural tube, since nucleotide substitutions abolishing DNA binding of the DE RARE in vitro (Figure 3A) prevented their anterior neural expres sion (Figure 4E and G, constructs 4 and 6, respectively). When transgenic mice containing construct 3 (the Hoxb8 promoter/lacZ, DE and posterior enhancer BH1100; Figure 4A and H) were examined in a Raldh2-null background, the anterior neural expression domain was completely abolished, apparently due to RA deficiency (Figure 4I compared with H).
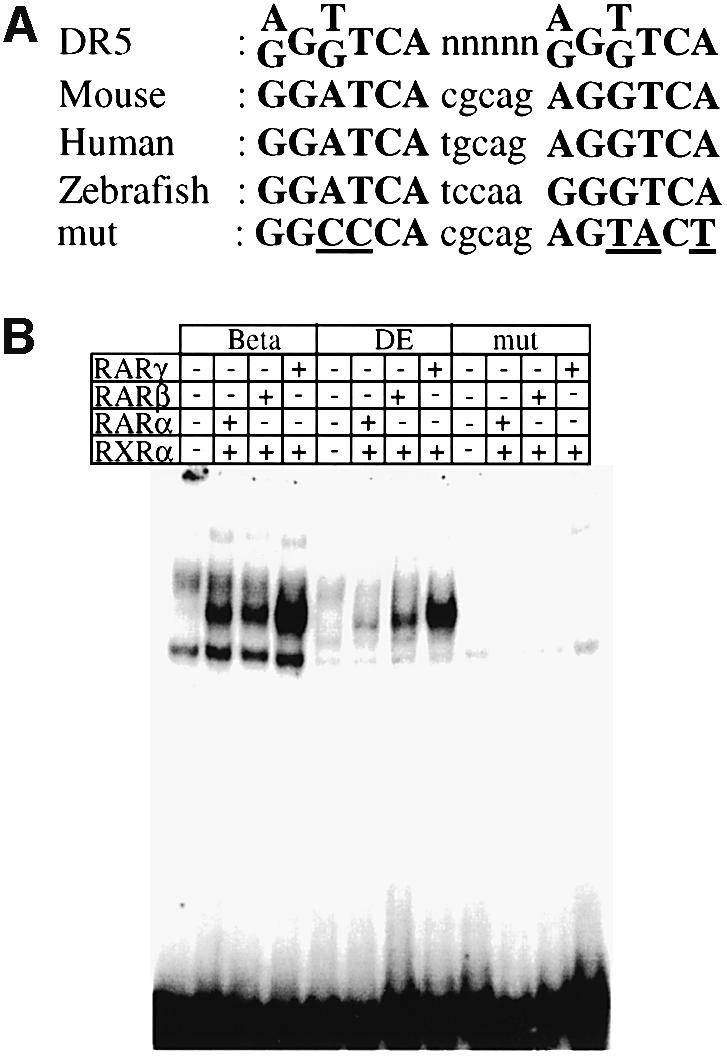
Fig. 3. (A) Sequence of the RARE consensus (Mader et al., 1993), and of the DE RARE (DR5) in the mouse, and in the human and zebrafish genome. The mutations experimentally introduced in the mouse DE RARE are also indicated. (B) EMSA: RAR–RXR heterodimers bind to the wild-type but not to the mutated DE RARE. The top column gives the three different labelled probes tested: β, the well-characterized DR5 RARE derived from the RARβ2 promoter; DE, the DR5 RARE of the DE; and DEmut, a mutated DE RARE (see A). The probes were tested with different combinations of RARs and RXRα proteins (indicated by + or – in the lower columns) present in whole-cell extracts (WCEs) of transfected COS-1 cells. In the lanes where no RA receptors were added, probes were incubated with WCE of COS-1 cells transfected with the empty expression vector. Note that the specific complexes formed on the RARβ2 RARE run at the identical position to those formed on the DE RARE.
Fig. 4. The DE RARE mediates an RA-dependent rostral shift of 5′ Hoxb gene expression into the hindbrain. (A) Reporter constructs. The physical map of the Hoxb cluster with the Hoxb5–Hoxb8 genes is shown at the top. Regulatory sequences relevant for this study are shown as lines above the cluster. Below: lines depict the regions that are included in the reporter constructs. Black triangles represent the insertion site of the lacZ gene. The mutations in the two half-sites of the RARE are indicated by an asterisk. DE, distal element; LPM, lateral plate mesoderm; B, BamHI; Hd, HindIII; Bg, BglII; N, NcoI; C, ClaI; H, HincII; R, EcoRI; K, KpnI. (B–E) In vivo properties of the DE combined with the Hoxb8 promoter and proximal regulatory elements. Construct numbers are indicated on the lower left of the panels. (B) E11.5 embryos expressing construct 2 (Hoxb8BH1100); (C) construct 1 (Hoxb8DE); (D) construct 3 (Hoxb8BH1100 + DE); and (E) construct 4 (Hoxb8BH1100 + DE with mutated RARE). (F and G) In vivo properties of the DE combined with the Hoxb5 promoter and a proximal mesoderm-specific element (Sharpe et al., 1998). (F) E11.5 embryos expressing construct 5 (Hoxb5LPM + DE); and (G) E11.5 embryos expressing construct 6 (Hoxb5LPM + DE with mutated RARE). (H and I) RA dependence of the DE activity. E11.5 embryo expressing construct 3 in the presence (I) or absence (H) of the Raldh2-null mutation.
In summary, these results show that the DE element contains a functional RARE that is necessary and sufficient to activate expression of the Hoxb8/lacZ and Hoxb5/lacZ transgenes in the posterior hindbrain.
The DE RARE is active in a spatio-temporal window compatible with late rostral expansion of 5′ Hoxb gene expression in the neural tube
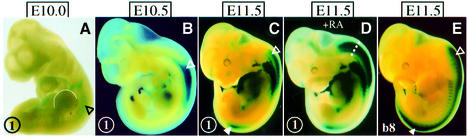
β-galactosidase activity in the neural tube of Hoxb8/lacZDE transgenic mice (Figure 4A, construct 1) appeared between the 27 and 32 somite stages (E10.0) at the level of the forelimb bud (Figure 5A). Expression at the level of the rostral spinal cord then intensified (E10.5, Figure 5B), to label the caudal hindbrain strongly by E11.5 (Figure 5C) and, more weakly, the spinal cord at the level of the hindlimb buds. These two regions of transgene expression driven by the DE in the neural tube are reminiscent of the expression driven by the RARβ2 promoter (Shen et al., 1992), and correspond to the areas of most intense Hoxb8 expression (Figure 5E). The anteriormost of the two regions spans the most rostral part of the Hoxb8 expression domain in the posterior hindbrain, which is generated between E10.5 and E11.5. It overlaps both spatially and temporally with the late anterior extension of the neural expression domain of Hoxb5 and Hoxb6 described above. The DE also mediates a response of construct 1 to exogenous RA, resulting in an anterior shift of its expression domain (Figure 5D compared with C).
Fig. 5. Expression dynamics of a Hoxb8/lacZ reporter construct driven by the DE (construct 1). (A–C) E10.0, E10.5 and E11.5 embryos, respectively. (D) Expression pattern of the Hoxb8/lacZ DE after in utero RA exposure from E10.5 on. (E) Endogenous Hoxb8 expression as revealed by lacZ expression of a Hoxb8/lacZ knockin allele (van den Akker et al., 1999). Open triangles, anterior domain of stronger expression; filled triangles, posterior domain of stronger expression.
In summary, the DE regulatory element activates transcription of reporter genes with temporal and spatial kinetics matching the late anterior expansion of 5′ Hoxb gene expression in the posterior hindbrain in wild-type embryos.
Neural expression of Hoxb5–Hoxb8 becomes sequentially sensitive to exogenous RA within the activity period of the DE RARE
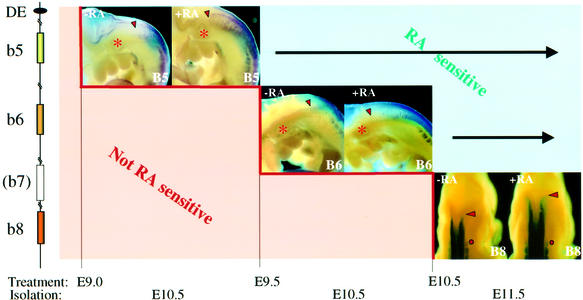
We analysed and compared the timing of RA sensitivity of the DE RARE coupled to the Hoxb8 promoter, with the RA sensitivity of endogenous Hoxb4–Hoxb8.
Transgenic embryos carrying a lacZ reporter driven by the Hoxb8 promoter and the DE (construct 1, Figure 4A) are RA sensitive upon treatment starting at E9.5 or E10.5 (Figure 5C and D). The expression domain of Hoxb4, known to depend on a more 3′ RARE-containing early neural enhancer (Gould et al., 1998), expanded rostrally after in utero exposure of the embryos to RA at E8.5, thus before the DE RARE is active (Gould et al., 1998; data not shown). Hoxb5–Hoxb8 did not respond to such an early treatment, but only to RA applied at successively later time points. Hoxb5 and Hoxb6 exhibited their earlier response to RA treatment at E9.0 and E9.5, respectively (Figure 6). No alteration in endogenous Hoxb8 expression occurred during E8.5–9.5 RA treatments. Only at E10.5 were RA-induced rostral shifts in Hoxb8 expression first observed (Figure 6). These responses fit within the window of sensitivity of the DE RARE.
Fig. 6. Sequential windows of RA sensitivity of Hoxb5 (B5), Hoxb6 (B6) and Hoxb8 (B8). Depicted on the left is a schematic representation of the 5′ part of the Hoxb cluster between Hoxb8 and the DE 3′ of Hoxb5. Time points of RA treatment and of embryo collection are indicated below. For each gene, the left panel represents the endogenous expression pattern in normal conditions (with solvent treatment), and the right panel shows the expression after RA treatment. The expression patterns shown correspond to the beginning of the window of RA sensitivity.
Hoxb5, Hoxb6 and Hoxb8 respond rapidly to exogenous RA
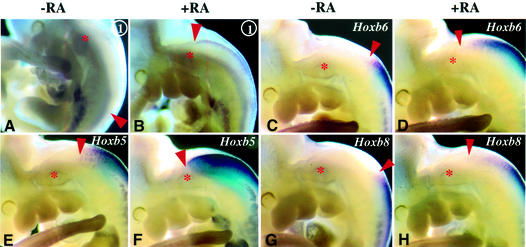
A prerequisite for a possible direct involvement of the DE RARE in the regulation of 5′ Hoxb genes would be rapid responsiveness to exogenous RA exposure. RA treatment of Hoxb8/lacZDE (construct 1, Figure 4A) transgenic embryos at E10.5 resulted in transcriptional induction within 6 h (Figure 7A and B). Under these conditions, the expression domains of endogenous Hoxb5, Hoxb6 and Hoxb8 also clearly extended rostrally in RA-treated embryos (Figure 7C–H). This is in agreement with previous studies showing that RA can activate Hoxb8 in the absence of protein synthesis in chicken limb bud cells (Lu et al., 1997).
Fig. 7. RA treatments result in a rapid sequential activation of the Hoxb5, Hoxb6 and Hoxb8 genes. (A–H) RA treatment from E10.5 to E10.75 (6 h) results in mRNA induction of Hoxb8/lacZ driven by the DE (construct 1, A and B), Hoxb6 (C and D), Hoxb5 (E and F) and Hoxb8 (G and H). The asterisk gives the position of the otic vesicle, the red dot the position of DRG C2, and the red arrowheads the anterior boundary of gene expression.
Discussion
The late regulatory phase of 5′ Hoxb genes and posterior hindbrain patterning
The expression domains of the 3′ Hoxb genes, Hoxb1–Hoxb4, reach their rostralmost boundaries before rhombomeric segmentation (Wilkinson et al., 1989; Gould et al., 1998). Several regulatory elements containing binding sites for RAR/RXR have been identified and characterized for these genes, and shown to contribute to the establishment of their early expression domains prior to and during segment formation in the rhombencephalon (Studer et al., 1994; Gavalas et al., 1998; Huang et al., 1998; see Gould et al., 1998; Gavalas and Krumlauf, 2000). In some cases, a later maintenance of Hox expression in specific rhombomeres is ensured by a different element receiving auto- and cross-regulatory Hox signals (Ogura and Evans, 1995; Pöpperl et al., 1995; Gould et al., 1998). The restricted reporter gene expression directed by the early neural enhancers of Hoxb1 and Hoxb4 is an integral part of the complete expression domain of these genes, and has a rostral boundary identical to the initial boundary of the endogenous gene [Hoxb1 (Marshall et al., 1994) and Hoxb4 (Gould et al., 1998)]. The 5′ Hoxb genes Hoxb5, Hoxb6 and Hoxb8 are expressed in domains that do not reach their definitive rostral boundaries in the posterior hindbrain until after rhombomeric segmentation. By E9.5, their anterior boundary of expression in the neural tube is at the level of somite 4 (Hoxb5), somite 5 (Hoxb6) and the S5–S6 boundary (Hoxb8). Thereafter, expression spreads rostrally in the neural tube, to reach successively their gene-specific anteriormost boundaries in the posterior hindbrain. Their definitive anterior boundaries are specified in rhombomeres 7/8 (r7/r8) in the hindbrain by E11.5, long after pre-otic rhombomere identity has been determined. Ontogenesis of the late rostral expression domains of these 5′ Hoxb genes between the upper spinal cord and the caudal hindbrain therefore occurs relatively late. It may correspond to a late function of 5′ Hoxb genes in the CNS, possibly contributing to specifying the identity of r7 and 8 (much less obvious morphologically than r1–r6).
It is interesting to put these observations side by side with the recent data on the graded role of RA signalling in hindbrain patterning in chick (Dupé and Lumsden, 2001). These studies showed that RA dependency of the specification of rhombomere identity is lost progressively in an anterior to posterior sequence between the definitive streak stage and the 16 somite stage, and that the post-otic neural tube between r5/r6 and the sixth somite belongs to the hindbrain and is anteriorized by abrogating RA signalling.
Comparison between the kinetics of the DE RARE and the sequential acquisition of definitive expression boundaries of 5′ Hoxb genes
The present study demonstrates that the late rostral extension of the expression of Hoxb8 and other 5′ Hoxb genes in the CNS depends on endogenous RA. A regulatory sequence containing a functional RARE was identified 3′ of Hoxb5. Our previous transgenic analyses (Charité et al., 1995; Valarché et al., 1997; our unpublished data) showed that the DE is the only sequence between Hoxb9 and Hoxb4 that can extend the expression of a Hoxb8 transgene rostrally into the posterior hindbrain. We now show that the RARE located within the DE is required for expression of Hoxb8/lacZ transgenes in the posterior hindbrain. This RARE, analysed using Hoxb8 and Hoxb5 transgenes, exhibits a window of spatio-temporal RA sensitivity encompassing that of the endogenous Hoxb8–Hoxb5.
A search in the Celera database for sites matching the RARE DR(1–5) consensus, as defined by Mader et al. (1993) (see also Figure 3A), did not reveal any such RARE between mouse Hoxb4 and 77 kb 5′ to Hoxb9. This sequence analysis revealed that the DE RARE, which we identified close to Hoxb5, was unique in the entire mouse Hoxb cluster. Although this information makes the DE RARE a very good candidate to account for RA sensitivity of Hoxb5–Hoxb8, we cannot rule out that other, divergent and unsuspected consensuses have escaped our analysis and are functionally involved in the RA-stimulated induction of the most rostral expression of these Hox genes. It will be necessary to inactivate the DE RARE in the mouse genome to understand its function definitely. The existence of a shared RARE regulatory element, which would act sequentially on successive 5′ Hoxb genes, might facilitate coordinated regulation of the establishment of their anteriormost expression boundaries in the neural tube, independently of their expression in the paraxial mesoderm. This mechanism would differ significantly from the regulation of 3′ Hoxb genes, which are controlled by several RAREs [Hoxb1 (Marshall et al., 1994; Ogura and Evans, 1995; Huang et al., 1998) and Hoxb4 (Gould et al., 1998)].
The newly identified RARE is evolutionarily conserved
Few binding sites for trans-acting factors have been characterized in the 5′ half of the Hox clusters so far, whereas binding sites for several trans-acting factors have been identified that control the expression of paralogous genes 1–4 (reviewed by Deschamps et al., 1999). The only sites that were found to bind trans-acting factors modulating the expression of 5′ Hoxb genes during their establishment were Cdx-binding sites (Charité et al., 1998; van den Akker et al., 2001). While Cdx proteins play a role during the ontogenesis of the Hox expression patterns during early embryogenesis (van den Akker et al., 2002), the RA-mediated control described in this work occurs later, between E9 and E11.5. The finding of strong conservation of the RARE sequence at corresponding positions of the mammalian and zebrafish genomes suggests a functional importance for this regulatory element. The relatively high conservation of the sequences flanking the RARE on both sides further suggests that essential co-activators may bind at positions flanking the RAR/RXR-binding site.
Endogenous retinoid signalling and 5′ Hoxb gene expression
RA distribution in developing embryos has been found to be restricted to posterior paraxial mesoderm, with a sharp anterior boundary at the level of the first somite (corresponding to r6/r7 in the hindbrain; Maden et al., 1998; Berggren et al., 1999; Swindell et al., 1999). Accordingly, Raldh2 expression is detected in cervical somites at E8.5, and in cervical mesenchyme by E9.5 (Niederreither et al., 1997). The endogenous RA regulating 5′ Hoxb genes in the posterior hindbrain between somite 6 and the r6/r7 region must therefore originate from that source between E9.5 and E11.5, and diffuse over a rather long distance (see also Dupé and Lumsden, 2001). The resulting RA gradient, together with a combination of other factors, including the Hox promoter-specific features of the RARE enhancer element, and differential availability of Hox genes for transcription (Kondo and Duboule, 1999; Kmita et al., 2000), might contribute to the sequential establishment of the definitive expression boundaries of the 5′ Hoxb genes.
Materials and methods
DNA constructs
Constructs 1 and 2 have been described previously (Charité et al., 1995; Valarché et al., 1997). Construct 3 was generated by cloning of a BamHI–BamHI fragment, containing BH1100 and Hoxb8/lacZ, from construct 2 into a plasmid containing the HincII–EcoRI distal element. Construct 4 was generated in an identical way with the DE containing a mutated RARE, GGCCCACGCAGAGTACT instead of the GGATCAC GCAGAGGTCA motif. The Hoxb5 reporter gene, in constructs 5 and 6, was generated by the in-frame fusion of the lacZ gene to the Hoxb5 gene, by making use of the NcoI site that includes the ATG start codon of the lacZ gene in PSDKlacZpA (a gift of J.Rossant) and the NcoI site in the first exon of Hoxb5. Constructs 5 and 6 include Hoxb5 upstream sequences up to the BglII site and, downstream from lacZ, the sequence from EagI in the Hoxb5 gene up to the BglII site downstream from Hoxb5, fused to the 3.5 kb ClaI–KpnI neural element (region E; Sharpe et al., 1998). Construct 6 contained the same mutation in the RARE as construct 4. All construct were linearized prior to microinjection of zygotes (Vogels et al., 1993).
DNA sequence analysis
The nucleotide sequence of the mouse DE (exactly 533 nucleotides) containing the RARE was determined and compared with its human equivalent using the human genome public database (web site: www.ensembl.org). The most conserved block of sequences spanning ∼160 nucleotides around the RARE was then used to screen the zebrafish genome database (web site: www.sanger.ac.uk), revealing 74% overall conservation along a 130 bp area surrounding the perfectly conserved RARE consensus, in the zebrafish Hoxba cluster, at a homologous position between Hoxb4 and Hoxb5. Mouse genomic sequences encompassing the Hoxb cluster were obtained from the Celera database and analysed using McDraw from DNAstar, Laser Gene.
Protein–DNA binding assays
Electropheretic mobility shift assays (EMSAs) were performed as described previously in Folkers et al. (1998). The oligonucleotides, labelled by filling in a 5′-AGG overhang, were as follows (without the overhang): β, AGGGTTCACCGAAAGTTCACTCGCA; DE, CGGG ATCACGCAGAGGTCAGCAGAC; and DEmut, CGGGCCCACG CAGAGTACTGCAGAC. The RARE sequences are shown in bold, and the mutations in DEmut are underlined.
X-gal staining and in situ hybridization
Whole-mount lacZ staining of embryos was performed as described previously (Hogan et al., 1994). Whole-mount in situ hybridization was performed according to Wilkinson (1992), with modifications in the post-hybridization washes (see Haramis et al., 1995), using the following probes: Hoxb1 (Conlon and Rossant, 1992), Krox20 (Conlon and Rossant, 1992; Seitanidou et al., 1997), Hoxb4 (Conlon and Rossant, 1992), Hoxb5 (Krumlauf et al., 1987), Hoxb6 (Schughart et al., 1988), Hoxb8 (SS420; Charité et al., 1998) and lacZ (Valarché et al., 1997).
In utero RA exposure of wild-type embryos
RA administration to pregnant mothers by oral gavage was performed as described previously by Conlon and Rossant (1992).
Early partial rescue of Raldh2-null embryos
Raldh2-null embryos die at E10.5 due to severe heart malformations (Niederreither et al., 1999), preventing the examination of later developmental events such as Hox gene regulation studied here. Whereas RA administration to Raldh2+/– pregnant mice by oral gavage resulted in teratogenic effects and a lower percentage of mutant survival (Nierderreither et al., 1999), RA supplementation in the food resulted in near normal development of Raldh2 mutant embryos (Nierderreither et al., 2001). Under food rescue conditions, the hindbrain of Raldh2-null embryos was morphologically normal, as indicated by the wild-type expression of 3′ Hoxb genes and Krox20 (Seitanidou et al., 1997; data not shown). These data exclude the possibility that early patterning defects in the hindbrain could have interfered with Hox gene expression at later stages of development. The landmarks for determining the position of the neural expression boundary of 5′ Hoxb genes, the dorsal root ganglia, were shown to be unaltered by histological inspection (data not shown). Finally, we ruled out that the Raldh2 mutation caused a loss of competence of the caudal hindbrain cells to express the 5′ Hoxb genes. Homozygous Raldh2 mutant embryos were completely rescued by supplementing their food with RA until the day of isolation. These experiments show that it is the late depletion of RA in Raldh2–/– embryos that causes the failure of 5′ Hoxb gene expression in the caudal hindbrain.
Acknowledgments
Acknowledgements
We thank Rolf Zeller for critical reading of the manuscript, Mark Reijnen and Jeroen Korving for microinjections, and Isabelle Valarché, Kirstie Lawson, Jeroen Charité, Antje Brouwer, Eric van den Akker, Lia Panman and Wim de Graaff for sharing materials and/or ideas. We are grateful to Carla Kroon for help with genotyping, and to Robb Krumlauf, Klaus Schughart and Patrick Charnay for probes. This work was supported by grants from the Dutch NWO-ALW (805.17.263 to F.M. and 809.38.05 to J.D.) and the CNRS, INSERM, Collège de France, Hôpitaux Universitaires de Strasbourg, Association pour la Recherche sur le Cancer, Fondation pour la Recherche Médicale and Bristol-Myers Squibb (K.N., P.D. and P.C.).
References
- Berggren K., McCaffery,K., Drager,U. and Forehand,C.J. (1999) Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme RALDH2. Dev. Biol., 210, 288–304. [DOI] [PubMed] [Google Scholar]
- Charité J., de Graaff,W., Vogels,R., Meijlink,F. and Deschamps,J. (1995) Regulation of the Hoxb-8 gene: synergism between multimerized cis-acting elements increases responsiveness to positional information. Dev. Biol., 171, 294–305. [DOI] [PubMed] [Google Scholar]
- Charité J., de Graaff,W., Consten,D., Reijnen,M.J., Korving,J. and Deschamps,J. (1998) Transducing positional information to the Hox genes: critical interaction of cdx gene products with position-sensitive regulatory elements. Development, 125, 4349–4358. [DOI] [PubMed] [Google Scholar]
- Conlon R.A. and Rossant,J. (1992) Exogenous retinoic acid rapidly induces anterior ectopic expression of murine Hox-2 genes in vivo. Development, 116, 357–368. [DOI] [PubMed] [Google Scholar]
- Deschamps J., van den Akker,E., Forlani,S., de Graaff,W., Oosterveen,T., Roelen,B. and Roelfsema,J. (1999) Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int. J. Dev. Biol., 43, 635–650. [PubMed] [Google Scholar]
- Dupé V. and Lumsden,A. (2001) Hindbrain patterning involves graded responses to retinoic acid signalling. Development, 128, 2199–2208. [DOI] [PubMed] [Google Scholar]
- Eid R., Koseki,H. and Schughart,K. (1993) Analysis of lacZ reporter genes in transgenic embryos suggests the presence of several cis-acting regulatory elements in the murine Hoxb-6 gene. Dev. Dyn., 196, 205–216. [DOI] [PubMed] [Google Scholar]
- Folkers G.E., van der Burg,B. and van der Saag,P.T. (1998) Promoter architecture, cofactors and orphan receptors contribute to cell-specific activation of the retinoic acid receptor β2 promoter. J. Biol. Chem., 273, 32200–32212. [DOI] [PubMed] [Google Scholar]
- Gavalas A., Studer,M., Lumsden,A., Rijli,F.M., Krumlauf,R. and Chambon,P. (1998) Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch. Development, 125, 1123–1136. [DOI] [PubMed] [Google Scholar]
- Gavalas A. and Krumlauf,R. (2000) Retinoid signalling and hindbrain patterning. Curr. Opin. Genet. Dev., 10, 380–386. [DOI] [PubMed] [Google Scholar]
- Gould A., Itasaki,N. and Krumlauf,R. (1998) Initiation of rhombomeric Hoxb4 expression requires induction by somites and a retinoid pathway. Neuron, 21, 39–51. [DOI] [PubMed] [Google Scholar]
- Greer J.M., Puetz,J., Thomas,K.R. and Capecchi,M.R. (2000) Maintenance of functional equivalence during paralogous Hox gene evolution. Nature, 403, 661–665. [DOI] [PubMed] [Google Scholar]
- Haramis A.G., Brown,J.M. and Zeller,R. (1995) The limb deformity mutation disrupts the SHH/FGF-4 feedback loop and regulation of 5′ HoxD genes during limb pattern formation. Development, 121, 4237–4245. [DOI] [PubMed] [Google Scholar]
- Hogan B., Beddington R., Constantini,F. and Lacy,E. (1994) Manipulating the Mouse Embryo. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Huang D., Chen,S.W., Langston,A.W. and Gudas,L.J. (1998) A conserved retinoic acid responsive element in the murine Hoxb-1 gene is required for expression in the developing gut. Development, 125, 3235–3246. [DOI] [PubMed] [Google Scholar]
- Kmita M., van der Hoeven,F., Zákány,J., Krumlauf,R. and Duboule,D. (2000) Mechanisms of Hox gene colinearity: transposition of the anterior Hoxb1 gene into the posterior HoxD complex. Genes Dev., 14, 198–211. [PMC free article] [PubMed] [Google Scholar]
- Kondo T. and Duboule,D. (1999) Breaking colinearity in the mouse HoxD complex. Cell, 97, 407–417. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. (1994) Hox genes in vertebrate development. Cell, 78, 191–201. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Holland,P.W., McVey,J.H. and Hogan,B.L. (1987) Developmental and spatial patterns of expression of the mouse homeobox gene, Hox2.1. Development, 99, 603–617. [DOI] [PubMed] [Google Scholar]
- Lu H.C., Revelli,J.P., Goering,L., Thaller,C. and Eichele,G. (1997) Retinoid signaling is required for the establishment of a ZPA and for the expression of Hoxb-8, a mediator of ZPA formation. Development, 124, 1643–1651. [DOI] [PubMed] [Google Scholar]
- Maden M., Sonneveld,E., van der Saag,P.T and Gale,E. (1998) The distribution of endogenous retinoic acid in the chick embryo: implications for developmental mechanisms. Development, 125, 4133–4144. [DOI] [PubMed] [Google Scholar]
- Mader S., Leroy,P., Chen,J.Y. and Chambon,P. (1993) Multiple parameters control the selectivity of nuclear receptors for their response elements. Selectivity and promiscuity in response element recognition by retinoic acid receptors and retinoid X receptors. J. Biol. Chem., 268, 591–600. [PubMed] [Google Scholar]
- Manzanares M., Bel-Vialar,S., Ariza-McNaughton,L., Ferretti,E., Marshall,H., Maconochie,M.M., Blasi,F. and Krumlauf, R (2001) Independent regulation of initiation and maintenance phases of Hoxa3 expression in the vertebrate hindbrain involve auto- and cross-regulatory mechanisms. Development, 128, 3595–3607. [DOI] [PubMed] [Google Scholar]
- Marshall H., Studer,M., Popperl,H., Aparicio,S., Kuroiwa,A., Brenner,S. and Krumlauf,R. (1994) A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature, 370, 567–571. [DOI] [PubMed] [Google Scholar]
- Nierderreither K., McCaffery,P., Drager,U.C., Chambon,P. and Dolle,P. (1997) Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mech. Dev., 62, 67–78. [DOI] [PubMed] [Google Scholar]
- Niederreither K., Subbarayan,V., Dolle,P. and Chambon,P. (1999) Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet., 21, 444–448. [DOI] [PubMed] [Google Scholar]
- Niederreither K., Vermot,J., Schuhbaur,B., Chambon,P. and Dolle,P. (2000) Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development, 127, 75–85. [DOI] [PubMed] [Google Scholar]
- Niederreither K., Vermot,J., Messaddeq,N., Schuhbaur,B., Chambon,P. and Dolle,P. (2001) Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development, 128, 1019–1031. [DOI] [PubMed] [Google Scholar]
- Ogura T. and Evans,R.M. (1995) A retinoic acid-triggered cascade of HOXB1 gene activation. Proc. Natl Acad. Sci. USA, 92, 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöpperl H., Bienz,M., Studer,M., Chan,S.-K., Aparicio,S., Brenner,S., Mann,R.S. and Krumlauf.,R. (1995) Segmental expression of Hoxb1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell, 81, 1031–1042. [DOI] [PubMed] [Google Scholar]
- Schughart K., Utset,M.F., Awgulewitsch,A. and Ruddle,F.H. (1988) Structure and expression of Hox-2.2, a murine homeobox-containing gene. Proc. Natl Acad. Sci. USA, 85, 5582–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitanidou T., Schneider-Maunoury,S., Desmarquet,C., Wilkinson,D.G. and Charnay,P. (1997) Krox-20 is a key regulator of rhombomere-specific gene expression in the developing hindbrain. Mech. Dev., 65, 31–42. [DOI] [PubMed] [Google Scholar]
- Sharpe J., Nonchev,S., Gould,A., Whiting,J. and Krumlauf,R. (1998) Selectivity, sharing and competitive interactions in the regulation of Hoxb genes. EMBO J., 17, 1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., van den Brink,C., Kruijer,W. and van der Saag,P.T. (1992) Embryonic stem cells stably transfected with mRARβ2-lacZ exhibit specific expression in chimeric embryos. Int. J. Dev. Biol., 36, 465–476. [PubMed] [Google Scholar]
- Simeone A., Acampora,D., Arcioni,L., Andrews,P.W., Boncinelli,E. and Mavilio,F. (1990) Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature, 346, 763–766. [DOI] [PubMed] [Google Scholar]
- Studer M., Popperl,H., Marshall,H., Kuroiwa,A. and Krumlauf,R. (1994) Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science, 265, 1728–1732. [DOI] [PubMed] [Google Scholar]
- Swindell E., Thaller,C., Sockanathan,S., Petkovitch,M., Jessel,T. and Eichele,G. (1999) Complementary domains of retinoic acid production and degradation in the early chick embryo. Dev. Biol., 216, 282–296. [DOI] [PubMed] [Google Scholar]
- Valarché I., de Graaff,W. and Deschamps,J. (1997) A 3′ remote control region is a candidate to modulate Hoxb-8 expression boundaries. Int. J. Dev. Biol., 41, 705–714. [PubMed] [Google Scholar]
- van den Akker E., Reijnen,M., Korving,J., Brouwer,A., Meijlink,F. and Deschamps,J. (1999) Targeted inactivation of Hoxb8 affects survival of a spinal ganglion and causes aberrant limb reflexes. Mech. Dev., 89, 103–114. [DOI] [PubMed] [Google Scholar]
- van den Akker E., Forlani,S., Chawengsaksophak,K., de Graaff,W., Beck,F., Meyer,B.I. and Deschamps,J. (2002) Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development, 129, 2181–2193. [DOI] [PubMed] [Google Scholar]
- van der Hoeven F., Zákány,J. and Duboule,D. (1996) Gene transpositions in the HoxD complex reveal a hierarchy of regulatory controls. Cell, 85, 1025–1035. [DOI] [PubMed] [Google Scholar]
- Vogels R., Charité,J., de Graaff,W. and Deschamps,J. (1993) Proximal cis-acting elements cooperate to set Hoxb-7 (Hox-2.3) expression boundaries in transgenic mice. Development, 118, 71–82. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.G., Bhatt,S., Chavrier,P., Bravo,R. and Charnay,P. (1989) Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature, 337, 461–464. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.G. (1992) Whole mount in situ hybridization of vertebrate embryos. In Wilkinson,D.G. (ed.), In Situ Hybridization. IRL Press at Oxford University Press, pp. 75–83.
- Zákány J., Gerard,M., Favier,B. and Duboule,D. (1997) Deletion of a HoxD enhancer induces transcriptional heterochrony leading to transposition of the sacrum. EMBO J., 16, 4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]