Abstract
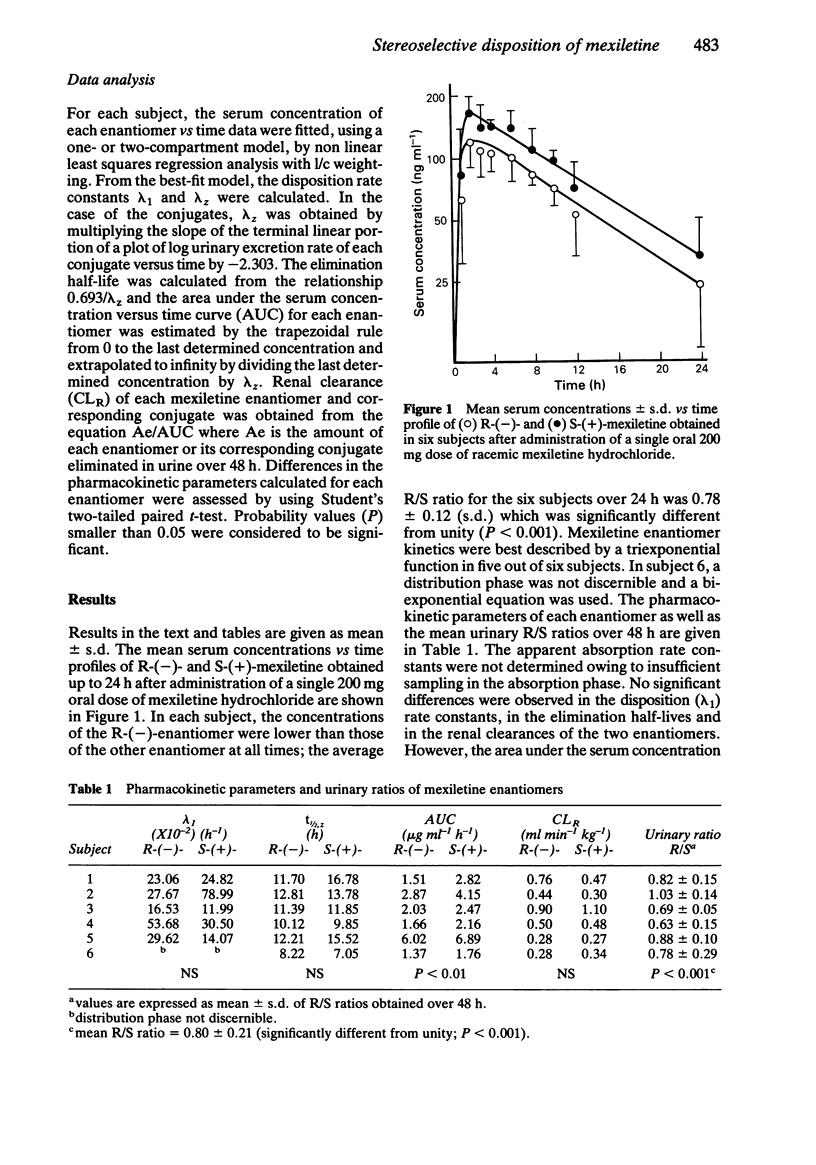
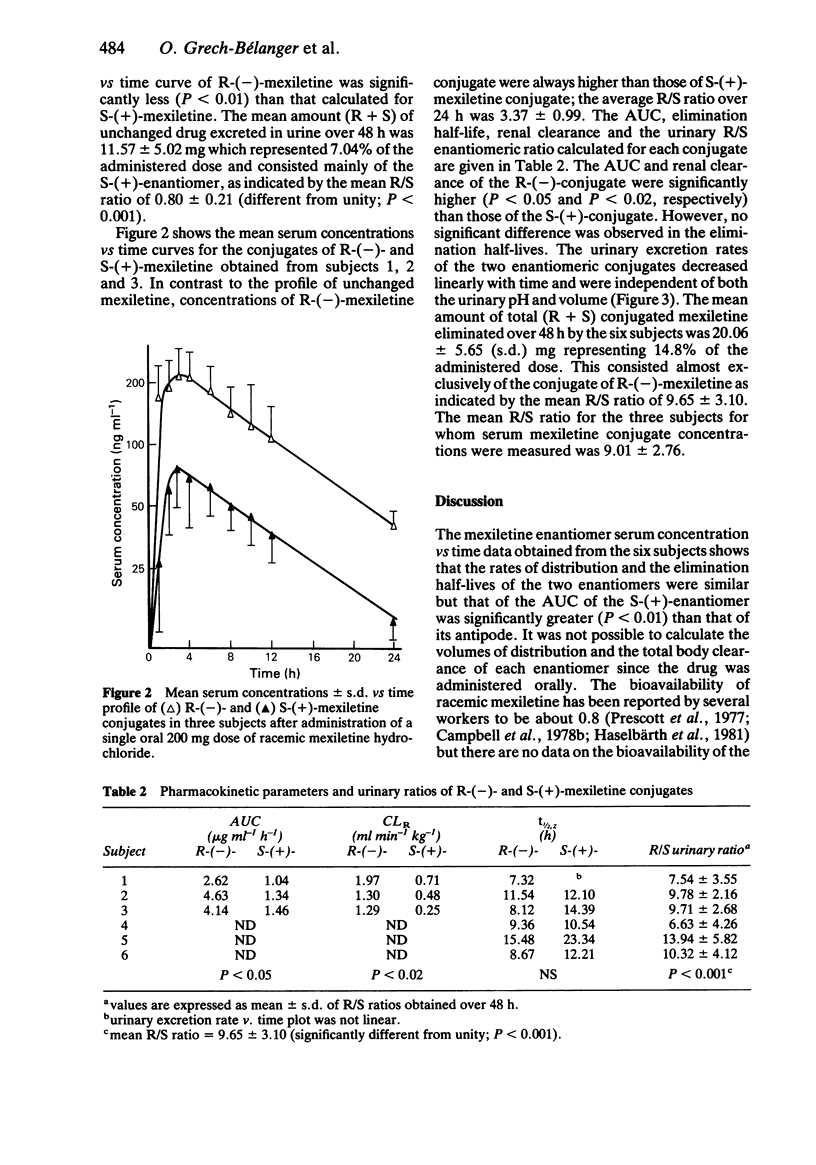
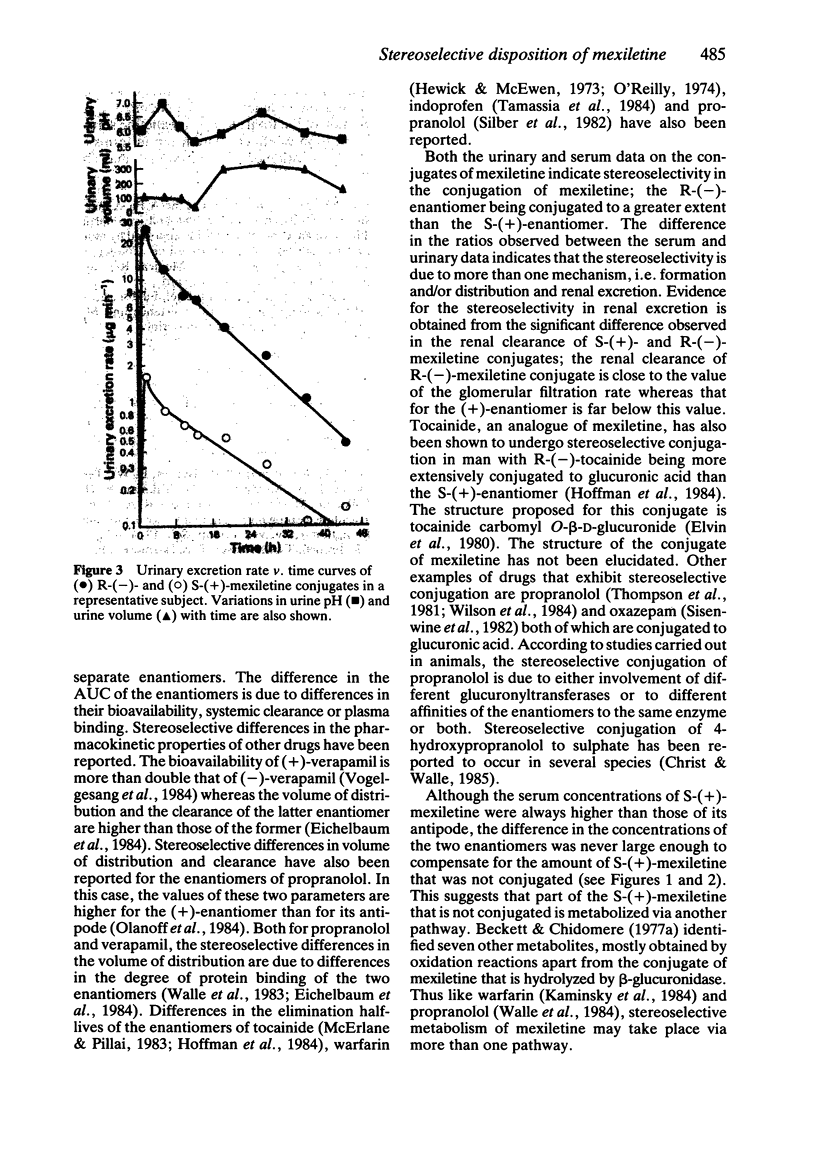
The pharmacokinetics of S-(+)- and R-(-)-mexiletine and of the corresponding conjugates were investigated in six healthy young volunteers after administration of a single 200 mg oral dose of racemic mexiletine hydrochloride. The values for the distribution rate constants as well as for the elimination half-lives of the two enantiomers were similar but the AUC of the S-(+)-enantiomer was always significantly higher (P less than 0.01) than that of the opposite enantiomer. The mean R/S ratios for unchanged mexiletine in serum and in urine were 0.78 +/- 0.12 (s.d.) and 0.80 +/- 0.21, respectively. Urinary excretion of mexiletine conjugates consisted mainly of the R-(-)-enantiomer; the mean R/S enantiomeric ratio over 48 h was 9.65 +/- 3.10. Serum concentrations of the conjugates were measured in three subjects. The mean R/S AUC ratio was 2.94 +/- 0.48 and the renal clearance of the R-(-)-enantiomer was significantly higher (P less than 0.02) than that of the S-(+)-enantiomer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariëns E. J. Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. Eur J Clin Pharmacol. 1984;26(6):663–668. doi: 10.1007/BF00541922. [DOI] [PubMed] [Google Scholar]
- Beckett A. H., Chidomere E. C. The distribution, metabolism and excretion of mexiletine in man. Postgrad Med J. 1977;53 (Suppl 1):60–68. [PubMed] [Google Scholar]
- Beckett A. H., Chidomere E. C. The identification and analysis of mexiletine and its metabolic products in man. J Pharm Pharmacol. 1977 May;29(5):281–285. doi: 10.1111/j.2042-7158.1977.tb11312.x. [DOI] [PubMed] [Google Scholar]
- Byrnes E. W., McMaster P. D., Smith E. R., Blair M. R., Boyes R. N., Duce B. R., Feldman H. S., Kronberg G. H., Takman B. H., Tenthorey P. A. New antiarrhythmic agents. 1. Primary alpha-amino anilides. J Med Chem. 1979 Oct;22(10):1171–1176. doi: 10.1021/jm00196a005. [DOI] [PubMed] [Google Scholar]
- Campbell N. P., Kelly J. G., Adgey A. A., Shanks R. G. Mexiletine in normal volunteers. Br J Clin Pharmacol. 1978 Oct;6(4):372–373. doi: 10.1111/j.1365-2125.1978.tb00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N. P., Kelly J. G., Adgey A. A., Shanks R. G. The clinical pharmacology of mexiletine. Br J Clin Pharmacol. 1978 Aug;6(2):103–108. doi: 10.1111/j.1365-2125.1978.tb00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N. P., Kelly J. G., Shanks R. G., Chaturvedi N. C., Strong J. E., Pantridge J. F. Mexiletine (Kö 1173) in the management of ventricular dysrhythmias. Lancet. 1973 Aug 25;2(7826):404–407. doi: 10.1016/s0140-6736(73)92271-x. [DOI] [PubMed] [Google Scholar]
- Chew C. Y., Collett J., Singh B. N. Mexiletine: a review of its pharmacological properties and therapeutic efficacy in arrhythmias. Drugs. 1979 Mar;17(3):161–181. doi: 10.2165/00003495-197917030-00003. [DOI] [PubMed] [Google Scholar]
- Christ D. D., Walle T. Stereoselective sulfate conjugation of 4-hydroxypropranolol in vitro by different species. Drug Metab Dispos. 1985 May-Jun;13(3):380–381. [PubMed] [Google Scholar]
- Eichelbaum M., Mikus G., Vogelgesang B. Pharmacokinetics of (+)-, (-)- and (+/-)-verapamil after intravenous administration. Br J Clin Pharmacol. 1984 Apr;17(4):453–458. doi: 10.1111/j.1365-2125.1984.tb02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin A. T., Keenaghan J. B., Byrnes E. W., Tenthorey P. A., McMaster P. D., Takman B. H., Lalka D., Manion C. V., Baer D. T., Wolshin E. M. Tocainide conjugation in humans: novel biotransformation pathway for a primary amine. J Pharm Sci. 1980 Jan;69(1):47–49. doi: 10.1002/jps.2600690113. [DOI] [PubMed] [Google Scholar]
- Gal J., French T. A., Zysset T., Haroldsen P. E. Disposition of (R,S)-tocainide. Some stereoselective aspects. Drug Metab Dispos. 1982 Jul-Aug;10(4):399–404. [PubMed] [Google Scholar]
- Gillis A. M., Kates R. E. Clinical pharmacokinetics of the newer antiarrhythmic agents. Clin Pharmacokinet. 1984 Sep-Oct;9(5):375–403. doi: 10.2165/00003088-198409050-00001. [DOI] [PubMed] [Google Scholar]
- Grech-Bélanger O. Gas chromatographic method for the routine serum monitoring of mexiletine. J Chromatogr. 1984 Jul 13;309(1):165–169. doi: 10.1016/0378-4347(84)80019-5. [DOI] [PubMed] [Google Scholar]
- Grech-Bélanger O., Gilbert M., Turgeon J., LeBlanc P. P. Effect of cigarette smoking on mexiletine kinetics. Clin Pharmacol Ther. 1985 Jun;37(6):638–643. doi: 10.1038/clpt.1985.103. [DOI] [PubMed] [Google Scholar]
- Grech-Bélanger O., Turgeon J., Gilbert M. High-performance liquid chromatographic assay for mexiletine enantiomers in human plasma. J Chromatogr. 1985 Jan 11;337(1):172–177. doi: 10.1016/0378-4347(85)80026-8. [DOI] [PubMed] [Google Scholar]
- Hewick D. S., McEwen J. Plasma half-lives, plasma metabolites and anticoagulant efficacies of the enantiomers of warfarin in man. J Pharm Pharmacol. 1973 Jun;25(6):458–465. doi: 10.1111/j.2042-7158.1973.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. J., Renberg L., Bärnhielm C. Stereoselective disposition of RS-tocainide in man. Eur J Drug Metab Pharmacokinet. 1984 Jul-Sep;9(3):215–222. doi: 10.1007/BF03189644. [DOI] [PubMed] [Google Scholar]
- Kaminsky L. S., Dunbar D. A., Wang P. P., Beaune P., Larrey D., Guengerich F. P., Schnellmann R. G., Sipes I. G. Human hepatic cytochrome P-450 composition as probed by in vitro microsomal metabolism of warfarin. Drug Metab Dispos. 1984 Jul-Aug;12(4):470–477. [PubMed] [Google Scholar]
- McErlane K. M., Pillai G. K. Gas--liquid chromatographic resolution and assay of tocainide enantiomers using a chiral capillary column and study of their selective disposition in man. J Chromatogr. 1983 May 13;274:129–138. doi: 10.1016/s0378-4347(00)84416-3. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. A. Studies on the optical enantiomorphs of warfarin in man. Clin Pharmacol Ther. 1974 Aug;16(2):348–354. doi: 10.1002/cpt1974162348. [DOI] [PubMed] [Google Scholar]
- Olanoff L. S., Walle T., Walle U. K., Cowart T. D., Gaffney T. E. Stereoselective clearance and distribution of intravenous propranolol. Clin Pharmacol Ther. 1984 Jun;35(6):755–761. doi: 10.1038/clpt.1984.107. [DOI] [PubMed] [Google Scholar]
- Prescott L. F., Pottage A., Clements J. A. Absorption, distribution and elimination of mexiletine. Postgrad Med J. 1977;53 (Suppl 1):50–55. [PubMed] [Google Scholar]
- Silber B., Holford N. H., Riegelman S. Stereoselective disposition and glucuronidation of propranolol in humans. J Pharm Sci. 1982 Jun;71(6):699–704. doi: 10.1002/jps.2600710623. [DOI] [PubMed] [Google Scholar]
- Sisenwine S. F., Tio C. O., Hadley F. V., Liu A. L., Kimmel H. B., Ruelius H. W. Species-related differences in the stereoselective glucuronidation of oxazepam. Drug Metab Dispos. 1982 Nov-Dec;10(6):605–608. [PubMed] [Google Scholar]
- Talbot R. G., Nimmo J., Julian D. G., Clark R. A., Neilson J. M., Prescott L. F. Treatment of ventricular arrhythmias with mexiletine (Kö 1173). Lancet. 1973 Aug 25;2(7826):399–404. doi: 10.1016/s0140-6736(73)92270-8. [DOI] [PubMed] [Google Scholar]
- Tamassia V., Jannuzzo M. G., Moro E., Stegnjaich S., Groppi W., Nicolis F. B. Pharmacokinetics of the enantiomers of indoprofen in man. Int J Clin Pharmacol Res. 1984;4(3):223–230. [PubMed] [Google Scholar]
- Vogelgesang B., Echizen H., Schmidt E., Eichelbaum M. Stereoselective first-pass metabolism of highly cleared drugs: studies of the bioavailability of L- and D-verapamil examined with a stable isotope technique. Br J Clin Pharmacol. 1984 Nov;18(5):733–740. doi: 10.1111/j.1365-2125.1984.tb02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T., Walle U. K., Wilson M. J., Fagan T. C., Gaffney T. E. Stereoselective ring oxidation of propranolol in man. Br J Clin Pharmacol. 1984 Nov;18(5):741–748. doi: 10.1111/j.1365-2125.1984.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle U. K., Walle T., Bai S. A., Olanoff L. S. Stereoselective binding of propranolol to human plasma, alpha 1-acid glycoprotein, and albumin. Clin Pharmacol Ther. 1983 Dec;34(6):718–723. doi: 10.1038/clpt.1983.240. [DOI] [PubMed] [Google Scholar]
- Wilson B. K., Thompson J. A. Glucuronidation of propranolol by dog liver microsomes. Effects of enantiomeric inhibition and detergent treatment on stereoselectivity. Drug Metab Dispos. 1984 Mar-Apr;12(2):161–164. [PubMed] [Google Scholar]


