Abstract
The tom2-1 mutation of Arabidopsis thaliana reduces the efficiency of intracellular multiplication of tobamoviruses. The tom2-1 mutant was derived from fast-neutron-irradiated seeds, and the original mutant line also carries ttm1, a dominant modifier that increases tobamovirus multiplication efficiency in a tobamovirus-strain-specific manner in the tom2-1 genetic background. Here, we show that the tom2-1 mutation involved a deletion of ∼20 kb in the nuclear genome. The deleted region included two genes named TOM2A and TOM2B that were both associated with the tom2-1 phenotype, whereas ttm1 corresponded to the translocation of part of the deleted region that included intact TOM2B but not TOM2A. TOM2A encodes a 280 amino acid putative four-pass transmembrane protein with a C-terminal farnesylation signal, while TOM2B encodes a 122 amino acid basic protein. The split-ubiquitin assay demonstrated an interaction of TOM2A both with itself and with TOM1, an integral membrane protein of A.thaliana presumed to be an essential constituent of tobamovirus replication complex. The data presented here suggest that TOM2A is also an integral part of the tobamovirus replication complex.
Keywords: Arabidopsis thaliana/chromosomal rearrangement/host factor/tobamovirus/TOM2
Introduction
Virus multiplication involves multiple host factors functioning in concert with factors encoded by the virus genome (for reviews, see Buck, 1996; Lai, 1998). While recent genetic and biochemical analyses have revealed some important host factors for virus multiplication (Diez et al., 2000; Yamanaka et al., 2000), information on such host factors remains limited.
Tobamovirus is a member of the alpha-like virus supergroup of positive-strand RNA viruses. The genome of tobamovirus is a single-stranded 5′-capped RNA encoding three non-structural proteins and the coat protein (CP). The 130K and 180K non-structural proteins with approximate molecular masses of 130 and 180 kDa, respectively, are directly translated from the genomic RNA and are involved in viral RNA replication via negative-strand RNA. The other non-structural protein, with an approximate molecular mass of 30 kDa, is necessary for viral cell-to-cell movement in plants and is referred to as the movement protein (MP). Both MP and CP are translated from respective subgenomic mRNAs (Dawson, 1992; Wilson and Davies, 1992).
The tobamovirus 130K protein harbors a methyltransferase domain implicated in 5′-capping and a helicase domain, which are conserved among alpha-like viruses. The 180K protein is synthesized by translational read-through of the termination codon of the 130K protein, and also harbors an RNA polymerase motif within the read-through region (reviewed in Buck, 1999). Osman and Buck (1996) have extracted template-dependent membrane-bound tobamovirus RNA-dependent RNA polymerase (RdRp) from infected plant tissue and shown that it catalyzes the complete replication cycle and contains both the 130K and 180K proteins. Further analyses with solubilized and purified tobamovirus RdRp revealed that the 130K and 180K proteins form a heterodimer (Watanabe et al., 1999) and that the RdRp also contains the Saccharomyces cerevisiae Gcd10p-related subunit of eukaryotic translation initiation factor 3 (Osman and Buck, 1997).
In order to identify host factors necessary for efficient multiplication of tobamoviruses, we have previously isolated two independent mutants of Arabidopsis thaliana named tom (tobamovirus multiplication) 1 and tom2 in which tobamovirus multiplication is reduced to low levels. The causal mutations are recessive and specifically affect intracellular multiplication of tobamoviruses, but not that of cucumber mosaic virus or turnip crinkle virus (TCV) (Ishikawa et al., 1991, 1993; Ohshima et al., 1998). By means of a map-based cloning approach, the TOM1 gene was identified and revealed to encode a putative seven-pass transmembrane protein (Yamanaka et al., 2000). We have demonstrated that the TOM1 protein can interact with the helicase domain polypeptide of tobamoviruses (Yamanaka et al., 2000) and that simultaneous inactivation of TOM1 and its homolog TOM3 completely inhibits tobamovirus multiplication (Yamanaka et al., 2002). Like the TOM1 protein, the TOM3 protein can also interact with the helicase domain polypeptide of a tobamovirus (Yamanaka et al., 2002). Based on these observations, we proposed a model in which TOM1 and TOM3 are essential constituents of the replication complex of tobamoviruses and function in parallel to tether the replication complex to membranes.
The tom2-1 mutant was isolated from an M2 population derived from fast-neutron-irradiated seeds (Ohshima et al., 1998). Although the tom2-1 mutant was also shown to carry a dominant modifier named ttm1 that increases the efficiency of tobamovirus multiplication in the tom2-1 genetic background (Ohshima et al., 1998), the nature of the tom2-1 and ttm1 mutations remained to be determined. Here, we demonstrate that these two mutations involve a rearrangement of the nuclear genome affecting two independent genes, TOM2A and TOM2B, both of which control the tobamovirus multiplication phenotype. Moreover, TOM2A is shown to encode a four-pass transmembrane protein that interacts both with itself and with the TOM1 integral membrane protein.
Results
Chromosomal rearrangement in the tom2-1 mutant genome
We have previously reported the isolation of the A.thaliana YS241 mutant strain, in which the intracellular multiplication of tobamoviruses is reduced to low levels, from an M2 population derived from fast-neutron-irradiated seeds (Ohshima et al., 1998). Genetic analyses suggested that YS241 harbored one major recessive mutation, tom2-1, which reduces the efficiency of tobamovirus multiplication, and a tobamovirus-strain-specific dominant modifier, ttm1, which increases the efficiency of tobamovirus multiplication in a tom2-1 background. In tom2-1 mutant plants that do not carry the ttm1 mutation (representative plant line B1-113), the multiplication of both TMV-Cg, a crucifer-infecting tobamovirus, and TMV-L, a tomato mosaic tobamovirus, is severely affected. In contrast, TMV-Cg multiplication in tom2-1 mutant plants carrying the ttm1 mutation (representative plant line B1-234) is slightly higher than that in B1-113 but lower than that in wild-type plants, and the level of TMV-L multiplication is similar to that in wild-type plants. Considering these observations, it was proposed that a single gene ‘TOM2’ was deleted in the tom2-1 plants (B1-113) and that ttm1 was a translocated and modified form of the TOM2 gene (Ohshima et al., 1998). According to this hypothesis, B1-113 should carry a chromosomal deletion that includes the TOM2 gene and B1-234 should carry this deleted region at least partly.
Based on the above prediction, we searched for a deletion in the B1-113 genome by the representational difference analysis (RDA) (Lisitsyn et al., 1993) using HindIII-digested genomic DNA from the parental wild-type plants as a ‘tester’ and HindIII-digested genomic DNA from B1-113 as a ‘driver’. After three rounds of subtraction and amplification by the PCR, a 570 bp DNA fragment (hereafter referred to as the RDA fragment) was obtained. Cloning and sequencing analysis revealed that the sequence of the RDA fragment represented a region of chromosome 1. PCR amplification analysis of this fragment showed that the region corresponding to the RDA fragment was present in both the wild-type and B1-234 genomes, but was absent in the B1-113 genome.
On the other hand, the tom2-1 mutation was previously mapped on chromosome 1 between restriction fragment length polymorphism (RFLP) markers m253 and m299 (Ohshima et al., 1998). Here, a contig encompassing m253 was constructed using yeast artificial chromosome (YAC) clones, and further RFLP mapping was performed with YAC end probes. This analysis suggested that the mutation is located between DNA markers CIC10D4R and yUP19F5R (Figure 1A and B). As expected, the RDA fragment hybridized with the YAC clones CIC3H11, yUP19F5 and CIC10D4 but not with CIC7G6 (Figure 1B), suggesting that the RDA fragment is tightly linked to the tom2-1 mutation.
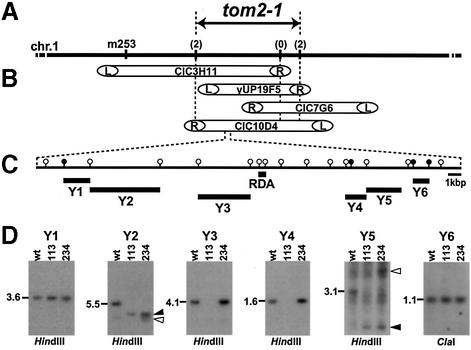
Fig. 1. Genetic mapping of tom2-1 and detection of chromosomal rearrangements. (A) A map of the top arm of chromosome 1 (not to scale). Vertical bars on chromosome 1 represent DNA markers. In the previous work (Ohshima et al., 1998), tom2-1 was mapped near m253. The numbers in parentheses represent the number of recombination events between the markers and the tom2-1 locus per 132 chromatids examined. (B) A YAC contig encompassing the tom2-1 locus. The left (L) and right (R) ends of the YAC inserts were defined by the method described by Liu and Whittier (1995). (C) Restriction map around the RDA fragment. HindIII and ClaI sites are indicated by open and filled circles respectively. Bars under the map show the position of the RDA fragment and fragments used as probes to detect chromosomal rearrangements (Y1–Y6). (D) Genomic Southern hybridization analysis showing a chromosomal deletion and rearrangement around the RDA fragment in plant lines B1-113 (tom2-1) and B1-234 (tom2-1 ttm1). Genomic DNA from wild-type (wt), B1-113 and B1-234 plants was digested with HindIII (for Y1–Y5) or ClaI (for Y6) and subjected to Southern hybridization analysis using Y1–Y6 as probes. Positions and sizes (kbp) of DNA fragments expected from the wild-type genomic sequence are shown to the left of each panel. Filled arrowheads represent the bands appearing in B1-113 and B1-234 lanes but not in wt lanes, whereas open arrowheads represent the bands appearing only in B1-234 lanes.
To determine the status of the genomic DNA surrounding the RDA fragment in B1-113 and B1-234, Southern blot hybridization analysis was performed using DNA probes located near the RDA fragment (Y1–Y6 in Figure 1C). When Y1 or Y6 were used as probes, restriction fragments of the same size were detected in the genomic DNA from wild-type, B1-113 and B1-234 plants (Figure 1D). The Y3 and Y4 probes each detected restriction fragments of the same size in genomic DNA from wild-type and B1-234 plants, but failed to do so in the genomic DNA from B1-113 plants (Figure 1D). The Y2 probe detected the expected 5.5 kb DNA fragment in the genomic DNA from wild-type plants, which was not detected in B1-113 or B1-234. Instead, the Y2 probe detected a band of ∼4.0 kb in the genomic DNA from both B1-113 and B1-234 (marked by a filled arrowhead in Figure 1D). In the genomic DNA from B1-234, the Y2 probe also detected one more band of ∼3.9 kb (marked by an open arrowhead in Figure 1D). The Y5 probe detected multiple bands, suggesting that it contained a repetitive sequence, although the expected 3.1 kb DNA fragment was detected in the genomic DNA from wild-type plants and not in B1-113 or B1-234. Instead, the Y5 probe detected a band of ∼1.3 kb in the genomic DNA from both B1-113 and B1-234 (marked by a filled arrowhead in Figure 1D). The Y5 probe also detected one more band of ∼4.3 kb in the genomic DNA from B1-234 (marked by an open arrowhead in Figure 1D). These results suggested that a region containing Y3 and Y4 was deleted in the B1-113 genome and was translocated in the B1-234 genome, and that the borders of the deletion and translocation are located within the regions covered by Y2 and Y5. To analyze the nature of the translocation further, the presence of DNA sequences within the possible deleted/translocated region in the B1-234 genome was extensively examined by PCR amplification. This analysis, together with the sequencing analysis of the amplified DNA segments from the B1-234 genome, indicated that a region of 18 kbp was translocated in B1-234 and that this region did not carry rearrangements or mutations (Y.Tsujimoto, R.Ohsawa, S.Naito and M.Ishikawa, unpublished results).
Identification of the TOM2A and TOM2B genes
The deleted region detected in the genome of B1-113 contained several putative genes. To identify which gene within this region is involved in the multiplication of tobamoviruses, we transformed tom2-1 (B1-113) plants with wild-type genomic DNA fragments indicated in Figure 2A and examined the multiplication of TMV-Cg and TMV-L in the T2 plants (for T2 plants, see Materials and methods). Among these plants, transformants with the clones 3, 280 and 472 showed a level of CP accumulation of both TMV-Cg and TMV-L similar to that in the wild-type plants (for clone 472 see Figure 2C). When protoplasts isolated from 472-transformed B1-113 plants were inoculated with TMV-Cg or TMV-L, accumulation of virus-related RNAs were similar to that in wild-type protoplasts, although the accumulation level was slightly lower than in wild-type protoplasts for TMV-L (Figure 3A and C). Transformants with clones 278, 273, 511 and 1034 showed a level of CP accumulation of both TMV-Cg and TMV-L similar to that in B1-234 (for clone 511, see Figure 2D). When protoplasts isolated from 511-transformed B1-113 plants were inoculated with TMV-L, the accumulation of viral RNAs was similar to that in wild-type and B1-234 protoplasts (Figure 3D). When protoplasts isolated from 511-transformed B1-113 plants were inoculated with TMV-Cg, the accumulation of viral RNAs was only slightly higher than that in B1-113 protoplasts and similar to that in B1-234 protoplasts (Figure 3B). Transformants with the clones 427, 413, 26, 670 and 672 showed a level of CP accumulation of both TMV-Cg and TMV-L similar to that in B1-113 (data not shown). These results suggest that two separate genes present within clones 472 and 1034 are involved in tobamovirus multiplication.

Fig. 2. Identification of TOM2A and TOM2B by functional complementation. (A) T-DNA contig covering the deletion detected in the tom2-1 mutant. The arrows above chromosome 1 show the regions determined to be deleted or translocated. Lines under the chromosome show the position of the RDA fragment and the Y2 and Y5 probes appearing in Figure 1. T-DNA clones shown as gray boxes with plus (+) signs complemented the tom2-1 mutation, while those shown as open boxes with minus (–) signs did not. (B) Position, orientation and intron–exon structures of TOM2A and TOM2B. Coding and non-coding regions in exons are shown as filled and open boxes respectively, whereas introns are marked with diagonal lines. (C and D) Complementation of the tom2-1 mutation with the T-DNA clones 472 (C) and 511 (D). Each T-DNA clone was used to genetically transform B1-113 plants. T2 plants derived from each single T1 plant were inoculated with TMV-Cg or TMV-L. At 11 (for TMV-Cg) and 21 days (for TMV-L) post-inoculation, upper uninoculated leaves of inoculated plants were harvested and CP accumulation was examined by SDS–PAGE and Coomassie Blue staining (panels marked Cg CP or L CP). The positions of TMV-Cg and TMV-L CP are indicated. A representative result from one of the T1 lines for each construct is shown in each set of panels, whereas lanes labeled i–vi represent single individual T2 plants. Genomic DNA was also prepared from each T2 plant and a transgene-specific DNA fragment was amplified by PCR, separated by agarose gel electrophoresis and stained by ethidium bromide (panels marked T-DNA). As T1 plants should carry transgenes heterozygously, transgene segregation was observed in the T2 generation. Therefore most T2 plants carry transgenes but some do not. Note that complementation occurs only if the T2 plants carry the transgenes.
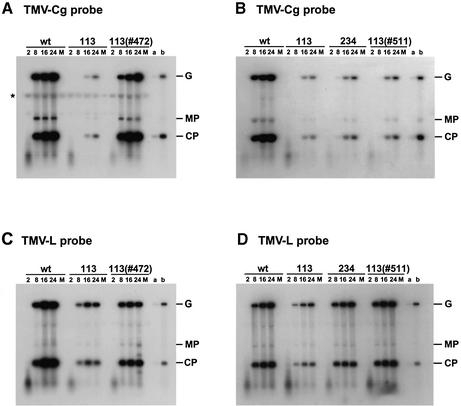
Fig. 3. Complementation of the tom2-1 mutation by T-DNA clones 472 and 511 at the protoplast level. Time courses of the accumulation of tobamovirus-related RNAs in wild type (wt), B1-113, B1-234 and B1-113-derived transformants with the T-DNA clones 472 (A and C) or 511 (B and D) are shown. Protoplasts were isolated from T3 transgenic plants that carried the respective transgene at a single locus homozygously. Protoplasts were then inoculated with TMV-Cg (A and B) or TMV-L (C and D) virion RNA by electroporation. Total RNA was extracted at 2, 8, 16 or 24 h post-inoculation (h.p.i.) and analyzed by northern blot hybridization. A 32P-labeled RNA probe synthesized by SP6 RNA polymerase from EcoRI-digested pCgP2 (Ishikawa et al., 1993) was used to detect positive-strand RNAs of TMV-Cg in (A) and (B). A 32P -labeled RNA probe synthesized by T3 RNA polymerase from HindIII-digested pT732 (Ishikawa et al., 1991) was used to detect positive-strand RNAs of TMV-L in (C) and (D). Positions of the viral genomic RNA (G) and subgenomic mRNAs for MP and CP are indicated to the right of each panel. A band representing a cross-hybridization of the TMV-Cg probe to A.thaliana RNA is indicated by an asterisk. The quality of protoplasts in each preparation was confirmed by similar levels of accumulation of TCV-related RNAs (data not shown). A representative result of three independent repeats is shown. M, mock-inoculation; a, 100-fold dilution of RNA extracted at 24 h.p.i. from wild-type protoplasts inoculated with the respective virion RNA; b, 10-fold dilution of RNA extracted at 24 h.p.i. from wild-type protoplasts inoculated with the respective virion RNA. Note that the degree of viral RNA reduction in B1-113 protoplasts is weaker than that for CP shown in Figure 2, which was measured in systemically infected leaves. This is consistent with our previous observation (Ohshima et al., 1998) and may be due to differences in host defense functions between protoplasts and intact plants.
To identify these genes, reverse-transcription PCR and 5′- and 3′-rapid amplification of cDNA ends (RACE) was carried out. These analyses identified a single transcription unit within each region. The 1293 nucleotide mRNA from the region corresponding to clone 472 consisted of six exons (Figure 2B) and contained an open reading frame (ORF) encoding a 280 amino acid polypeptide (Figure 4A). We refer to this gene as TOM2A. PCR amplification of a genomic DNA region containing the truncated point of the TOM2A gene in B1-113 and B1-234 (Siebert et al., 1995) and subsequent sequencing analysis indicated that the TOM2A gene is truncated at the position corresponding to the 141st amino acid residue in the mutants (Y.Tsujimoto, S.Naito and M.Ishikawa, unpublished results). Since a band of TOM2A mRNA could not be detected by northern blot hybridization in B1-113 and B1-234 (data not shown), it is likely that the function of the TOM2A gene is lost completely in the tom2-1 mutant lines. Although abnormal TOM2A mRNA in the mutants is likely to be synthesized, it would be degraded by mRNA surveillance systems (reviewed in Hilleren and Parker, 1999). A 488 nucleotide mRNA consisting of four exons (Figure 2B) and containing an ORF encoding a 122-amino-acid polypeptide (Figure 4C) was identified from the region corresponding to clone 1034. This gene, referred to as TOM2B, was completely deleted in B1-113, but existed intact in B1-234. The tom2-1 mutation corresponds to the simultaneous defect of TOM2A and TOM2B, whereas ttm1 is the translocated form of TOM2B.
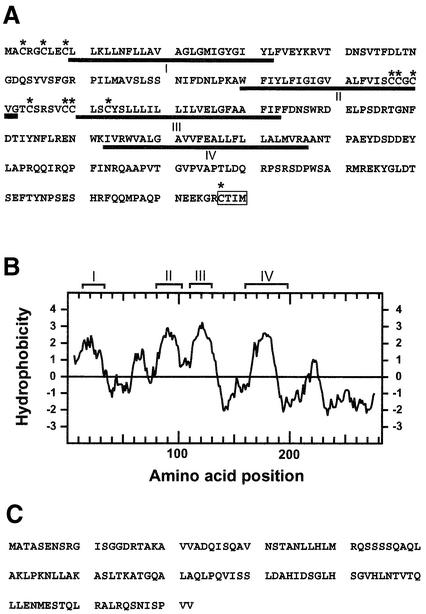
Fig. 4. Analysis of deduced amino acid sequences of TOM2A and TOM2B. (A) Deduced amino acid sequence of TOM2A. Underlined amino acid residues represent regions (numbered I–IV) predicted to be in membrane-spanning regions by the SOSUI program (Hirokawa et al.,1998). The program PSORT (Nakai and Horton, 1999) did not list region I as a transmembrame region. The putative farnesylation signal is boxed. Cysteine residues are marked with asterisks. (B) Hydropathy plot for the deduced amino acid sequence of TOM2A. The hydropathy plot was created by the method described (Kyte and Doolittle, 1982). The regions predicted to be membrane-spanning in (A) are indicated above the plot. (C) Deduced amino acid sequence of TOM2B.
The deduced amino acid sequence of TOM2A contained several hydrophobic regions (Figure 4A and B) and was predicted by the SOSUI program (Hirokawa et al., 1998) to be a four-pass transmembrane protein with both termini cytoplasmic. The Sos recruitment assay was utilized to test this possibility. This system is based on the observation that the S.cerevisiae cdc25-2 mutation is complemented by the expression of a fragment of human Sos protein (5′Sos) only if the 5′Sos is recruited to the cytoplasmic side of the plasma membrane (Aronheim et al., 1997). Confirming the prediction that both the N- and C-termini of TOM2A are cytoplasmic, we found that the S.cerevisiae cdc25-2 mutation was complemented by the expression of both N- and C-terminal fusion proteins of TOM2A with the 5′Sos polypeptide (data not shown). The C-terminus of TOM2A contained a ‘CaaX’ farnesylation signal (Clarke, 1992). TOM2A also contained multiple ‘CxxC’ sequences in regions predicted to be in or close to the cytoplasm (amino acids 3–6, 6–9, 97–100 and 110–113, Figure 4A). The CxxC motif is implicated in redox reactions (Holmgren, 1985) and the N-terminal MxCxxC sequence is also implicated in metal-binding ability (Harrison et al., 1999). Sequence comparisons carried out using the BLAST program at NCBI (Altschul et al., 1990) revealed that neither TOM2A nor TOM2B share significant homology with any genes of known function.
Possible interaction between TOM2A and TOM1
We have previously demonstrated, using the Sos recruitment assay, that TOM1 interacts with the helicase domain polypeptide of tobamovirus replication proteins (Yamanaka et al., 2000). No interaction could be detected between the TOM2A integral membrane protein and tobamovirus replication proteins in similar tests using the Sos recruitment system (M.Takahashi, T.Meshi, S.Naito and M.Ishikawa, unpublished results). Here, we examined the possibility that TOM2A functions through an interaction with TOM1 using the split-ubiquitin system recently developed by Stagljar et al. (1998) that allows interactions between two membrane proteins to be examined in yeast.
In the split-ubiquitin system, the C-terminal fragment of ubiquitin (Cub) is linked to a protein A-LexA-VP16 (PLV) reporter polypeptide and fused to the C-terminal cytoplasmic tail of one of the membrane proteins (e.g. TOM1-Cub-PLV in Figure 5D). If expressed alone, the complete fusion protein remains attached to the membranes and the PLV reporter polypeptide cannot enter the nucleus to induce LexA-VP16-dependent transcription. If the membrane protein fused to Cub-PLV interacts with another membrane protein fused to a mutant form of the ubiquitin N-terminal fragment (NubG) (e.g. NubG-TOM2A in Figure 5D), quasi-native ubiquitin is reconstituted and recognized by ubiquitin-specific proteases (UBPs). This results in the release of the PLV moiety from the membrane complex (as shown in Figure 5D), which is converted into β-galactosidase activity in the yeast strain L40 that harbors a β-galactosidase expression cassette driven by a LexA-dependent promoter. If the membrane proteins fused to Cub-PLV and NubG do not interact, the cleavage of Cub-PLV does not occur even if the fusion proteins are present on the same membrane (Stagljar et al., 1998).
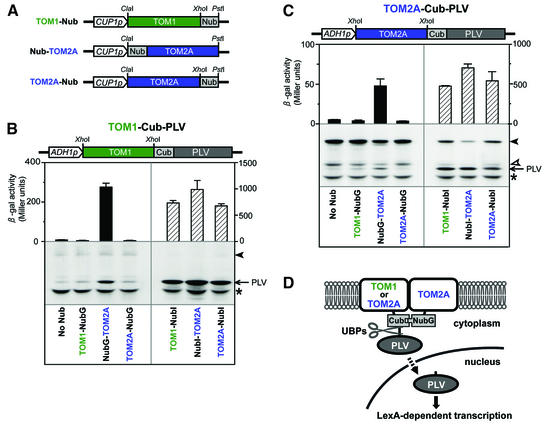
Fig. 5. Analysis of interactions between TOM1 and TOM2A using the split-ubiquitin system. (A) Schematic diagram of the Nub fusion constructs (see Materials and methods). Constructs containing the mutant form of the ubiquitin N-terminal fragment (NubG) were used to test for interactions between TOM1 and TOM2A, whereas control constructs contained the wild-type ubiquitin N-terminal fragment (NubI). (B) Split-ubiquitin assay using the TOM1-Cub-PLV construct. β-galactosidase activities (upper panel) in L40 cells expressing either the TOM1-Cub-PLV fusion protein alone or together with one of the NubI (solid bars) or NubG (hatched bars) fusion proteins described in (A) were determined as described in the text. Averages and standard deviations for three independent yeast transformants are shown. These results were confirmed in three independent experiments. Western blot analysis of the PLV moiety cleaved by UBPs is also shown (lower panel). Protein samples prepared from L40 cells expressing either the TOM1-Cub-PLV fusion protein alone or together with one of the NubI or NubG fusion proteins described in (A) were analyzed. The cleaved PLV moiety was detected using a rabbit IgG–horseradish peroxidase conjugate (indicated by an arrow). Membranes were pretreated with anti-yeast Pgk protein antibody to confirm similar amounts of protein loading (marked with an asterisk). The position of the full-length TOM1-Cub-PLV fusion protein is indicated by a filled arrowhead. A representative result from three western blotting experiments with independent sets of transformants is shown. (C) Split-ubiquitin assay using the TOM2A-Cub-PLV construct. β-galactosidase activities (upper panel) and western blot analysis of the cleaved PLV moiety (lower panel) in L40 cells expressing either the TOM2A-Cub-PLV fusion protein alone or together with one of the NubG or NubI fusion proteins are shown as in (B). The cleaved PLV moiety is indicated by an arrow, whereas the yeast Pgk protein loading control band is marked with an asterisk. Positions of the full-length TOM2A-Cub-PLV fusion protein and non-specific degradation products of TOM2A-Cub-PLV are indicated by filled and open arrowheads respectively. (D) A model explaining the results of the split-ubiquitin assay. In this model, TOM1 or TOM2A moieties in the Cub-PLV fusion proteins interact non-covalently with the TOM2A moiety of NubG-TOM2A. This interaction induces the formation of quasi-native ubiquitin, which is recognized by UBPs. The PLV moiety is then cleaved off the membrane and transported into the nucleus to activate LexA-dependent transcription, resulting in β-galactosidase production (Stagljar et al., 1998).
Because the C-terminus of TOM1 and the N- and C-termini of TOM2A were suggested to be cytoplasmic, gene cassettes were constructed to express TOM1 or TOM2A fused at their C-termini with the Cub-PLV module (TOM1-Cub-PLV and TOM2A-Cub-PLV; Figure 5B and C). TOM1 was also separately fused at the C-terminus with NubG (TOM1-NubG; Figure 5A), whereas TOM2A was separately fused at the N- or C-termini with NubG (NubG-TOM2A and TOM2A-NubG; Figure 5A). Similar control constructs were also generated using the wild-type form of the ubiquitin N-terminal fragment, NubI (referred to as TOM1-NubI, NubI-TOM2A and TOM2A-NubI respectively). Unlike NubG, NubI is able to self-associate with the Cub moiety when localized to the same membrane without requiring direct interaction between the respective membrane proteins (Stagljar et al., 1998).
One each of the above Cub-PLV and NubG or NubI constructs were introduced into the yeast L40 strain in all possible combinations. High β-galactosidase activity was observed in all the control experiments containing the NubI constructs (Figure 5B and C), suggesting that these TOM2A and TOM1 fusion proteins were localized on the same membrane and that the Cub-PLV and Nub moieties of the fusion proteins were at least partly exposed to the cytoplasm. Among the combinations for the NubG constructs, L40 co-expressing NubG-TOM2A and either TOM1-Cub-PLV (Figure 5B) or TOM2A-Cub-PLV (Figure 5C) showed β-galactosidase activity significantly higher than that in L40 expressing the respective Cub-PLV fusion protein alone. In contrast, L40 cells co-expressing other combinations of Cub-PLV and NubG constructs showed similar levels of β-galactosidase activity to those in L40 harboring the corresponding Cub-PLV construct alone (Figure 5B and C). Western blot analysis also showed that the accumulation level of cleaved PLV was higher in yeast co-expressing NubG-TOM2A and either TOM1-Cub-PLV (Figure 5B) or TOM2A-Cub-PLV (Figure 5C) than that in L40 expressing the respective Cub-PLV fusion protein alone, consistent with the respective β-galactosidase activities. Taken together, these results suggest that the TOM2A integral membrane protein can interact both with itself and with TOM1 (Figure 5D).
Discussion
In this study, we have characterized a fast-neutron-induced A.thaliana mutant in which intracellular multiplication of tobamoviruses is affected. The mutation was shown to involve an ∼20 kbp deletion in chromosome 1 and translocation of an ∼18 kbp segment of the deleted region to a locus genetically dissectable from the original locus. Sequence analysis of the break points suggested that the alterations also involved sequences from chromosome 4 and were more complex than a simple cut and ligation (Y.Tsujimoto, R.Ohsawa, S.Naito and M.Ishikawa, unpublished results). Ionizing radiation is known to cause chromosomal deletions or complex inter- or intra-chromosomal rearrangements (Shirley et al., 1992; Sun et al., 1992; Nambara et al., 1994; Bruggemann et al., 1996), and our results represent a typical example of such mutations.
Previous genetic analyses suggested that, in addition to the recessive tom2-1 mutation that reduces the multiplication levels of tobamoviruses, the tom2-1 mutant also contains a tobamovirus-strain-specific dominant genetic element, ttm1, which increases the multiplication levels of tobamoviruses in the tom2-1 genetic background (Ohshima et al., 1998). Based on the complementation analysis, we identified two genes referred to as TOM2A and TOM2B that are involved in the intracellular multiplication of tobamoviruses. In B1-113, the tom2-1 mutant that does not carry ttm1, both TOM2A and TOM2B were inactivated. On the other hand, in B1-234, the tom2-1 mutant that carries ttm1, TOM2A was inactivated but the TOM2B gene remained intact. Thus the nature of tom2-1 was revealed as the simultaneous deletion of the TOM2A and TOM2B genes, while that of the ttm1 mutation was revealed as the translocation of the TOM2B gene.
Introduction of either TOM2A or TOM2B into the tom2-1 mutant (B1-113) resulted in increased accumulation of TMV-Cg and TMV-L (compare B1-113 with B1-113::472 or B1-113::511; see Figures 2C, D and 3), demonstrating that TOM2A and TOM2B both play positive roles in the multiplication of TMV-Cg and TMV-L although the contribution of TOM2B to TMV-Cg multiplication may be small (compare B1-113 with B1-234 or B1-113::511; see Figures 2D and 3B). On the other hand, inactivation of TOM2A alone did not affect the multiplication of TMV-L (compare wild type with B1-234 or B1-113::511; see Figures 2D and 3D), whereas inactivation of TOM2B alone did not drastically affect the multiplication of either TMV-Cg or TMV-L (compare wild type with B1-113::472; see Figures 2C, 3A and C). In a similar example, we have previously found that tobamovirus multiplication is not affected by inactivation of the TOM3 gene, which functions in parallel with TOM1 (Yamanaka et al., 2002). In the tom2a (for TMV-L only), tom2b and tom3 single mutants, reduced efficiency of each process in tobamovirus multiplication would still be higher than the minimal efficiency required for wild-type levels of overall multiplication. In the case of TMV-Cg, loss of TOM2A alone reduced the efficiency of the relevant multiplication process to a level below the minimum required for this strain. TMV-Cg and TMV-L are distantly related tobamoviruses that are distinct at the sequence level (Yamanaka et al., 1998). The fact that these strains respond differently to the tom2a and tom2b mutations demonstrates that, although they share similar host factors, the relative contribution of specific host factors to TMV-Cg and TMV-L multiplication is distinct.
Inactivation of neither TOM2A nor TOM2B resulted in a complete inhibition of tobamovirus multiplication (B1-234, B1-113::472, B1-113::511; see Figures 2C, D and 3), indicating that they function mainly to enhance the efficiency of tobamovirus multiplication. Alternatively, the functions of TOM2A and TOM2B could indeed be essential for tobamovirus multiplication, and a set of homologs may exist in A.thaliana to reserve the multiplication of tobamoviruses in the mutants. In fact, for TOM2A, we have found several nucleotide sequences potentially encoding homologous proteins in the A.thaliana genome database (Y.Tsujimoto, S.Naito and M.Ishikawa, unpublished results).
The replication complexes of eukaryotic positive-strand RNA viruses are formed on the cytoplasmic surface of membranes (reviewed in Buck, 1996). In the case of tobamoviruses, it is suggested that a host integral membrane protein TOM1 interacts with the helicase domain polypeptide of the tobamovirus-encoded replication proteins (Yamanaka et al., 2000), thus playing an important role in membrane association of the tobamovirus replication complex. In this article, we have shown that TOM2A is able to interact with TOM1, suggesting that TOM2A is also a constituent of the tobamovirus replication complex along with TOM1 and tobamovirus-encoded 130K and 180K replication proteins. Therefore TOM2A may facilitate the binding of the tobamovirus replication proteins to TOM1 on membranes, or may be important for the formation or maintenance of the replication complex. Alternatively, TOM2A may function to recruit other host factors necessary for tobamovirus replication.
The replication complex of brome mosaic virus (BMV) is a spherule budding into the endoplasmic reticulum that contains positive- and negative-strand RNAs and multiple molecules of BMV replication proteins 1a and 2a (Schwartz et al., 2002). A wide variety of positive-strand RNA viruses form replication complexes with similar morphology to that of BMV (Schwartz et al., 2002, and references cited therein), suggesting that these viruses form replication complexes with similar molecular architecture. The BMV 1a protein contains methyltransferase and helicase domains and is related to the 130K protein of tobamoviruses. Protein 1a interacts with itself (O’Reilly et al., 1998) and is the only viral protein essential for the formation of the spherule (Schwartz et al., 2002). For tobamoviruses, the helicase domain polypeptide of the 130K and 180K proteins interacts with the intervening region between the methyltransferase and helicase domains of the same protein, potentially enabling 130K and 180K proteins to form homo- or heteromultimers. This interaction has also been shown to be important for virus multiplication (Goregaoker et al., 2001). Therefore, although a spherule-like replication complex has not been reported for tobamoviruses, it is possible that the tobamovirus replication complex contains multimeric 130K and 180K proteins. Formation of such a tobamovirus replication complex containing multimeric 130K and 180K proteins on membranes may require the TOM1 protein, which tethers the 130K and 180K proteins to membranes, to be present locally at high concentrations in order to bind the multiple 130K/180K proteins. We have shown here that TOM2A interacts both with TOM1 and with itself in yeast. If it is assumed that these interactions also occur in plant cells and that the TOM2A–TOM2A interaction does not inhibit the interactions with additional TOM2A or with TOM1, TOM2A and TOM1 would plausibly form a multimer on membranes. It is interesting to speculate that TOM2A functions to facilitate tobamovirus multiplication through the formation of TOM1–TOM2A multimers on membranes that are necessary or advantageous for the formation of the tobamovirus replication complex.
Materials and methods
Viruses and plants
Arabidopsis thaliana (L.) Heynh. ecotype Columbia carrying a gl1 mutation was used as the wild-type strain. The mutant YS241 was described previously (Ohshima et al., 1998). TMV-Cg, a crucifer strain of tobamovirus (Yamanaka et al., 1998), TMV-L, a tomato mosaic tobamovirus (Ohno et al., 1984), and TCV strain B, a member of the carmo-like virus supergroup (Heaton et al., 1989), were used for inoculation. The conditions of plant growth and purification of virus particles and virion RNAs were as described previously (Ishikawa et al., 1991, 1993).
Genetic mapping of the tom2-1 mutation
We have previously found that the tom2-1 mutation is tightly linked to the RFLP marker m253 on chromosome 1 (Ohshima et al., 1998). Therefore a YAC contig encompassing m253 was constructed (Figure 1) and further RFLP mapping was performed using terminal regions of the insert DNA harbored by the YACs. YAC end probes were prepared by the thermal asymmetric interlaced PCR method (Liu and Whittier, 1995), and Southern hybridization was performed by using the Gene Images labeling and detection system (Amersham Pharmacia).
Representational difference analysis
RDA was carried out essentially as described previously (Lisitsyn et al., 1993) using a GeneFisher PCR Subtraction System (Takara Syuzo, Tokyo, Japan). HindIII-digested genomic DNA from wild-type plants was used as a tester and HindIII-digested genomic DNA from B1-113 plants as a driver. Because the kit was originally constructed for the analysis of mammalian genomic DNA, the concentration of tester DNA in subtraction hybridization was reduced six-fold in view of the genome size of A.thaliana.
Complementation analysis of the tom2-1 mutation
A contig was constructed using a P1 library (Liu et al., 1995) covering a region of chromosome 1 between the DNA markers CIC10D4R and yUP19F5R (Figure 1). One of the clones belonging to the P1 contig, N20L, hybridized with the RDA fragment and was subcloned in pCLD04541 (Flanders et al., 1998) to generate the T-DNA clones shown in Figure 2A. Details of subcloning will be provided upon request. The T-DNA clones were electroporated into Agrobacterium tumefaciens C58C1 (pGV2260 or pMP90), which was then used to transform A.thaliana plants by the vacuum infiltration (Bechtold and Pelletier, 1998) or floral dip (Clough and Bent, 1998) methods. Seeds harvested from the plants treated with A.tumefaciens were sown on agar plates containing 50 µg/ml kanamycin, and kanamycin-resistant transformants (T1 plants) were selected. T1 plants were allowed to self-pollinate to obtain T2 seeds. For each T-DNA construct, six independent T1 plants were isolated and 10 T2 plants from each T1 line were analyzed. The tobamovirus multiplication phenotype was determined by inoculating the T2 plants with TMV-Cg or TMV-L and examining the respective CP accumulation in each plant by SDS–PAGE and Coomassie Blue staining (Ishikawa et al., 1991). Genomic DNA was also extracted from representative T2 plants by the cetyltrimethylammonium bromide method and the presence of transgenes was confirmed by PCR (Yamanaka et al., 2000). Preparation of protoplasts from homozygous T3 transgenic plants, inoculation of protoplasts with viral RNAs by electroporation and subsequent RNA analysis were carried out as described previously (Ishikawa et al., 1993).
Isolation of TOM2A and TOM2B cDNAs and sequence analysis
Total RNA was extracted from frozen plant tissues by using ISOGEN LS (Nippon Gene, Toyama, Japan) according to the manufacturer’s instructions. The full-length cDNA sequences of TOM2A and TOM2B (DDBJ/EMBL/GenBank accession Nos. AB085684 and AB085685, respectively) were identified by 5′-and 3′-RACE using a SMART PCR cDNA Synthesis kit (CLONTECH) and by reverse-transcription PCR using a One Step PCR kit (Takara Syuzo, Tokyo, Japan). A 377 DNA sequencer (Applied Biosystems) and BigDye Terminator Sequencing kit (Perkin–Elmer) were used for sequencing.
The split-ubiquitin system
Plasmids pRS305(Δwbp1-Cub-PLV), pOST1-NubG, pOST1-NubI, pNubG-ALG5 and pNubI-ALG5 and the yeast strain L40 have been described by Stagljar et al. (1998). pTN-TOM1-Cub-PLV and pTN-TOM2A-Cub-PLV were constructed by replacing the XhoI fragment of pRS305(Δwbp1-Cub-PLV) containing the Δwbp1 region with PCR-amplified DNA fragments containing the TOM1 and TOM2A ORFs, respectively, under the control of the ADH1 promoter (Figure 5B and C). These constructs contained an 11 amino acid linker (LESGGSTLSGG) between each TOM and the Cub ORFs. Nub-TOM2A-encoding DNA fragments (Nub fused to the N-terminus) were obtained by the overlap-extension PCR method (Ho et al., 1989) using TOM2A cDNA and pNubG-ALG5 or pNubI-ALG5 as templates. These sequences contained a four-amino-acid linker (GGST) between the Nub and the TOM2A ORFs. pTN-NubG-TOM2A and pTN-NubI-TOM2A were then constructed by replacing the ClaI-PstI fragment of pNubI-ALG5 (containing the NubI-ALG5 region) with the fragment encoding the NubI-TOM2A and NubG-TOM2A fusion proteins respectively (Figure 5A). To obtain the TOM-Nub constructs (Nub fused to the C-terminus), the ClaI–PstI digested pNubI-ALG5 vector (NubI-ALG5 removed) was ligated with a PCR-amplified and ClaI–XhoI-digested DNA fragment containing either TOM1 or TOM2A ORFs and a XhoI–PstI fragment containing either NubG (from pOST1-NubG) or NubI ORFs (from pOST1-NubI). The resulting plasmids pTN-TOM1-NubG and pTN-TOM1-NubI encoded the TOM1-NubG and TOM1-NubI fusion proteins, respectively, whereas pTN-TOM2A-NubG and pTN-TOM2A-NubI encoded the TOM2A-NubG and TOM2A-NubI fusion proteins, respectively (Figure 5A). These constructs contained a seven-amino-acid linker (LESGGST) between the TOM and the Nub ORFs. All Nub fusion protein genes were driven by the CUP1 promoter. L40 yeast was first transformed with Cub-PLV plasmids (pTN-TOM1-Cub-PLV or pTN-TOM2A-Cub-PLV) linearized with ClaI to integrate into the leu2 locus and transformants with a single-copy integration were selected for further transformation with Nub 2µ plasmids. Following the second transformation, yeast cells were grown at 30°C to OD600 of 0.5 to 1.0 in a defined synthetic glucose medium (Burke et al., 2000) lacking appropriate amino acids in order to maintain the plasmids. Cells were harvested from 0.5 ml culture, washed, suspended in 100 µl of Z-buffer (60 mM NaH2PO4, 40 mM NaH2PO4, 1 mM MgSO4, 10 mM KCl pH 7.0) and lysed by three freeze–thaw cycles in liquid nitrogen. The lysate was then mixed with 700 µl of Z-buffer containing 0.27% 2-mercaptoethanol and 160 µl of 4 mg/ml o-nitrophenyl β-d-galactopyranoside in Z-buffer, and incubated for 1–20 h at 30°C. The reaction was stopped by adding 400 µl of 1 M Na2CO3, and OD420 was measured after removal of cell debris by centrifugation. β-galactosidase activity was calculated as described previously (Miller, 1972). Protein samples for western blot analysis were prepared from yeast essentially as described previously (Horvath and Riezman, 1994). Briefly, one OD600 unit of cells were suspended in 150 µl of 1.85 M NaOH and incubated on ice for 10 min. The same volume of 50% trichloroacetic acid was added and incubated on ice for 10 min. Protein was precipitated by centrifugation, suspended in 50 µl of SDS–PAGE sample buffer containing 8 M urea and 20 µl of 1 M Tris–HCl pH 8.0, and dissolved by incubation at 37°C for 1.5 h. Samples were centrifuged for 2 min and 0.5 µl of supernatant was used for 11% SDS–PAGE followed by western blot analysis. Membranes were probed with anti-yeast Pgk-mouse IgG as the primary antibody, followed by the detection of signals using anti-rat IgG–rabbit IgG–horseradish peroxidase conjugate and the ECL system (Amersham Pharmacia Biotech).
Acknowledgments
Acknowledgements
We thank Daisuke Shibata for the A.thaliana genomic P1 library, Igor Stagljar for the split-ubiquitin system and Tetsuo Meshi for critical reading of the manuscript. We used the facilities of the Biopolymer Analysis Laboratory in the Faculty of Agriculture, Hokkaido University, and the Research Center for Molecular Genetics at Hokkaido University. This work was supported in part by grants from the Japan Society for the Promotion of Science to M.I.
References
- Altschul S.F., Gish,W., Miller,W., Myers,E.W. and Lipman,D.J. (1990) Basic local alignment search tool. J. Mol. Biol., 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Aronheim A., Zandi,E., Hennemann,H., Elledge,S.J. and Karin,M. (1997) Isolation of an AP-1 repressor by a novel method for detecting protein–protein interactions. Mol. Cell. Biol., 17, 3094–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N. and Pelletier,G. (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol., 82, 259–266. [DOI] [PubMed] [Google Scholar]
- Bruggemann E., Handwerger,K., Essex,C. and Storz,G. (1996) Analysis of fast neutron-generated mutants at the Arabidopsis thaliana HY4 locus. Plant J. 10, 755–760. [DOI] [PubMed] [Google Scholar]
- Buck K.W. (1996) Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 47, 159–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck K.W. (1999) Replication of tobacco mosaic virus RNA. Philos. Trans. R. Soc. Lond. B Biol. Sci., 354, 613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Dawson,D. and Stearns,T. (2000) Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual (2000 Edition). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Clarke S. (1992) Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu. Rev. Biochem., 61, 355–386. [DOI] [PubMed] [Google Scholar]
- Clough S.J. and Bent,A.F. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J., 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dawson W.O. (1992) Tobamovirus-plant interactions. Virology, 186, 359–367. [DOI] [PubMed] [Google Scholar]
- Diez J., Ishikawa,M., Kaido,M. and Ahlquist,P. (2000) Identification and characterization of a host protein required for efficient template selection in viral RNA replication. Proc. Natl Acad. Sci. USA, 97, 3913–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders D.J., Weng,S., Petel,F.X. and Cherry,J.M. (1998) AtDB, the Arabidopsis thaliana database, and graphical-web-display of progress by the Arabidopsis genome initiative. Nucleic Acids Res., 26, 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goregaoker S.P., Lewandowski,D.J. and Culver,J.N. (2001) Identification and functional analysis of an interaction between domains of the 126/183-kDa replicase-associated proteins of tobacco mosaic virus. Virology, 282, 320–328. [DOI] [PubMed] [Google Scholar]
- Harrison M.D., Jones,C.E. and Dameron,C.T. (1999) Copper chaperones: function, structure and copper-binding properties. J. Biol. Inorg. Chem., 4, 145–153. [DOI] [PubMed] [Google Scholar]
- Heaton L.A., Carrington,J.C. and Morris,T.J. (1989) Turnip crinkle virus infection from RNA synthesized in vitro. Virology, 170, 214–218. [DOI] [PubMed] [Google Scholar]
- Hilleren P. and Parker,R. (1999) Mechanisms of mRNA surveillance in eukaryotes. Annu. Rev. Genet., 33, 229–260. [DOI] [PubMed] [Google Scholar]
- Hirokawa T., Boon-Chieng,S. and Mitaku,S. (1998) SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics, 14, 378–379. [DOI] [PubMed] [Google Scholar]
- Ho S.N., Hunt,H.D., Horton,R.M., Pullen,J.K. and Pease,L.R. (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene, 77, 51–59. [DOI] [PubMed] [Google Scholar]
- Holmgren A. (1985) Thioredoxin. Annu. Rev. Biochem., 54, 237–271. [DOI] [PubMed] [Google Scholar]
- Horvath A. and Riezman,H. (1994) Rapid protein extraction from Saccharomyces cerevisiae. Yeast, 10, 1305–1310. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Obata,F., Kumagai,T. and Ohno,T. (1991) Isolation of mutants of Arabidopsis thaliana in which accumulation of tobacco mosaic virus coat protein is reduced to low levels. Mol. Gen. Genet., 230, 33–38. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Naito,S. and Ohno,T. (1993) Effects of the tom1 mutation of Arabidopsis thaliana on the multiplication of tobacco mosaic virus RNA in protoplasts. J. Virol., 67, 5328–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J. and Doolittle,R.F. (1982) A simple method for displaying the hydropathic character of a protein. J. Mol. Biol., 157, 105–132. [DOI] [PubMed] [Google Scholar]
- Lai M.M.C. (1998) Cellular factors in the transcription and replication of viral RNA genomes: a parallel to DNA-dependent RNA transcription. Virology, 244, 1–12. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N., Lisitsyn,N. and Wigler,M. (1993) Cloning the differences between two complex genomes. Science, 259, 946–951. [DOI] [PubMed] [Google Scholar]
- Liu Y.G. and Whittier,R.F. (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics, 25, 674–681. [DOI] [PubMed] [Google Scholar]
- Liu Y.G., Mitsukawa,N., Vazquez-Tello,A. and Whittier,R.F. (1995) Generation of a high-quality P1 library of Arabidopsis suitable for chromosome walking. Plant J., 7, 351–358. [Google Scholar]
- Miller J.H. (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Nakai K. and Horton,P. (1999) PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci., 24, 34–35. [DOI] [PubMed] [Google Scholar]
- Nambara E., Keith,K., McCourt,P. and Naito,S. (1994) Isolation of an internal deletion mutant of the Arabidopsis thaliana ABI3 gene. Plant Cell Physiol., 35, 509–513. [PubMed] [Google Scholar]
- Ohno T., Aoyagi,M., Yamanashi,Y., Saito,H., Ikawa,S., Meshi,T. and Okada,Y. (1984) Nucleotide sequence of the tobacco mosaic virus (tomato strain) genome and comparison with the common strain genome. J. Biochem., 96, 1915–1923. [DOI] [PubMed] [Google Scholar]
- Ohshima K., Taniyama,T., Ishikawa,M. and Naito,S. (1998) Isolation of a mutant of Arabidopsis thaliana carrying two simultaneous mutations affecting tobacco mosaic virus multiplication within a single cell. Virology, 243, 472–481. [DOI] [PubMed] [Google Scholar]
- O’Reilly E.K., Wang,Z., French,R. and Kao,C.C. (1998) Interactions between the structural domains of the RNA replication proteins of plant-infecting RNA viruses. J. Virol., 72, 7160–7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman T.A.M. and Buck,K.W. (1996) Complete replication in vitro of tobacco mosaic virus RNA by a template-dependent, membrane-bound RNA polymerase. J. Virol., 70, 6227–6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman T.A.M. and Buck,K.W. (1997) The tobacco mosaic virus RNA polymerase complex contains a plant protein related to the RNA-binding subunit of yeast eIF-3. J. Virol., 71, 6075–6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Chan,J., Janda,M., Sullivan,M., den Boon,J. and Ahlquist,P. (2002) A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell, 9, 505–514. [DOI] [PubMed] [Google Scholar]
- Shirley B.W., Hanley,S. and Goodman,H.M. (1992) Effects of ionizing radiation on a plant genome: analysis of two Arabidopsis transparent testa mutations. Plant Cell, 4, 333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert P.D., Chenchik,A., Kellogg,D.E., Lukyanov,K.A. and Lukyanov,S.A. (1995) An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res., 23, 1087–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagljar I., Korostensky,C., Johnsson,N. and te Heesen,S. (1998) A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc. Natl Acad. Sci. USA, 95, 5187–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T.-P., Goodman,H.M. and Ausubel,F.M. (1992) Cloning the Arabidopsis GA1 locus by genomic subtraction. Plant Cell, 4, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Honda,A., Iwata,A., Ueda,S., Hibi,T. and Ishihama,A. (1999) Isolation from tobacco mosaic virus-infected tobacco of a solubilized template-specific RNA-dependent RNA polymerase containing a 126K/183K protein heterodimer. J. Virol., 73, 2633–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.M.A. and Davis,J.W. (1992) Genetic Engineering with Plant Viruses. CRC Press, Boca Raton, FL, pp. 149–186.
- Yamanaka T., Komatani,H., Meshi,T., Naito,S., Ishikawa,M. and Ohno,T. (1998) Complete nucleotide sequence of the genomic RNA of tobacco mosaic virus strain Cg. Virus Genes, 16, 173–176. [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Ohta,T., Takahashi,M., Meshi,T., Schmidt,R., Dean,C., Naito,S. and Ishikawa,M. (2000) TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein. Proc. Natl Acad. Sci. USA, 97, 10107–10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T., et al. (2002) Complete inhibition of tobamovirus multiplication by simultaneous mutations in two homologous host genes. J. Virol., 76, 2491–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]