Abstract
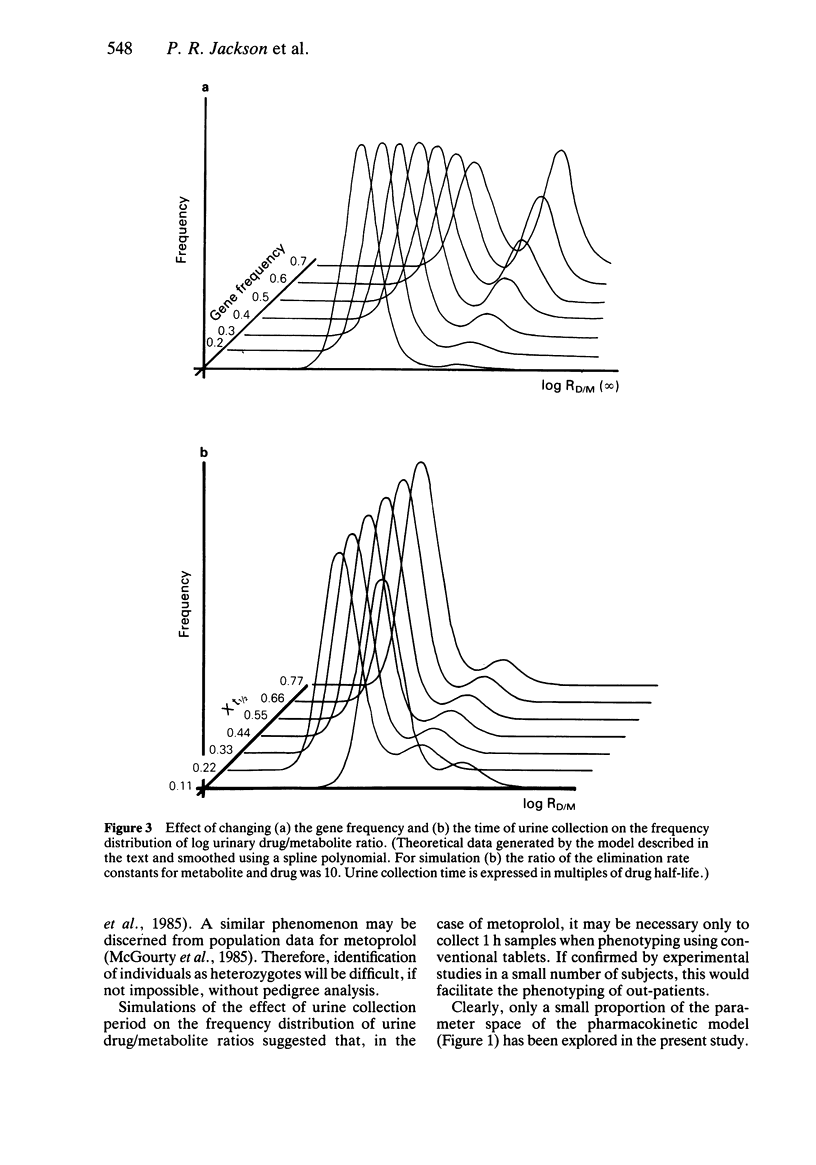
The pharmacokinetic basis for using various experimental indices, (urinary drug: metabolite and metabolite:drug + metabolite ratios, urinary metabolite recovery and AUC values), for detecting polymorphic oxidative drug metabolism was examined. Pharmacokinetic determinants in addition to partial metabolic clearance down the polymorphic route were identified in each index. The ability of the various indices to discriminate bimodality in population data was assessed using a computer simulation. With the exception of the AUC data, bimodality was apparent to varying extents in all of the frequency distributions and, in general, logarithmic transformation allowed clearer visualisation of the two phenotypic groups. Simulated distributions were compared with those observed experimentally for metoprolol and its alpha-hydroxy metabolite. Detailed pharmacokinetic data from controlled studies in small numbers of volunteers can form the basis of the input to the simulation programme. Inspection of the output may help in the design of further studies in larger numbers of subjects in whom only limited data collection is possible.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelo M., Dring L. G., Lancaster R., Latham A., Smith R. L. Proceedings: A correlation between the response to debrisoquine and the amount of unchanged drug excreted in the urine. Br J Pharmacol. 1975 Oct;55(2):264P–264P. [PMC free article] [PubMed] [Google Scholar]
- Cooper R. G., Evans D. A., Whibley E. J. Polymorphic hydroxylation of perhexiline maleate in man. J Med Genet. 1984 Feb;21(1):27–33. doi: 10.1136/jmg.21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M. Defective oxidation of drugs: pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 1982 Jan-Feb;7(1):1–22. doi: 10.2165/00003088-198207010-00001. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Mahgoub A., Sloan T. P., Idle J. R., Smith R. L. A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet. 1980 Apr;17(2):102–105. doi: 10.1136/jmg.17.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T., Otton S. V., Kalow W. Debrisoquine hydroxylation capacity: problems of assessment in two populations. Clin Pharmacol Ther. 1981 Feb;29(2):218–223. doi: 10.1038/clpt.1981.35. [DOI] [PubMed] [Google Scholar]
- Jack D. B., Wilkins M., Quarterman C. P. Lack of evidence for polymorphism in metoprolol metabolism. Br J Clin Pharmacol. 1983 Aug;16(2):188–190. doi: 10.1111/j.1365-2125.1983.tb04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K. A., Appelgren C., Borg K. O., Elofsson R. Binding of two adrenergic beta-receptor antagonists, alprenolol and H 93-26, to human serum proteins. Acta Pharm Suec. 1974 Sep;11(4):333–346. [PubMed] [Google Scholar]
- Lennard M. S., Silas J. H., Freestone S., Ramsay L. E., Tucker G. T., Woods H. F. Oxidation phenotype--a major determinant of metoprolol metabolism and response. N Engl J Med. 1982 Dec 16;307(25):1558–1560. doi: 10.1056/NEJM198212163072505. [DOI] [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- McGourty J. C., Silas J. H., Lennard M. S., Tucker G. T., Woods H. F. Metoprolol metabolism and debrisoquine oxidation polymorphism--population and family studies. Br J Clin Pharmacol. 1985 Dec;20(6):555–566. doi: 10.1111/j.1365-2125.1985.tb05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regårdh C. G., Borg K. O., Johansson R., Johnsson G., Palmer L. Pharmacokinetic studies on the selective beta1-receptor antagonist metoprolol in man. J Pharmacokinet Biopharm. 1974 Aug;2(4):347–364. doi: 10.1007/BF01061407. [DOI] [PubMed] [Google Scholar]
- Regårdh C. G., Johnsson G. Interindividual variations in metoprolol metabolism--some clinical and other observations. Br J Clin Pharmacol. 1984 Apr;17(4):495–496. doi: 10.1111/j.1365-2125.1984.tb02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silas J. H., Lennard M. S., Tucker G. T., Smith A. J., Malcolm S. L., Marten T. R. Why hypertensive patients vary in their response to oral debrisoquine. Br Med J. 1977 Feb 12;1(6058):422–425. doi: 10.1136/bmj.1.6058.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan T. P., Lancaster R., Shah R. R., Idle J. R., Smith R. L. Genetically determined oxidation capacity and the disposition of debrisoquine. Br J Clin Pharmacol. 1983 Apr;15(4):443–450. doi: 10.1111/j.1365-2125.1983.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner E., Iselius L., Alván G., Lindsten J., Sjöqvist F. A family study of genetic and environmental factors determining polymorphic hydroxylation of debrisoquin. Clin Pharmacol Ther. 1985 Oct;38(4):394–401. doi: 10.1038/clpt.1985.193. [DOI] [PubMed] [Google Scholar]
- Tucker G. T., Silas J. H., Iyun A. O., Lennard M. S., Smith A. J. Polymorphic hydroxylation of debrisoquine. Lancet. 1977 Oct 1;2(8040):718–718. doi: 10.1016/s0140-6736(77)90527-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. R., Shand D. G. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975 Oct;18(4):377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]


