Abstract
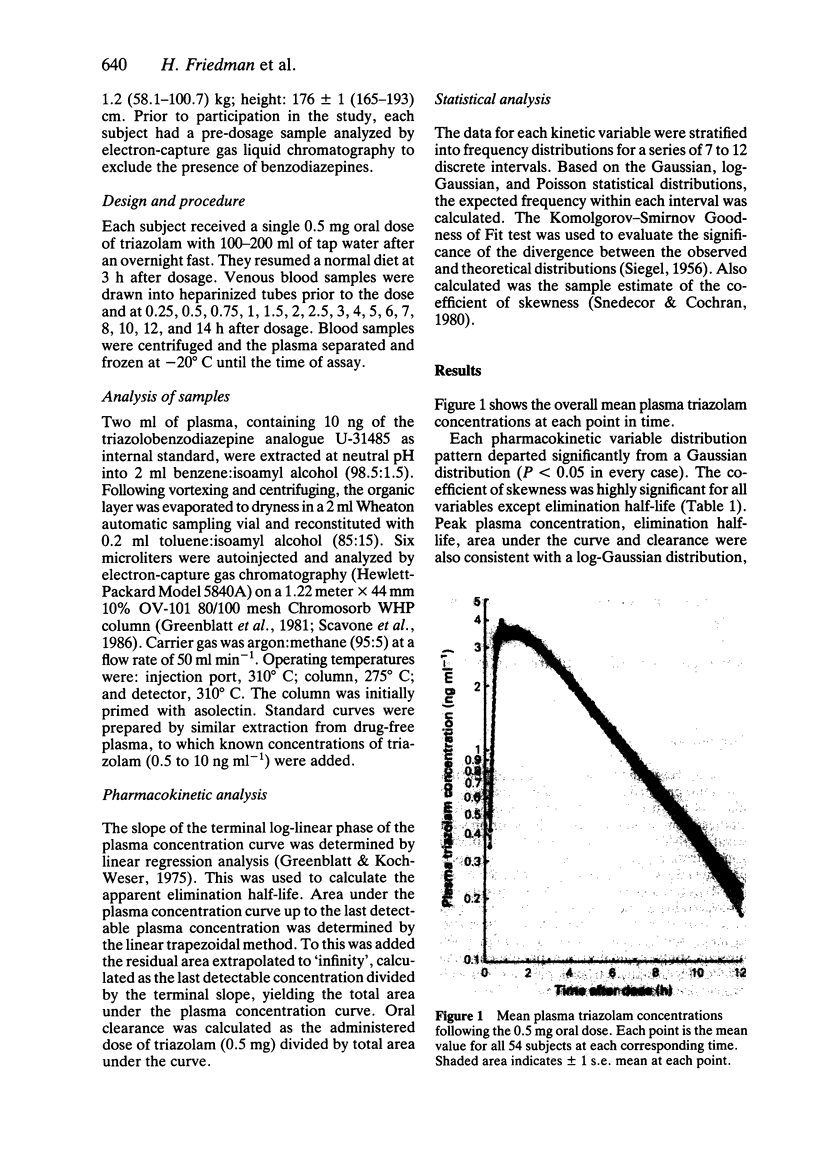
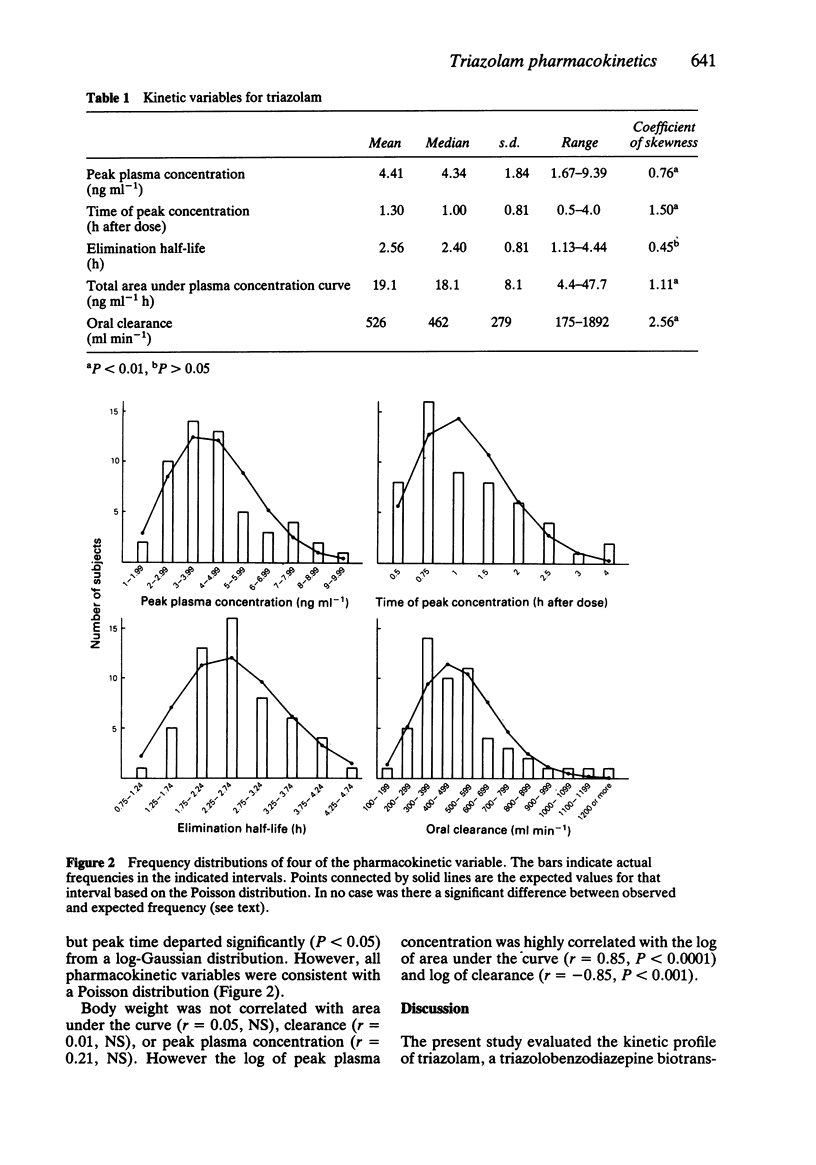
The kinetics of a single 0.5 mg oral dose of the triazolobenzodiazepine hypnotic triazolam, were studied in 54 healthy young men aged 20-44 years, with a mean body weight of 77 kg. Triazolam kinetics were determined from multiple plasma concentrations measured during 14 h post-dose. The overall mean +/- s.e. mean (with range) kinetic variables were: peak plasma concentration, 4.4 +/- 0.3 (1.7-9.4) ng ml-1; time of peak, 1.3 +/- 0.1 (0.5-4.0) h after dose; elimination half-life, 2.6 +/- 0.1 (1.1-4.4) h; total AUC: 19.1 +/- 1.1 (4.4-47.7) ng ml-1 h; oral clearance, 526 +/- 38 (175-1892) ml min-1. All kinetic variables were consistent with Poisson distributions, based on the Kolmogorov-Smirnov Goodness of Fit test. None of the variables fit normal distributions. Four of five were consistent with a log normal distribution. Peak plasma level was highly correlated with clearance (r = -0.85, P less than 0.0001), and AUC (r = 0.85, P less than 0.0001) but not with body weight (r = 0.21, NS). Clearance and body weight were not correlated (r = -0.01). Triazolam clearance may vary widely even within a homogeneous group of healthy young men.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernethy D. R., Greenblatt D. J., Divoll M., Smith R. B., Shader R. I. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984 Mar-Apr;9(2):177–183. doi: 10.2165/00003088-198409020-00005. [DOI] [PubMed] [Google Scholar]
- Beal S. L., Sheiner L. B. Estimating population kinetics. Crit Rev Biomed Eng. 1982;8(3):195–222. [PubMed] [Google Scholar]
- Eberts F. S., Jr, Philopoulos Y., Reineke L. M., Vliek R. W. Triazolam disposition. Clin Pharmacol Ther. 1981 Jan;29(1):81–93. doi: 10.1038/clpt.1981.14. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Divoll M., Abernethy D. R., Moschitto L. J., Smith R. B., Shader R. I. Reduced clearance of triazolam in old age: relation to antipyrine oxidizing capacity. Br J Clin Pharmacol. 1983 Mar;15(3):303–309. doi: 10.1111/j.1365-2125.1983.tb01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Divoll M., Moschitto L. J., Shader R. I. Electron-capture gas chromatographic analysis of the triazolobenzodiazepines alprazolam and triazolam. J Chromatogr. 1981 Sep 11;225(1):202–207. doi: 10.1016/s0378-4347(00)80261-3. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Koch-Weser J. Clinical pharmacokinetics (second of two parts). N Engl J Med. 1975 Nov 6;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- Roth T., Roehrs T. A., Zorick F. J. Pharmacology and hypnotic efficacy of triazolam. Pharmacotherapy. 1983 May-Jun;3(3):137–148. doi: 10.1002/j.1875-9114.1983.tb03237.x. [DOI] [PubMed] [Google Scholar]
- Scavone J. M., Greenblatt D. J., Friedman H., Shader R. I. Enhanced bioavailability of triazolam following sublingual versus oral administration. J Clin Pharmacol. 1986 Mar;26(3):208–210. doi: 10.1002/j.1552-4604.1986.tb02935.x. [DOI] [PubMed] [Google Scholar]
- Sheiner L. B., Beal S., Rosenberg B., Marathe V. V. Forecasting individual pharmacokinetics. Clin Pharmacol Ther. 1979 Sep;26(3):294–305. doi: 10.1002/cpt1979263294. [DOI] [PubMed] [Google Scholar]
- Smith R. B., Divoll M., Gillespie W. R., Greenblatt D. J. Effect of subject age and gender on the pharmacokinetics of oral triazolam and temazepam. J Clin Psychopharmacol. 1983 Jun;3(3):172–176. [PubMed] [Google Scholar]


