Abstract
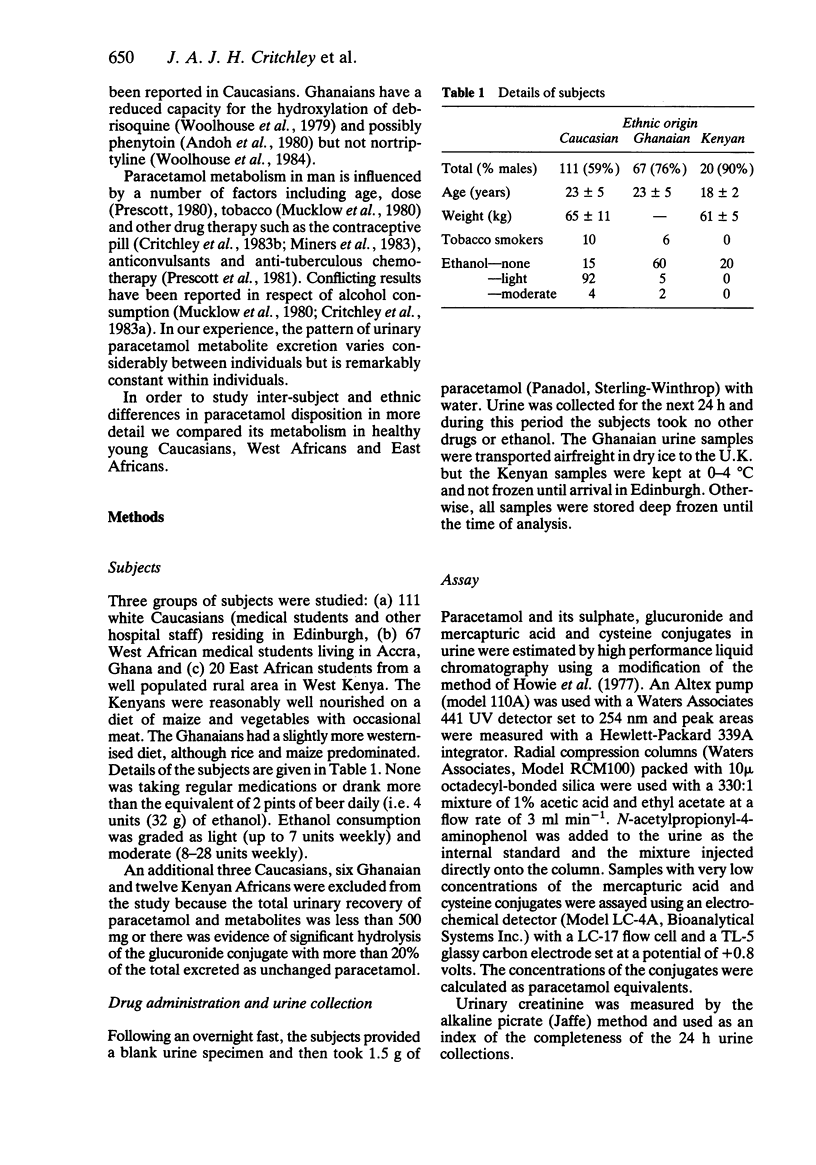
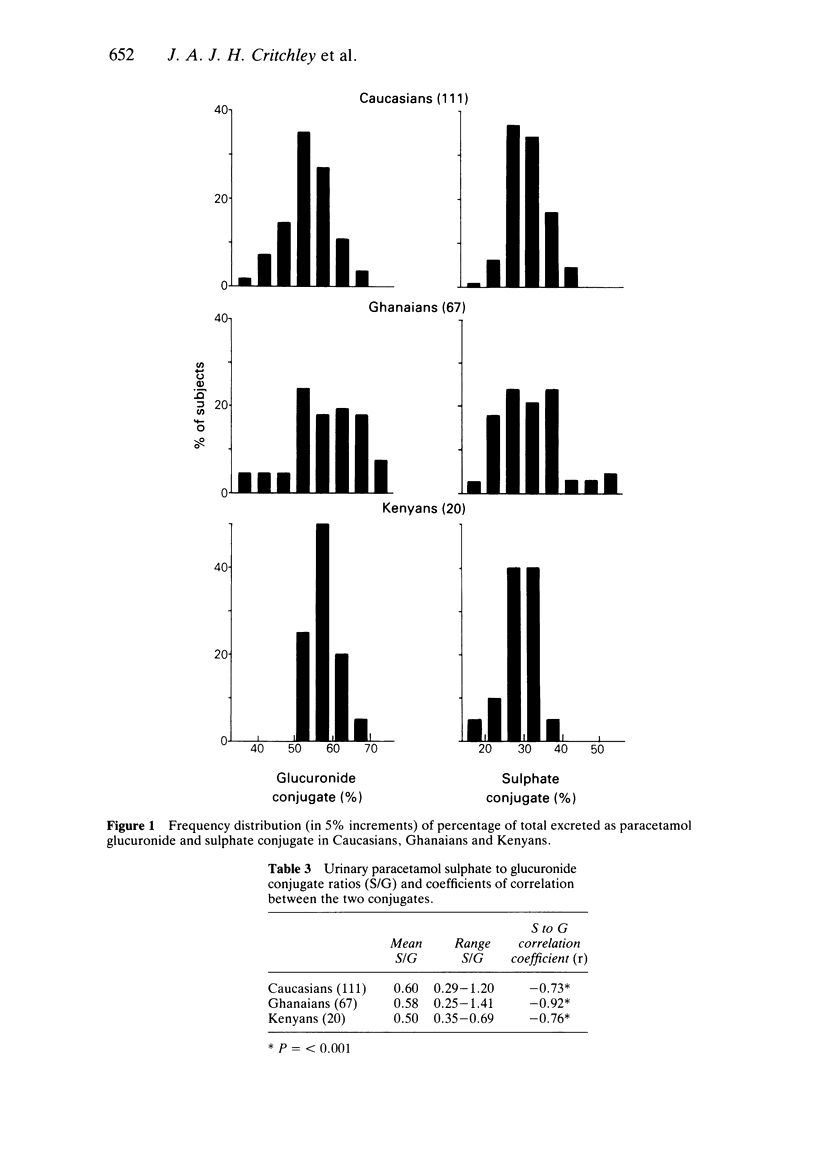
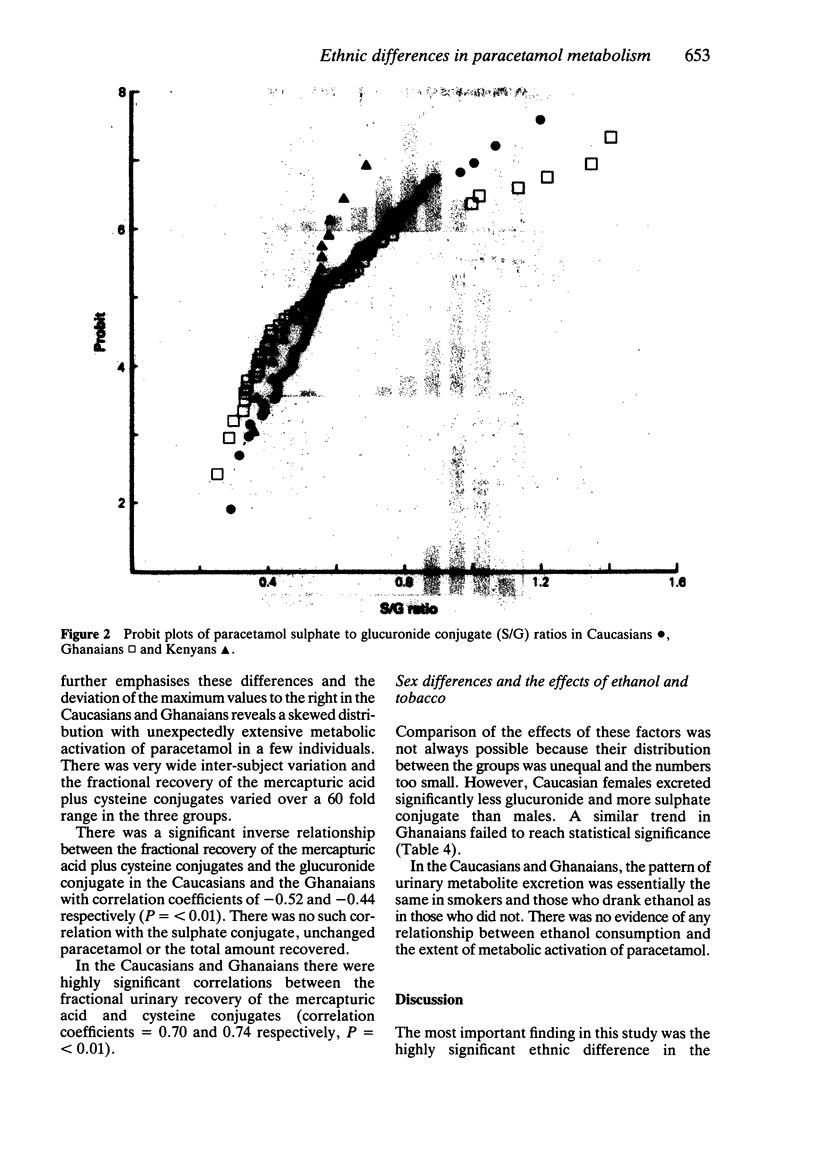
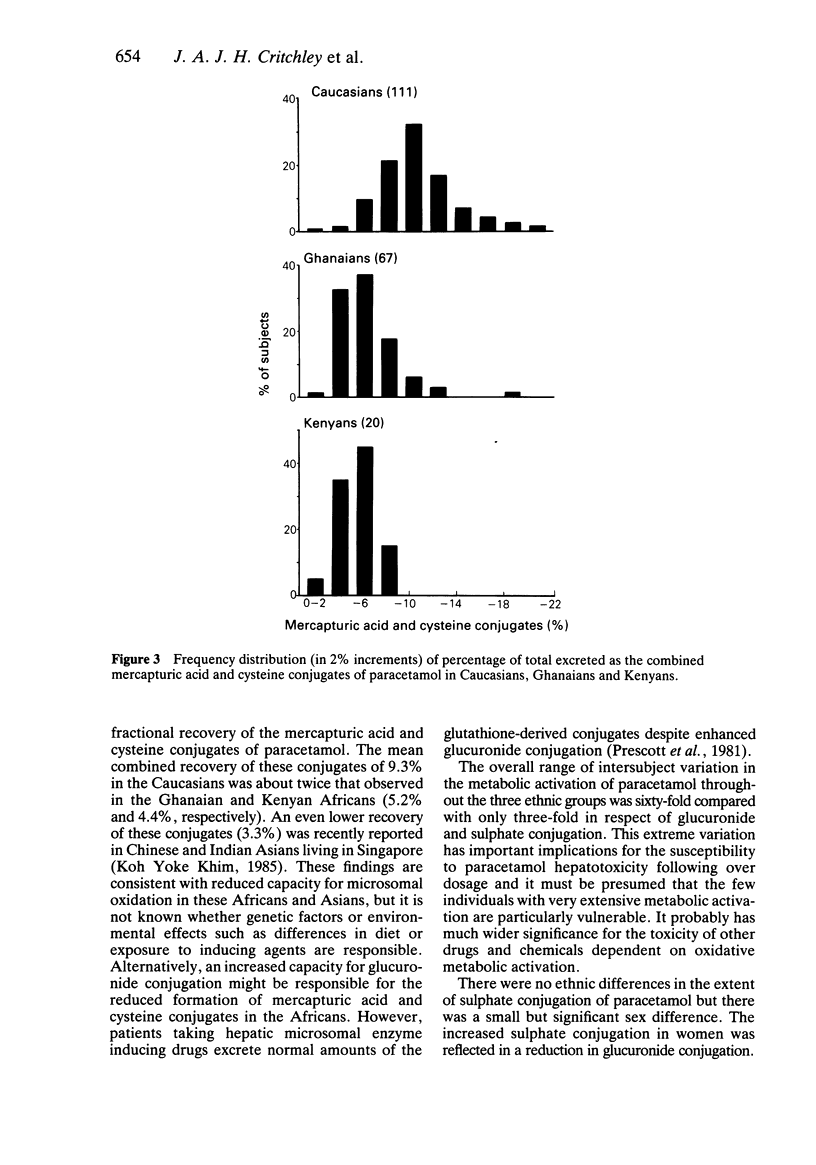
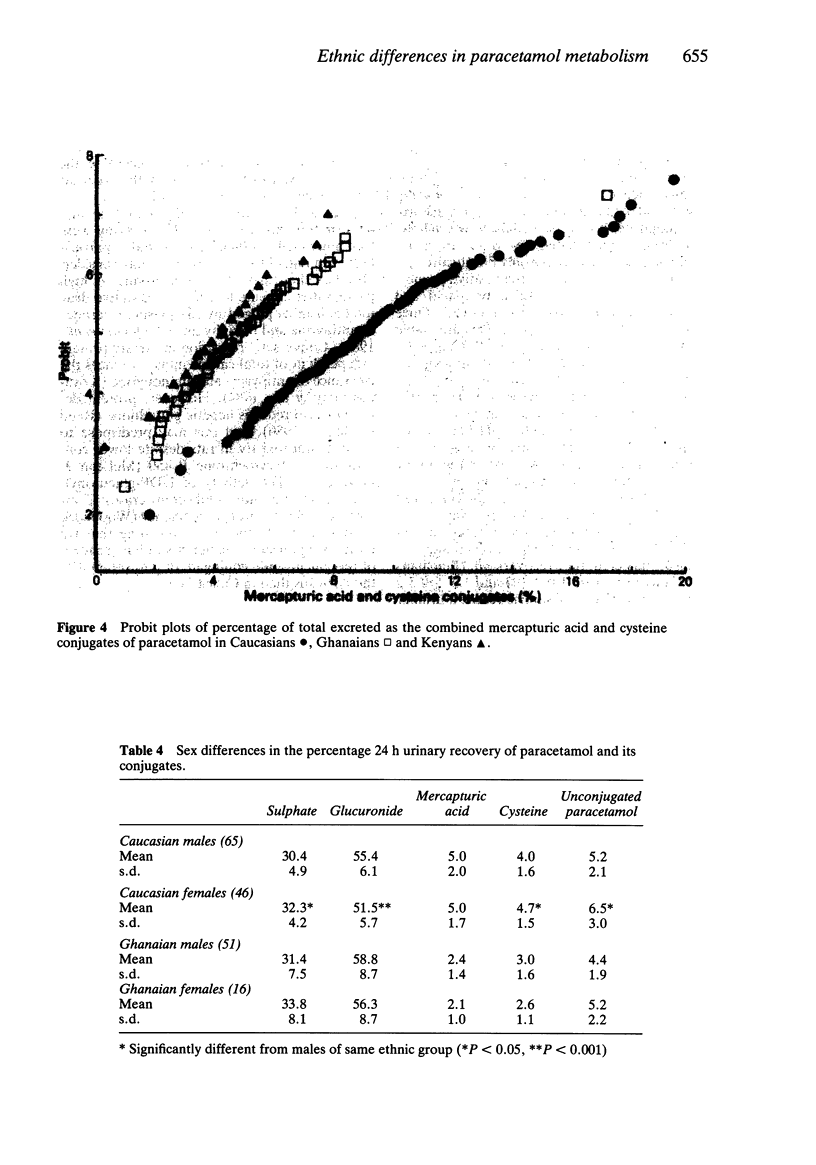
The 24 h urinary excretion of paracetamol and its metabolites following a single oral dose of 1.5 g was compared in 111 Caucasians (Scotland), 67 West Africans (Ghana) and 20 East Africans (Kenya). The fractional recovery of the mercapturic acid and cysteine conjugates of paracetamol was 9.3% in the Caucasians compared with only 5.2% and 4.4% in the Ghanaians and Kenyans respectively (P = less than 0.0005). This probably indicates markedly reduced metabolic activation of paracetamol in the Africans. There were no ethnic differences in the sulphate conjugation of paracetamol, but the mean fractional recovery of the glucuronide conjugate in Caucasians (54%) was significantly less than in the Africans (58%). The sulphate conjugation of paracetamol was increased and glucuronide conjugation reduced in Caucasian females compared with males. A similar trend was seen in the Ghanaians but there were no other significant sex differences. The range of intersubject variation in the metabolic activation of paracetamol was sixty fold compared with only a three fold variation in glucuronide and sulphate conjugation. This has important implications for susceptibility to paracetamol hepatotoxicity following overdosage especially in a small subgroup showing extensive metabolic activation. These ethnic differences in paracetamol metabolism may be related to genetic or environmental factors including differences in diet and protein intake.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Conney A. H., Kappas A. Nutritional influences on chemical biotransformations in humans. Nutr Rev. 1982 Jun;40(6):161–171. doi: 10.1111/j.1753-4887.1982.tb05298.x. [DOI] [PubMed] [Google Scholar]
- Andoh B., Idle J. R., Sloan T. P., Smith R. L., Woolhouse N. Inter-ethnic and inter-phenotype differences among Ghanaians and Caucasians in the metabolic hydroxylation of phenytoin [proceedings]. Br J Clin Pharmacol. 1980 Mar;9(3):282P–283P. doi: 10.1111/j.1365-2125.1980.tb04842.x. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Effects of nutritive factors on metabolic processes involving bioactivation and detoxication of chemicals. Annu Rev Nutr. 1984;4:207–231. doi: 10.1146/annurev.nu.04.070184.001231. [DOI] [PubMed] [Google Scholar]
- Howie D., Adriaenssens P. I., Prescott L. F. Paracetamol metabolism following overdosage: application of high performance liquid chromatography. J Pharm Pharmacol. 1977 Apr;29(4):235–237. doi: 10.1111/j.2042-7158.1977.tb11295.x. [DOI] [PubMed] [Google Scholar]
- Kalow W. Ethnic differences in drug metabolism. Clin Pharmacokinet. 1982 Sep-Oct;7(5):373–400. doi: 10.2165/00003088-198207050-00001. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy K., Kalamegham R., Naidu N. A. Dietary influences on the kinetics of antipyrine and aminopyrine in human subjects. Br J Clin Pharmacol. 1984 Feb;17(2):139–146. doi: 10.1111/j.1365-2125.1984.tb02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Smith R. L. A population and familial study of the defective alicyclic hydroxylation of debrisoquine among Egyptians. Xenobiotica. 1979 Jan;9(1):51–56. doi: 10.3109/00498257909034703. [DOI] [PubMed] [Google Scholar]
- McLean A. E., Day P. A. The effect of diet on the toxicity of paracetamol and the safety of paracetamol-methionine mixtures. Biochem Pharmacol. 1975 Jan 1;24(1):37–42. doi: 10.1016/0006-2952(75)90310-x. [DOI] [PubMed] [Google Scholar]
- Miners J. O., Attwood J., Birkett D. J. Influence of sex and oral contraceptive steroids on paracetamol metabolism. Br J Clin Pharmacol. 1983 Nov;16(5):503–509. doi: 10.1111/j.1365-2125.1983.tb02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. R., Jollows D. J. Progress in hepatology. Metabolic activation of drugs to toxic substances. Gastroenterology. 1975 Feb;68(2):392–410. [PubMed] [Google Scholar]
- Mitchell J. R., Thorgeirsson S. S., Potter W. Z., Jollow D. J., Keiser H. Acetaminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther. 1974 Oct;16(4):676–684. doi: 10.1002/cpt1974164676. [DOI] [PubMed] [Google Scholar]
- Mucklow J. C., Fraser H. S., Bulpitt C. J., Kahn C., Mould G., Dollery C. T. Environmental factors affecting paracetamol metabolism in London factory and office workers. Br J Clin Pharmacol. 1980 Jul;10(1):67–74. doi: 10.1111/j.1365-2125.1980.tb00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott L. F., Critchley J. A., Balali-Mood M., Pentland B. Effects of microsomal enzyme induction on paracetamol metabolism in man. Br J Clin Pharmacol. 1981 Aug;12(2):149–153. doi: 10.1111/j.1365-2125.1981.tb01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott L. F., Critchley J. A. The treatment of acetaminophen poisoning. Annu Rev Pharmacol Toxicol. 1983;23:87–101. doi: 10.1146/annurev.pa.23.040183.000511. [DOI] [PubMed] [Google Scholar]
- Prescott L. F. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980 Oct;10 (Suppl 2):291S–298S. doi: 10.1111/j.1365-2125.1980.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers D. K., van Staden D. A., Moncrieff J., Schoeman H. S. Paracetamol metabolism in African villagers. Hum Toxicol. 1985 Jul;4(4):385–389. doi: 10.1177/096032718500400404. [DOI] [PubMed] [Google Scholar]
- Spedding M. Functional interactions of calcium-antagonists in K+-depolarized smooth muscle. Br J Pharmacol. 1983 Nov;80(3):485–488. doi: 10.1111/j.1476-5381.1983.tb10719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock B. G., Wood G. C. Effect of protein-free diet on UDP-glucuronyltransferase and sulphotransferase activities in rat liver. Biochem Pharmacol. 1971 Oct;20(10):2703–2713. doi: 10.1016/0006-2952(71)90180-8. [DOI] [PubMed] [Google Scholar]
- Woolhouse N. M., Adjepon-Yamoah K. K., Mellström B., Hedman A., Bertilsson L., Sjöqvist F. Nortriptyline and debrisoquine hydroxylations in Ghanaian and Swedish subjects. Clin Pharmacol Ther. 1984 Sep;36(3):374–378. doi: 10.1038/clpt.1984.190. [DOI] [PubMed] [Google Scholar]
- Woolhouse N. M., Andoh B., Mahgoub A., Sloan T. P., Idle J. R., Smith R. L. Debrisoquin hydroxylation polymorphism among Ghanaians and Caucasians. Clin Pharmacol Ther. 1979 Nov;26(5):584–591. doi: 10.1002/cpt1979265584. [DOI] [PubMed] [Google Scholar]


