Abstract
Several micro-scale chromatography-based procedures for purification of the β-galactosidase from the yeast Kluyveromyces lactis were assayed. Purified enzyme was suitable to be used as antigen to induce polyclonal antibodies production. Specific staining of non-denaturing PAGE gels with chromogenic substrates allowed the determination of the number of subunits forming the native enzyme.
Keywords: Kluyveromyces, Glucosidases
Introduction
The lactase or β-galactosidase (β-D-galactoside galactohydrolase, EC 3.2.1.23) from Kluyveromyces lactis is an enzyme which has attracted our attention since it represents an essential material to convert the waste product cheese whey into a substrate valuable for biotechnology industries (1). Our previous research focused on several different aspects about this enzyme such as production (2,3), immobilization (4), use of whole cells as catalytic agents (5, 6) and, more recently, release by autolytic mutants (7) in order to reduce the cost associated to preparation. As this research progressed, the need for purified Kluyveromyces lactis β-galactosidase arose, for example, to use it as antigen to induce specific antibodies. Therefore, we assayed several purification procedures looking for a quick and simple method which allowed us to obtain enough purified enzyme in only one step and minimizing as much as possible the amount of crude extract needed. The best results regarding Kluyveromyces lactis β-galactosidase purification have been published very recently (8). Here we describe the methods in more detail and comment briefly on some complementary aspects of the results which are not shown in the previous article.
Materials and Methods
Some of the methods here described in detail were briefly related in references (7, 8).
Preparation of crude extract
The cells cultured in 1 l of YPL (10 g/l yeast extract, 5 g/l peptone and 40 g/l lactose) up to an A600 nm of 2 were harvested by centrifugation at 7000 rpm for 5 minutes at 4°C and washed once with distilled water. They were suspended in 20 mM Tris-ClH, pH 7.8, 300 mM (NH4)2SO4, 10 mM MgCl2, 1 mM EDTA, 10% glycerol buffer with 0.1 mM PMSF, 4 mM Pepstatin, 4 mM Leupeptin and 2 μM β-Mercaptoethanol and broken using a sonicator at 16 microns for 20 minutes at 4°C making 5 minutes intervals after each 5 minutes exposure. Cell debris was removed by centrifugation at 40,000 rpm for 90 minutes at 4°C. The supernatant, cell-free extract, was stored at -80°C.
Purification of β-Galactosidase
Purification of the β-galactosidase of K. lactis strain NRRL-Y1140 from the protein extract described above and from a K. lactis commercial lactase preparation, Maxilact LX-5000 (Gist Brocades, France/The Netherlands), was performed using different chromatography techniques. All purification steps were carried out at 4°C. The enzymatic activity was assayed in the fractions obtained from chromatography. Active fractions were pooled and, when required, concentrated by filtration in Amicon Microcon-100 YM membranes.
Gel filtration chromatography.
The FPLC Smart system from Pharmacia was used. The column Superose 12 PC 3.2/30 (2.4 ml) prepacked with Superose 12, a highly cross-linked, 12% agarose-based medium, was equilibrated and further eluted with 50 mM Sodium Phosphate, pH 7.0, 0.15 M NaCl. Absorbance (280 nm) was measured on line. The elution rate was 40 ml/min and the eluate was collected in 0.1 ml aliquots. The column was calibrated using the following molecular weight standards (Sigma Chemical, USA): Jack Bean Urease 545,000 Da (hexamer) and 272,000 Da (trimer) and Bovine Serum Albumin 132,000 Da (dimer) and 66,000 Da (monomer). The molecular mass of the native protein was extrapolated from a plot of the logarithm of the molecular mass versus the elution volume.
Ion-Exchange chromatography.
The FPLC Smart system from Pharmacia was used. The column Mono Q PC 1.6/5 (0.10 ml) prepacked with Mono Q (Quarternary amino ethyl), a strong anion exchanger based on a beaded hydrophilic polymer, was equilibrated with 20 mM Trietanolamine, pH 7.5. Proteins were eluted with a linear gradient of NaCl from 0 to 1.0 M in 20 minutes at a flow rate of 100 ml/min, and the eluate was collected in 0.1 ml aliquots.
Affinity chromatography.
The column with 5 ml agarose-p-aminophenyl-β-D-thiogalactoside (Sigma Chemical, USA) was equilibrated with 50 mM phosphate buffer, and the enzyme was eluted with 0.1 M borate buffer, pH 10 (9). 1 ml aliquots were collected at a flow rate of 100 ml/min and pH was neutralized to avoid denaturation.
β-Galactosidase activity
β-Galactosidase activity was determined by a modification of the method of Guarante (1983) (10). The enzyme solution was incubated at 30°C for several minutes in 2.0 ml of Z-buffer (0.1 M Sodium Phosphate, 10 mM KCl, 1 mM MgSO4 and 50 mM 2-mercaptoethanol, pH 7.0) with 440 ml of orthonitrophenyl-β-D-galactopyranoside (4 mg/ml). The reaction was stopped by adding 0.5 ml of 1 M Na2CO3. Released o-nitrophenol (ONP) was measured spectrophotometrically at 420 nm. The molar extinction coefficient of o-nitrophenol under these conditions is 4.5x103l mol-1cm-1 (11). One enzyme unit (EU) is defined as the quantity of enzyme that catalyzes the release of 1 mmol of ONP from orthonitrophenyl-β-D-galactopyranoside (ONPG) per minute under assay conditions.
Protein Determination
Protein concentration was determined according to the procedure of Bradford (12) using bovine serum albumin as the standard.
Polyacrylamide gel electrophoresis.
Denaturing acrylamide gels (10% polyacrylamide gels) containing sodium dodecyl sulfate (SDS) were run according to the procedure of Laemmli (13), using a Bio-Rad Mini Protean II apparatus. Protein samples were solubilized by boiling for 5 min in 2.5% SDS and 5% 2-Mercaptoethanol. Protein was concentrated in the gel with a voltage of 100V, then the voltage was increased to 150V and kept constant for 2 hours. Gels were fixed in 12% trichloroacetic acid and protein was stained with a modified and more sensitive Coomassie brilliant blue G-250 dye-binding method (14). Molecular weight was determined using a molecular weight marker kit obtained from Sigma Chemical (USA), containing Rabbit Muscle Myosin (205,000 Da), E. coli β-galactosidase (116,000 Da), Rabbit Muscle Phosphorylase b (97,000 Da), Rabbit Muscle Fructose-6-phosphate Kinase (84,000 Da), Bovine Serum Albumin (66,000 Da), Bovine Liver Glutamic Dehydrogenase (55,000 Da), Chicken Egg Ovoalbumin (45,000 Da), Rabbit Muscle Glyceraldehyde-3-phosphate Dehydrogenase (36,000 Da).
Native electrophoresis was performed at 4°C, using gradient gels (5-15%) without SDS. The molecular mass was extrapolated from a plot of the logarithm of the molecular mass versus the logarithm of the percentage of polyacrylamide. The following standards (Sigma Chemical, USA) were used: bovine serum albumin monomer and dimer (66,000 and 132,000 Da), urease trimer and hexamer (272,000 and 545,000 Da).
When detection of β-galactosidase activity was required after a non-denaturating electrophoresis process, three different techniques were used. A) a non-fixed gel was plunged in Z-buffer containing ONPG (4 mg/ml) for several minutes at 30°C until a yellow band appeared (15). B) the active bands in the native gels were also stained following the technique of Erickson and Steers (16), using 0.025 BNG (6-bromo-2-naphthyl-β-D-galactopyranoside) from Sigma Chemical (USA) as substrate in 10% methanol (v/v), 0.01 M Tris-ClH (pH 7.4), 0.01 M NaCl and 0.01 M MgCl2. The incubation time was several minutes at room temperature, followed by 1 to 2 minutes in 1 mg/ml diazo-blue B and the appearance of brown band indicated the presence of an enzymatically active form of β -galactosidase. The reaction was stopped by rinsing in water and fixing the gel in 7.5% acetic acid. C) An alternative stain was used (17), gels to be stained were placed in substrate solution (5 mM methylumbelliferyl-β-D-galactoside, MUG, in 50 mM sodium phosphate buffer, pH 7.0) and incubated for 2 to 5h at room temperature. Gels were transferred to a UV transilluminator for visualization.
Immunological studies.
To obtain antiserum against K. lactis β-galactosidase BALB/c mice and a Californian rabbit were used. In the first case, BALB/c mice were intraperitoneally immunized with 0.2 ml of a 1:1 (v/v) mixture of Freunds complete adjuvant (FCA) and PBS containing 50 mg of the K. lactis β-galactosidase (Maxilact LX-5000, Gist Brocades, France/The Netherlands). The same dose in 125 ml (but without FCA) was injected via the retrobulbar venous plexus 21 days post-primary immunization. Mice were bled 14 days post-secondary immunization and the sera was separated by centrifugation at 2,000g for 10 min., mixed 1:1 (v/v) with glycerol and stored at -20°C until use.
In the second case, a primary dose of 50 μg of the K lactis β-galactosidase 124 kDa band cut out from denaturing PAGE gels and emulsified in Freund's complete adjuvant was administered subcutaneously at multiple sites in a healthy Californian rabbit of 2-3 kg body weight. The rabbit was given a booster injection after 8-10 weeks (50 μg of the same band but electroeluted from the gel). Two weeks after the second booster dose, the animal was bled and antiserum was collected. Serum was separated by centrifugation at 5,000g for 5 minutes, mixed 1:1 with glycerol and stored at -20°C until use.
To avoid secondary reactions, antisera, both from mice and rabbit, were adsorbed 24 hours at 4°C 1:1 (v/v) with a crude proteic extract obtained as described above by sonical disruption of K. lactis β-galactosidase defficient cells (MW-190-9B strain, from Dr. Wlowski-Louvel's collection) cultured in YPD (10 g/l yeast extract, 5 g/l peptone and 20 g/l glucose). Both antisera showed a similar band pattern in western blots.
Serological reaction between enzyme and antiserum was determined by Ouchterlony immunodiffusion method (18) using 1% agarose in 0.2M Tris-ClH, pH 8.3, 3% PEG 6000 and 0.01% sodium azide.
Enzyme-linked immunosorbent assay (ELISA)
Indirect ELISA was carried out as described in Iglesias et al. (19). Extracts containing b-galactosidase were bound to PVC microtitre plates (0.5-2 mg of protein/well) in 100 ml/well of carbonate-bicarbonate buffer (pH 9.6) overnight at 4°C. Plates were then washed three times with TBS (50 μM Tris, 0.15 M NaCl; pH 7.4) and blocked for two hours at 37°C with 5% solution of non-fat dry milk in TBS containing 0.2% Tween 20 (TBS-Tween). The plates were incubated for two hours at 37 °C with the test sera diluted in TBS-Tween containing 1% non-fat dry milk, washed with TBS containing 0.05% Tween (5x5 minutes) and incubated for one hour at 37°C with peroxidase-conjugated rabbit anti-mouse immunoglobulin (Ig) polyclonal antibody (Dakopatts A/S, Glostrup, Denmark) diluted in TBS-Tween containing 3% polyethylene glycol 6000. The plates were then washed with TBS (5x5 minutes), then 100 ml of substrate containing 0.04% o-phenylenediamine (Sigma Chemical, USA) and 0.001% hydrogen peroxide in phosphate-citrate buffer (pH 5.0) was added. The reaction was stopped after 20 minutes by adding 25 ml of 3 N sulfuric acid. Optical density at 492 nm was measured on a microtitre plate reader (Titertek Multiskan, Labsystems, Finland).
Immunoblotting
Following electrophoresis, gels were electotransferred onto a nitrocellulose membrane at a constant voltage of 15 V for one hour and immunostained. The membrane was washed with TBS (50 mM Tris, 0.15 M NaCl, pH 7.4) stained with Ponceau S to verify transfer, then dried and blocked overnight at 4°C with TBS containing 0.2% Tween-20 and 5% non-fat dry milk, and finally incubated 2 hours with a 1:500 dilution of mouse immune serum or 1:250 of rabbit immune serum. Membrane was washed with TBS containing 0.2% Tween-20 and incubated with a 1:1600 dilution of peroxidase-conjugated rabbit anti-mouse Ig (Dakopats, Denmark) 2000 of peroxidase-conjugated goat anti-rabbit Ig (Sigma ImmunoChemicals, USA), respectively. The immunoreactant bands were stained by adding TBS containing 0.003% H2O2, 0.06% diaminobenzidine tetrahydrochloride and 0.03% NiCl2 . The reaction was stopped by thorough washing in TBS.
Results and Discussion
The purification of β-galactosidase from a crude protein extract from K. lactis strain NRRL-Y1140 by gel filtration chromatography on Superose 12 PC 3.2/30 resulted in a purification factor of 1.8-fold having an overall yield, based on total enzyme units, of about 50 %. The purification factor reached 2.3-fold when this technique was applied to partially purified extracts. Representative chromatograms are shown in figure 1.
Fig. 1.
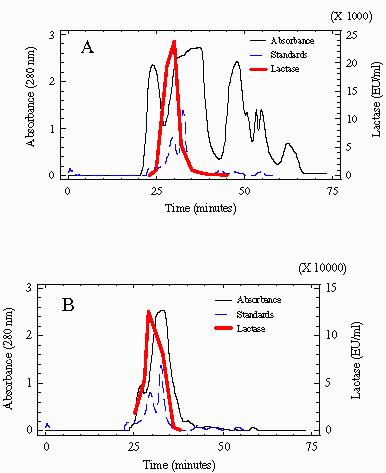
Profiles of β-Galactosidase activity and protein obtained after gel filtration (A). Chromatography of 2.4 mg of a crude extract from K. lactis. (B) Chromatography of 3mg of partially purified extracts from Maxilact LX-5000. The profile of molecular weight standards used for calibration are shown. Absorbance values of standards are multiplied by 100. Fraction showing the peak of maximum lactase activity was estimated to correspond to a molecular weight of 250 kDa in the crude extract and 260 kDa in Maxilact LX-5000.
Ion-exchange chromatography turned out to be less effective than gel filtration. When it was applied, as described in the Materials and Methods section, both to the active fractions pooled from gel filtration chromatography of a crude yeast protein extract and directly to the Maxilact LX-5000 preparation, a purification factor of 1.6-fold was obtained. Yield, based in recovered enzyme units, reached 8% in the first case and 33% in the second one. Sometimes a slight reduction in specific activity was observed, it was due to unknown reasons and probably related to enzyme denaturation produced during the chromatography process. Another unexplained result was that when this technique was used both directly with the crude protein extract or with the active fractions pooled from the gel filtration chromatography, enzyme activity profiles repeatedly showed two peaks, whereas when Maxilact LX-5000 was used, only one peak of activity was observed (Figure 2).
Fig. 2.
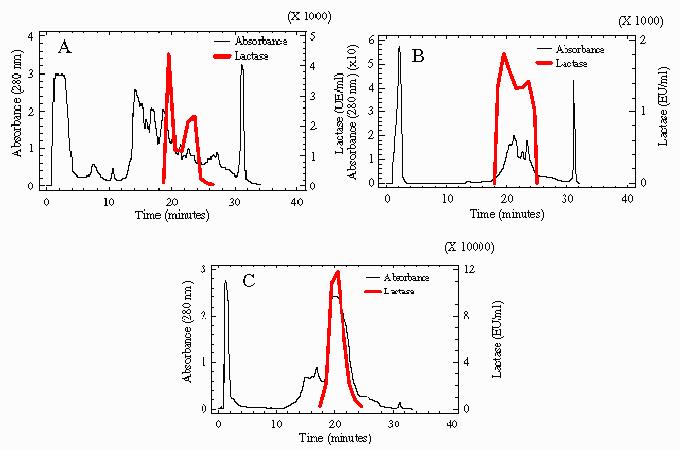
Profiles of β-Galactosidase activity and protein obtained after ion-exchange chromatography. (A) Profile of 2.4 mg of a crude extract from K. lactis. (B) Chromatography of 0.6 mg of active fractions pooled from gel filtration of a crude yeast protein extract. (C) Chromatography of 2.4 mg protein of Maxilact LX-5000.
Absorbance (280 nm) The FPLC systems show the advantage that proteins may be purified from low quantities of crude preparations if compared to traditional higher-scale chromatography systems. For instance, in this study, about 7 mg of total protein in the crude extract were enough for a purification process including two gel filtration steps, one ion-exchange step and one ultrafiltration step. An 11-fold purification factor was achieved after the whole process (Table 1 of reference 8). Although this purification factor may seem low, it is in accordance with the antibodies-reactivity showed by a crude protein extract versus the purified enzyme and, moreover, the specific activity was very high (8).
Affinity chromatography on the substrate-analogue agarose-p-aminophenyl-β-D-thiogalactoside was also assayed for β-galactosidase purification both from crude K. lactis protein extracts and from Maxilact LX-5000. A representative chromatogram has been shown in the previous article (8). The purification factor was 2.5 and 2.1-fold, respectively. Therefore, we consider this technique more effective than each single gel filtration and ion-exchange chromatography step. Moreover, the affinity purified b-galactosidase turned out to be homogeneous in denaturing polyacrilamide gel electrophoresis and western-blotting analysis (Figure 3A). A main band with an approximate molecular weight of 124 kDa was observed.
Fig. 3.
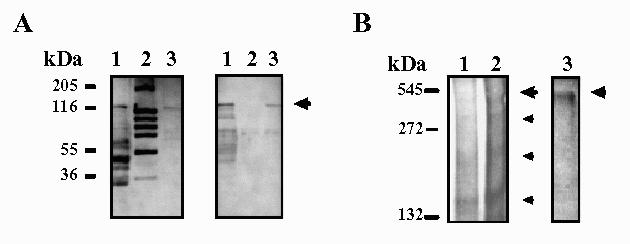
SDS-PAGE (left) and immunoblotting (right) of purified β-Galactosidase from K. lactis (A). β-Galactosidase is indicated by an arrow. Western-blot was incubated with mouse immune serum. Lane 1: 75 μg of a crude extract from K. lactis; lane 2: Molecular weight markers; lane 3: 5 μg of β- galactosidase from K. lactis purified by affinity chromatography. (B) Electrophoresis on a non-denaturing gradient gel (5-15%) stained with ONPG and later Coomassie brilliant blue G250 (left) and with BNG (right). Bands with β-Galactosidase activity are indicated by higher arrows. Different forms of β-Galactosidase are shown by smaller arrows. Different concentrations of b-Galactosidase from Maxilact LX-5000 were loaded. Lane 1:20 μg; lane 2:40 μg; lane 3:80 μg.
Sometimes, other additional bands of smaller molecular weight were also present, more notoriously in the case of Maxilact LX-5000, and are probably attributable to β-galactosidase degradation products; their intensity increased when the 124 kDa band decreased and they were also detected in Western blotting analysis with the anti-K.lactis-&beta-galactosidase antibodies (both with rabbit and with mouse antisera). This occurred for the three purification techniques employed in this work. In fact, one of the major difficulties encountered in the purification was the increasing instability of the enzyme during the different purification steps, and also for the purified enzyme even if it was stored in a buffer containing glycerol.
Relatively high amounts of enzyme (40-80 μg of purified protein) were needed in order to detect the active band after electrophoresis on a 5-15% nondenaturing acrylamide gel. In this case β-galactosidase purified from Maxilact LX-5000 was used. As has been previously described (8) among the several Coomassie brilliant blue-staining bands observed, only the one with an estimated molecular weight corresponding to the tetrameric form always showed enzyme activity on an identical unstained native gel incubated with the chromogenic substrates ONPG (data not shown) or BNG (Figure 3B). Bands stained with ONPG were more intense, but also less stable after storage, than those obtained with the BNG solution. In our hands, the technique using MUG as substrate was less sensitive than the other ones and did not show active bands.
Polyclonal anti-K.lactis-β-galactosidase antibodies were prepared. Specificity of these antibodies was tested by ELISA (Figure 4). Secondary non-specific reactions were considered negligible. The most outstanding results concerning the immunological characterization of K. lactis β-galactosidase have been discussed in the previous paper (8).
Fig. 4.
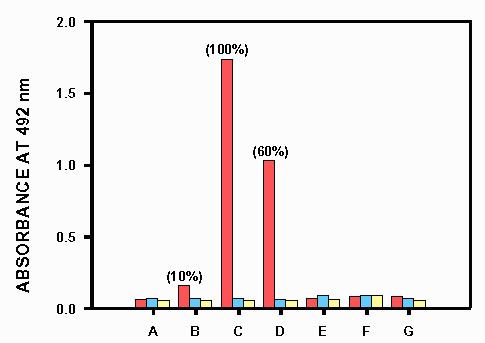
Results of cross-reactivity obtained in the ELISA performed with the specific anti K.lactis β-galactosidase antibodies prepared in this work (red bars) versus (A) a crude protein extract of the K.lactis β-galactosidase deficient strain MW-190-9B, (B) a crude protein extract of K.lactis NRRL-Y1140, (C) purified Maxilact LX-5000 β-galactosidase, (D) partially purified K. fragilis β-galactosidase, (E) Aspergillus niger β-galactosidase, (F) bovine liver β-galactosidase, (G) Escherichia coli β-galactosidase. Blue bars are controls of absence of reactivity when the serum of a non-immunized mouse was used instead of the specific anti K.lactis β-galactosidase antibodies. Yellow bars are controls of absence of reactivity when these primary antibodies are not added.
Therefore, we may conclude that the purification procedures assayed in this work, which are easy and need small amounts of crude extract, allowed us to obtain enough purified K.lactis β-galactosidase forseveral uses, for example, antigen to induce antibodies. Comparing these techniques, affinity chromatography gave the highest purification factor although gel filtration in the FPLC system has demonstrated to be faster (each run takes about 1 hour whereas each affinity chromatography run takes about 8 hours) and very accurate and reproducible. Ion-exchange chromatography is the fastest procedure (each run takes about half an hour) but purification factor is lower than with the other two techniques.
Acknowledgments
Maxilact LX-5000 was a generous gift from Gist-Brocades. The authors would like to thank Dr J. Leiro for his help with immunological techniques and Dr. M. Wlowski-Louvel for providing the MW-190- 9B strain. M. Becerra was the recipient of a predoctoral fellowship from the Xunta de Galicia (Spain) during 1995-97 and is the recipient of a fellowship from the Instituto Danone (Spain). This research was funded by grants BIO94-0961 from the CICYT (Spain) and XUGA-10305A93 from the Xunta de Galicia (Spain).
References
- González Siso MI. The biotechnological utilization of cheese whey: a review. Biores Technol. 1996;57:1–11. [Google Scholar]
- González Siso MI. β-galactosidase production by Kluyveromyces lactis on milk whey: batch versus fed-batch cultures. Proc Biochem. 1994;29:565–568. [Google Scholar]
- Becerra M, González Siso MI. Yeast β-galactosidase in solid-state fermentations. Enz Microb Technol. 1996;19:33–44. [Google Scholar]
- González Siso MI, Freire Picos A, Ramil E, Rodríguez Belmonte E, Rodríguez Torres A, Cerdán E. Covalent immobilization of β-galactosidase on corn grits. A system for lactose hydrolysis without diffusional resistance. Proc Biochem. 1994;29:7–12 . [Google Scholar]
- González Siso MI, Cerdán E, Freire Picos MA, Rodríguez Belmonte E, Rodríguez Torres AM. Permeabilization of Kluyveromyces lactis cells for milk whey saccharification: a comparison of different treatments. Biotechnol Techniques. 1992;6:289–292. [Google Scholar]
- González Siso MI, Suárez Doval S. Kluyveromyces lactis immobilization on corn grits for milk whey lactose hydrolysis. Enzyme Microb Technol. 1994;16:303–310. [Google Scholar]
- Becerra M, Cerdán E, González Siso MI. Heterologous Kluyveromyces lactis β-galactosidase production and release by Saccharomyces cerevisiae osmotic-remedial thermosensitive autolytic mutants. Biochim Biophys Acta. 1997;1335:235–241. doi: 10.1016/s0304-4165(97)00048-2. [DOI] [PubMed] [Google Scholar]
- Becerra M, Cerdán E, González Siso MI. Micro-scale purification of β-galactosidase from Kluyveromyces lactis reveals that dimeric and tetrameric forms are active. Biotechnol Techniques. 1998;12:253–256. [Google Scholar]
- Nader de Macias ME, Perdigon G, Oliver G, de Ruiz Holgado AAP. Immunological relationships among β-galactosidases in members of the genus Lactobacillus. Int J Sys Bacteriol. 1985;35:103–110. [Google Scholar]
- Guarante L. Yeast promoters and LacZ fusions designed to study expression of cloned genes in yeasts. Meth Enzymol. 1983;101:181–189. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Inchaurrondo VA, Yantorno OM, Voget CE. Yeast growh and β-galactosidase production during aerobic batch cultures in lactose limited synthetic medium. Proc Biochem. 1994;29:47–54. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neuhoff V, Stamm R, Eibk M. Clear background and highly sensitive protein staining with Coomassie blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis. 1985;6:427–448. [Google Scholar]
- Dickson RC, Dickson LR, Markin JS. Purification and properties of an inducible β-galactosidase isolated from the yeast Kluyveromyces lactis. J Bacteriol. 1979;137:51–61. doi: 10.1128/jb.137.1.51-61.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RP, Steers JRE. Comparative study of isoenzyme formation of bacterial β-Galactosidase. J Bacteriol. 1970;102:79–84. doi: 10.1128/jb.102.1.79-84.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady D, Marchant R, McHale L, McHale AP. Isolation and partial characterization of β-galactosidase activity produced by a thermotolerant strain of Kluyveromyces marxianus during growth on lactose-containing media. Enzyme Microb Technol. 1995;17:696–699. [Google Scholar]
- Pritchard DI, Maizels RM, Behnke JM, Appleby P. Stage-specific antigens of Nematospiroides dubius. Immunology. 1984;53:325–335. [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Leiro J, Ubeira FM, Santamarina MT, Sanmartín ML. Anisakis simplex: antigen recognition and antibody production in experimentally infected mice. Parasite Immunol. 1993;15:243–250. doi: 10.1111/j.1365-3024.1993.tb00607.x. [DOI] [PubMed] [Google Scholar]


