Abstract
cAMP response element-binding protein (CREB) is important for the formation and facilitation of long-term memory in diverse models. However, to our knowledge, involvement of CREB in age-associated memory impairment has not been reported. Here, we use a recombinant adeno-associated virus vector to obtain stable transgenic expression of CREB as well as the inducible cAMP early repressor (ICER) in the hippocampus of adult rats. In a longitudinal study, we show that somatic gene transfer of both CREB and ICER does not alter long-term memory in young (3-month-old) rats. However, at 15 months of age, the same CREB-transduced rats show significantly better long-term memory in spatial-navigation and passive-avoidance tasks compared with their equally aged control littermates, and a threshold effect is evident. In contrast, the aged ICER-transduced rats demonstrate significantly reduced memory in comparison with the same control group. Hippocampal CREB gene transfer prevented the aging-related decrease in long-term memory found in the control rats. These data suggest that elevation of CREB protein levels in a subset of hippocampal neurons as achieved by somatic cell gene transfer might compensate for general deficits in molecular mechanisms underlying age-related memory loss in rats and, therefore, attenuate long-term-memory impairment during normal aging.
Keywords: adeno-associated virus vector, inducible cAMP early repressor, long-term memory, hippocampus
Deficits in hippocampus-dependent long-term memory, which accompany normal aging, have been reported for many mammal species, including rats (1, 2), mice (3), monkeys (4), and humans (5). However, molecular mechanisms underlying such age-related changes are still unknown.
Studies by Kandel and colleagues (6) using in vitro cultures of mollusk neurons revealed the involvement of the transcription factor cAMP response element-binding protein (CREB) in the molecular mechanisms underlying long-term facilitation. Further behavioral analysis in Drosophila (7, 8) and mice (9, 10) demonstrated that CREB is necessary for long-term-memory formation both in nonmammalian and mammalian species. Also, pharmacological antagonism and genetic disruption of CREB signaling prevents or attenuates long-term-memory consolidation in these model systems.
CREB is constitutively expressed in cells, with the phosphorylation of Ser-133 generally believed to be the main mechanism of regulation of its transcriptional activity (11). However, several studies indicate that the CREB protein concentration might be critical in some aspects of long-term-memory formation. A transgenic fly that overexpresses an active form of CREB shows a lower threshold for the consolidation of long-term memory (8). Also, transiently increasing WT CREB levels in the basolateral amygdala by means of herpes simplex virus vector-mediated gene transfer facilitates long-term-memory formation after massed fear training (12).
There is no direct evidence that implicates CREB in age-related memory impairment. However, electrophysiological studies in mice (3) have shown that spatial-memory deficits in aged animals are correlated with a reduced late phase of hippocampal long-term potentiation and can be attenuated by drugs that act to facilitate the cAMP signaling pathway. Also, a recent report (13) shows that absolute CREB protein levels are decreased within the hippocampus of aged rats with spatial-memory impairments. These finding are purely associational and do not indicate relative CREB deficiency as a cause of aging-related memory impairment. However, they do suggest the possibility that elevation of absolute CREB levels may ameliorate aging-related cognitive decline.
Here, we used the nonpathogenic recombinant adeno-associated virus (rAAV) vector to achieve long-lasting and stable transgene expression within the hippocampus of adult rats. We sought to determine whether stable CREB overexpression (limited to this specific region in the adult brain) would have a positive impact on spatial long-term memory in adult rats and attenuate spatial-memory impairments during normal aging.
Results
Construction and Expression Analysis of rAAV Vectors.
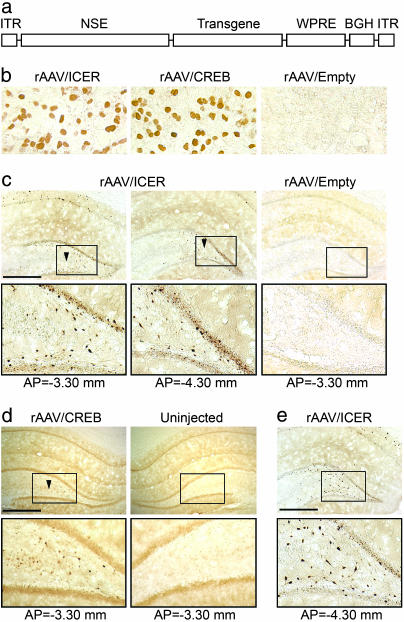
We constructed and packaged rAAV vectors expressing full-length rat CREB (rAAV/CREB), rat inducible cAMP early repressor (ICER) (rAAV/ICER), or an empty vector (rAAV/Empty) containing the identical expression cassette with no transgene. The CREB and ICER cDNA coding regions were N-terminally tagged with influenza virus hemagglutinin (HA) antigenic epitope sequence. The expression cassette for these vectors consisted of an 1,800-bp fragment of rat neuron-specific enolase (NSE) promoter; the transgene as described above; and, at the 3′ end, a 650-bp woodchuck hepatitis virus tripartite postregulatory element (WPRE) (14), followed by the bovine growth hormone polyadenylation signal (Fig. 1a).
Fig. 1.
Immunohistochemical analysis of rAAV vector expression. (a) rAAV expression cassette. ITR, inverted terminal repeats of AAV (120 bp), NSE (1,800 bp), and WPRE (650 bp); BGH, bovine growth hormone polyadenylation signal (250 bp). (b) HA immunoreactivity in human embryonic kidney 293 cells transduced with rAAV/CREB, rAAV/ICER, or rAAV/Empty vectors. (c and d) HA immunoreactivity within the rat brains that were transfected with rAAV/ICER, rAAV/Empty, or rAAV/CREB vectors. Animals were injected unilaterally into the hippocampus 4 weeks before immunohistochemistry analysis. Low-magnification (Upper) and high-magnification (Lower) images of the sections from different parts of the brain are shown. (e) Transgenic HA-ICER expression in aged animals (13 months after vector transfection). Anterior/posterior (AP) coordinates were measured from bregma. Arrows indicate the projection of the injection site with AP = −3.80 mm. (Scale bar, 1 mm.)
We have shown (15) that intraparenchymal rAAV vector infusion (2 μl, 5 × 109 particles) results in highly efficient neuronal transduction (≈5 × 104 cells) within a region extending 1–2 mm from the injection site. Expression of control GFP and Luc transgenes that are driven by the NSE-WPRE cassette reaches peak levels within 2–3 weeks and then remains stable for ≥15 months. Also, intracerebral infusion of rAAV is devoid of significant immunogenicity or toxicity, with no cell infiltration or inflammation at injection sites (15).
All rAAV vectors that were used in this study had similar physical and genomic titers (4 × 1012 particles per ml and ≈2 × 1012 genomes per ml, respectively). rAAV/CREB and rAAV/ICER vectors also demonstrate a similar transgene expression level in human embryonic kidney 293 (HEK293) cells at 3 days after transfection (Fig. 1b).
In a preliminary experiment, we also analyzed the in vivo transgene expression that was provided by the rAAV vectors injected unilaterally into the rat hippocampus by using both immunohistochemistry and Western blotting techniques. At 4 weeks after injection in rAAV/ICER-injected animals, transgene expression was robust and widespread throughout the hippocampus, including pyramidal cells in the CA fields, dentate granule cells (DGC), and hilar neurons, with transduced cells being found at distances of ≤1.5 mm from the injection site but confined to the dorsal hippocampus (Fig. 1c). Immunocytochemical detection of the transgenic CREB protein levels of rAAV/CREB transduced rats (as determined with an HA Ab) showed a similar distribution of expression with transduced cells in CA1, CA3, the hilus, and DGC layer; however, the number of immunoreactive cells was considerably less than that obtained in the ICER animals (Fig. 1d).
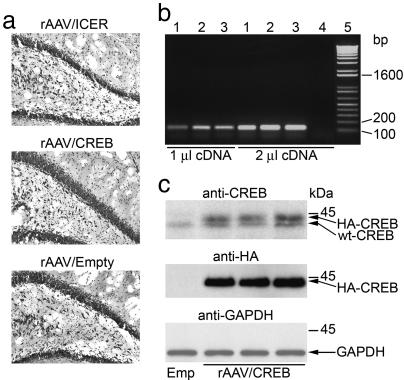
End-point RT-PCR analysis using primers specific to WPRE (a 3′ element that is common to all vectors) showed that transgenic mRNA level within the hippocampi of rAAV/CREB-injected animals is comparable with that of rAAV/ICER and rAAV/Empty groups (Fig. 2b). Cresyl violet staining also indicated no difference in numbers and the morphology of hippocampal neurons in the different groups of animals (Fig. 2a). Thus, the difference in in vivo expression of ICER vs. CREB is likely caused by nonsaturated degradation of CREB that compensates for its increased transcription and translation, thus maintaining relatively constant protein levels. To estimate the concentration of transgenic HA-CREB relative to endogenous CREB, we performed more sensitive Western blot analysis of extracts derived from the dorsal hippocampi of rAAV/CREB-injected animals by using both HA and WT CREB Abs. The analysis indicated that transgenic HA-CREB levels were comparable with those of endogenous WT CREB (Fig. 2c). Hence, a single bilateral injection of a high titer rAAV vector stock leads to an ≈2-fold increase in total CREB protein levels within the dorsal hippocampus.
Fig. 2.
In vivo rAAV vector expression analysis. (a) Cresyl violet staining in the hippocampus of animals that were transfected with rAAV/CREB, rAAV/ICER, or rAAV/Empty vectors. (b) End-point RT-PCR analysis of mRNAs that were derived from rat hippocampi injected with rAAV/Empty (lane 1), rAAV/ICER (lane 2), and rAAV/CREB (lane 3), or from an intact one (lane 4) by using 1 or 2 μl of cDNA templates and PCR primers specific to a 120-bp WPRE fragment along with the 1-kb Plus DNA ladder (GIBCO/BRL; lane 5). (c) Western blot analysis of protein extracts derived from the hippocampi of three rats injected with rAAV/CREB vectors with rAAV/Empty as a control by using CREB polyclonal Ab or HA or GAPDH mAbs. Representative hippocampal sections and electrophoretic patterns are shown.
Behavioral Analysis in Rats Transduced with rAAV Vectors.
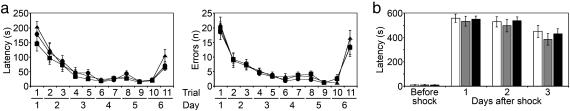
Three groups of young (8-week-old) adult male rats were injected bilaterally into the dorsal hippocampus with rAAV/CREB (n = 12), rAAV/ICER (n = 12), or rAAV/Empty vectors (n = 12). After 4 weeks, rats were tested blindly in both the Barnes circular table (1) and passive-avoidance response (16), which are tasks that are known to depend on an intact hippocampus. At the time of the analysis, animals were 3 months of age (referred to as young animals). All groups had similar ingestive behavior, body weight, and general motor activity as defined by line crossings in an open field (data not shown). Also, there was no difference in performance between the rAAV/CREB, rAAV/ICER, and rAAV/Empty groups in both the Barnes and passive-avoidance tasks (Fig. 3).
Fig. 3.
Behavioral analysis in young (3 months of age) animals. (a) Barnes circular-platform test latency (Left) and errors (Right). •, rAAV/CREB rats; ■, rAAV/ICER rats; ▴, control animals. (b) Passive-avoidance test. White bar, rAAV/CREB rats; gray bar, rAAV/ICER rats; black bar, control animals. (n = 12 per group for all tasks.)
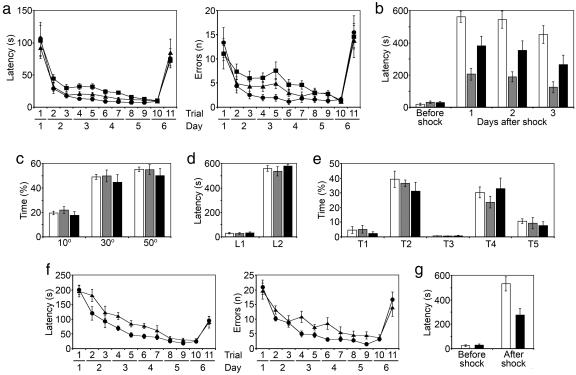
All animals were maintained for an additional 12 months under identical standard housing conditions (two rats per cage). At this time, with the rats being 15 months of age (referred as to old, or aged, animals), we repeated the behavioral analysis by using both the Barnes circular-table and passive-avoidance-response tasks. Also, all animals underwent an auditory cue fear conditioning test (17), a task that is largely independent of the hippocampus. Note that the older rAAV/CREB rats performed significantly better this time, whereas the performance of the rAAV/ICER animals was significantly worse compared with their equally aged empty vector controls in both the Barnes circular-table and passive-avoidance tasks (Fig. 4a and b). Statistical analysis of the Barnes data showed rAAV/CREB animals had reduced latencies to finding the escape hole during the first trials of days 3 and 4, compared with control group [ANOVA; trial 4, F(1,22) = 4.55, P = 0.044; trial 6, F(1,22) = 4.38, P = 0.048]. In contrast, rAAV/ICER rats had significantly longer latencies during the same trials [ANOVA; trial 4, F(1,22) = 10.55, P = 0.004; trial 6, F(1,22) = 8.10, P = 0.009]. The number of errors within each group during the experiment mirrored the latency results, but, because of greater variance, the data did not quite reach statistical significance [ANOVA for rAAV/CREB vs. control group; trial 4, F(1,22) = 3.169, P = 0.089; trial 6, F(1,22) = 3.459, P = 0.076]. During the final probe trial (day 6, trial 11) in which the spatial location of the escape dark box was rotated by 140°, both latency and number of errors were increased with no significant difference between the groups (Fig. 4a). Remarkably, all groups of animals spent >50% of time during this trial in the sector where the escape box was previously located (Fig. 4c). This observation indicates that all groups of animals had equal motivation and ability to locate a newly positioned escape chamber.
Fig. 4.
Behavioral analysis in old (15 months of age) animals. (a) Barnes circular-platform test latency (Left) and errors (Right). (b) Passive-avoidance test. (c) Time spent by old animals searching for escape box during the last probe trial in the sectors of 10°, 30°, and 50° symmetrically covering previous location of the escape box. (d) Short-term memory in old rats in passive-avoidance test. L1, initial latency before shock; L2, latency to enter dark 24 h after shock. (e) Auditory cue fear conditioning test in old rats. Time spent freezing is indicated as follows: T1, during the auditory cue (a 70-dB tone) delivered for 28 s and preceding the electric shock; T2, within the 1-min interval immediately after the 2-s shock cued by the tone; T3, during the 1-min interval in new context 24 h after the electric shock; T4, during the 2 min of cued tone given 24 h after the electric shock in new context; and T5, within the 2-min interval after the cued tone and 24 h after the electric shock in new context. (f and g) Barnes circular-platform (f) and passive-avoidance (g) tests in rats injected with rAAV vectors at 4 weeks of age and subjected to the test for the first time at 15 months of age. •, rAAV/CREB rats; ■, rAAV/ICER rats; ▴, control animals. White bar, rAAV/CREB rats; gray bar, rAAV/ICER rats; black bar, control animals. (n = 12 per group for all tasks.)
In the passive-avoidance response, animals learn to associate an aversive stimulus with the surrounding context. Aged rAAV/CREB-injected animals also demonstrated better memory in this task, such that there was a significantly longer latency to enter the dark (previously shocked) chamber compared with control group (P < 0.05; Fig. 4b). In contrast, the latencies of the ICER animals were lower than those of the control groups, consistent with poor memory. The differences between groups remained significant at both 24 h and 3 days after the initial exposure.
Long-term memory in the passive-avoidance task usually lasts in rats for 1–2 weeks, with the latency dropping to basal levels within this period. This finding allowed us to repeat the task with the aged rats 3 weeks later to check their short-term memory. We found no difference between the groups in latency to enter the dark chamber at 10 min after the shock (Fig. 4d).
In the cued-fear-conditioning task, a non-hippocampus-dependent paradigm, there was no difference between the ability of all groups of rats to associate the aversive stimulus with the auditory cue (Fig. 4e). Also, this last test suggests that there is no difference in pain sensitivity, and, coupled to the open-field behavior, it suggests that the CREB rats have no significant changes in motor, sensory, or motivational behavior.
Behavioral Analysis in Aged Rats That Did Not Undergo Previous Testing at 3 Months of Age.
The decrease in the first trial average latency in aged rats (Figs. 3a and 4a) could be caused by previous training and the development of an improved search strategy.
To investigate this possibility we performed an additional experiment in which two groups of animals (n = 12) were also injected with the rAAV/CREB or rAAV/Empty control vectors at 4 weeks of age. However, in contrast to the previous experiment, these animals were subjected to the Barnes and passive-avoidance tasks for the first time when they reached 15 months of age. Fig. 4 f and g show that, in this experiment, the aged rAAV/CREB animals also performed better than the control group in both tasks. CREB-injected animals demonstrated significantly lower latencies and numbers of errors during trials 4 and 6 (the first trials of days 3 and 4) in the Barnes task (Fig. 4f). In the passive-avoidance test, they had significantly greater latency to enter the dark chamber at 24 h after the aversive stimulus (Fig. 4g).
Transgene Expression Analysis in Aged Rats.
After completion of the behavioral tests, we performed expression analysis in the brains of aged rats injected with CREB and ICER rAAV vectors by using rAAV/Empty rats as a control.
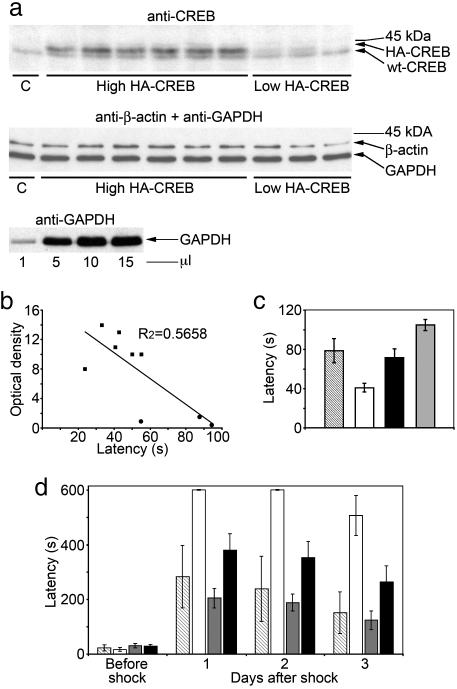
Immunohistochemical analysis showed that ICER transgene expression in the hippocampi of aged rats remained robust and widespread even 13 months after gene transfer with an expression pattern similar to those observed in young animals analyzed at 4 weeks after vector injection (Fig. 1e). For quantitative analysis of transgene HA-CREB expression in aged animals, we used Western blot analysis. Cells extracts that were derived from the whole hippocampi of nine rats that were transduced with rAAV/CREB vector were analyzed by using both CREB and HA Abs. We found that six of nine animals had high levels of HA-CREB in their hippocampi (High HA-CREB subgroup), whereas transgenic CREB levels within the three remaining rats was extremely low (Low HA-CREB subgroup; Fig. 5a). Such large differences in transgenic CREB expression between the animals provided the opportunity to perform a correlational analysis to determine whether CREB protein levels might be associated with the individual performance of the rats in the behavioral tasks. To do so, we combined individual latencies that were shown by each of the rAAV/CREB-transduced rats on the Barnes maze during trials 2, 4, 6, and 8 (the first trials of days 2–5), which indicate the long-term memory of the animals. Within the entire group of nine rAAV/CREB-transduced rats there was a strong negative correlation (r = −0.75) of CREB protein levels with individual combined latencies (Fig. 5b). In contrast, within the High or Low HA-CREB subgroups, which included animals with relatively high or low transgenic CREB expression respectively, there was no correlation (r = 0.06 and r = −0.09, respectively) of combined latencies with the endogenous CREB protein levels (Fig. 5b). Thus, the strong negative correlation within the entire CREB group is due to significant differences in CREB protein levels and combined latencies between the subgroups. We performed statistical analysis of combined latency in those two subgroups as well as in rAAV/ICER and rAAV/Empty groups. We found significant difference in combined latencies between two subgroups of rAAV/CREB with high and low CREB expression [ANOVA; F(1,7) = 12.69, P = 0.01)], although the Low CREB subgroup contained only three animals (Fig. 5c). More importantly, the difference between the High CREB subgroup and control animals was even more significant [ANOVA; F(1,16) = 6.28, P = 0.023] compared with the differences shown by entire CREB group in any one of the single trials during the Barnes test. The difference in combined latencies between ICER and control groups was also significant [ANOVA, F(1,22) = 9.65665329, P = 0.005; Fig. 5c].
Fig. 5.
Postbehavioral in vivo transgene expression analysis. (a) Western blot analysis of hippocampus extracts derived from the rAAV/CREB-transfected animals with CREB or β-actin and GAPDH Abs. Animals expressing high or low levels of transgenic HA-CREB are indicated along with the control rAAV/Empty-transfected animal (C). Immunoblotting of increasing amounts of a hippocampus lysate with GAPDH Ab is also shown. (b) Correlation analysis of relative concentration of transgenic HA-CREB in rAAV/CREB-transfected rats with their individual combined latencies shown in the Barnes test. (c) Statistical analysis of combined latencies shown by all groups of animals in the Barnes task. (d) Comparative analysis of the latencies shown in the passive-avoidance task by rats expressing high or low levels of transgenic HA-CREB along with the other groups of animals. ■, high HA-CREB animals; •, low HA-CREB animals. Hatched bar, rAAV/CREB rats expressing low levels of HA-CREB; white bar, rAAV/CREB rats expressing high levels of HA-CREB; gray bar, rAAV/ICER rats; black bar, control animals.
Also, we compared the performance of both HA-CREB subgroups in the passive-avoidance task. There were significant differences between the High CREB subgroup and the Low CREB subgroup [ANOVA; F(1,7) = 14.32, P < 0.01], ICER [ANOVA; F(1,22) = 8.49, P < 0.01], or control [ANOVA; F(1,16) = 7.46, P < 0.01] groups (Fig. 5d). Similar to the results of the Barnes maze performance, in the passive-avoidance test, the High CREB subgroup demonstrated an even bigger difference in latency compared with the ICER and control groups than that found with the total CREB group analysis (Fig. 4b).
Discussion
A study using an amplicon herpes simplex virus system (12) has shown that transient overexpression of CREB in the basolateral complex of the amygdala facilitates long-term-memory formation in the fear-potentiated startle paradigm after massed training, which normally induces short-term memory only. However, more robust long-term memory produced by spaced training in control animals was not further increased by the CREB overexpression.
In our study using different behavioral paradigms, we have shown that CREB overexpression within the hippocampus does not alter spatial long-term memory in young (3 months of age) rats. However, at 15 months of age, CREB-transduced animals performed significantly better than control aged rats. A recent report has shown that there is a reduction in CREB protein levels in the hippocampus of aged rats with impaired spatial memory (13). These data suggest that CREB gene transfer might simply compensate for the decrease in CREB protein concentration within the cell that occurs during normal aging. However, the actual molecular mechanism of age-related long-term-memory impairment is likely to be more complicated. For example, deficits in the late phase of long-term potentiation can be attenuated by drugs positively affecting the cAMP signaling pathway without changing absolute CREB protein concentration (3). Expression of CREB-binding protein (CBP), one of the critical components of CREB-mediated transcription, whose expression is not directly regulated by CREB, is also reduced in the aged brain (18). Also, a recent study (19) in rats and monkeys indicates that the cAMP/PKA pathway is activated within the prefrontal cortex of aged animals with cognitive deficits. Thus, the attenuation of the memory changes associated with normal aging achieved in this study by elevation of CREB protein above normal levels is most likely to be due to compensation for deficits in the activities of many important components of the long-term-memory machinery and may be hippocampus-specific.
We found that a subgroup of aged rAAV/CREB-transduced rats that expressed relatively high levels of the transgene CREB demonstrated improved performance in the Barnes task, with significantly lower latencies compared with both the control group and those rAAV/CREB transduced animals with low levels of transgenic CREB expression. However, within the High HA-CREB subgroup there was no correlation of CREB protein concentrations with the combined latencies. These data suggest the effect of saturation, or a threshold effect, in CREB protein concentrations that is required for up-regulation of long-term-memory formation. We hypothesize that young animals are “saturated” with most of the critical components that are involved in the process of long-tem-memory formation. Decline in activities in one or more of these components occur during normal aging and might be compensated for by suprathreshold CREB protein concentration obtained by means of somatic-cell gene transfer.
It is of special interest that ICER overexpression also did not change long-term memory in young animals. Although the transgenic ICER expression within the rAAV/ICER-transduced rats was robust and widespread it still did not involve the entire population of neurons within the hippocampus. In young animals, those untransfected neurons (or even those with low ICER expression) may have enough capacity to maintain normal function of the long-term-memory machinery. However, in old animals, which likely experience global deficits in activities of CREB signaling, the expression of ICER interferes with long-term memory because the nontransduced cells are no longer are able to compensate, consistent with the theory of a reduced memory reserve or capacity in aged animals.
In our longitudinal study design, we did not compare spatial memory in aged CREB-transduced rats vs. a young control group directly. However, the latencies showed by the aged CREB-transduced rats in the passive-avoidance task were comparable with those of young animals that were analyzed 12 months earlier (Figs. 3b and 4b). Unfortunately, it was not possible to use the Barnes maze for such a comparison, because this task recruits two different types of animals’ performance, spatial navigation memory and an effective search strategy, both of which influence the latency and number of errors. In general, the aged animals showed lower latency and number of errors in this task compared with their performance as young rats (Figs. 3a and 4a). This decrease in the first trial average latency in aged rats could be caused by previous training and the development of an improved search strategy. Nevertheless, in aged rats who never underwent prior training, the rAAV/CREB animals also performed better than the control group in both tasks (Fig. 4 f and g). Note that the average Barnes platform latencies of both groups were higher than the corresponding latencies of the previously trained aged rats (Fig. 4a) and were similar to those of their young untrained counterparts (Fig. 3a). It is also important to note that during the last probe trial when the position of the escape box was rotated by 140°, all groups of both young and aged animals demonstrated similar average latencies in all Barnes tests.
These results suggest that previous training in the Barnes task leads to the development of an improved search strategy, which might influence the average latency in finding the escape box. However, such training does not affect the differences between the rAAV/CREB and control groups, which remain significant regardless of whether the animals were trained previously or not. This experiment also shows that prior training on the Barnes platform at a young age does not significantly contribute to the attenuation of age-related memory loss.
A major finding of this study is that elevated hippocampal CREB levels achieved by somatic gene transfer can prevent memory loss during normal aging. However, one of the most important remaining questions is whether this positive effect is a result of long-term plasticity associated with prolonged CREB overexpression during the life of the animals or whether a similar effect could also be achieved by acute CREB gene transfer into aged rats that already exhibit impaired spatial memory. CREB, as a transcription factor, acts by altering the expression of many genes, including other transcription factors. The identification of genes whose expression is modulated by CREB gene transfer, coupled with a combined genetic and behavioral approach to determine which downstream genes mediate the effect of hippocampal CREB gene transfer, remains to be accomplished.
Note that the increased transgene CREB transcription evidenced by RT-PCR analysis was not reflected in a similar increase in protein immunoreactivity under basal conditions (Fig. 2). The recent finding of phosphorylation-dependent CREB protein ubiquitination and proteasomal degradation in hypoxic epithelial cells (20) shows that cellular levels of CREB protein may be regulated tightly and maintained at a relatively constant level. However, even as minor an increase in CREB protein as achieved in our experiments resulted in a strong positive effect on spatial long-term memory in aged rats.
Together, our studies further support for the hypothesis that CREB functions to link environmental tasks to long-term brain plasticity and memory consolidation, and they raise the possibility that strategies leading to increased hippocampal CREB expression may be useful in treating neurodegenerative disorders of learning and memory.
Methods
Generation of Transducing rAAV Vectors.
Packaging of rAAV transducing vectors was carried out by using standard calcium phosphate cotransfection of human embryonic kidney 293 cells with the following three plasmids: the rAAV expression vector; the packaging plasmid, ACG2; and the helper plasmid pXX6, which contains all of the genes necessary for AAV helper-free packaging (21). Virus purification included ultrasonic cell destruction in the presence of Freon 113, followed by low-speed centrifugation (5,000 × g for 10 min) and filtration of virus-containing supernatant through a 0.2-μm membrane filter.
Animal Surgery and in Vivo Gene-Expression Analysis.
The experiments were carried out in accordance with standard guidelines for animal care and use of the University of Auckland Animal Ethics Committee. Male Wistar rats (280–300 g; 8–10 weeks old) were anesthetized with ketamine/xylazine (70:7 mg/kg, i.p.). We injected 2 μl of vector (5 × 109 viral particles) plus 1 μl of 20% mannitol bilaterally into the hippocampus. The coordinates were measured from bregma by using the Paxinos and Watson rat brain atlas at −3.8 mm AP, 2.0 mm mediolateral, and −3.4 mm dorsoventral from the skull. The animals were left to recover for 4 weeks before behavioral analysis. Antigen detection in 40-μm coronal sections was performed as described in ref. 22. Synthetic primers complementary to WPRE (common DNA element for all expression cassettes) were used for RT-PCR analysis of transgene mRNA expression.
Western Blot Analysis.
Rat hippocampi were dissected and subjected to ultrasonic disintegration in saline solution, followed by centrifugation at 20,000 × g. Supernatants were analyzed by Western blotting using enhanced chemiluminescence detection reagents (Amersham Pharmacia Biotech). As internal protein controls, we used both β-actin and GAPDH, which were detected with specific mAbs (Abcam, Cambridge, MA). The quantitative analysis of the images was carried out by using quantity one software (version 4.1.1; Bio-Rad).
RT-PCR Analysis.
Total mRNA was isolated from dissected rat hippocampi by using TRIzol reagent (GIBCO/BRL). For crudely quantitative analysis of transgene mRNA levels in rat hippocampi, we used end-point RT-PCR assay as described in the SuperScript II protocol (GIBCO/BRL). Especially designed primers provided amplification of a 120-bp fragment from WPRE, which is an element common for all expression vectors. Number of PCR cycles as well as a concentration of cDNA template was optimized to avoid product saturation and obtain more precise quantification.
Behavioral Tests.
All tests were carried out blindly with the same groups of animals. The Barnes circular-maze task was conducted in accordance with the methods originally described and validated by Barnes (1). Testing lasted for 6 days, with the first day consisting of one trial and all subsequent days consisting of two trials per day, each separated by a 1-min interval. The final trial was a probe trial, in which the spatial location of the escape box was rotated by 140°. In the second Barnes test with the aged animals (which was carried out 13 months later), the initial position of the escape box was rotated by 270° and then by an additional 140° during the last probe trial. The apparatus for the passive-avoidance task (16) consisted of two identical chambers, one illuminated and one dark. Rats were initially placed in the light chamber, and the latency to enter the dark chamber was recorded. When the four paws of the rat were inside the chamber, an electric shock of 0.8 mA (duration, 2 s) was delivered. After 24 h, the rat was returned to the light chamber, and the latency to enter the dark chamber was recorded. In the auditory-cue fear-conditioning test (17), rats were placed in a fear-conditioning box where they received a shock of 0.8 mA (duration, 2 s), which was preceded by a tone (70 dB; duration, 28 s). After 24 h, rats were placed in a novel chamber. After the first minute, the tone was sounded for 2 min, followed by an additional 2 min of silence. Freezing was scored as the percentage of 5-s intervals that were spent frozen within each period.
Acknowledgments
We thank Dr. J. Lipski for helpful suggestions, technical advice, and manuscript review. We also thank Drs. M. Montminy (Salk Institute, La Jolla, E CA) for rat CREB cDNA, J. G. Sutcliffe (Scripps Clinic, La Jolla, CA) for the rat NSE promoter fragment, T. Hope (Salk Institute) for the WPRE plasmid, and X. Xiao (University of Pittsburgh, Pittsburgh) and R. J. Samulski (University of North Carolina, Chapel Hill) for the pXX6 and pACG-2 plasmids. This work was supported in by the New Zealand Marsden Fund, the New Zealand Health Research Council, and the National Institutes of Health.
Abbreviations
- CREB
cAMP response element-binding protein
- ICER
inducible cAMP early repressor
- rAAV
recombinant adeno-associated virus
- HA
hemagglutinin
- AP
anterior/posterior
- NSE
neuron-specific enolase
- WPRE
woodchuck hepatitis virus tripartite postregulatory element.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Barnes C. A. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher M., Rapp P. R. Annu. Rev. Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- 3.Bach M. E., Barad M., Son H., Zhuo M., Lu Y. F., Shih R., Mansuv I., Hawkins R. D., Kandel E. R. Proc. Natl. Acad. Sci. USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapp P. R., Kansky M. T., Roberts J. A. NeuroReport. 1997;8:1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- 5.Newman M. C., Kaszniak A. W. Aging Neuropsychol. Cogn. 2000;7:86–93. [Google Scholar]
- 6.Dash P. K., Hochner B., Kandel E. R. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 7.Yin J., Wallach J. S., Del Vecchio M., Wilder E. L., Zhou H., Quinn W. G., Tully T. Cell. 1994;81:107–115. [Google Scholar]
- 8.Yin J., Del Vecchio M., Shou H., Tully T. Cell. 1995;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 9.Bourtchuladze R., Frenguelli B., Blendy J., Cioffi D., Schutz G., Silva A. J. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger C., Huang Y. Y., Paletzki R. F., Bourtchouladze R., Scanlin H., Vronskaya S., Kandel E. R. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez G. A., Montminy M. R. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 12.Josselyn S. A., Shi C., Carlezon W. A., Jr, Neve R. L., Nestler E. J., Davis M. J. Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brightwell J. J., Gallagher M., Colombo P. J. Neurobiol. Learn. Mem. 2004;81:19–26. doi: 10.1016/j.nlm.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Donello J. E., Loeb J. E., Hope T. J. J. Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu R., Janson C. G., Mastakov M., Lawlor P., Young D., Mouravlev A., Fitzsimons H., Choi K.-L., Ma H., Dragunow M., et al. Gene Ther. 2001;8:1323–1332. doi: 10.1038/sj.gt.3301529. [DOI] [PubMed] [Google Scholar]
- 16.Cammarota M., Bevilaqua L. R., Ardenghi P., Paratcha G., Levi de Stein M., Izquierdo I., Medina J. H. Brain Res. Mol. Brain Res. 2000;76:36–46. doi: 10.1016/s0169-328x(99)00329-0. [DOI] [PubMed] [Google Scholar]
- 17.Bach M. E., Hawkins R. D., Osman M., Kandel E. R., Mayford M. Cell. 1995;81:905–915. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 18.Chung Y. H., Kim E. J., Shin C. M., Joo K. M., Kim M. J., Woo H. W., Cha C. I. Brain Res. 2002;956:312–318. doi: 10.1016/s0006-8993(02)03562-x. [DOI] [PubMed] [Google Scholar]
- 19.Ramos B. P., Birnbaum S. G., Lindenmayer I., Newton S. S., Duman R. S., Arnsten A. F. T. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 20.Taylor C. T., Furuta G. T., Synnestvedt K., Colgan S. P. Proc. Natl. Acad. Sci. USA. 2000;97:12091–12096. doi: 10.1073/pnas.220211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao X., Li J., Samulski R. J. J. Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young D., Lawlor P. A., Leone P., Dragunow M., During M. J. Nat. Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]