Abstract
Background
The aim of the present study was to determine whether stage of invasiveness of bladder cancer cell lines contributes to alterations in glycan pattern of their cadherins.
Results
Human non-malignant epithelial cell of ureter HCV29, v-raf transfected HCV29 line (BC3726) and transitional cell cancers of urine bladder Hu456 and T24 were grown in cell culture. Equal amounts of protein from each cell extracts were separated by SDS-PAGE electrophoresis and were blotted on an Immobilon P membrane. Cadherins were immunodetected using anti-pan cadherin mAb and lectin blotting assays were performed, in parallel. N-oligosaccharides were analysed by specific reaction with Galanthus nivalis agglutinin (GNA), Sambucus nigra agglutinin (SNA), Maackia amurensis agglutinin (MAA), Datura stramonium agglutinin (DSA), Aleuria aurantia agglutinin (AAA), Phaseolus vulgaris agglutinin (PHA-L) and wheat germ agglutinin (WGA). The cadherin from HCV29 cell line possessed bi- and/or 2,4-branched triantennary complex type glycans, some of which were α2,6-sialylated. The cadherin from BC3726 cell line exhibited exclusively high mannose type glycans. Cadherins from Hu456 and T24 cell lines expressed high mannose type glycans as well as β1,6-branched oligosaccharides with poly-N-acetyllactosamine structures and α2,3-linked sialic acid residues. Additionally, the presence of fucose and α2,6-sialic acid residues on the cadherin from T24 cell line was detected.
Conclusions
These results indicate that N-glycosylation pattern of cadherin from bladder cancer cell line undergoes modification during carcinogenesis.
Background
Cadherins comprise a family of calcium-dependent transmembrane cell-cell adhesion molecules, generally thought to be homophilic cell adhesion proteins [1]. The homophilic binding of cadherins is regulated by extracellular and intracellular signals, which modulate cadherin activity [2] without a concomitant changes in cadherin expression. Nevertheless, the signals that modulate cadherin activity are not completely characterized.
Being cell surface proteins, cadherins are glycosylated [3]. Protein-linked carbohydrates determine protein stability, activity and specificity of interaction, and they are also involved in cell-cell and cell-matrix recognition [4]. Disturbances in cadherin-based adhesion contribute to tumor progression in a range of epithelium tumors [5]. Yoshimura et al. [6] have shown that the suppression of metastasis in murine melanoma B16-hm cells expressing N-acetylglucosaminyltransferase III was at least partly due to increased level of glycosylated E-cadherin. The authors imply that glycosylation is one of the important events in the process of metastasis.
Despite the increasing number of studies on structure and biological function of cadherins, little is known about carbohydrate chains structure of these glycoproteins and its role. It is indispensable for us to know the changes in glycosylation pattern of adhesion molecules, for example cadherins, in both normal and malignant tissues in order to promote a better understanding of the roles of these carbohydrate structures in physiological and pathological processes. Thus, the aim of our study was to perform a preliminary characterization of carbohydrate structure of cadherins from human non-malignant epithelial cells of ureter (HCV29), v-raf transfected HCV29 cell line (BC 3726) and human transitional cell cancers of urinary bladder (Hu456 and T24) using highly specific digoxigenin-labeled lectins.
Results
The results of glycan chain analysis of cadherins are shown in Fig. 1. The specificities of the lectins used are summarized in Table 1. The results revealed some differences in glycosylation patterns of cadherins from normal and cancer cell lines. The presence of high mannose type oligosaccharides was ascertained in cadherins from BC3726, Hu456 and T24 cell lines as indicated by the positive reaction with GNA. The specific reaction with PHA-L indicated the existence of GlcNAcβ1,6-branched triantennary and/or tetraantennary complex type glycans on cadherins from Hu456 and T24 cell lines. The DSA binding to cadherin from HCV29 cell line indicated the presence of terminal disaccharide(s) Galβ1,4GlcNAc (N-acetyllactosamine unit) in biantennary complex type and/or in 2,4-branched triantennary species. Moreover, positive reaction with both DSA and PHA-L, observed for cadherins from bladder cancer Hu456 and T24 cell lines, suggested the presence of poly-N-acetyllactosamine units on 2,6-branched triantennary and/or tetraantennary structures. The sialic acid residue(s) occupying the terminal position in N-linked oligosaccharides of cadherins were found to be in Galα2,3-linkage in Hu456 and T24 cell lines (positive reaction with MAA), or in Galα2,6-linkage in HCV29 and T24 cell lines (positive reaction with SNA). Additionally, positive reaction with AAA confirmed the existence of fucose residue(s) on cadherin from T24 cell line. AAA binds Fucα1,6-linked to the proximal GlcNAc residue as well as a Fucα1,2Galβ1,4GlcNAc- sequence (blood group H(O) determinant) and a Galβ1,4(Fucα1,3)GlcNAc- sequence (Lex determinant) [7]. No evidence for the presence of bisected species and/or branching poly-N-acetyllactosamine species on examined cadherins from all cell lines was found (negative reaction with WGA, data not shown) [7].
Figure 1.
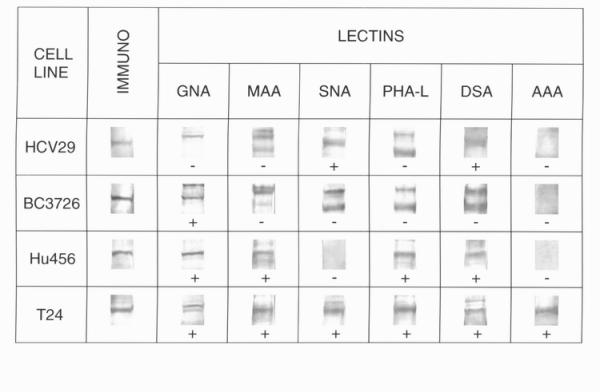
Extracts from cell lines: HCV29, BC3726, Hu456 and T24 (100 μg of total protein) were run on 8% PAGE/SDS and blotted onto Immobilon P membrane and probed with lectins: GNA, MAA, SNA, PHA-L, DSA and AAA. Immuno-cadherin probed with anti-pan cadherin mAbs. (+) Positive reaction with lectin, (-) negative reaction with lectin.
Table 1.
Sugar binding specificities of lectin used for lectin blotting studies (lectin assays).
| Lectin | Lectin-specific glycan structure |
| GNA | Manα1,2Man- |
| Manα1,6Man- | |
| Manα1,3Man- | |
| SNA | NeuAcα2,6Gal- |
| MAA | NeuAcα2,3Gal- |
| DSA | Galβ1,4GlcNAc- |
| AAA | Fucα1,6GlcNAc-Asn |
| Fucα1,2Galβ1,4GlcNAc- | |
| Galβ1,4(Fucα1,3)GlaNAc- | |
| PHA-L | Galβ1,4GlcNAcβ1,6(Galβ1,4GlcNAcβ1,2)Manα- |
| WGA | GlcNAcβ1,4Man- |
| NeuAαGal- | |
| GlcNAcβ1,4GlcNAc- |
Immunodevelopment of the Western blots of protein cell extracts with anti-pan cadherin antibodies revealed a position of cadherin molecules on the blots and it allowed to estimate the molecular weight of examined glycoproteins. Cadherin from non-malignant HCV29 cell line showed lower apparent molecular weight (130 kDa) than its cancer counterparts (131 kDa in Hu456 and 135 kDa in BC3726 and T24). These findings are consistent with well-documented phenotypic alternation of the transformed cells [8].
Discussion
Previously, we had established that the adhesion molecules, expressed in all mentioned above cell lines, which reacted with anti-pan cadherin monoclonal antibodies were N-cadherins except the HCV29 non-malignant ureter cell line [9]. In this cell line only trace amounts of N-cadherin were detected. Moreover, neither this nor any other examined cancer cell lines expressed E-cadherin [9]. Frixen et al. [10] found that differentiated human cancer cell lines, including bladder cell lines, generally expressed E-cadherin and were noninvasive in vitro, whereas dedifferentiated cell lines did not express this cell-cell adhesion molecule and were invasive. It is well-documented that, in a wide range of cancers, E-cadherin expression is loss or downregulated, resulting in a reduced level of intracellular adhesion, and perturbation in E-cadherin-mediated cell adhesion is involved in tumor progression and metastasis [7,11-15]. Giroldi et al. [16] showed that N-cadherin become predominantly expressed in bladder-cancer cell lines that have lost E-cadherin expression.
Mialhe et al. [17] observed that T24 cells were poorly differentiated but had yet a typical epithelial morphology. T24 cells possessed an N-cadherin-dependent adhesivness. The authors found that T24 cells lacked completely E-cadherin expression. These findings suggested that N-cadherin could play a role in bladder carcinogenesis, especially in E-cadherin-negative, poorly differentiated cells. In various cancers [18-20]), including bladder cancer reduced E-cadherin expression has been shown to correlate with progression of disease. Mialhe et al. [17] hypothesized that, N-cadherin expressed in T24 bladder cell, a highly invasive tumor, could play a major role in acquisition of invasive phenotype. Thus, it is not surprising, that bladder cancer cell line which we examined did not express E-cadherin molecules. However, the lack of E-cadherin expression in the non-malignant HCV29 cell line was not expected since normal epithelium strongly expresses E-cadherin [21]. It is conceivable that a lack of E-cadherin may be a phenomenon characteristic for in vitro culture of HCV29 cell line [9].
It is well known that tumorigenesis and metastasis are frequently associated with altered structure and expression of oligosaccharides on cell surface glycoproteins and glycolipids [8,22-25]. Therefore, we suspected that the cadherins from human cancers cell lines originated from ureter and bladder tissues, might represent, in comparison with cadherin from non-malignant HCV29 cell line different glycosylation patterns. Our study confirmed this expectation and demonstrated that cadherin from non-malignant HCV29 cell line possessed bi- and/or 2,4-branched triantennary complex type glycans, some of which were α2,6-sialylated. The glycosylation pattern observed for N-cadherin from BC3726 cell line was, in comparison with cadherin from parental HCV29 cell line, dramatically different. Interestingly, this N-cadherin possessed exclusively high mannose type glycans. This finding indicated that v-raf transfection of parental cells suppressed the synthesis of complex type glycans on N-cadherin molecules. This may cause disturbance in intercellular interaction mediated by these adhesion molecules.
On the contrary, bladder cancer cells of Hu456 and T24 cell lines showed ability to generate more complex glycans on cadherins than HCV29 cell line. The basic N-oligosaccharide structures recognised on cadherins from bladder cancer Hu456 and T24 cell lines were high mannose type as well as 2,6-branched tri- and/or tetrantennary poly-N-acetyllactosamine complex type oligosaccharides. Some of complex species were α2,3- or α2,3- and α2,6-sialylated in N-cadherins from Hu456 or T24 cell lines, respectively. Additionally, N-cadherin from highly invasive tumor T24, possessed also core fucosylated and/or Fucα1,2 and Fucα1,3 substituted carbohydrates. It has been demonstrated that the presence of poly-N-acetyllactosamine structures appears to be essential for metastatic potential of lymphoid tumor cell line and sublines of human colon cancers [26]. Also, increased branching of N-linked glycans is a common feature of most malignant cells. In several model systems, malignant transformation, tumor cell invasiveness and metastatic potential were shown to be associated with increased levels of GlcNAcβ6Manα6Manβ4-R branches of complex N-glycans [27]. It was suggested that poly-N-acetyllactosamine chains contributed to the metastatic potential by diminishing cell-substratum adhesion and thereby facilitating tumor cell invasion [27]. Examining the glycosylation pattern of Hu456 and T24 we could observe that the higher-grade classification had a cell line in which the glycosylation pattern was more changed. The donor of Hu456 cells was a male patient, aged 72, with urinary bladder cancer grade I [28], whereas the line T24 was obtained from a 82-year-old female patient with urinary bladder cancer grade III [29]. Both cancer lines were enriched in high mannose type glycans, but only cadherins from T24 were core fucosylated and also possessed α2-6 linked glycans. Thus, fucosylation and α2,6-sialylation in bladder cancer correlate with poor prognosis and patient survival. We found quite similar results for N-cadherins from human metastatic melanoma cell lines [30].
At present we can only speculate on the physiological role that the cadherins glycans play. Although it is well established that covalentely linked oligosaccharide chains can be involved in such fundamental biological process as cellular adhesion, their role in the case of individual glycoprotein is usually enigmatic. Moreover, more invasive cell lines are characterized by fewer cell-to-cell junctions. It was suggested that N-cadherin mediates a less stable or more dynamic intercellular adhesion than that of E-cadherin and may make possible detachment and heterotypic interactions with surrounding cells. We postulate that altered glycosylation can repel intercellular interaction and sterically prevent cell adhesion molecules such as cadherins from achieving intermolecular distances necessary for effective interactions.
Materials and Methods
Subjects
Human bladder cancer cell lines (Hu456, T24, BC3726) and human non-malignant ureter epithelium cell line (HCV29) were kindly donated by Prof. Danuta Duś, Institute of Immunology and Experimental Therapy, PAN, Wrocław, Poland. All cell lines were cultured and cell extract proteins were prepared as described in [9].
SDS-PAGE and Western blotting
Equal amounts of total protein (100 μg) from all cell extracts were electrophoresed on 8% SDS-polyacrylamide gels under reducing conditions according to Laemmli [31]. Western-blotting on PVDF membranes (Millipore) was performed according to [32] at 250 mA for 18 h at 4°C.
Immunodetection of cadherins
The immunodetection of cadherins was performed with a 1/500 dilution of mouse anti-pan cadherin monoclonal antibodies (Sigma) in 0.1% Tween/TBS, 1% BSA for 18 hs at room temperature and with alkaline phosphate coupled goat anti-mouse immunoglobulin (Roche) (a 1/500 dilution of IgG in 0.1% Tween/TBS, 1% BSA) for 1 h at room temperature.
Lectin assays
Glycan chains analysis of cadherins was performed according to the procedure described by the manufacturer of the Glycan Differentiation Kit (Roche).
Acknowledgments
Acknowledgements
This work was supported by the State Committee for Scientific Research (KBN, Poland) grant No. 6 P04A 021 20, and the Institute of Zoology, Jagiellonian University (BW/IZ/2001, DS/22/IZ/2002).
Contributor Information
Małgorzata Przybyło, Email: kloc@zuk.iz.uj.edu.pl.
Dorota Hoja-Lukowicz, Email: hoja@zuk.iz.uj.edu.pl.
Anna Lityńska, Email: lita@zuk.iz.uj.edu.pl.
Piotr Laidler, Email: mblaidle@cyf-kr.edu.pl.
References
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Williams CL. Regulation of cadherin activity by G protein and the actin cytoskeleton (DSG). In: Cowin C, Klymkowsky MW, editor. Cytoskeletal-membrane interactions and signal transduction. Austin, R. G. Landes Company and Chapman & Hall; 1997. pp. 111–126. [Google Scholar]
- Gahmberg CG, Tolvenen M. Why mammalian cell surface proteins are glycoprotein. TiBS. 1996;21:308–311. doi: 10.1016/0968-0004(96)10034-7. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:1297–1340. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS. The morphogenic role of cadherin cell adhesion molecules in human cancer: a thematic review. Cancer Invest. 1998;16:252–261. doi: 10.3109/07357909809039774. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Ihara Y, Matsuzawa Y, Taniguchi N. Aberrant glycosylation of E-cadherin enhances cell-cell binding to supress metastasis. J Biol Chem. 1996;271:13811–13815. doi: 10.1074/jbc.271.34.20265. [DOI] [PubMed] [Google Scholar]
- Mareel M, Noë V, Vermeulen S, Bracke M. Anti-invsive therapy: manipulation of the E-cadherin/catenin/cytoskeleton complex. Anti-Cancer Drugs. 1996;7:149–156. [Google Scholar]
- Laidler P, Lityńska A. Tumor cell N-glycans in metastasis. Acta Biochem Pol. 1997;44:343–358. [PubMed] [Google Scholar]
- Laidler P, Gil D, Pituch-Noworolska A, Ciołczyk D, Książek D, Przybyło M, Lityńska A. Expression of β1-integrins and N-cadherin in bladder cancer and melanoma cell lines. Acta Biochim Pol. 2000;47:1159–1170. [PubMed] [Google Scholar]
- Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, A Warda, Löchner D, Birchmeier W. E-Cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RE, Porter WH, Whelan P, Smith PH, Selby PJ. Soluble forms of the adhesion molecule E-cadherin in urine. J Clin Pathol. 1995;48:179–180. doi: 10.1136/jcp.48.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareel M, Berx G, van Roy F, Bracke M. Cadherin/catenin complex: a target for antiinvasive therapy? J Cell Biol. 1996;61:524–530. doi: 10.1002/(SICI)1097-4644(19960616)61:4<524::AID-JCB5>3.3.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Croix BS, Sheehan C, Rak JW, Florens VA, Slingerland JM, Kerbel RS. E-cadherin-dependent growth supression is mediated by the cyclin-dependent kinase inhibitor p27KIP1. J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiannakis A, Syrigos KN, Chatzigianni E, Papanikolaou S, Karatzas G. E-cadherin expression as a differentiation marker in gastric cancer. Hepato-Gastroenterology. 1998;45:2437–2442. [PubMed] [Google Scholar]
- Noë V, Willems J, Vanderkerckhove J, van Roy F, Bruyneel E, Mareel M. Inhibition of adhesion and induction of epithelial cell invasion by HAV-containing E-cadherin-specific peptides. J Cell Sci. 1999;112:127–135. doi: 10.1242/jcs.112.1.127. [DOI] [PubMed] [Google Scholar]
- Giroldi LA, Bringuier PP, Shimazui T, Jansen K, Schalken JA. Changes in cadherin-catenin complexes in the progression of human bladder carcinoma. Int J Cancer. 1999;82:70–76. doi: 10.1002/(SICI)1097-0215(19990702)82:1<70::AID-IJC13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Mialhe A, Levacher G, Champelovier P, Martel V, Serres M, Knudsen K, Seigneurin D. Expression of E-, P-, N-cadherins and catenins in human bladder carcinoma cell lines. J Urol. 2000;164:826–835. doi: 10.1097/00005392-200009010-00057. [DOI] [PubMed] [Google Scholar]
- Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S, Takeichi M, et al. Expresion of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53:1696–1701. [PubMed] [Google Scholar]
- Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, Klöppel G, Lemoine NR. Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade and advanced stage. J Pathol. 1994;174:243–248. doi: 10.1002/path.1711740403. [DOI] [PubMed] [Google Scholar]
- Umbas R, Isaacs WB, Bringuier PP, Schaafsma HK, Karthaus HFM, Oosterhof GON, Debruyne FMJ, Schalken JA. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54:3929–3933. [PubMed] [Google Scholar]
- Wakatsuki S-ji, Watanabe R, Saito K, Saito T, Katagiri A, Sato S, Tomita Y. Loss of human E-cadherin (ECD) correlated with invasiveness of transitional cell cancer in the renal pelvis, ureter and urinary bladder. Cancer Lett. 1996;103:11–17. doi: 10.1016/0304-3835(96)04194-8. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Laferte S, Warghorne C, Breitman ML, Kerbel RS. β1-6 Branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1986;236:582–584. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Dali'olio F. Protein glycosylation in cancer biology: An overview. Clin Mol Pathol. 1996;49:126–135. doi: 10.1136/mp.49.3.m126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda F. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996;56:2237–2244. [PubMed] [Google Scholar]
- Ørntoft TF, Wolf H. Molecular alternation in bladder cancer. Urol Res. 1998;26:223–233. doi: 10.1007/s002400050050. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Wei-Chun W, Lotan R, Fukuda M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1992;267:5700–5711. [PubMed] [Google Scholar]
- Ørntoft TF, Vestergaard EM. Clinical aspects of altered glycosylation of glycoproteins in cancer. Electrophoresis. 1999;20:362–371. doi: 10.1002/(SICI)1522-2683(19990201)20:2<362::AID-ELPS362>3.3.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Vilien M, Christensen B, Wolf H, Rasmussen F, Hou-Jensen C, Povlsen CO. Comparative studies on normal, 'spontaneously' transformed and malignant human urothelium cells in vitro. Eur J Cancer Clin Oncol. 1983;19:775–789. doi: 10.1016/0277-5379(83)90010-x. [DOI] [PubMed] [Google Scholar]
- Bubenik J, Baresova M, Viklicky V, Jakoubkova J, Sainerova H, Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973;11:765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- Ciołczyk-Wierzbicka D, Gil D, Hoja-Lukowicz D, Lityńska A, Laidler P. Carbohydrate moieties of N-cadherin from human melanoma cell lines. Acta Biochem Pol. [PubMed]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Towbin H, Stachelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheet: procedure and some application. Proc Natl Acad Sci USA. 1979;76:4350–4355. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]


