Abstract
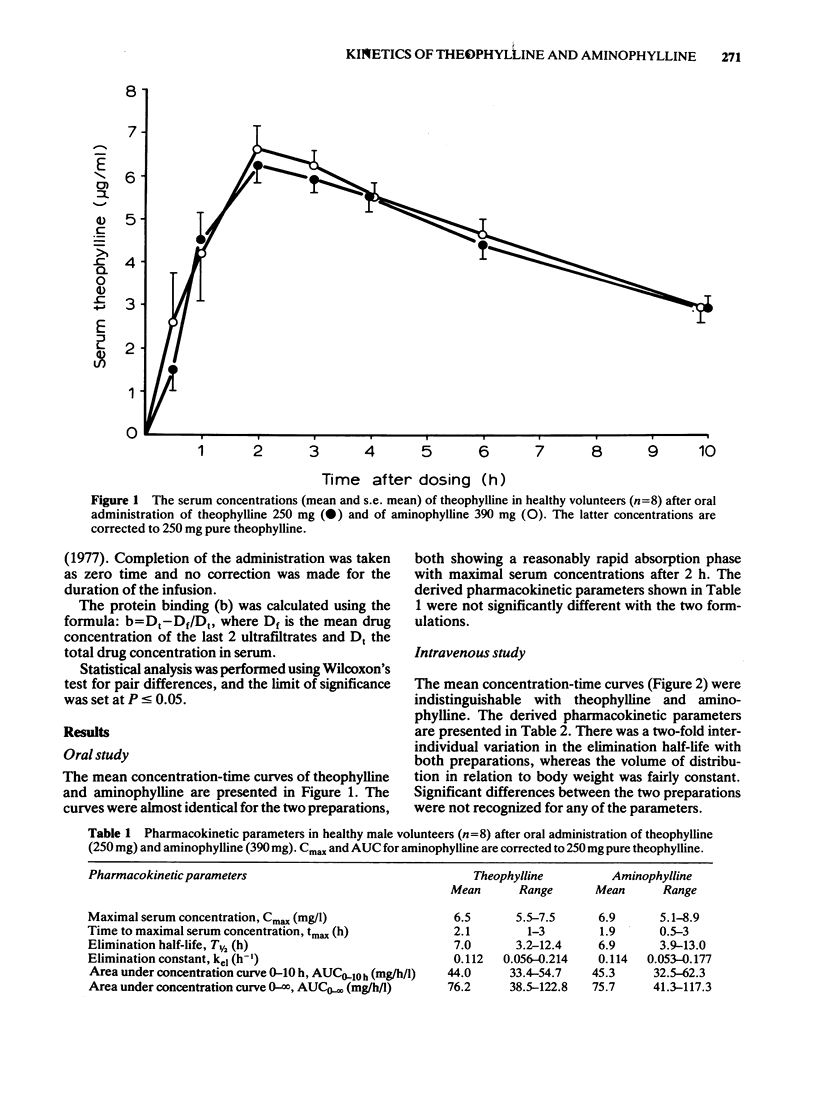
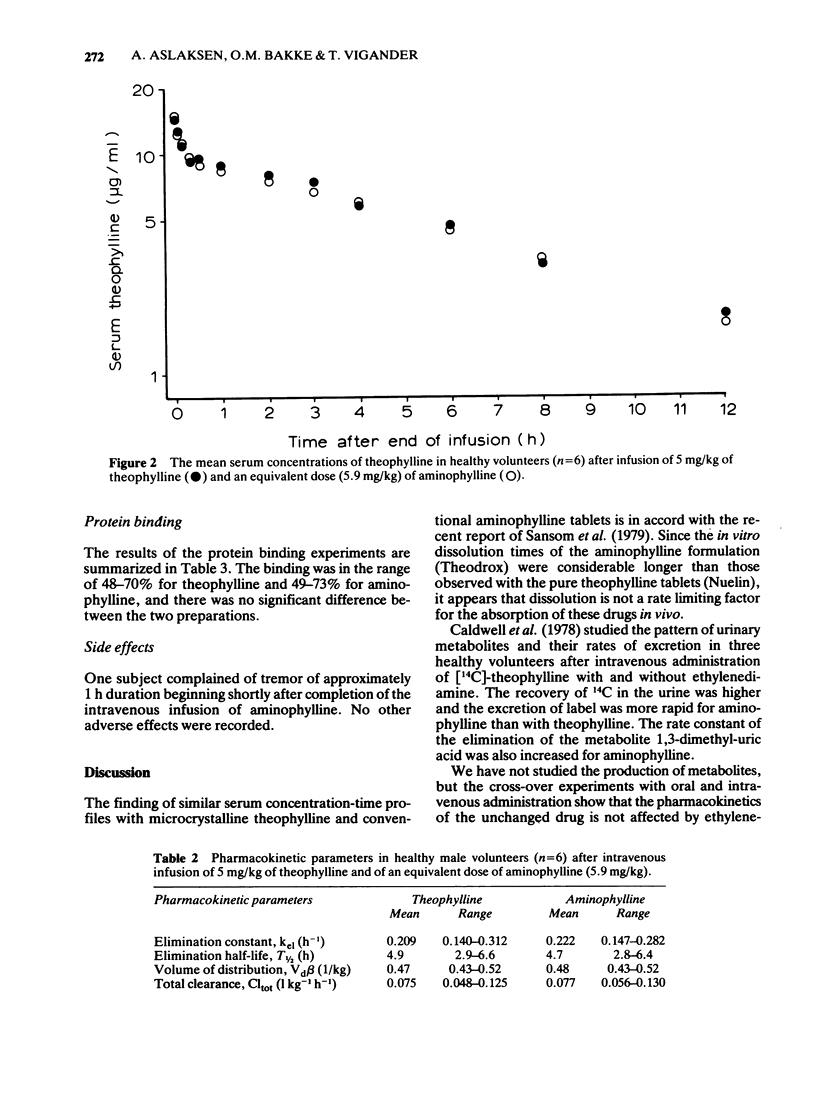
1 The pharmacokinetics of theophylline and aminophylline was compared after oral administration and intravenous infusion. 2 Theophylline (250 mg) and aminophylline (390 mg) were taken orally by eight healthy volunteers in a randomized cross-over study. 3 In another cross-over study theophylline and aminophylline were administered intravenously to six healthy volunteers at a dose corresponding to 5 mg/kg pure theophylline. 4 The protein binding of the theophylline in serum collected during the intravenous study was studied by ultrafiltration. The serum concentration of theophylline was measured by high pressure liquid chromatography. 5 Almost identical concentration-time curves were found for theophylline and aminophylline in both of the studies. No significant difference was found in the pharmacokinetic parameters or protein binding with the two preparations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell J., Monks T. J., Smith R. L. A comparison of the metabolism and pharmacokinetics of intravenously administered theophylline and aminophylline in man [proceedings]. Br J Pharmacol. 1978 Jun;63(2):369P–370P. [PMC free article] [PubMed] [Google Scholar]
- Foster T. S., Bourne D. W. Use of a programmable hand-held calculator for clinical pharmacokinetics. Am J Hosp Pharm. 1977 Jan;34(1):70–75. [PubMed] [Google Scholar]
- Grygiel J. J., Wing L. M., Farkas J., Birkett D. J. Effects of allopurinol on theophylline metabolism and clearance. Clin Pharmacol Ther. 1979 Nov;26(5):660–667. doi: 10.1002/cpt1979265660. [DOI] [PubMed] [Google Scholar]
- Hendeles L., Weinberger M., Bighley L. Absolute bioavailability of oral theophylline. Am J Hosp Pharm. 1977 May;34(5):525–527. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ogilvie R. I. Clinical pharmacokinetics of theophylline. Clin Pharmacokinet. 1978 Jul-Aug;3(4):267–293. doi: 10.2165/00003088-197803040-00002. [DOI] [PubMed] [Google Scholar]
- Orcutt J. J., Kozak P. P., Jr, Gillman S. A., Cummins L. H. Micro-scale method for theophylline in body fluids by reversed-phase, high-pressure liquid chromatography. Clin Chem. 1977 Mar;23(3):599–601. [PubMed] [Google Scholar]
- Petrozzi J. W., Shore R. N. Generalized exfoliative dermatitis from ethylenediamine. Arch Dermatol. 1976 Apr;112(4):525–526. [PubMed] [Google Scholar]
- Piafsky K. M., Sitar D. S., Rangno R. E., Ogilvie R. I. Theophylline disposition in patients with hepatic cirrhosis. N Engl J Med. 1977 Jun 30;296(26):1495–1497. doi: 10.1056/NEJM197706302962603. [DOI] [PubMed] [Google Scholar]
- Provost T. T., Jillson O. F. Ethylenediamine contact dermatitis. Arch Dermatol. 1967 Sep;96(3):231–234. [PubMed] [Google Scholar]
- Sansom L. N., Milne R. W., Cooper D. Comparative bioavailability of a microcrystalline theophylline tablet and uncoated aminophylline tablets. Eur J Clin Pharmacol. 1979;16(6):417–421. doi: 10.1007/BF00568203. [DOI] [PubMed] [Google Scholar]
- Simons K. J., Simons F. E., Briggs C. J., Lo L. Theophylline protein binding in humans. J Pharm Sci. 1979 Feb;68(2):252–253. doi: 10.1002/jps.2600680238. [DOI] [PubMed] [Google Scholar]
- Talseth T., Boye N. P., Bredesen J. E. Clinical and pharmacological observations on a new microcrystalline theophylline preparation. Scand J Respir Dis. 1979 Dec;60(6):358–366. [PubMed] [Google Scholar]
- White M. I., Douglas W. S., Main R. A. Contact dermatitis attributed to ethylenediamine. Br Med J. 1978 Feb 18;1(6110):415–416. doi: 10.1136/bmj.1.6110.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


