Abstract
In budding yeast, the silent information regulator Sir2p is a nuclear NAD-dependent deacetylase that is essential for both telomeric and rDNA silencing. All eukaryotic species examined to date have multiple homologues of Sir two (HSTs), which share a highly conserved globular core domain. Here we report that yeast Hst2p and a mammalian Hst2p homologue, hSirT2p, are cytoplasmic in yeast and human cells, in contrast to yHst1p and ySir2p which are exclusively nuclear. Although yHst2p cannot restore silencing in a sir2 deletion, overexpression of yHst2p influences nuclear silencing events in a SIR2 strain, derepressing subtelomeric silencing while increasing repression in the rDNA. In contrast, a form of ySir2p carrying a point mutation in the conserved core domain disrupts both telomeric position effect (TPE) and rDNA repression at low expression levels. This argues that non-nuclear yHst2p can compete for a substrate or ligand specifically required for telomeric, and not rDNA repression.
Keywords: homologues of Sir2p/nucleolus/SIR2/telomeric silencing/yeast
Introduction
Chromatin-mediated silencing converts specific domains of the yeast genome into a transcriptionally inactive state that shares several characteristics with heterochromatin. Notably, silent chromatin generally contains underacetylated histones, replicates late in S phase and is refractory to the transcriptional apparatus and DNA-modifying enzymes (reviewed in Lustig, 1998). In several species, this reduced accessibility has been shown to correlate with a characteristic subnuclear localization; for example, in both Drosophila and yeast, silent domains on different chromosomes cluster together near the nuclear periphery. Similarly, in mammalian B cells, the association of genes with centromeric heterochromatin correlates with an inactive transcriptional state (reviewed in Cockell and Gasser, 1999; Marshall and Sedat, 1999).
In budding yeast, silencing requires a protein complex that contains balanced proportions of the silent information regulators 2–4 (Sir proteins; Rine and Herskowitz, 1987; Aparicio et al., 1991). This complex interacts with underacetylated N-termini of histones H3 and H4, as well as with sequence-specific DNA-binding factors that recruit and nucleate its binding (reviewed in Lustig, 1998). Sir-mediated repression occurs at three different loci: at the two silent mating type cassettes on ChrIII (HMR and HML), and adjacent to the telomeric TG1–3 repeat (called the telomeric position effect or TPE; Gottschling et al., 1990). A related type of repression occurs in the repetitive rDNA array on ChrXII (Bryk et al., 1997; Fritze et al., 1997; Smith and Boeke, 1997), although Sir2p is the only Sir protein required for the repressive chromatin structure in the nucleolus. Cross-linking assays show that Sir2p is associated with silenced reporter genes both at telomeres and in the rDNA, and overexpression studies indicate that Sir2p levels are limiting at both sites (Gotta et al., 1997; Strahl-Bolsinger et al., 1997; Cockell et al., 1998; Smith et al., 1998).
Despite its dependence on Sir2p, the mechanism of rDNA repression is clearly distinct from that of TPE. First, the Sir2p-dependent rDNA chromatin structure also suppresses homologous recombination among the tandemly repeated rDNA copies (Gottlieb and Esposito, 1989; Fritze et al., 1997; Smith and Boeke, 1997). Secondly, in the nucleolus, Sir2p is complexed with the nucleolar protein Net1p, and a telophase-regulating phosphatase, Cdc14p, which is released in late metaphase (Shou et al., 1999; Straight et al., 1999). Finally, overexpression of either Sir4p or domains of Sir3p, which derepress silencing at telomeres (Cockell et al., 1998; Gotta et al., 1998; Smith et al., 1998), actually increases repression of a PolII reporter gene in the rDNA. In comparison with TPE, nucleolar repression is relatively unstable, fluctuating in response to chromatin-modifying proteins that act at non-nucleolar sites (Bryk et al., 1997; Smith et al., 1999).
The protein encoded by the SIR2 gene is a member of a highly conserved family of proteins called homologues of Sir two in Saccharomyces cerevisiae (yHst1–4p; Brachmann et al., 1995) or SirTuins in humans (hSirT1-7; Frye, 1999, 2000). The hallmark of the Sir2-like family is a conserved globular core of ∼250 amino acids containing a four-Cys Zn2+ finger motif. Recently, yeast Sir2p and Hst2p (hereafter ySir2p and yHst2p) as well as mouse Sir2-like proteins have been shown to catalyse an NAD-dependent deacetylation reaction in vitro using acetylated histone tails as substrate (Imai et al., 2000; Landry et al., 2000). A weak ribosyl transferase activity was also detected under some conditions, and may reflect an intermediate state during NAD hydrolysis (Frye, 1999; Tanny et al., 1999). Unlike other histone deacetylases, that of Sir2p activity is stoichiometrically coupled to NAD hydrolysis (Tanner et al., 2000) and both enzymatic activities are abrogated by mutations within the core domain that correlate with a loss of silencing (Tanny et al., 1999; Imai et al., 2000). Although this suggests that a major function of ySir2p in vivo is enzymatic, essential non-enzymatic silencing functions have also been assigned to its N- and C-terminal domains (Cockell et al., 2000). It remains unclear whether Sir2p prefers acetylated histones over other acetylated substrates, and whether histones are indeed the physiological targets of these enzymes.
Several Sir2p homologues may also modulate chromatin structure, since overexpression of yHst1p, the protein most similar to ySir2p itself, restores silencing at HMRa in a sir2-deficient strain (Brachmann et al., 1995; Derbyshire et al., 1996). In addition, yHst1p forms a complex with Sum1p and represses meiosis-specific sporulation genes during mitotic growth (Xie et al., 1999). Less is known about the functions of HST3 and HST4, although the hst3hst4 double mutant has increased chromosome instability and strongly reduced TPE (Brachmann et al., 1995).
In related yeasts, such as Kluyveromyces lactis, the loss of Sir2p renders cells hypersensitive to the DNA-intercalator ethidium bromide and reduces both mating and sporulation efficiency (Chen and Clark-Walker, 1994), while in Candida albicans a Sir2-like protein is implicated in the control of phenotypic switching (Perez-Martin et al., 1999). Finally, in fission yeast, the mutations of hst4 correlate with slow growth and fragmented DNA, as well as a decrease in chromatin-mediated repression in subtelomeric and centromeric domains (Freeman-Cook et al., 1999). At least partial cross-species complementation for the loss of TPE in S.cerevisiae strains lacking Sir2p was shown for genes from these three distantly related fungi, although higher eukaryotic homologues were unable to complement silencing (Chen and Clark-Walker, 1994; Freeman-Cook et al., 1999; Perez-Martin et al., 1999). Indeed, no cellular function has been assigned to any of the seven mammalian SirTuins.
Here we have analysed the function of yHst2p, the least well characterized yet most universally conserved member of the yeast Sir2 family (Figure 1). yHst2p has a robust deacetylase activity and is more active than ySir2p on histone substrates in vitro (Landry et al., 2000). Indeed, in yeast cell extracts, yHst2p accounts for the majority of detectable NAD-dependent deacetylase activity (Smith et al., 2000). To examine potential roles for both yHst2p and the human homologue hSirT2p in silencing, we have monitored their subcellular localization and their effects on TPE and rDNA silencing in yeast. Surprisingly, we find that both yHst2p and hSirT2p are cytoplasmic enzymes. Nonetheless, elevated levels of yeast Hst2p can modulate TPE and rDNA repression in yeast, albeit in opposite ways. This demonstrates that Sir2-like enzymes can influence silencing without being targeted to the site of repression. Although loss-of-function mutations suggest that ySir2p and yHst2p have distinct physiological roles, we propose that these two homologues share a limiting substrate or ligand, other than NAD, that is necessary for telomeric, but not rDNA repression.
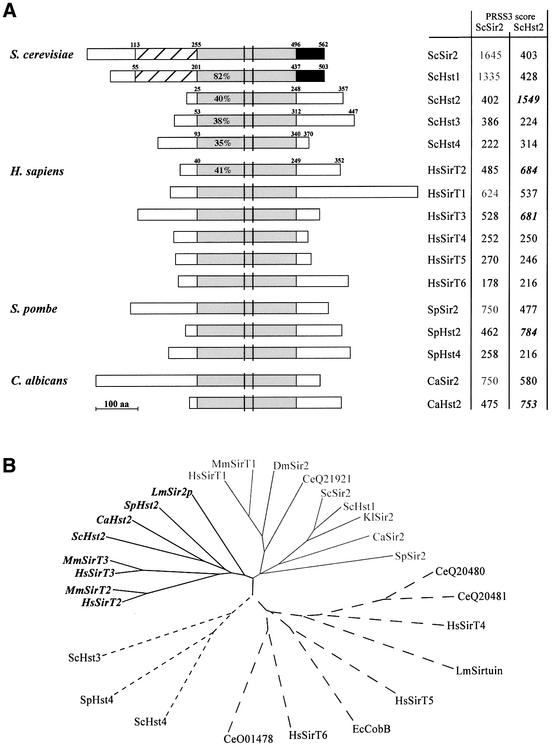
Fig. 1. Comparative alignments and phylogenetic tree of Sir2 family members. (A) Diagrammatic representations of Sir2p homologues are aligned with respect to the evolutionarily conserved core domain (light grey boxes). The PRSS3 score is indicated for the pairwise comparison of the indicated core domain with that of the yeast Sir2p or Hst2p (labelled ScSir2 and ScHst2). The PRSS3 program (http://www.ch.embnet.org/software/PRSS) calculates the optimal score of a protein sequence alignment and evaluates the significance of this score. Scores with higher homology to ySir2p are indicated in grey and those closer to yHst2p are in italics. The percentage amino acid identity shared with the ySir2p core domain is calculated by the Gap program of GCG (with ran = 100, gap = 12, gap extension = 2), and is indicated for the yHst family and the Homo sapiens SirT2 protein. Unshaded boxes are regions of no significant identity (<20%) with ySir2p. Cross-hatched boxes indicate 50% identity in N-terminal extensions, and black boxes indicate 55% identity to ySir2p. The amino acid boundaries of the domains selected for comparison are indicated. Vertical black bars represent cysteine pairs of a putative zinc finger motif. We have used the nomenclature of Frye (1999) for human SirTuins 1–5, and named a previously unreported human cDNA, HsSirT6. The HsSirT2 is identical to the gene called hSir2L (Afshar and Murnane, 1999) and hSir2A (Sherman et al., 1999). (B) The phylogenetic unrooted tree of eukaryotic Sir2 homologues was generated using CLUSTALW (Higgins et al., 1996) and TREEVIEW (Page, 1996), which compare the core domain sequences of homologues identified in cDNA and unigene libraries from Escherichia coli, S.cerevisiae, K.lactis, Schizosaccharomyces pombe, C.albicans, Leishmania major, H.sapiens, Mus musculus, Caenorhabditis elegans and Drosophila melanogaster. The database accession No. for each gene listed is given in Materials and methods. The Sir2 subfamily is indicated in grey and the Hst2 subfamily in italics.
Results
BLAST searches of genomic and expressed sequence tag (EST) databases using either the full-length SIR2 sequence or the region that encodes the ySir2p core domain (amino acids 255–496, shaded grey in Figure 1A) have identified additional Sir2 family members in vertebrate, fly, worm, yeast and bacterial species, allowing for a more complete alignment of homologous genes. The five related genes in budding yeast (encoding ySir2p and yHst1–4p; Brachmann et al., 1995) all share strong sequence identity in a core domain (between 35 and 82% identity). In addition, yHst1p and ySir2p have N- and C-terminal extensions that share 50 and 55% identity, while yHst2p and yHst3p lack N-terminal extensions and have unrelated C-terminal domains. At least some bacterial species have more than one homologue of Sir2p (e.g. Archeoglobus fulgibus), although the prokaryotic Sir2p-like proteins generally lack the N- and C-terminal extensions that help distinguish the eukaryotic forms. Intriguingly, in all eukaryotic species examined to date we find members that are much more closely related to the core domain of the yHst2p protein than to the core domain of ySir2p itself (see values in italics for PRSS scores, Figure 1A). These include a Sir2p family member from Schizosaccharomyces pombe, C.albicans, Leishmania (LmSir2rp; Yahiaoui et al., 1996) and chicken, as well as the human hSirT2p and hSirT3p, and the closely related mouse proteins, MmSirT2p and MmSirT3p (see Hst2 subfamily in italics, Figure 1B; for accession numbers see Materials and methods). We propose to designate this group as Hst2-like, as it forms an independent branch within the Sir2 family tree.
In addition to the Hst2-like and the Hst1/Sir2 subfamilies, our phylogenetic analysis confirms the designation of a third subfamily that includes yHst3p, yHst4p and the S.pombe hst4+, as previously suggested (Sherman et al., 1999). Cross-species complementation has been demonstrated among these genes, although their physiological roles are not yet fully understood (Figure 1B; Freeman-Cook et al., 1999).
Despite its ubiquitous character, no functional information has been published on any member of the Hst2 subfamily, although a mutated form of a human homologue, hSirT2, has been cloned as a melanoma antigen (T.Woelfel, personal communication). Comparison of the sequence encoding the tumour-specific antigen with the corresponding region of the wild-type gene (identical to hSir2L in Afshar and Murnane, 1999; or hSir2A in Sherman et al., 1999) revealed a single point mutation that converts a conserved proline at amino acid 182 to a leucine. This mutation, which will be called hsirT2P182L, is responsible for the observed autoantigenicity (T.Woelfel, unpublished data). It is not known whether hsirT2P182Lp contributes to the cellular transformation events that led to this melanoma, but previously identified mutations responsible for melanoma antigens have been correlated with oncogenesis (Woelfel et al., 1995). Motivated by our identification of a highly conserved Hst2 subfamily, and by the potential medical relevance of the hsirT2P182L mutation, we examined whether overexpression of yHst2p, hSirT2p, or mutant forms of these proteins, would modulate silencing functions in yeast.
Dominant-negative effects of yHst2p on TPE
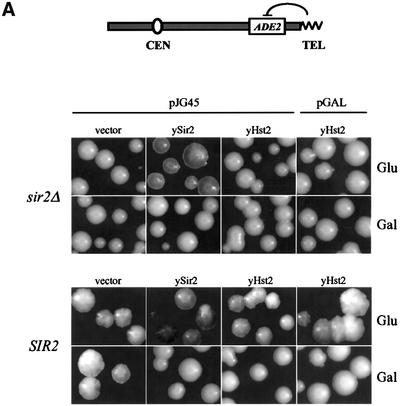
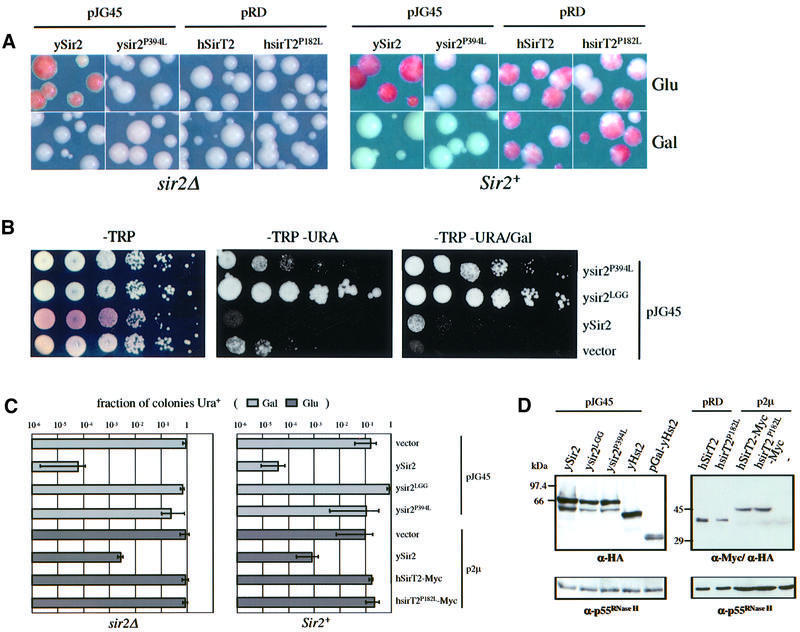
Previous studies have shown that normal levels of ySir2p are limiting in the nucleus, since low level overexpression of SIR2 could improve both TPE and rDNA repression (Cockell et al., 1998; Smith et al., 1998). In contrast, high levels of ySir2p are dominant-negative at telomeres, presumably due to disruption of the Sir complex. No similar disruption or titration occurs in the rDNA, even at the highest levels of ySir2p overexpression (Cockell et al., 2000). Although published studies indicated that yHst2p is not essential for mating type or telomeric silencing (Brachmann et al., 1995), it was not tested whether overexpressed yHst2p complements a sir2::HIS3 strain for TPE, or whether it modulates repression in a wild-type background. Because yHst2p has no readily identifiable nuclear localization signal (NLS), we tested constructs in which full-length yHst2p is fused to a functional NLS (pJG45-yHst2) and one in which it is not (pGAL-yHst2). Both constructs are expressed under the control of the GAL10 promoter on high copy number (2µ) vectors (pJG45 or pGAL, see Materials and methods). Growth on glucose medium confers a low, but non-negligible level of expression from these vectors, since pJG45-ySir2 is able to complement a sir2::HIS3 strain for TPE on glucose (Figure 2A; repression of the telomere-proximal reporter TelVR::ADE2 is monitored by an accumulation of red pigment; see Materials and methods).
Fig. 2. yHst2p at high levels of overexpression is dominant-negative for TPE. The effects of ectopic expression of yeast Hst2p on silencing of the TelVR::ADE2 reporter gene (Singer and Gottschling, 1994) were determined after growth on glucose (Glu, low expression levels) and galactose (Gal, high expression levels). Isogenic sir2::HIS3 (GA427) and SIR2 (GA426) strains, carrying the TelVR::ADE2 reporter gene, were used in (A). (A) The plasmid indicated above each panel was introduced into the sir2::HIS3 strain GA427 labelled sir2Δ or in the SIR2 strain GA426, and were grown under limiting adenine conditions (see Materials and methods). pJG45, pJG45-ySir2 and pJG45-yHst2 express the indicated yeast protein fused at their N-termini to the B42-NLS-HA peptide. pGAL-yHst2 encodes an HA-Hst2p fusion. All fusions are expressed under the inducible GAL10 pomoter, and are expressed at low levels on glucose and induced at least 40-fold on galactose. Both on glucose and galactose media, sir2Δ colonies carrying the parental vectors (shown here only for pJG45) are white, indicating ADE2 expression, whereas Sir2+ colonies carrying the parental vectors alone (shown here only for pJG45) have a red/white sectored appearance like the untransformed strain, indicating metastable ADE2 silencing. (B) The effects of strong ectopic expression of yHst2p, with and without a fused SV40 NLS, on silencing of RDN1::mURA3/HIS3, was determined in isogenic sir2::kanMX4 (GA759) and SIR2 (GA758) strains. Strains were transformed with the plasmids indicated at the right of the graph and described above in (A). Galactose induces strong expression from the GAL10 promoter. Five-fold serial dilutions of each transformant were plated on both –TRP and –TRP–URA media with the indicated carbon source. URA3 expression is calculated as described (see Materials and methods). The sir2::kanMX4 strain (GA759) carrying either vector alone gives a value of ∼1 for this calculation (100% of the colonies express URA3), while the SIR2 strain (GA758) with either vector gives a value of ∼0.1 (10% of the cells express URA3). The mean and standard deviation were calculated from at least three independent transformants of each plasmid.
In Figure 2A, we show that neither pJG45-yHst2 nor pGAL-yHst2 is able to complement a sir2::HIS3 strain for TPE, whether expressed at low or high levels (glucose versus galactose, respectively). However, when HST2 is induced in a Sir2+ strain, which is normally pink or red-sectored, the colonies become completely white, demonstrating a dominant-negative effect of HST2 induction on telomeric repression (Gal, Figure 2A). This effect is similar to that observed for ySir2p overexpression, is independent of the presence of the NLS (compare pJG45-ySir2 with either pJG45- or pGAL-yHst2; Figure 2A) and does not reflect a non-specific effect of galactose (see vector control). As reported for overexpression of the core domain of ySir2p (Cockell et al., 2000), only high levels of yHst2p impair TPE. The simplest interpretation of this result is that yHst2p interferes with the activity of ySir2p by binding to or modifying an essential silencing factor, or by forming a non-productive complex with a common substrate.
yHst2p influences telomeric and rDNA silencing in opposite ways
Although it is well established that ySir2p is essential for rDNA silencing, it has not been reported how the deletion of HST1 and HST2 affects the expression of PolII reporters in the rDNA. To this end, we created single and double hst1 and hst2 deletions in strains containing the RDN1::mURA3-HIS3 insert (see Table I). 5-fluoro-orotic acid (FOA) resistance monitors repression of the mURA3 reporter, while the loss rate for the mURA3 reporter indicates recombination rates. A complete deletion of hst1 has a very minor effect on rDNA silencing, producing a 3-fold reduction in FOA resistance, as compared with the >106-fold drop that correlates with sir2 deletion (Table II). Only a third of the FOA-resistant hst1 colonies reflect excision of the mURA3 reporter, whereas recombination appears to account for all of the resistance in a sir2 null (Table I). Previous results also showed no significant increase in recombination rates in the absence of yHst1p (Derbyshire et al., 1996). Since disruption of hst2 alone, or in combination with hst1, incurs no significant change in rDNA repression or recombination (Table II), we conclude that neither homologue is essential for nucleolar silencing. The minor changes in the hst1 mutant are likely to result from indirect effects, as suggested by the immunostaining data presented below, which localize yHst1p to the non-nucleolar nucleoplasm.
Table I. Yeast strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| GA194 | MATa/MATα ade2/ADE2 trp1/trp1 his3-11/his3 ura3-1/ura3-52 can1-100/can1-100 leu2-3, 112/leu2, sir2::HIS3/ sir2::HIS3 | (Gotta et al., 1997) |
| GA225 | MATa/MATα ade2/ADE2 trp1/trp1 his3-11, 15/his3 ura3-1/ura3-52 can1-100/can1-100 | (Gotta et al., 1997) |
| GA424 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ1 leu2-Δ1 his3-Δ200 ppr1::HIS3 adh4::URA3-TEL | (formerly UCC111; D.Gottschling) |
| GA426 | MATa ade2::hisG can1::hisG his3-11 leu2 trp1Δ ura3-52 TelVR::ADE2 | (Stone and Pillus, 1996) |
| GA427 | MATa ade2::hisG can1::hisG his3-11 leu2 trp1Δ ura3-52 TelVR::ADE2 sir2::HIS3 | (Gotta et al., 1997) |
| GA503 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 leu2-Δ1 his3-Δ200 ppr1::HIS3 adh4::URA3-TEL TelVR::ADE2 | (formerly UCC3505; Singer and Gottschling, 1994) |
| GA758 | MATα his3Δ200 leu2Δ1 trp1Δ63 ura3-167 RDN1::mURA3/HIS3 | (Smith et al., 1998) |
| GA759 | MATα his3Δ200 leu2Δ1 trp1Δ63 ura3-167 RDN1::mURA3/HIS3 sir2::kanMX4 | (J.Smith, unpublished) |
| GA917 | GA758 with hst1::LEU2 | this study |
| GA918 | GA758 with hst2::TRP1 | this study |
| GA921 | GA758 with hst1::LEU2, hst2::TRP1 | this study |
| GA1154 | GA426 with HST1-13Myc-kanMX6 | this study |
| GA1155 | GA427 with HST1-13Myc-kanMX6 | this study |
| GA1229 | GA427 with HST2-13Myc-TRP1 | this study |
| GA1275 | GA426 with SIR4-13Myc-kanMX6 | this study |
| GA1276 | GA426 with HST2-13Myc-kanMX6 | this study |
| GA1281 | GA758 with sir2::TRP1 | this study |
Table II. Disruption of hst1 and hst2 does not significantly affect rDNA repression.
| Genotype | “Repression (FOAr) |
Recombination | |
|---|---|---|---|
| Mean | Normalized | ||
| HST1 HST2 SIR2 | 0.393 (0.29–0.621) | 1.0 | 0.067 |
| hst1 HST2 SIR2 | 0.128 (0.054–0.34) | 0.33 | 0.3 |
| HST1 hst2 SIR2 | 1.0 (0.1–1.04) | 2.54 | 0.0 |
| hst1 hst2 SIR2 | 0.110 (0.062–0.17) | 0.28 | 0.3 |
| HST1 HST2 sir2 | 6.4 × 10–7 (4.7–14.3 × 10–7) | 1.6 × 10–6 | 1.0 |
SIR2, HST1 and HST2 genes were disrupted in the strain GA758. Growth on FOA-containing media monitors the efficiency of repression of the RDN1::mURA3-HIS3 reporter construct as described in Materials and methods. The left hand column shows the mean and numerical spread of the fraction of a total of 30 colonies in which URA3 is not expressed. The value for the wild-type strain is used to normalize values to 1. The fraction of the Ura– cells that have lost URA3 due to recombination events is indicated in the right hand column (see Materials and methods).
To see if HST2 overexpression might influence rDNA silencing, isogenic sir2::kanMX4 (GA759) and SIR2 (GA758) strains containing the RDN1::mURA3 insert were used (Figure 2B; see Materials and methods). We confirm that both low and high level expression from pJG45-ySir2 improves rDNA silencing in a Sir2+ background, while neither form of yHst2p (i.e. yHst2p or NLS–Hst2p) can complement the sir2::kanMX4 strain for rDNA repression (Figure 2B). In striking contrast to the effects at telomeres, however, overexpression of either form of yHst2p significantly improves rDNA silencing (1000-fold; see Figure 2B), almost as efficiently as overexpressed ySir2p in a Sir2+ strain.
yHst2p is cytoplasmically localized, even at high levels of expression
To understand better the differential effects of the Sir2p homologues in the different silencing assays, we investigated the subcellular distributions of ySir2p, yHst1p and yHst2p under conditions of low and high level expression. To this end, the endogenous copies of HST1 and HST2 were fused to a sequence encoding a 13 Myc epitope tag, in otherwise isogenic sir2::HIS3 and SIR2 strains. Although yHst1p has 61% overall identity to ySir2p and has the N- and C-terminal extensions characteristic of ySir2p, it is not enriched in the nucleolus, but is localized to the non-nucleolar nucleoplasm (Figure 3). This is true in both the presence and absence of Sir2p (Figure 3; see inset), consistent with the minor effects HST1 deletion has on rDNA repression.
Fig. 3. yHst1p is enriched in the non-nucleolar nucleoplasm and yHst2p is cytoplasmic. The indicated proteins were localized by indirect immunofluorescence on fixed yeast cells as described in Materials and methods. In all cases, the nucleolar marker Nop1p was localized with anti-Nop1 (rabbit antiserum or mouse monoclonal antibody, as appropriate; Gotta et al., 1997) and a Cy5-coupled secondary antibody. This is shown in the first panel of each row and is red in the merged images. In the first row, localization of ectopically expressed ySir2p (α-Sir2, green in merged image) was examined in a diploid sir2::HIS3 strain (GA194) after transformation with pADH-ySir2. The inset shows the localization of ySir2p to the telomeric foci and the nucleolus, when the cells have been washed, after fixation, in 1% Triton–0.02% SDS to improve accessibility (see Gotta et al., 1997). yHst1-Myc is detected by the monoclonal 9E10 (α-Myc) in the haploid strain GA1154 (SIR2) and the isogenic sir2::HIS3 strain GA1155 (inset). yHst2-Myc was examined in GA1276 (SIR2) and the isogenic sir2::HIS3 strain GA1229 (inset). Both fusions are genomic and under their endogenous promoters. The NLS-containing HA-tagged yHst2p expressed from pJG45-yHst2 was examined in transformants of the diploid wild-type strain GA225 expressing either low or high levels of the protein after 4 h of galactose induction, as indicated. The localization of the HA-tagged yHst2 fusion protein, which is encoded by pGAL-yHst2 and lacks a detectable NLS, was examined in transformants of GA225 under conditions of low and high expression, as indicated. The merge is shown in colour, with Nop1p in red and ySir2p, c-Myc or HA epitopes in green. Coincidence of the two signals is yellow. Bar = 2 µm.
In contrast to yHst1p, however, yHst2p expressed under its endogenous promoter gives a weak but almost entirely cytoplasmic signal (Figure 3, yHst2-myc). Since yHst2p affects both TPE and rDNA repression at high expression levels, we next examined the localization of yHst2p and NLS–Hst2p when they were induced on galactose as pGAL or pJG45 constructs. We first note that in the absence of an NLS, even at very high levels of expression, yHst2p remains cytoplasmic (Figure 3; pGAL-yHst2, low or high). Thus, if its dominant-negative effect on TPE results from competition for a ligand or substrate, that ligand must spend time in both the nucleus and the cytoplasm. Intriguingly, the NLS–Hst2p fusion is also retained in the cytoplasm, except in cells expressing very high amounts (compare pJG45-yHst2 low and high) where it becomes largely nuclear. This suggests that NLS–Hst2p may associate with a cytoplasmic complex that only becomes limiting when the fusion protein is in great excess. Importantly, loss of TPE occurs equally efficiently when either yHst2p or NLS–Hst2p is overexpressed.
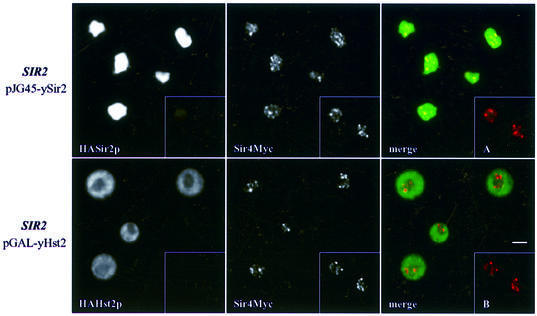
The dual effect of yHst2p on silencing might have been explained by an increase in the free pool of ySir2p following the disruption of complexes involved in TPE, analogous to the increase in rDNA repression observed when Sir4p subdomains are overexpressed (Kennedy et al., 1997; Smith et al., 1998). However, immunolabelling shows that, unlike the situation when ySir2p is overexpressed, ySir4p is not significantly delocalized by high levels of yHst2p (Figure 4). Moreover, there was no detectable increase in non-telomeric ySir2p (data not shown). This is consistent with the fact that yHst2p lacks the N-terminal domain of ySir2p that binds ySir4p (Cockell et al., 2000), and argues that the dominant-negative effect of yHst2p overexpression does not simply reflect disruption of the Sir2/3/4 complex.
Fig. 4. Telomeric foci remain intact at the nuclear periphery despite yHst2p-mediated disruption of silencing. Upper panels: ySir4p-Myc was localized in SIR2 cells (GA1275) transformed with pJG45-ySir2 after 4 h induction on galactose, using mouse anti-Myc antibodies (red signal in the merge). Nuclear localization of the highly overexpressed HA-Sir2p is demonstrated by immunostaining with anti-HA in the first panel (visualized in green in the merge). ySir4p-Myc is partially delocalized as compared with the punctate pattern observed in cells that do not overexpress ySir2p (GA1275, inset A). Lower panels: ySir4-Myc was localized in the same cells transformed with pGAL-yHst2 and induced for 4 h on galactose. The cytoplasmically localized HA-yHst2p is visualized in green; anti-Myc (ySir4p-Myc) is in red in the merged image. The inset B shows control cells that do not overexpress yHst2p. Bar = 2 µm.
Human SirT2p is also cytoplasmically localized
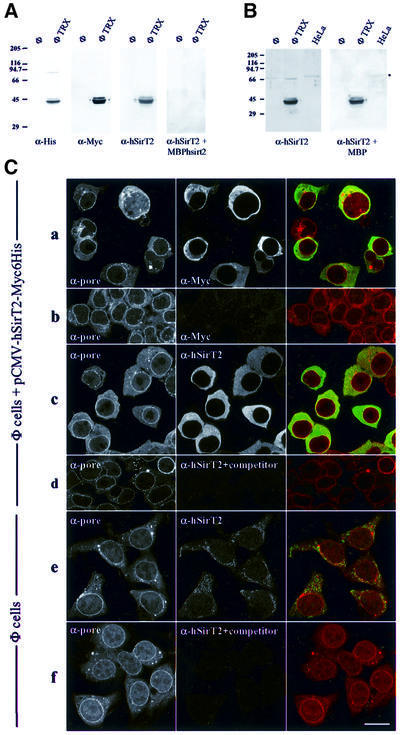
The unexpected finding that the most active NAD-dependent Sir2-like deacetylase in yeast is cytoplasmic led us to ask whether other members of the Hst2 subfamily share this characteristic. Wild-type and mutant forms of the hSIRT2 gene were cloned and sequenced during the analysis of a human melanoma antigen (T.Woelfel, personal communication). Both the wild-type and the mutant gene (carrying the Pro182 to leucine transition) were fused to both His6 and Myc epitope tags under the control of a cytomegalovirus (CMV) promoter, for transfection into mammalian cells (see Materials and methods). In transfected Phoenix cells, the protein is expressed at high levels. Immunostaining with an anti-Myc epitope reveals a strong cytoplasmic signal (Figure 5C), which does not coincide significantly with nuclear DNA nor with nuclear pores (red, anti-pore; green, hSirT2p; Figure 5C). An identical localization, which is highly reminiscent of that of yHst2p, was obtained for both the wild-type and P182L form of hSirT2p (data not shown).
Fig. 5. hSirT2 is cytoplasmic in human cells. (A) Whole-cell extracts of a human embryonic kidney cell line (Phoenix cells, labelled Φ, see Materials and methods) and of the same cells transfected with pCMV-hSirT2-Myc6His (labelled ΦTRX) were analysed by western blotting using anti-His, anti-Myc, column-purified anti-hSirT2 (see Materials and methods) and the same anti-hSirT2 mixed with an excess of a bacterial extract expressing MBP–hSirT2, to demonstrate the specificity of the anti-hSirT2 antibody. Each lane was loaded with ∼40 µg of total protein. Molecular weight markers (kDa) are indicated on the left of the blot. (B) Protein samples are as in (A), with the addition of a total cell extract from HeLa cells. A 40 µg aliquot of protein was analysed in each lane by western blotting using affinity-purified anti-hSirT2 and the same purified antibody mixed with bacterial extract expressing MBP, to identify endogenous hSirT2p. Molecular weight markers (kDa) are indicated on the left of the blot. (C) hSirT2-Myc6His and endogenous hSirT2p are cytoplasmic in Phoenix cells. Row a: Phoenix cells transfected with pCMV-hSirT2-Myc6His were stained with anti-pore (detected by Cy5-conjugated secondary antibodies, red in the merge) and anti-Myc (detected by DTAF-conjugated secondary antibodies, green in the merge). Row b: to test the specificity of the anti-Myc, non-transfected Phoenix cells were stained with anti-pore and anti-Myc. Row c: Phoenix cells transfected with pCMV-hSirT2-Myc6His were stained with anti-pore (red in the merge) and column-purified anti-hSirT2 (green in the merge). Row d: as c, except that the column-purified anti-hSirT2 antibodies were pre-incubated with an excess of extract from bacteria overexpressing MBP–hSirT2p. The immune complexes were removed by centrifugation prior to staining the fixed cells. Row e: non-transfected Phoenix cells were stained with anti-pore (red in the merge) and column-purified anti-hSirT2 (green in the merge). To detect the low level signal of endogenous hSirT2, the laser intensity (488 nm) was increased 40-fold over the scanning conditions used in a–d. Row f: the specificity of column-purified anti-hSirT2 is demonstrated by pre-mixing an excess of bacterial extract expressing MBP–hSirT2 with the purified antibody, prior to staining non-transfected Phoenix cells with anti-pore (red in the merge) and the depleted anti-hSirT2 (green in the merge). Bar = 15 µm.
To see if the endogenous hSirT2p is also cytoplasmic, polyclonal antibodies were raised against hSirT2p fused to the bacterial maltose-binding protein (MBP). The resulting antiserum was purified against recombinant antigen that was either denatured (on nitrocellulose strips) or native (by affinity chromatography). The specificity was confirmed by showing that both strip-purified and column-purified anti-hSirT2p (Figure 5A and B, respectively) react strongly with the epitope-tagged hSirT2p expressed in transfected Phoenix cells. The bands coincide with those labelled by reaction with anti-Myc and anti-His6 monoclonals (Figure 5A), while in non-transfected cells this band is absent. The endogenous hSirT2p is below the level of detection by western blotting in the Phoenix cell line, although a weak signal at ∼68 kDa in HeLa cell extracts resists competition with MBP, and may represent the endogenous hSirT2 protein (Figure 5B, right panel).
Finally, immunofluorescence performed with column-purified anti-hSirT2 antibodies on non-transfected Phoenix cells revealed a general cytoplasmic staining, which is lost when competed by a bacterial extract containing the MBP–hSirT2p fusion (Figure 5C). Similar results were obtained with nitrocellulose-purified antibodies on HeLa cells, human kidney carcinoma cells (RCC7680), in mouse NIH-3T3 and in the human Mel4 fibroblasts, which carry the sirT2P182L mutation (T.Laroche and S.M.Gasser, data not shown). Since an excess of MBP–hSirT2 antigen can compete for the cytoplasmic staining pattern, we conclude that the localization of both wild-type and mutant hSirT2p in mammalian cells is cytoplasmic, like that of yHst2p.
A proline to leucine mutation in ySir2p abrogates both rDNA and telomeric silencing in a dominant-negative manner
Does either hSirT2p or its mutant form mimic the effects of yHst2p on silencing in yeast? As shown in Figure 6A, neither low nor high levels of hSirT2p or hsirT2P182Lp can complement the sir2 phenotype in TPE (Figure 6A, sir2Δ), nor were dominant-negative effects detected when hSirT2p or its mutant form was overexpressed in a wild-type background (Figure 6A, Sir2+, Gal). Western blots confirm that the galactose-induced human proteins are stable and readily detected in yeast cell extracts (Figure 6D). Since hSirT2p expression had no phenotype, we decided to introduce the equivalent melanoma-associated point mutation into the yeast SIR2 gene, producing a full-length ysir2P394Lp (Pro394 to leucine). The analogous mutation could not be made in yHst2p, since the relevant proline is not conserved; moreover, no silencing phenotypes have been correlated with inactivation of Hst2p to date.
Fig. 6. The ysir2P394L mutant form is non-functional and disrupts TPE in wild-type cells. (A) The effects of ectopic expression of yeast Sir2p mutants and human SirT2 on silencing of the TelVR::ADE2 reporter gene (Singer and Gottschling, 1994) was determined after growth on glucose (Glu, low expression) and galactose (Gal, high expression). Isogenic sir2::HIS3 (GA427) and Sir2+ (GA426) strains were transformed with the plasmids indicated above each panel and were grown under limiting adenine conditions such that colonies accumulate red pigment when ADE2 is repressed. The plasmids pJG45-ySir2 and pJG45-ysir2P394L express the indicated yeast protein fused at their N-termini to the B42-NLS-HA peptide. pRD-hSirT2 and pRD-hsirT2P182L encode HA epitope-tagged hSirT2p and hsirT2P182Lp. All are under GAL10 control. Although these hSirT2 constructs lack their first 33 amino acids, we obtained identical results by expressing a full-length hSirT2 clone fused to a C-terminal Myc epitope under ADH control (data not shown and see below). (B) The effects of wild-type and mutated yeast Sir2p (plasmids pJG45-ysir2P394L and pJG45-ysir2LGG) on silencing of the TelVIIL::URA3 reporter were determined in a Sir2+ strain carrying the TelVR::ADE2 marker (GA503). GA503 was transformed with the plasmids indicated on the right: pJG45-ysir2P394L, pJG45-ysir2LGG, pJG45-ySir2 and the vector alone. Ten-fold serial dilutions starting with 5 × 105 cells of each transformant were plated on glucose plates lacking tryptophan (–TRP), tryptophan and uracil (–TRP–URA), and on galactose plates with the same selectivity (growth on –TRP/Gal was identical to that observed on –TRP, data not shown). Growth on –URA indicates loss of TPE on glucose or galactose in the presence of the mutated forms of ySir2p, while low level expression of wild-type ySir2p improves TPE. On –TRP plates, the reddish colour indicates subtelomeric repression of the TelVR::ADE2 marker, whereas white indicates full derepression. (C) The effect of ectopic expression of wild-type and mutants of ySir2p and hSirT2p on silencing of a RDN1::mURA3 reporter was determined in isogenic sir2::kanMX4 (GA759) and Sir2+ (GA758) strains, transformed with the plasmids indicated on the right of the graph. Plasmids pJG45, pJG45-ySir2, pJG45-ysir2LGG and pJG45-ysir2P394L are described in (B), and are all galactose inducible. Five-fold serial dilutions of each transformant were plated on both –TRP and –TRP–URA plates on the indicated carbon source. The fraction of colonies in which the URA3 gene is expressed is described in Materials and methods. For sir2::kanMX4 strain (GA759), transformation with either vector alone gives a value of ∼1 (100% of colonies express URA3) while the Sir2+ strain (GA758) with either vector alone gives ∼0.1 (∼10% of cells express URA3). Standard deviations and means were calculated from at least three independent transformants of each plasmid. (D) Epitope-tagged proteins expressed in GA426 after transformation with the plasmid indicated above each lane were detected by western blots of whole-cell extracts of each transformant. For all constructs, except those labelled p2µ, proteins were extracted after growth on galactose/raffinose. Approximately 30 µg of protein extract was loaded per lane. Equal loading was checked by blotting with a constitutively expressed protein, p55RNase H (Karwan et al., 1990).
The sir2P394L mutation is compared in all assays with a previously characterized triple mutant called sir2LGG, which has two arginine to glycine substitutions at amino acids 403 and 404, together with the Pro394 to leucine mutation (Cockell et al., 2000). Neither pJG45-sir2LGG nor the single mutant pJG45-sir2P394L can complement TPE in a sir2::HIS3 strain on glucose (low level expression) or galactose (high level expression; Figure 6A and Cockell et al., 2000). In contrast, the same vector expressing wild-type Sir2p on glucose under control of the GAL10 promoter restores repression in a sir2Δ strain (Figure 6A). Thus, both mutant forms inactivate SIR2 function at telomeres. Unlike wild-type ySir2p, we observed that low level expression of the mutant sir2P394L is strongly dominant-negative for TPE in a Sir2+ strain. This is evidenced by the loss of the red/pink sectors in both GA426 (Sir2+; Figure 6A) and GA503, the latter carrying two independent subtelomeric reporters (URA3 and ADE2; see –TRP, Figure 6B). Since pigment accumulation is qualitative, derepression of the subtelomeric URA3 reporter was quantified to compare the dominant-negative effects of the two mutant forms. In contrast to pJG45-ySir2 or pJG45-yHst2, which only affect silencing at high levels of expression, the single point mutation confers an ∼10-fold loss of TPE on glucose and a 104-fold effect at high expression levels, while the triple sir2LGG mutant derepresses ∼103-fold on glucose and up to 104-fold on galactose (compare ysir2P394L, ysir2LGG and pJG45 on –TRP–URA /±Gal, Figure 6B). All the yeast proteins are expressed at approximately equal levels, as shown by a western blot in which a cytoplasmic protein (p55RNase H) serves as a loading control (Figure 6D). In summary, when the mutation correlated with the human melanoma antigen is introduced into ySir2p, we obtain a gain of function that disrupts TPE even at low levels of expression. Intriguingly, both the single and triple mutations tested here render Sir2p entirely deficient for the NAD-dependent deacetylation reaction in vitro (J.Landry, R.Sternglanz, M.M.Cockell, S.Perrod and S.M.Gasser, unpublished results).
To examine whether the rDNA silencing behaves similarly in the presence of hSirT2p or the mutant forms of ySir2p, we introduced the expression plasmids into a strain carrying the RDN1::mURA3 reporter construct. Neither the wild-type nor the mutant forms of hSirT2p (hSirT2-Myc or hsirTP182L-Myc) complement ySir2p for rDNA silencing, nor do they disrupt rDNA repression in a SIR2 strain (sir2Δ and Sir2+ panels, Figure 6C). Similarly, the mutant ySir2p forms are unable to complement a sir2 deficiency for rDNA repression (sir2Δ panel, Figure 6C). In Sir2+ strains, however, we see that overexpression of ysir2LGGp partially disrupts rDNA repression (a 5- to 10-fold increase in the fraction of Ura+ colonies on galactose) while overexpression of ysir2P394Lp has no significant effect. Neither mutant form is excluded from the nucleolus as determined by immunostaining (Cockell et al., 2000; S.Perrod, data not shown); thus, we conclude that the strong dominant-negative effect that correlates with the enzymatically inactive ysir2P182Lp mutant at telomeres is not manifest in rDNA repression. This, together with the differential effects of yHst2p overexpression, distinguishes the repression mechanism at telomeres from that in the rDNA. Since the enzymatically inactive sir2 mutants do not improve rDNA repression like yHst2p overexpression, we propose that they modulate silencing by different mechanisms (see Table III).
Table III. Effects on transcriptional silencing in SIR2 and sir2Δ strains.
| Genotype |
SIR2 |
sir2Δ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus monitored |
TelVIIL::URA3 |
TelVR::ADE2 |
RDN1::mURA3 |
HMLα |
TelVR::ADE2 |
RDN1::mURA3 |
|||||
| Media | Glu | Gal | Glu | Gal | Glu | Gal | Glu | Gal | Glu | Gal | |
| Protein expressed | |||||||||||
| none (vector) | wt | wt | sectored | sectored | wt | wt | – | – | – | – | – |
| ySir2 | ++ | – | red | white/pink | + | ++ | + | + | – | + | + |
| ysir2LGG | – | — | white | white | – | – | – | – | – | – | – |
| ysir2P394L | – | — | white | white | wt | wt | + | – | – | – | – |
| yHst2 | wt | (–/+)a | sectored | white | wt | ++ | – | – | – | – | – |
| NLS–Hst2 | wt | (–/+)a | sectored | white | wt | ++ | – | – | – | – | – |
| yHst1 | – | – | white/pink | white/pink | nd | nd | nd | nd | nd | nd | nd |
| hSirT2 | wt | wt | sectored | sectored | wt | wt | nd | – | – | – | – |
| hSirT2P182L | wt | wt | sectored | sectored | wt | wt | nd | – | – | – | – |
Telomeric silencing was assayed in GA424 (SIR2, TelVIIL::URA3), GA503 (SIR2, TelVIIL::URA3, TelVR::ADE2), GA426 (SIR2, TelVR::ADE2)and GA427 (sir2::HIS3, TelVR::ADE2), while rDNA repression was measured in GA758 (SIR2, RDN1::mURA3) and GA759 (sir2::kanMX4, RDN1::mURA3). Wt, for wild-type levels of silencing based on Sir2+ strains transformed with the empty vector; +, repression better than wt; –, repression less than wt; —, a very pronounced reduction of silencing; (–/+), derepression that is coupled with slow growth and reduced viability.
aCells of GA503 and GA424, which overexpress either yHst2p or NLS–yHst2p, grow ∼10 times less efficiently, possibly due to a negative effect of yHst2p on pathways required for growth on galactose. This effect is less pronounced in GA758 in which we test rDNA silencing.
Discussion
The number of genes characterized as members of the highly conserved Sir2 family continues to expand, and currently includes seven SIR2-like enzymes in humans [see Figure 1 for hSirT1-6p; and Frye (2000) for hSirT7]. The initial speculation that Sir2 family members might generally be involved in the modification of chromatin structure (Brachmann et al., 1995) has been strengthened by the fact that both mammalian and yeast Sir2p-related homologues have an NAD-dependent deacetylase activity in vitro (Imai et al., 2000; Landry et al., 2000; Smith et al., 2000). Because mutations that eliminate this activity correlate with a loss of TPE, it appears likely that the enzymatic activity is involved in the repression mechanism. Moreover, mutations in other enzymes that affect NAD levels abrogate rDNA repression efficiently (Smith et al., 2000). Here, we describe in detail the localization and silencing phenotypes of yHst2p, which is a member of a distinct but broadly conserved subfamily of Sir2p homologues, and which constitutes the major NAD-dependent deacetylase in yeast.
ySir2p, yHst1p and yHst2p have distinct patterns of subcellular localization
In yeast, ySir2p, yHst1p and yHst2p each have a unique subcellular distribution. Like ySir2p, yHst1p is nuclear, yet it is not enriched at telomeres or in the nucleolus. In contrast, yHst2p and its closest homologue in human cells are cytoplasmic. For yHst2p, the cytoplasmic localization is observed for the protein whether it is expressed at low levels or induced from the GAL10 promoter. Moreover, when a functional NLS is fused artificially to yHst2p, the protein remains cytoplasmic until it is highly overexpressed, suggesting that cytoplasmic retention competes for nuclear translocation. This is consistent with the localization of the epitope-tagged form of hSirT2 in transiently transfected human cells (Figure 5C and Afshar and Murnane, 1999) and with the diffuse cytoplasmic localization detected for endogenous hSirT2p using affinity-purified antibodies on fixed cultured cells. Taken together with a Leishmania Sir2-like protein that also appears to be cytoplasmic (Zemzoumi et al., 1998), we suggest that the entire Hst2 branch will be cytoplasmic NAD-dependent deacetylases.
Cytoplasmic yHst2p may compete for a telomere-specific silencing ligand
We show that null alleles of HST1 or HST2 have only minor effects on rDNA repression, although overexpression of Hst2p in a Sir2+ strain disrupts telomeric silencing while significantly improving repression in the rDNA. We interpret these findings in light of recent studies that show that Sir2-like proteins share one or more enzymatic functions (Frye, 1999; Imai et al., 2000; Landry et al., 2000; Tanny et al., 1999). A robust NAD-dependent deacetylation activity was demonstrated for ySir2p, yHst2p and mouse SirT1p (i.e. mSir2α), and mutations in yeast Sir2p that eliminate deacetylase activity were shown to result in derepression in vivo (Imai et al., 2000). Since rDNA silencing drops when cellular NAD levels fall (Smith et al., 2000), and since hSirT2p itself is able to bind NAD (Frye, 1999), it is unlikely that the loss of TPE elicited by the overexpression of yHst2p reflects titration of NAD. Rather, the dosage-dependent, dominant-negative phenotype suggests that the conserved core domain shared among the Sir2-like enzymes can compete for a ligand or substrate that can be present in both nuclear and cytoplasmic compartments. Importantly, we find that the telomeric localization of ySir4p is not affected by yHst2p overexpression, making it unlikely that yHst2p acts through modulation of the Sir complex. Consistently, yHst2p lacks the ySir2p domain that binds ySir4p and does not interact with ySir4p by two-hybrid analysis (Cockell et al., 2000; and data not shown).
Several lines of evidence suggest that histone tails may be a physiological target for deacetylation by ySir2p and yHst2p. First, overexpression of ySir2p resulted in global hypoacetylation of histones (Braunstein et al., 1996). Secondly, both yHst2p and ySir2p proteins deacetylate bulk histones efficiently in vitro (Landry et al., 2000). Finally, Imai et al. (2000) have reported a preference of ySir2p for an acetylated K16 of the histone H4 peptide. In this case, however, substrates other than histone N-terminal fragments were not tested and the target histone was not assembled into nucleosomes. If histones are the critical substrate of ySir2p and overexpressed yHst2p, then it follows from our results that the pattern of deacetylated lysines on the histone H4 tail might have opposite effects on rDNA and telomeric silencing. Consistent with this possibility, mutation of Arg17 to glycine in the histone H4 tail was found to improve rDNA repression while disrupting TPE (H.Renauld, unpublished observations). Clearly additional studies are required to determine which proteins involved in silencing are common targets of the different Sir2 family members.
Although their distinct subcellular distribution suggests that yHst2p might sequester a ySir2p substrate in the cytoplasm, this appears not to be the case. The relocalization of overexpressed yHst2p to the nucleus through its fusion to the SV40 NLS does not abrogate its dominant-negative effect. Alternatively, a silencing-relevant ligand may be modified inappropriately, or bound irreversibly to yHst2p. The proposal that yHst2p modifies a range of substrates is consistent with the observation that cell viability in some yeast backgrounds drops up to 10-fold when HST2 is overexpressed (data not shown).
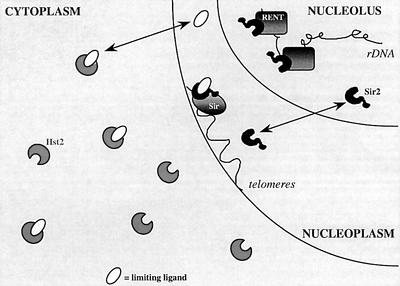
From the silencing results and immunolocalization studies, we propose the following model for the effects of yHst2p on gene silencing (Figure 7). yHst2p may have a significant affinity for what is normally a ySir2p-specific ligand required for proper telomeric repression by the Sir complex. Normal levels of cytoplasmic yHst2p are probably not sufficient to compete for this ligand. However, a ligand required for Sir2/3/4-mediated silencing could become modified or bound by overexpressed yHst2p before assembly at its normal site of action in the nucleus. In its absence, nuclear ySir2p would no longer be able to promote Sir complex propagation at telomeres. This might then permit the relocalization of some fraction of the ySir2p pool to the nucleolus to improve rDNA repression. In this way, weak but overlapping substrate specificities between yHst2p and ySir2p could suffice to explain the phenotypes associated with yHst2p overexpression. This demonstrates the importance of the enzymatic activity per se in silencing, and dissociates it from the role of ySir2p in Sir complex formation. On the other hand, unlike a Gal4 DNA binding domain fusion to ySir2p, the targeting of an equivalent yHst2p fusion protein to a reporter gene is not sufficient to promote silencing (M.M.Cockell, unpublished data), indicating that even the targeting of a Sir2-like deacetylation activity is not sufficient to promote transcriptional repression.
Fig. 7. Model for yHst2p effects on silencing in yeast. A model is shown to account for the dominant-negative effects of cytoplasmic yHst2p on silencing in yeast. yHst2p is cytoplasmic at both low and high levels of expression, and its absence has no effect on TPE or rDNA silencing. At high levels of expression, cytoplasmic yHst2p affects TPE and rDNA silencing much like highly overexpressed ySir2p: rDNA silencing improves while TPE is disrupted. We propose that yHst2p sequesters or modifies a ligand of ySir2p that is essential for TPE, and which shuttles between the nucleoplasm and cytoplasm. By sequestering or modifying this unknown ligand, yHst2p may disrupt TPE, releasing a pool of ySir2p that can relocalize to the nucleolus and improves rDNA silencing. The limiting ySir2p/yHst2p ligand must not be necessary for rDNA repression. In this model, fluctuations in levels of cytoplasmic ySir2-like proteins, as well as changes in the amount of this ligand are predicted to influence Sir protein function at telomeres and at the rDNA.
Inactivating point mutations can render ySir2p dominant-negative for silencing
In contrast to the yeast HST2, the introduction of a melanoma-associated point mutation into yeast SIR2 provokes the loss of both telomeric and rDNA silencing functions, and renders the proteins strongly dominant-negative for telomeric repression, even at low expression levels. These observations indicate that even in heteroallelic human melanoma cells, the mutant form of hSirT2p could influence patterns of gene expression. We have found that the sir2P394L and sir2LGG mutations in S.cerevisiae inactivate the gene product’s enzymatic function (J.Landry, R.Sternglanz, M.M.Cockell, S.Perrod and S.M.Gasser, data not shown). These mutations eliminate a function required for both SIR3/4-dependent and -independent silencing, but also sequester or inhibit silencing factors in Sir2+ strains. This may provide an example of how a single mutant allele of a chromatin factor can have strong repercussions on patterns of gene expression in differentiated cells.
The fact that a non-nuclear NAD-dependent deacetylase can disrupt silencing suggests that there are common substrates for yHst2p and ySir2p, and most probably substrates other than histones, that are likely to influence silencing events. Characterization of these substrates and mutagenesis of the target sites will be needed to decipher the cellular functions of this large and ubiquitous family of enzymes in higher eukaryotes.
Materials and methods
Plasmid constructions
Plasmids used in the study are listed below along with a brief description of their relevant characteristics. The following abbreviations are used: NLS, nuclear localization signal; GAL10, the UAS and promoter sequence of the GAL10 gene; ADH1, the UAS and promoter sequence of the ADH1 gene; B42, the bacterial B42 activation domain; HA, the haemagglutinin epitope; and Myc, the c-Myc epitope. pJG45 (2µARS, TRP1, expresses B42-NLS-HA under control of GAL10); pJG45-ySir2 (identical to pJG45 but with an in-frame fusion of B42-NLS-HA to full-length SIR2); pJG45-ysir2P394L (identical to pJG45-Sir2 but with a Pro394 to leucine substitution); pJG45-ysir2LGG (identical to pJG45-sir2P394L but with two additional mutations of Arg403 and Arg404 to glycines); pJG45-yHst2 (identical to pJG45 but with an in-frame fusion of B42-NLS-HA to full-length HST2); pGAL (2µARS, TRP1, GAL10 promoter); pGAL-yHst2 (identical to pGAL with full-length HST2 fused to HA); pRD (or pRD54C with CEN3 ARS1, URA3 and the HA epitope under GAL10 control); pRD-hSirT2 (as pRD with hSIRT2 lacking the N-terminal 33 amino acids fused in-frame to HA); pRD-hsirT2P182L (as pRD-hSirT2 with a Pro182 to leucine substitution); p2µ (equivalent to pRS424, with 2µARS, TRP1 and ADH1); p2µ-hSirT2-Myc (p2µ with the full-length hSIRT2 fused to a Myc epitope at its C-terminus); p2µ-hsirT2P182L-Myc (identical to p2µ-hSirT2 but with the Pro182 to leucine substitution). These latter were constructed by subcloning PmeI fragments from pcDNA3.1/Myc-HisB-hSirT2/hsirT2P182L into SmaI sites of p2µ. p2µ-ySir2 was constructed by ligating an EcoRI–XhoI fragment, encoding SIR2 subcloned from the pJG45-ySir2 vector into EcoRI–XhoI sites of p2µ.
The plasmids pJG45-ySir2, pJG45-ysir2LGG (Cockell et al., 2000) and pJG45-ysir2P394L (obtained from pJG45-ySir2 by PCR mutagenesis; this study) were constructed by in-frame ligation of the appropriate SIR2 fragment into the EcoRI and XhoI sites of the vector pJG45. An EcoRI–XhoI fragment containing HST2 was also obtained by PCR and cloned in-frame using the EcoRI and XhoI sites of pJG45 (Golemis et al., 1996). A PstI–ClaI fragment encoding HA-yHst2 was obtained from pJG45-yHst2 by PCR, and then cloned into the PstI and ClaI sites of pGAL. All constructs were verified by DNA sequence analysis, and western blots on extracts of the yeast transformants verified the correct size of each fusion protein.
pRD-hSirT2 and pRD-hsirT2P182L (hSIRT2, 960 bp) were constructed by ligating RT–PCR (from cDNA libraries of a melanoma patient, taking melanoma cells and normal cells of this same patient; T.Woelfel, personal communication) EcoRI–HindIII fragments encoding hSirT2 and hsirT2P182L, respectively, into EcoRI–HindIII sites of pRD (i.e. pRD54C). pCMV-hSirT2-Myc6His (full-length hSIRT2, 1059 bp) is also called pcDNA3.1-hSirT2-Myc6His (gift of T.Woelfel and U.Mainz). This was constructed by ligating an ApaI–XbaI fragment of hSIRT2 obtained by RT–PCR (as above) into pcDNA3.1/Myc-HisB (Invitrogen).
Accession numbers
EcSir2, SW:P75961; ScSir2, SW:P06700; ScHst1, SW:P53685; ScHst2, SW:P53686; ScHst3, SW:P53687; ScHst4, SW:P53688; KlSir2, SW:P33294; HsSirT1, EM:AF083106; HsSirT2, EM:AF083107; HsSirT3, EM:AF083108; HsSirT4, EM:AF083109; HsSirT5, EM:AF083110; HsSirT6, TREMBL NEW:AAD15478; SpSir2, sp_tr:O94640; SpHst2, emnew:AL121807; SpHst4, emnew:AF117324; CaSir2, SW:O59923; CaHst2, sp_tr:O94066; LmSir2p, SW:G25337; LmSirTuin, emnew:AL117324; DmSir2, sp_tr:O96505; MmSirT1, EM:AF214646; MmSirT2, munigene:Mm25161; MmSirT3, munigene:Mm41625; CeQ20480, sp_tr:Q20480; CeQ20481, sp_tr:Q20481; CeO01478, sp_tr:O01478; CeQ20480, sp_tr:Q219221; GgSirT2, EM:AI981091.1; GgSirT6, EM:AI981030.1.
Yeast methods and tagging of HST1, HST2 and SIR4
The genotypes of the yeast strains used in this study are indicated in Table I. Media and standard yeast genetic methods were as described (Rose et al., 1990). Limiting adenine medium contains 10 µg/ml adenine sulfate, and all yeast incubations were performed at 30°C.
HST1, HST2 and SIR4 open reading frames were fused in-frame at their C-termini to a 13 Myc epitope as described (Longtine et al., 1998). Clones expressing kanMX6 were selected by growth on YPAD medium containing 200 µg/ml G418, whereas clones expressing TRP1 were selected by growth on SC-trp. Correct insertion of the tag was verified by PCR and immunoblotting in each case.
Silencing assays
Expression of the subtelomeric TelVR::ADE2 reporter gene, the TelVIIL::URA3 reporter and the RDN1::mURA3/HIS3 construct (Smith and Boeke, 1997) have been described previously (Cockell et al., 1998, 2000). For the assay in Table II, repression of RDN1::mURA3/HIS3 was monitored by comparing growth on YPD ± 0.1% FOA. A total of 30 colonies were used for each strain in six different experiments. Recombination events that excise URA3 were monitored by streaking eight FOAr colonies from each strain on medium lacking uracil.
Protein extraction and western blots
Yeast strains were grown to 1–2 × 107 cells/ml and cells were recovered by centrifugation to form a pellet of ∼100 µl. Cell extracts were obtained by glass bead breakage in the presence of 50 mM Tris–HCl pH 7.5, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/ml leupeptin, 2 µg/ml antipain, 300 µg/ml benzamidine, 1 µg/ml pepstatin A, 100 µg/ml 1-chloro-3-tosylamido-7-l-2-heptanone (TLCK), 50 µg/ml N-tosyl-l-phenylalanine chloromethylketone (TPCK), 1% Trasylol. Protein concentrations were calculated by Bradford assay, and 30–40 µg of protein extract were separated on a 10% SDS–polyacrylamide gel and western blotted by standard enhanced chemiluminescence protocols (ECL, Amersham).
Immunofluorescence microscopy on yeast cells and preparation of antibodies
Yeast immunofluorescence methods and antibodies were described previously (Gotta et al., 1996, 1997). Rabbit anti-hSirT2 was raised against recombinant MBP fused to hSirT2p expressed in E.coli by standard procedures (New England Biolabs). All rabbit antisera were affinity purified against bacterially expressed fusion proteins transferred to nitrocellulose strips. In the case of anti-MBP-hSirT2, antibodies were affinity purified in two ways: either on nitrocellulose strips or by binding and elution from native MBP–hSirT2 protein that was covalently bound to cyanogen bromide-activated Sepharose beads. For affinity purification, antisera were first depleted for anti-MBP antibodies by incubation with MBP alone. Other antibodies used are: anti-Myc (monoclonal 9E10), anti-Nop1 (A66, gift of John P.Aris, University of Florida, Gainesville, FL; Aris and Blobel, 1988), rabbit anti-Nop1 (RP1-5, gift of Ed Hurt, Heidelberg), anti-HA (HA.11, clone 16B12 monoclonal from BABCO, Berkeley, CA), Cy5-coupled anti-mouse secondary antibody and 5-(4,6-dichlorotriazinyl)aminofluorescein (DTAF)-coupled anti-rabbit secondary antibody (both Milan Analytica). Secondary antibodies were pre-absorbed against fixed yeast spheroplasts prior to use. No cross-reactivity among these reagents has been detected, and controls using secondary antibodies alone were carried out. For western blot standardization, a rat antibody that recognizes an abundant RNase H (p55) was used (Karwan et al., 1990).
Confocal microscopy was performed on a Zeiss Laser Scanning Microscope 410 and 510 with a 63× Plan-Apochromat objective (1.4 oil), as previously described (Gotta et al., 1996). No signal from one fluorochrome could be detected on the other filter set, and image capture and background subtraction were carried out uniformly on all images to allow direct comparisons.
Cell culture and immunofluorescence microscopy on mammalian cells
ΦNXA cells (Phoenix amphotropic packaging lines, 293T packaging cell line, human embryonic kidney line transformed with adenovirus E1a) were maintained in high glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL) with 10% fetal calf serum (FCS) and HEPES. Transfections were done with calcium phosphate precipitates, as described by Jordan et al. (1996). Cells were grown on coverslips which were washed twice in phosphate-buffered saline (PBS) and fixed in 4% freshly prepared paraformaldehyde for 15 min at room temperature. All subsequent steps were carried out at this temperature. Cells were washed once with PBS and permeabilized in 0.1% Triton X-100/PBS for 10 min. Cells were washed once in PBS, sites were saturated for 30 min in 2% bovine serum albumin (BSA)/0.1% Tween 20/PBS and subsequently washed twice in PBS. Primary and secondary antibodies were incubated in 1% BSA/0.1% Triton X-100/PBS with washes of 3 × 5 min with PBS (primary antibodies for 2 h, secondary antibodies for 45 min). Images were captured as described above. For samples with competitor, 10 µl of whole bacterial extract dissolved in Laemmli buffer lacking β-mercaptoethanol and bromophenol blue were added to 90 µl of antibody adjusted to 1% Triton X-100. After 2 h, complexes were sedimented by centrifugation, and the supernatant was used to stain the coverslips.
Acknowledgments
Acknowledgements
We thank J.Smith (Johns Hopkins, Baltimore), J.Broach (Princeton University, Princeton), D.Moazed (Harvard Medical Schools, Boston), D.Shore (University of Geneva, Geneva) and M.Amacker (ISREC, Epalinges) for strains, plasmids and protocols. We are grateful to Dr T.Woelfel (University of Mainz, Mainz) for the pCMV-hSIRT2 plasmids and for sharing unpublished results. We thank R.Sternglanz, D.Shore and members of the Gasser laboratory for stimulating discussions and the latter for critical readings of the manuscript. Research was funded by the Swiss National Science Foundation, the Swiss Cancer League and Human Frontiers Science Program grants to S.M.G.
References
- Afshar G. and Murnane,J.P. (1999) Characterization of a human gene with sequence homology to Saccharomyces cerevisiae SIR2. Gene, 234, 161–168. [DOI] [PubMed] [Google Scholar]
- Aparicio O.M., Billington,B.L. and Gottschling,D.E. (1991) Modifiers of position effect are shared between telomeric and silent mating-type loci in S.cerevisiae. Cell, 66, 1279–1287. [DOI] [PubMed] [Google Scholar]
- Aris J.P. and Blobel,G. (1988) Identification and characterization of a yeast nucleolar protein that is similar to a rat liver nucleolar protein. J. Cell Biol., 107, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann C.B., Sherman,J.M., Devine,S.E., Cameron,E.E., Pillus,L. and Boeke,J.D. (1995) The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression and chromosome stability. Genes Dev., 9, 2888–2902. [DOI] [PubMed] [Google Scholar]
- Braunstein M., Sobel,R.E., Allis,C.D., Turner,B.M. and Broach,J.R. (1996) Efficient transcriptional silencing in S.cerevisiae requires a heterochromatin histone acetylation pattern. Mol. Cell. Biol., 16, 4349–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk M., Banerjee,M., Murphy,M., Knudsen,K.E., Garfinkel,D.J. and Curcio,M.J. (1997) Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev., 11, 255–269. [DOI] [PubMed] [Google Scholar]
- Chen X.J. and Clark-Walker,G.D. (1994) sir2 mutants of Kluyveromyces lactis are hypersensitive to DNA-targeting drugs. Mol. Cell. Biol., 14, 4501–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell M. and Gasser,S.M. (1999) Nuclear compartments and gene regulation. Curr. Opin. Genet. Dev., 9, 199–205. [DOI] [PubMed] [Google Scholar]
- Cockell M., Gotta,M., Palladino,F., Martin,S.G. and Gasser,S.M. (1998) Targeting Sir proteins to sites of action: a general mechanism for regulated repression. Cold Spring Harb. Symp. Quant. Biol., 63, 401–412. [DOI] [PubMed] [Google Scholar]
- Cockell M., Perrod,S. and Gasser,S.M. (2000) Analysis of Sir2p domains required for rDNA and telomeric silencing. Genetics, 154, 1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire M.K., Weinstock,K.G. and Strathern,J.N. (1996) HST1, a new member of the SIR2 family of genes. Yeast, 12, 631–640. [DOI] [PubMed] [Google Scholar]
- Freeman-Cook L.L., Sherman,J.M., Brachmann,C.B., Allshire,R.C., Boeke,J.D. and Pillus,L. (1999) The Schizosaccharomyces pombe hst4(+) gene is a SIR2 homologue with silencing and centromeric functions. Mol. Biol. Cell, 10, 3171–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze C.E., Verschueren,K., Strich,R. and Esposito,R.E. (1997) Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J., 16, 6495–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R.A. (1999) Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun., 260, 273–279. [DOI] [PubMed] [Google Scholar]
- Frye R.A. (2000) Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun., 273, 793–798. [DOI] [PubMed] [Google Scholar]
- Golemis E.A., Gyuris,J. and Brent,R. (1996) Interaction trap/two hybrid system to identify interacting proteins. In Ausubel,F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (eds), Current Protocols in Molecular Biology. John Wiley & Sons, Inc., Vol. 3, pp. 20.21.21–20.21.23.
- Gotta M., Laroche,T., Formenton,A., Maillet,L., Scherthan,H. and Gasser,S.M. (1996) The clustering of telomeres and colocalization with Rap1, Sir3 and Sir4 proteins in wild-type Saccharomyces cerevisiae. J. Cell Biol., 134, 1349–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta M., Strahl-Bolsinger,S., Renauld,H., Laroche,T., Kennedy,B.K., Grunstein,M. and Gasser,S.M. (1997) Localization of Sir2p: the nucleolus as a compartment for silent information regulators. EMBO J., 16, 3243–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta M., Palladino,F. and Gasser,S.M. (1998) Functional characterization of the N terminus of Sir3p. Mol. Cell. Biol., 18, 6110–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S. and Esposito,R.E. (1989) A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell, 56, 771–776. [DOI] [PubMed] [Google Scholar]
- Gottschling D.E., Aparicio,O.M., Billington,B.L. and Zakian,V.A. (1990) Position effect at S.cerevisiae telomeres: reversible repression of Pol II transcription. Cell, 63, 751–762. [DOI] [PubMed] [Google Scholar]
- Higgins D.G., Thompson,J.D. and Gibson,T.J. (1996) Using CLUSTAL for multiple sequence alignments. Methods Enzymol., 266, 383–402. [DOI] [PubMed] [Google Scholar]
- Imai S.I., Armstrong,C., Kaeberlein,M. and Guarente,L. (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature, 403, 795–800. [DOI] [PubMed] [Google Scholar]
- Jordan M., Schallhorn,A. and Wurm,F.M. (1996) Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res., 24, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwan R.M., Laroche,T., Wintersberger,U., Gasser,S.M. and Binder,M. (1990) Ribonuclease H(70) is a component of the yeast nuclear scaffold. J. Cell Sci., 96, 451–459. [DOI] [PubMed] [Google Scholar]
- Kennedy B.K. et al. (1997) Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S.cerevisiae. Cell, 89, 381–391. [DOI] [PubMed] [Google Scholar]
- Landry J., Sutton,A., Tafrov,S.T., Heller,R.C., Stebbins,J., Pillus,L. and Sternglanz,R. (2000) The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl Acad. Sci. USA, 97, 5807–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M.S., McKenzie,A.,III, Demarini,D.J., Shah,N.G., Wach,A., Brachat,A., Philippsen,P. and Pringle,J.R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in S.cerevisiae. Yeast, 14, 953–961. [DOI] [PubMed] [Google Scholar]
- Lustig A.J. (1998) Mechanisms of silencing in Saccharomyces cerevisiae. Curr. Opin. Genet. Dev., 8, 233–239. [DOI] [PubMed] [Google Scholar]
- Marshall W.F. and Sedat,J.W. (1999) Nuclear architecture. In Ohlsson,R. (ed.), Genomic Imprinting. Springer-Verlag Berlin, Vol. 25, pp. 283–301. [DOI] [PubMed]
- Page R.D. (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci., 12, 357–358. [DOI] [PubMed] [Google Scholar]
- Perez-Martin J., Uria,J.A. and Johnson,A.D. (1999) Phenotypic switching in Candida albicans is controlled by a SIR2 gene. EMBO J., 18, 2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J. and Herskowitz,I. (1987) Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics, 116, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M.D., Winston,F. and Hieter,P. (1990) Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sherman J.M., Stone,E.M., Freeman-Cook,L.L., Brachmann,C.B., Boeke,J.D. and Pillus,L. (1999) The conserved core of a human SIR2 homologue functions in yeast silencing. Mol. Biol. Cell, 10, 3045–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou W. et al. (1999) Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell, 97, 233–244. [DOI] [PubMed] [Google Scholar]
- Singer M.S. and Gottschling,D.E. (1994) TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science, 266, 404–409. [DOI] [PubMed] [Google Scholar]
- Smith J.S. and Boeke,J.D. (1997) An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev., 11, 241–254. [DOI] [PubMed] [Google Scholar]
- Smith J.S., Brachmann,C.B., Pillus,L. and Boeke,J.D. (1998) Distribution of a limited Sir2 protein pool regulates the strength of yeast rDNA silencing and is modulated by Sir4p. Genetics, 149, 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.S., Caputo,E. and Boeke,J.D. (1999) A genetic screen for ribosomal DNA silencing defects identifies multiple DNA replication and chromatin-modulating factors. Mol. Cell. Biol., 19, 3184–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.S. et al. (2000) A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl Acad. Sci. USA, 97, 6658–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E.M. and Pillus,L. (1996) Activation of an MAP kinase cascade leads to Sir3p hyperphosphorylation and strengthens transcriptional silencing. J. Cell Biol., 135, 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl-Bolsinger S., Hecht,A., Luo,K. and Grunstein,M. (1997) SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev., 11, 83–93. [DOI] [PubMed] [Google Scholar]
- Straight A.F., Shou,W., Dowd,G.J., Turck,C.W., Deshaies,R.J., Johnson,A.D. and Moazed,D. (1999) Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell, 97, 245–256. [DOI] [PubMed] [Google Scholar]
- Tanner K.G., Landry,J., Sternglanz,R. and Denu,J.M. (2000) Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc. Natl Acad. Sci. USA, 97, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanny J.C., Dowd,G.J., Huang,J., Hilz,H. and Moazed,D. (1999) An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell, 99, 735–745. [DOI] [PubMed] [Google Scholar]
- Woelfel T. et al. (1995) A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science, 269, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Xie J., Pierce,M., Gailus-Durner,V., Wagner,M., Winter,E. and Vershon,A.K. (1999) Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in S.cerevisiae. EMBO J., 18, 6448–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahiaoui B., Taibi,A. and Ouaissi,A. (1996) A Leishmania major protein with extensive homology to Sir2 of Saccharomyces cerevisiae. Gene, 169, 115–118. [DOI] [PubMed] [Google Scholar]
- Zemzoumi K., Sereno,D., Francois,C., Guilvard,E., Lemesre,J.L. and Ouaissi,A. (1998) Leishmania major: cell type dependent distribution of a 43 kDa antigen related to Sir2 family. Biol. Cell, 90, 239–245. [DOI] [PubMed] [Google Scholar]