Abstract
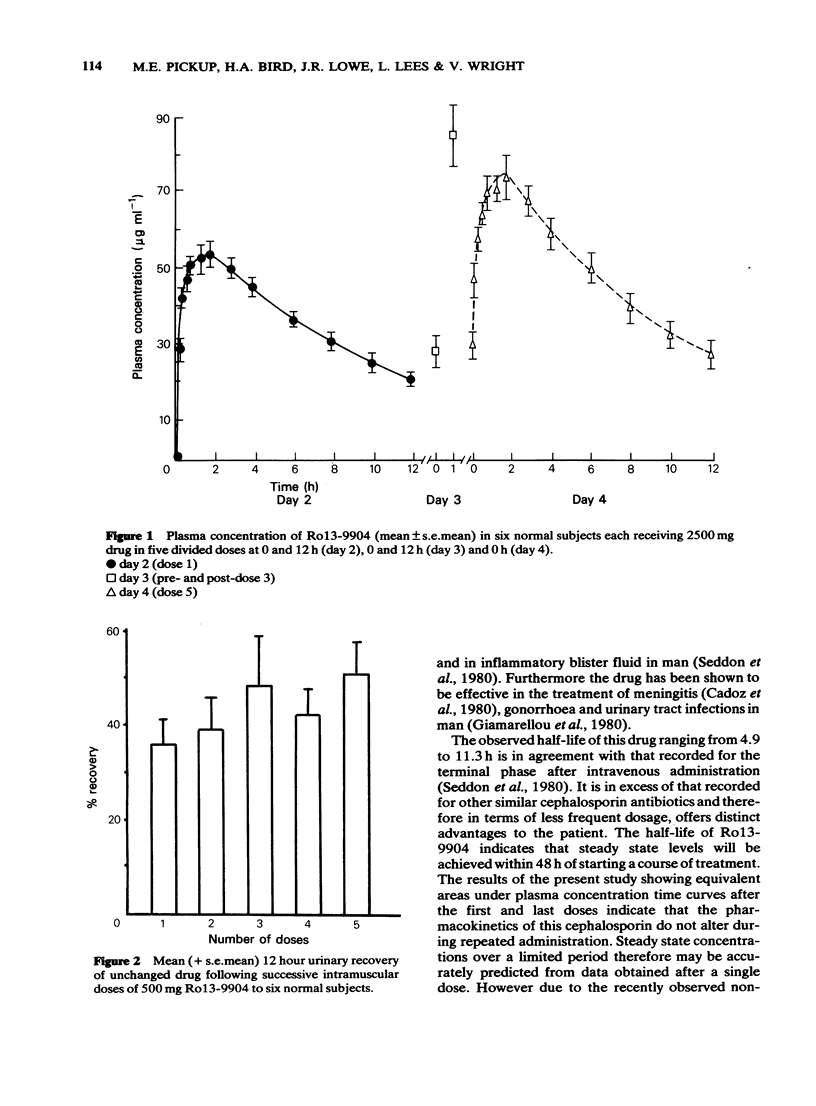
1 Six healthy male volunteers received a total of 2500 mg of a new cephalosporin antibiotic Ro13-9904 by intramuscular injection in five divided doses at intervals of 12 h. 2 No significant systemic side-effects were observed and this was confirmed haematologically and biochemically. 3 The drug was distributed following intramuscular injection reaching a mean peak plasma concentration of 55 micrograms ml-1 (range 46-66) 1 to 2 h after the first injection. 4 Monoexponential elimination of drug was demonstrated. No significant difference was recorded in the plasma half-life after the initial dose (mean 6.7 h) and at steady state (mean 6.7 h). The half-life is long compared with other cephalosporin antibiotics. 5 On the basis of the observed half-life, steady state should be reached within 48 h. A mean peak plasma concentration of 74 micrograms ml-1 (range 65-87) was recorded at steady state. Steady state plasma concentrations of Ro13-9904 with a dose of 500 mg every 13 h may be predicted from the pharmacokinetics of a single dose.
Full text
PDF

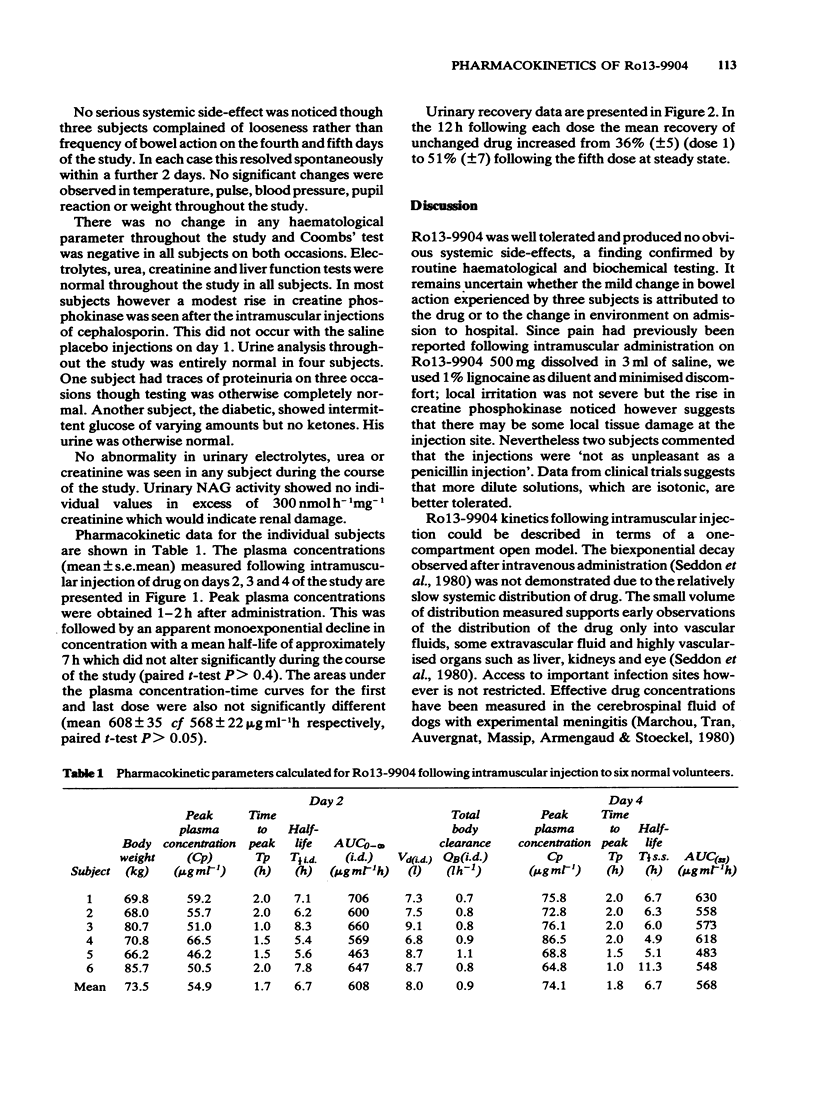

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Seddon M., Wise R., Gillett A. P., Livingston R. Pharmacokinetics of Ro 13-9904, a broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1980 Aug;18(2):240–242. doi: 10.1128/aac.18.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K., King A., Warren C., Phillips I. In vitro antibacterial activity and susceptibility of the cephalosporin Ro 13-9904 to beta-lactamases. Antimicrob Agents Chemother. 1980 Aug;18(2):292–298. doi: 10.1128/aac.18.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S. M., Boyd P. J., Thompson A. E., Price R. G. Automated assay of N-acetyl-beta-glucosaminidase in normal and pathological human urine. Clin Chim Acta. 1975 Jul 23;62(2):333–339. doi: 10.1016/0009-8981(75)90245-4. [DOI] [PubMed] [Google Scholar]
- Wise R., Gillett A. P., Andrews J. M., Bedford K. A. Ro 13-9904: A cephalosporin with a high degree of activity and broad antibacterial activity: an invitro comparative study. J Antimicrob Chemother. 1980 Sep;6(5):595–600. doi: 10.1093/jac/6.5.595. [DOI] [PubMed] [Google Scholar]


