Abstract
The translocation of secretory polypeptides into the endoplasmic reticulum (ER) occurs at the translocon, a pore-forming structure that orchestrates the transport and maturation of polypeptides at the ER membrane. In yeast, targeting of secretory precursors to the translocon can occur by two distinct pathways that are distinguished by their dependence upon the signal recognition particle (SRP). The SRP-dependent pathway requires SRP and its membrane-bound receptor, whereas the SRP-independent pathway requires a separate receptor complex consisting of Sec62p, Sec63p, Sec71p, Sec72p plus lumenal Kar2p/BiP. Here we demonstrate that Sec63p and Kar2p are also required for the SRP-dependent targeting pathway in vivo. Furthermore, we demonstrate multiple roles for Sec63p, at least one of which is exclusive to the SRP-independent pathway.
Keywords: endoplasmic reticulum/Kar2p/Sec63p/signal recognition particle/yeast
Introduction
The initial step in the biogenesis of secretory proteins is their targeting to, and subsequent translocation across the membrane of the endoplasmic reticulum (ER). All cells examined to date possess a signal recognition particle (SRP), which binds to nascent secretory polypeptides and targets them as ribosome-associated complexes to the ER via a specific membrane receptor (SRP receptor; SR) (for a review see Walter and Johnson, 1994). The membrane translocation reaction then requires the trimeric Sec61 complex (comprising Sec61α, β and γ subunits), which forms an aqueous transmembrane channel, or translocon (for a review see Johnson and van Waes, 1999). The Sec61 complex regulates the release of the nascent chain– ribosome complex from SRP–SR (Song et al., 2000) and ensures the specificity of the targeting reaction by interacting specifically with the signal sequence before initiating the translocation reaction (Jungnickel and Rapoport, 1995; Plath et al., 1998). The translating ribosome interacts with the Sec61 complex to create a tight seal at the cytosolic surface of the translocon, enabling the growing polypeptide to be extended directly into the Sec61 channel (Gorlich et al., 1992; Liao et al., 1997). There are conflicting reports as to the requirement for lumenal components in this co-translational translocation reaction (Gorlich and Rapoport, 1993; Nicchitta and Blobel, 1993; Dierks et al., 1996). However, recent evidence indicates that, prior to initiation of translocation, the translocon is sealed at its lumenal face in a manner that is dependent upon BiP and ATP hydrolysis (Hamman et al., 1998). Whether this lumenal ‘gating’ mechanism is essential for the translocation process is not yet clear, nor is it known how BiP is recruited to the Sec61 complex in order to perform this function.
In yeast, the SRP-dependent co-translational targeting pathway is conserved, but an alternative pathway has also been defined. This second pathway is independent of SRP/SR and is not obligately co-translational, but rather is capable of efficient post-translational targeting/translocation of completed polypeptide chains. The SRP-independent pathway requires cytosolic chaperones (for a review see Wilkinson et al., 1997), a heptameric membrane protein complex plus the lumenal components Kar2p (yeast BiP) (Vogel et al., 1990) and Lhs1p (Craven et al., 1996; Tyson and Stirling, 2000). The heptameric ‘Sec complex’ comprises the Sec61 trimer (Sec61p, Sss1p and Sbh1p) plus the tetrameric Sec63 complex, which is composed of two essential proteins (Sec62p and Sec63p) plus two non-essential proteins (Sec71p and Sec72p) (Brodsky and Schekman, 1993; Panzner et al., 1995). The Sec complex plus Kar2p are sufficient for the post-translational translocation of pre-pro-α-factor into reconstituted proteoliposomes in vitro (Panzner et al., 1995). A homologous Sec complex has been identified in mammals but its role in protein translocation has yet to be established (Meyer et al., 2000; Tyedmers et al., 2000). In yeast, post-translationally translocated precursors bind to the cytosolic surface of the ER membrane in an ATP-independent manner. This binding involves interactions with the Sec61, Sec62 and Sec72 proteins (Lyman and Schekman, 1997; Matlack et al., 1997; Plath et al., 1998), but occurs only in the context of the intact heptameric complex (Pilon et al., 1998). Whilst yeast has two ER-targeting pathways, it appears that individual secretory precursors fall into three classes with respect to their targeting. They are SRP dependent, SRP independent or able to follow either pathway, and are distinguished by differences in their signal sequences (Ng et al., 1996).
Here we report the isolation of novel alleles of sec63 that are defective in the translocation of an SRP-dependent precursor. We have gone on to show that Sec63p and Kar2p, but not Sec62p, are required for the translocation of SRP-dependent precursors in vivo. Our studies further demonstrate that Sec63p plays two distinct roles in ER membrane translocation. The first is required for stability of the heptameric complex, and is exclusive to the post-translational mechanism, whereas the second is required for co-translational translocation of an SRP-dependent precursor protein. Overall, these findings indicate that the minimal functional translocon comprises the Sec61 complex plus Sec63p/Kar2p. These findings offer a unifying hypothesis predicting a common gating mechanism regulating the yeast ER translocon in both the co- and post-translational translocation reactions.
Results
A genetic selection for mutants defective in SRP-dependent protein translocation
The ability to select directly for translocation mutants in Saccharomyces cerevisiae has been instrumental in identifying components of the translocation apparatus (see Wilkinson et al., 1997). Such selections typically involve the use of a fusion protein consisting of a normally cytoplasmic reporter protein attached to a defined signal sequence. In wild-type cells, the translocation of fusion protein into the ER effectively sequesters the reporter protein within the ER lumen, where it is inactive. Any mutant cells with defects in translocation that lead to mislocalization of fusion protein to the cytoplasm can then be selected by virtue of the reporter protein’s activity. Most recently, Ng et al. (1996) fused the signal sequence from pre-procarboxypeptidase Y (pre-proCPY) to the cytosolic enzyme Ura3p to select for mutants defective in the SRP-independent targeting/translocation pathway. We have adapted this approach in order to isolate mutants specifically defective in the translocation of the SRP-dependent precursors. For this, we fused the N-terminal signal anchor domain from the SRP-dependent type II integral membrane protein dipeptidyl-aminopeptidase B (DPAP B) to the Ura3p reporter (pRC7; see Materials and methods). When this fusion was expressed in a Δura3 strain, the cells remained phenotypically Ura–, being unable to grow on uracil-free medium. They did, however, express an N-glycosylated form of the fusion protein, consistent with its correct insertion into the ER membrane (data not shown). The targeting of fusion protein was examined in mutant cells defective either in a subunit of SRP (sec65-1), or a component of the heptameric complex required for post-translational translocation (sec62-1). Both mutants are conditional lethal, being viable at 24°C but exhibiting lethal translocation defects at 37°C. CSY135 (sec65-1) cells transformed with pRC7 were found to grow on uracil-free medium at 24°C, and further exhibited a complete defect in fusion protein translocation at 37°C (not shown). In contrast, CSY221 (sec62-1) cells transformed with the same plasmid remained Ura– at 24°C, and had no detectable defect in fusion protein translocation at any temperature (not shown). These results indicate that the DPAP B–URA3p fusion protein was translocated via the same SRP-dependent mechanism as native DPAP B.
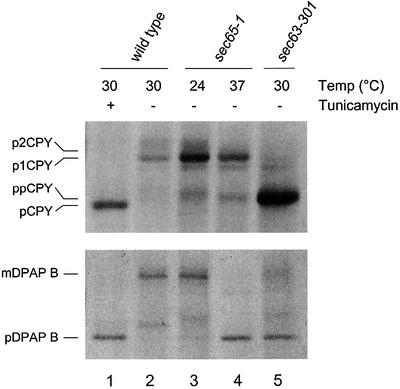
Wild-type cells (RCY3; Δura3) transformed with pRC7 were mutagenized and then Ura+ mutants were selected on minimal medium (see Materials and methods). A total of 82 recessive mutants were isolated, which fell into six complementation groups. Two of the largest groups corresponded to novel alleles of sec61 and sec63 (14 and 45 alleles, respectively). The novel sec63 alleles were of particular interest since there have been no reports of Sec63p being required for the translocation of an SRP-dependent precursor. This defect might be specific to the fusion protein construct and so cells were first cured of pRC7 before being examined by pulse-labelling and immunoprecipitation in order to determine the extent of any defect in the translocation of endogenous protein precursors. The results obtained from one such mutant, sec63-301, are shown in Figure 1. This strain exhibited a severe constitutive defect in post-translational translocation, as indicated by the accumulation of the unglycosylated precursor form of CPY (upper panel, lane 5). However, sec63-301 cells also accumulated the majority of newly synthesized DPAP B in its precursor form, indicating a major defect in SRP-dependent translocation (lower panel, lane 5). In these experiments, the temperature-sensitive SRP mutant, sec65-1, serves as a control confirming that translocation of CPY occurs independently of SRP, whilst DPAP B is an entirely SRP-dependent precursor.
Fig. 1. A sec63 allele defective in SRP-dependent translocation. Translocation defects in sec63-301 cells. Cultures were grown in minimal medium at 24 or 30°C as indicated: lanes 1 and 2, wild-type cells (W303-3d); lanes 3 and 4, sec65-1 cells (BWY501) grown at 24°C and shifted to 37°C (lane 4) for 1 h prior to labelling; lane 5, sec63-301 cells (RCY45a). Cells were harvested and pulse-labelled with [35S]l-methionine and processed as described in Materials and methods. Whole-cell extracts were immunoprecipitated with DPAP B- or CPY-specific antiserum and two OD600 equivalents were resolved by 7.5% (DPAP B) or 10% (CPY) SDS–PAGE. Tunicamycin treatment was performed by addition of the drug to 10 µg/ml 90 min before labelling. CPY forms are indicated as follows: ppCPY (pre-proCPY), pCPY (proCPY), p1CPY and p2CPY (ER and Golgi modified forms, respectively). The precursor and mature forms of DPAP B are labelled pDPAP B and mDPAP B, respectively.
Depletion of Sec63p leads to accumulation of SRP-dependent and -independent precursors in vivo
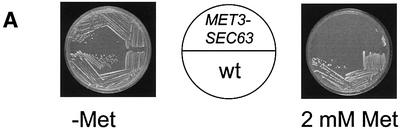
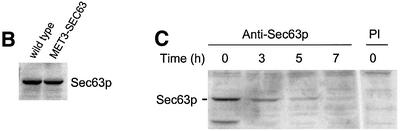
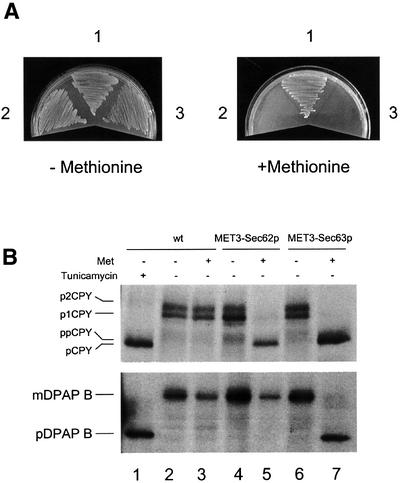
The finding that sec63-301 mutant cells are substantially defective in SRP-dependent translocation might define a novel role for Sec63p in this pathway. Alternatively, it may reflect an indirect consequence of this particular mutation, perhaps involving a dominant-negative gain of function relating to the role of Sec63p in the post-translational reaction. The sec63-301 mutation is recessive, thus excluding any dominant effect, but, in order to eliminate the possibility of an indirect consequence of this specific allele, we next examined protein translocation in cells depleted of native Sec63p. The SEC63 gene is essential for viability (Sadler et al., 1989) and so null mutant cells have never been analysed for their translocation activity. In order to do so, we constructed a strain in which the genomic copy of the SEC63 gene was placed under the control of the repressible MET3 promoter (PMET3-SEC63; see Materials and methods). Genes under the control of this promoter can be transcriptionally repressed by the addition of methionine to the growth media (Cherest et al., 1985). Cells carrying the PMET3-SEC63 allele grew well on minimal medium lacking methionine, but were unable to grow on medium supplemented with methionine (Figure 2A). In contrast, an otherwise isogenic strain carrying the wild-type SEC63 allele grew well on both media (Figure 2A). Immunoblotting analysis revealed that, in the absence of methionine, PMET3-SEC63 cells express a level of Sec63p similar to that seen in wild-type cells (Figure 2B). However, the levels of Sec63p antigen were found to decline rapidly upon addition of methionine, becoming almost undetectable after 7 h (Figure 2C).
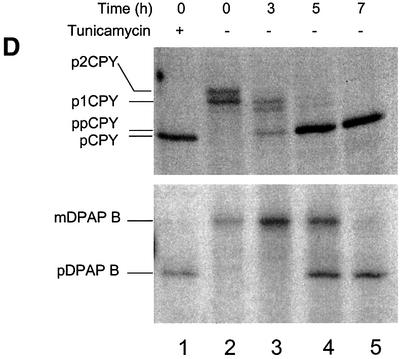
Fig. 2. Depletion of Sec63p inhibits translocation of SRP-dependent and -independent precursors. (A) Wild-type and PMET3-SEC63 cells were plated on minimal medium in either the absence or presence of 2 mM methionine as indicated and incubated at 30°C. (B) Expression of Sec63p from the MET3 promoter. Wild-type (W303-3d) and PMET3-SEC63 (BYY5) cultures were grown in minimal medium lacking methionine at 30°C. Whole-cell extracts were prepared and 0.5 OD600 equivalents were resolved by 10% SDS–PAGE. Immunodetection was performed using antiserum against Sec63p. (C) Depletion of Sec63p. PMET3-SEC63 cells (BYY5) were grown in minimal medium at 30°C to early log phase, whereupon methionine was added to a final concentration of 2 mM. Whole-cell yeast extracts were prepared at the times indicated and 0.5 OD600 equivalents were resolved by 10% SDS–PAGE. Immunoblotting was performed using antiserum against Sec63p or pre-immune (PI) serum as indicated, followed by ECL detection. (D) PMET3-SEC63 cells (BYY5) were harvested at various time points following addition of methionine as shown and were pulse-labelled with [14C]l-amino acid cocktail as described in Materials and methods. After immunoprecipitation, proteins were resolved by 7.5% (DPAP B) or 10% (CPY) SDS–PAGE, and visualized by autoradiography. Tunicamycin treatment (lane 1) was performed by addition of the drug to 10 µg/ml 90 min before harvesting cells.
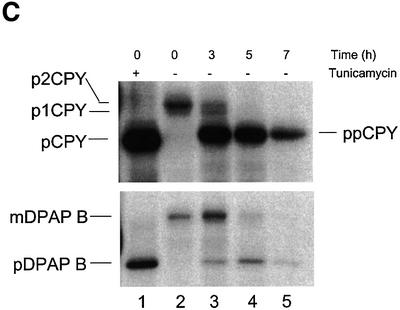
We next analysed the ability of cells to translocate various precursor proteins during depletion of Sec63p. Cells were pulse-labelled for 5 min with a cocktail of [14C]l-amino acids at various time points following addition of unlabelled methionine. Labelled proteins were immunoprecipitated from whole-cell extracts and then analysed by SDS–PAGE. We found that PMET3-SEC63 cells exhibited no defects in the translocation of either CPY or DPAP B when grown in the absence of methionine (Figure 2D). However, 3 h after the addition of methionine, we observed slight accumulation of pre-proCPY, with a complete defect in CPY translocation after 7 h (Figure 2D). Depleted cells also accumulated the precursor form of DPAP B, but the onset of this defect occurred later than for pre-proCPY. No defect in DPAP B translocation was evident after 3 h depletion but, after 5 h, >50% of this protein was found to have accumulated in the precursor form (Figure 2D, lanes 3 and 4). Following 7 h depletion, the defect in DPAP B translocation was complete (Figure 2D, lane 5). Significantly, we found that >95% of cells could recover following 5 h depletion if they were then plated onto methionine-free medium. Thus, the onset of the DPAP B translocation defect is not due to an indirect consequence of cell death. These results confirm that the post-translational translocation reaction is dependent upon Sec63p, but further demonstrate that Sec63p is also required for the translocation of SRP-dependent precursors in vivo.
Accumulation of SRP-dependent precursors in Sec63p-depleted cells is not a consequence of a block in post-translational protein translocation
One possible explanation for the accumulation of DPAP B in Sec63p-depleted cells is that inhibition of the post-translational pathway prevents translocation of a factor that is required for successful SRP-dependent translocation. To address this issue, we analysed the effect of depleting the other essential component of the Sec63 complex, Sec62p. As expected, a PMET3-SEC62 strain was unable to grow on media containing methionine (Figure 3A). The time course of Sec62p depletion was similar to that observed for Sec63p, with no detectable antigen present 7 h after addition of methionine (data not shown). When PMET3-SEC62 cells were pulse-labelled at this time point, we found that pre-proCPY translocation was completely blocked, whilst translocation of DPAP B remained unaffected (Figure 3B, lane 5). These results demonstrate that the defect in SRP-dependent translocation observed in PMET3-SEC63 is not due to a general defect in post-translational protein targeting to the ER.
Fig. 3. Defects in SRP-independent targeting and translocation do not block SRP-dependent translocation. (A) Strains were inoculated on minimal medium in either the absence or presence of methionine (0.1 mM) as indicated and then incubated at 30°C. (1) W303-3d (SEC63), (2) BYY5 (PMET3-SEC63) and (3) PRY26 (PMET3-SEC62). (B) Translocation defects in cells depleted of Sec62p or Sec63p. Strains were grown for 7 h in either the absence or presence of methionine before being pulse-labelled with [14C]amino acids, after which either DPAP B or CPY was immunoprecipitated and resolved by SDS–PAGE as before. Lanes 1–3, W303-3d; lanes 4 and 5, PRY26; lanes 6 and 7, BYY5. No pre-DPAP B was detectable in lanes 2–6 even after prolonged exposure times. (C) BYY12 cells (PMET3-SEC62 and PMET3-SEC63) were depleted of Sec62p and Sec63p simultaneously by addition of methionine (0.1 mM). CPY and DPAP B biogenesis were analysed essentially as described in Figure 2D.
We next examined the possibility that depletion of Sec63p might lead to abortive translocation of SRP-independent precursors that may block the available translocons, thus creating an indirect effect on the SRP-dependent pathway. To do this, it was necessary to analyse the effect of Sec63p depletion under condi tions where SRP-independent targeting was blocked prior to the interaction of precursor with the translocon. This was achieved by combining the PMET3-SEC63 and PMET3-SEC62 alleles to permit simultaneous depletion of both Sec62p and Sec63p. Since Sec62p is essential for targeting of SRP-independent precursors to the ER membrane (Lyman and Schekman, 1997), then its depletion would result in the accumulation of precursors at a stage prior to their interaction with the Sec61 translocon. This would be predicted to alleviate any indirect effect on DPAP B translocation caused by the occupation of translocons by SRP-independent precursors. As expected, we found that simultaneous depletion of Sec62p and Sec63p led to a more rapid onset of the CPY translocation defect (compare Figure 2D with Figure 3C). DPAP B translocation was also affected in these cells (Figure 3C), with the onset of the defect being slightly earlier than upon Sec63p depletion alone (see Figure 2D). Our data clearly show that the DPAP B defect observed upon Sec63p depletion cannot be alleviated by a reduction in the Sec62p-dependent targeting of precursors to the membrane. We therefore conclude that the co-translational translocation of DPAP B is directly dependent on an activity of Sec63p.
Functional Kar2p is required for translocation of SRP-dependent precursors in vivo
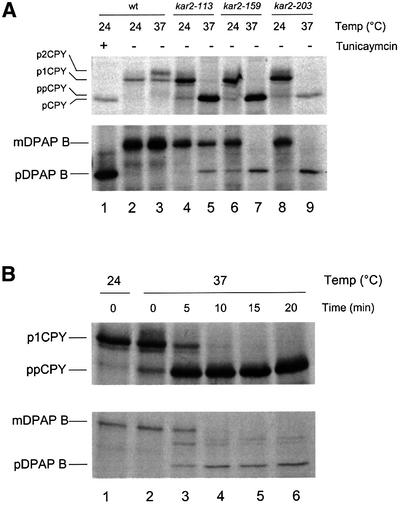
A range of studies indicate that the role of Sec63p in translocation is closely entwined with that of Kar2p (Brodsky and Schekman, 1993; Scidmore et al., 1993; Corsi and Schekman, 1997). We therefore tested whether the observed role for Sec63p in SRP-dependent translocation might also involve Kar2p. To this end, we examined various temperature-sensitive kar2 alleles to determine the extent of any defect in translocation of DPAP B in vivo. Cells carrying either the kar2-113, kar2-159 or kar2-203 alleles were grown at their permissive temperature of 24°C, before being shifted to their restrictive temperature of 37°C for 30 min. As expected, all kar2 strains showed a severe defect in pre-proCPY translocation at the restrictive temperature (Figure 4A). However, all three mutants were also defective in the translocation of DPAP B, with the most pronounced defects evident in kar2-159 and kar2-203 cells (Figure 4A, lanes 7 and 9). These results indicate a role for Kar2p in SRP-dependent protein translocation in vivo.
Fig. 4. Translocation defects in kar2 mutant strains. (A) Temperature-sensitive defects in kar2 strains. Strains were grown in minimal medium at 24°C to late log phase. Cells were shifted to the indicated temperature for 30 min and pulse-labelled for 5 min with [35S]l-methionine at the same temperature. DPAP B and CPY were immunoprecipitated and visualized by SDS–PAGE essentially as described in Figure 1. Strains were as follows: lanes 1–3, W303-3d; lanes 4 and 5, MS543; lanes 6 and 7, MS177; lanes 8 and 9, MS1030. (B) Kinetics of translocation defects in kar2-159 cells. MS177 cells were grown at 24°C to late log phase, after which 12 OD600 equivalents were harvested, resuspended in 3 ml of culture supernatant and split into six equal aliquots. After 5 min incubation at 24°C, five aliquots were shifted to 37°C for varying lengths of time (as indicated) whilst one aliquot was retained at 24°C. Cells were then pulse-labelled for 5 min with 40 µCi of [35S]l-methionine. CPY and DPAP B were immunoprecipitated from whole-cell extracts and visualized by SDS–PAGE.
The kar2-159 allele is particularly useful since the associated defect in post-translational translocation occurs very rapidly following a shift to the restrictive temperature (Vogel et al., 1990). If Kar2p plays a direct role in SRP-dependent translocation, then one would expect to see a similarly rapid onset of the accumulation of DPAP B in these cells. Cells grown at 24°C were shifted to 37°C and then pulse-labelled after increasing intervals at this restrictive temperature. Immunoprecipitation and SDS–PAGE analysis were again used to monitor the processing of pre-proCPY and DPAP B. Within 5 min at 37°C, substantial accumulation of precursor forms of both CPY and DPAP B was evident, with both defects being nearly complete after 10 min (Figure 4B). The rapid onset of precursor accumulation at 37°C is consistent with a direct role for Kar2p in both translocation pathways.
Multiple roles for Sec63p
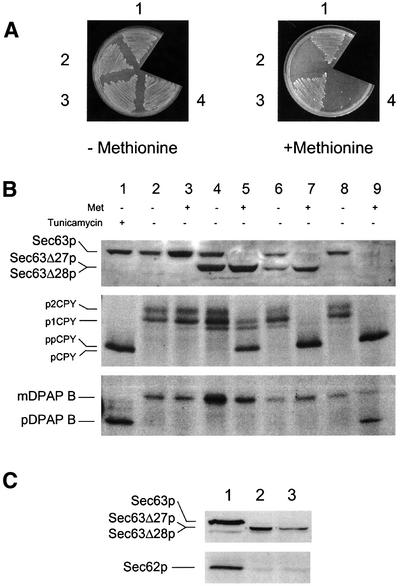
The finding that Sec62p is not required for SRP-dependent translocation suggests that the role of Sec63p in this pathway might be independent of its interaction with the other components of the ‘Sec63 complex’. In order to investigate this further, we examined the sec63-201 allele, which expresses a truncated form of Sec63p lacking the C-terminal 27 amino acid residues and which exhibits an impaired ability to interact with Sec62p (Ng and Walter, 1996). We noted with interest that while the sec63-201 mutant is viable, Feldheim et al. (1992) have shown that a Sec63–invertase fusion protein lacking the C-terminal 28 residues of Sec63p was unable to complement the sec63 null mutation. The failure of this fusion to function might reflect an inhibitory effect of the invertase domain itself, or might indicate a vital role for the threonine residue at position –28. We therefore engineered strains that expressed truncated forms of Sec63p lacking either the C-terminal 27 or 28 amino acid residues (Sec63Δ27p and Sec63Δ28p). These were constitutively expressed in cells containing the PMET3-SEC63 allele in order to complement any potentially lethal deletion (see Materials and methods). Cells expressing Sec63Δ27p grew on methionine-containing medium as expected, whereas those expressing Sec63Δ28p did not (Figure 5A). These results indicate an essential function of Sec63p associated with the C-terminal 28 residues. We then assayed the translocation phenotypes of these strains and found that cells expressing either of the truncated proteins exhibited SRP-independent translocation phenotypes (Figure 5B, lanes 5 and 7) in the absence of wild-type Sec63p. Interestingly, sec63Δ28 cells exhibited a greater defect in CPY translocation than those expressing Sec63Δ27p, which may account for the difference in viability of the two strains. However, neither of the truncated proteins was defective in SRP-dependent translocation of DPAP B (Figure 5B).
Fig. 5. Truncations in the C-terminus of Sec63p specifically block SRP-independent translocation. (A) Strains were inoculated on minimal medium in either the absence or presence of methionine (0.1 mM) as indicated and incubated at 30°C. (1) W303-3d (SEC63), (2) BYY5 (PMET3-SEC63), (3) BYY9 (PMET3-SEC63/SEC63Δ27) and (4) BYY8 (PMET3-SEC63/SEC63Δ28). (B) Translocation defects of Sec63p C-terminal truncation mutants. Strains were analysed by immunoblotting (for Sec63p) or by pulse-labelling and immunoprecipitation essentially as described in Figure 2. Cells were grown at 30°C for 7 h in either the absence or presence of methionine. Proteins were visualized by 10% SDS–PAGE (Sec63p and CPY) or 7.5% SDS–PAGE (DPAP B). Lanes 1–3, W303-3d (SEC63); lanes 4 and 5, BYY9 (PMET3-SEC63/SEC63Δ27); lanes 6 and 7, BYY8(PMET3-SEC63/SEC63Δ28); lanes 8 and 9, BYY5 (PMET3-SEC63). (C) Truncations in the C-terminal region of Sec63p perturb the stability of Sec62p in membranes. Wild-type (lane 1), BYY9 (lane 2) or BYY8 (lane 3) cells were grown in minimal medium and then harvested 7 h after addition of methionine. Membranes were prepared as described by Wilkinson et al. (2000) and equal aliquots (0.1 OD280 equivalents) resolved by 11% SDS–PAGE followed by immunodetection of Sec62p or Sec63p as indicated.
As a simple test for the ability of the truncated pro teins to form the heptameric Sec complex, microsomal membrane fractions were prepared from cells expressing full-length or truncated Sec63p. The abundance of Sec62p was then analysed by western blotting and shown to be substantially reduced in cells expressing only truncated Sec63p (Figure 5C). These findings indicate that the deleted portions of Sec63p are important for the stability of Sec62p in membranes. In contrast, the depletion of Sec63p had no detectable effect on the steady-state level of Sec61p, or of components of the SRP-targeting machinery (Srp102p and Sec65p; data not shown). Taken together, these results demonstrate multiple roles for Sec63p, with its activity in SRP-dependent translocation being independent of its role in stabilizing the heptameric post-translational translocon.
The cytosolic domain of Sec63p is required for SRP-dependent translocation
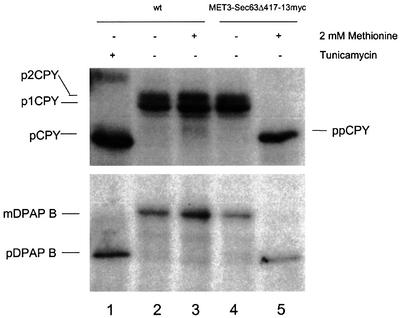
The topology of Sec63p gives clues to its potential roles in translocation. The presence of a large (423 residues) cytosolic domain suggests a prominent site for cytosolic interactions. Our results show that the extreme C-terminal 28 residues are not required for SRP-dependent translocation. In order to test whether the remaining 395 residues are required for the SRP-dependent reaction, a strain was constructed in which the entire cytosolic domain of Sec63p was deleted and replaced with a 13-Myc epitope. Again, a copy of full-length SEC63 under the MET3 promoter was present to complement the predicted lethal phenotype of the deletion. Analysis of translocation in these cells showed that after methionine depletion of wild-type Sec63p, both CPY and DPAP B translocation are completely blocked (Figure 6, lane 5). These phenotypes are identical to those seen after the complete depletion of Sec63p, suggesting that the cytosolic domain of Sec63p plays a further role that is essential for SRP-dependent translocation.
Fig. 6. Deletion of the C-terminal cytosolic domain of Sec63p blocks SRP-dependent and -independent translocation. Strains were grown for 7 h in either the absence or presence of methionine (2 mM) before being pulse-labelled with [14C]amino acids, after which either DPAP B or CPY was immunoprecipitated and resolved by SDS–PAGE as before. Lanes 1–3, W303-3d (SEC63); lanes 4 and 5, BYY14 (PMET3-SEC63/SEC63Δ417-13myc).
Discussion
Previous studies have clearly shown that both Sec63p and Kar2p are required for post-translational translocation in yeast (Rothblatt et al., 1989; Vogel et al., 1990). In addition, one interesting study found that sec63-1 and kar2-159 membranes are defective in the co-translational import of a truncated form of yeast invertase in vitro (Brodsky et al., 1995). However, the in vitro translocation of truncated invertase is not obligatorily co-translational (Hansen and Walter, 1988; Brodsky et al., 1995), nor is the targeting of full-length invertase entirely SRP dependent in vivo (Rothblatt et al., 1989; Stirling et al., 1992). Indeed, whilst sec63-1 cells are severely defective in the translocation of invertase and CPY (Rothblatt et al., 1989), they exhibit only a very marginal defect in translocation of the SRP-dependent precursor DPAP B (Stirling et al., 1992). The SRP-dependent translocation of native DPAP B into yeast membranes has not yet been reconstituted in vitro. We therefore chose to examine the role of Sec63p and Kar2p in the SRP-dependent pathway by monitoring processing of DPAP B in vivo. Our analysis revealed severe defects in the translocation of both SRP-dependent and -independent precursors in novel sec63 mutants and in various kar2 mutant strains. These findings represent the first demonstration of an essential role for Sec63p/Kar2p in SRP-dependent protein translocation.
During a time course of Sec63p depletion, we noted that pre-proCPY accumulation began before the DPAP B defect was evident (Figure 2B). This raised the possibility that the defect in SRP-dependent translocation might have arisen as an indirect consequence of the defect in SRP-independent translocation in these cells. The most obvious mechanism for this was to suggest that Sec63p depletion led to a failure to translocate post-translationally some factor that was required for SRP-dependent translocation. We addressed this issue by using a sec62 mutant that is specifically defective in the SRP-independent targeting pathway. Like Sec63p, depletion of Sec62p led to a complete block in CPY translocation (Figure 3B), but in this case no defect in DPAP B processing was observed. From these results, it is evident that inhibition of SRP-independent targeting had no effect on the SRP-dependent pathway. The role of Sec63p/Kar2p in driving post-translational translocation across the bilayer suggested a second possible mechanism by which an indirect effect on DPAP B processing might arise. As Sec63p levels decline, there may come a point at which there is sufficient Sec63p present to initiate interaction of a precursor with the translocon, but not to complete translocation. This might lead to SRP-independent precursors remaining associated with the translocon, blocking access for all other precursors. We have addressed this possibility by simultaneous depletion of both Sec63p and Sec62p. The Sec62 protein is required for targeting SRP-independent precursors to the membrane and so its depletion would be expected to reduce the proportion of translocons that can be blocked by such precursors. As might be predicted, the double depletion led to a more rapid onset of the defect in CPY translocation, but this did not alleviate the accumulation of DPAP B associated with Sec63p depletion
We therefore conclude that the effect of Sec63p depletion on DPAP B translocation is due neither to a block in translocation of an SRP-independent factor nor to accumulation of blocked translocons, but rather reflects a direct requirement for Sec63p in the SRP-dependent translocation reaction. The earlier onset of the CPY defect compared with that for DPAP B during Sec63 depletion suggests that the two pathways have differing requirements for Sec63p. Consistent with this, we note that the CPY defect appeared at a time (3 h) when a significant level of Sec63p was still evident (Figure 2D, lane 3). This level of Sec63p is therefore sufficient for DPAP B translocation but has become limiting for CPY translocation. Only when the level of Sec63p had declined further (at 5–7 h) did it become limiting for DPAP B translocation (see Figure 2C and D).
The different requirements for Sec63p levels observed in SRP-dependent versus -independent translocation might suggest that it plays different roles in the two pathways. Indeed, our analysis of C-terminal truncation mutants has revealed evidence of an additional function for Sec63p that is specific to the SRP-independent pathway. The C-terminal 28 residues are required to stabilize Sec62p and thus for the formation of the heptameric Sec complex. As might be expected, the loss of this activity correlates with a lethal defect in post-translational translocation. However, this truncation had no effect on the activity of Sec63p that is required for the co-translational translocation reaction, demonstrating that Sec63p performs at least two distinct roles in ER translocation. Whilst the C-terminal 28 residues are not required for DPAPB translocation, the deletion of the entire C-terminal cytosolic domain does eliminate SRP-dependent translocation. These results suggest two roles for the cytosolic domain that, taken together with the characterized role of the lumenal J-domain, suggest that Sec63p is a functionally complex molecule.
Given the characterized interaction between Sec63p and Kar2p, we next asked whether various kar2 mutants might also be defective in SRP-dependent translocation. In both kar2-159 and kar2-203 cells, we observed a complete defect in DPAP B translocation under non-permissive conditions. The onset of the DPAP B accumulation defect was extremely rapid, and is entirely consistent with a direct role for Kar2p in this process. Interestingly, we found that kar2-113 cells displayed a complete defect in post-translational translocation of CPY, but only a partial defect in DPAP B translocation. This might indicate that, like Sec63p, Kar2p has multiple functions in translocation and that the kar2-113 mutation affects an activity required primarily in the post-translational pathway. The simplest hypothesis to explain the requirement for both Sec63p and Kar2p would be to propose that this involves their interaction via the lumenal J-domain of Sec63p; however, we cannot formally exclude alternative models at this time.
Current evidence indicates that Kar2p is required at two distinct stages during the post-translational pathway (Sanders et al., 1992; Lyman and Schekman, 1995, 1997). In the first step, it promotes the stable interaction of a precursor protein with Sec61p. In the second step, it is required to bind the incoming precursor in order to drive the import reaction (Matlack et al., 1999). The precise mechanism driving import is unknown, but all current models predict multiple cycles of precursor binding by Kar2p in an ATP-dependent reaction promoted by the J-domain of Sec63p. It follows from such models that Sec63p/Kar2p would be required at the translocon both during initiation and then throughout the import process.
Recent evidence from mammalian studies suggests that BiP acts to seal the lumenal face of the translocon prior to initiation of the co-translational translocation into mammalian membranes in vitro (Hamman et al., 1998). Yet it has also been reported that protein translocation can proceed in this system in the apparent absence of BiP (Gorlich and Rapoport, 1993). Our results offer clear evidence that yeast Kar2p (BiP) is essential for SRP-dependent protein translocation in vivo. The simplest interpretation of these results would be to suggest that Kar2p performs a similar gating reaction to that described in the mammalian ER, and that this gating role is essential in vivo where the integrity of the ER lumen would be paramount.
The BiP-dependent lumenal gating mechanism opens only after the cytosolic surface of the translocon has been sealed by tight binding of the ribosome (Hamman et al., 1998). It follows that the activity of the lumenal seal must be regulated by events occurring at the cytosolic face of the membrane, and indeed this has been shown to be true at least in the case of membrane protein integration (Liao et al., 1997). This requires some mechanism capable of transducing a signal across the ER membrane to co-ordinate the cytosolic and lumenal gates. The data we present here support the hypothesis that Sec63p mediates this communication between the cytosolic and lumenal face of the ER membrane. First, Sec63p possesses a large cytosolic domain that is essential for co-translational translocation. Thus there is the potential for interaction with cytosolic components, such as the ribosome. Secondly, the lumenal domain of Sec63p is capable of interacting with BiP and stimulating its ATPase activity. It has been proposed that the transition of BiP from an ATP- to an ADP-bound state may correlate directly with its ability to bind to the translocon (Hamman et al., 1998). Therefore, Sec63p is potentially capable of modulating the interaction of Kar2p with the translocon. It will be interesting to determine whether the cytosolic domain of Sec63p can influence the activity of the protein’s lumenal J-domain.
Materials and methods
Materials
DNA restriction and modification enzymes were purchased from Roche Molecular Biochemicals. [35S]methionine and [14C]l-amino acid cocktail were from NEN Life Science Products. All other reagents were from Roche Molecular Biochemicals, Sigma, British Drug House or Melford Labs (Suffolk, UK) at analytical grade.
To create plasmid pPR33, a blunt-ended 498 bp EcoRV–XbaI fragment from pHAM8 containing the MET3 promoter (Mountain and Korch, 1991) was inserted into the blunt-ended SalI site of pFA6a (Wach et al., 1994). Plasmid pRC7 was generated by subcloning the promoter region plus codons 1–349 of the DAP2 open reading frame from pRG1 (Roberts et al., 1989) as a blunt-ended BamHI–ClaI fragment into the SmaI site of YEp351 (Hill et al., 1986) to give pRC1. A 1.1 kb BamHI fragment encoding Ura3p was then subcloned from pNKY48 (Alani and Kleckner, 1987) into pRC1 to give pRC7.
Yeast strains are listed in Table I. The SEC63 gene was placed under the control of the MET3 promoter (strain BYY5) by transforming W303 with a linear kanMX4-SEC63::PMET3 cassette, giving diploid strain BYY4. The cassette was generated by PCR amplification of pPR33 using primers 5′-CACTAGCCTCATCATACTCGTAATTTGTAGGCATTGTGCTCGGCCGCCAGCTGAAGC-3′ and 5′-ACTGAACGAATAAAAGATGCGACTGGAACAATAGTCAGTTCCAGCATTCACATACGATTG-3′. Selection was performed on YPD containing 200 µg/ml G418, and correct integration confirmed by PCR analysis. A haploid strain carrying the PMET3-SEC63 allele, BYY5, was generated by sporulation and tetrad dissection onto minimal medium lacking methionine. Strain PRY26, which contains the SEC62 gene under the control of the MET3 promoter, was generated in a fashion similar to BYY5, except that wild-type strain TR1 was used and primers for generation of the kanMX4-PMET3 cassette were 5′-CTGACATGTTCTCCTGTGTTTGCTCGGCTACCATACTGCACGGCCGCCAGCTGAAGC-3′ and 5′-ATCGTGTGCGACGGAATAGACGTGTCGTTTTCCCAATACTCCAGCATTCACATACGATTG-3′. Strain BYY8, containing a 28 amino acid truncation of SEC63 and the full-length copy of SEC63 under the MET3 promoter, was generated by transforming the His– strain BYY4 with a HIS3MX6::sec63Δ28 cassette. This cassette was generated by PCR of a module from pFA6a-GFP(S65T)-HIS3MX6 (Longtine et al., 1998) that contains the his5+ gene from Schizosaccharomyces pombe, and a stop codon followed by the ADH1 transcription termination region. Primers 5′-TTCTGAGGAGGATGATGAGTACTCTACTGATGACGACGAATGAGGCGCGCCACTTCTAAA-3′ and 5′-CTATTCTGGTGATTCATCATCTTCAGCTTCTGTATCCGTAGAATTCGAGCTCGTTTAAAC-3′ were used, which contain 5′ homology to the C-terminal region of SEC63 and 3′ homology to pFA6a-GFP(S65T)-HIS3MX6, such that integration of the cassette results in the insertion of the HIS3MX6 gene along with a stop codon and the ADH1 terminator so that the predicted polypeptide translated from the gene is truncated by 28 amino acids at the C-terminus. Strain BYY9 was generated by transformation of strain BYY5 to tryptophan prototrophy with a plasmid encoding a 27 amino acid C-terminal deletion of Sec63p (pDN259; Ng and Walter, 1996). Strain BYY12 was generated by first crossing BYY5 and PRY26 to yield BYY10, followed by sporulation and tetrad dissection on minimal media as before. BWY501 is a sec65-1 strain derived from CSY126 (Stirling and Hewitt, 1992) backcrossed extensively to W303 haploid strains. Strain BYY14, containing a 417 amino acid truncation of SEC63 fused N-terminally to 13 copies of the myc epitope and the full-length copy of SEC63 under the MET3 promoter, was generated by transforming the His– strain BYY4 with a HIS3MX6::sec63Δ417-13myc cassette. This cassette was generated by PCR amplification of a module from pFA6a-13-Myc-HIS3MX6 (Longtine et al., 1998) that contains the his5+ gene from S.pombe, and a 13-Myc epitope. Primers 5′-CTAGGTTTAATCTTGCCATATTTTGTTAGTAGATGGTGGGCAAGACGGATCCCCGGGTTAATTAA-3′ and 5′-CTATTCTGGTGATTCATCATCTTCAGCTTCTGTATCCGTAGAATTCGAGCTCGTTTAAAC-3′ were used, such that integration of the cassette results in a predicted polypeptide where the C-terminal 417 amino acids are replaced with the 13-Myc epitope.
Table I. Saccharomyces cerevisiae strains.
| Strain | Genotype | Source or reference |
|---|---|---|
| W303 | MATa/MATα ade2/ade2 his3/his3 leu2/leu2 ura3/ura3 trp1/trp1 CAN1-100/CAN1-100 | Thomas and Rothstein (1989) |
| W303-3d | MATα ade2 his3 leu2 trp1 CAN1-100 | Thomas and Rothstein (1989) |
| TR1 | MATa/MATα ade2/ade2 his3/his3 lys2/lys2 ura3/ura3 trp1/trp1 | Parker et al. (1988) |
| MS543 | MATα ade2 trp1 ura3 kar2-113 | Scidmore et al. (1993) |
| MS1030 | MATα ade2 leu2 trp1 ura3 kar2-203 | Scidmore et al. (1993) |
| MS177 | MATα ade2 ura kar2-159 | Vogel et al. (1990) |
| RCY3 | MATα leu2 his3 trp1 ura3::TRP1 | this study |
| RCY4 | MATa ade2 leu2 trp1 ura3::TRP1 | this study |
| RCY45a | MATα leu2 his3 trp1 ura3::TRP1 sec63-301 | this study |
| PRY26 | MATa ade2 his3 lys2 trp1 ura3 kanMX4-PMET3-SEC62 | this study |
| BYY4 | as W303 but SEC63/kanMX4-PMET3-SEC63 | this study |
| BYY5 | MATα ade2 his3 leu2 trp1 can1 kanMX4-PMET3-SEC63 | this study |
| BYY8 | MATa/MATα ade2/ade2 his3/his3 leu2/leu2 ura3/ura3 trp1/trp1 CAN1-100/CAN1-100 kanMX4-PMET3-SEC63/sec63Δ28 | this study |
| BYY9 | MATα ade2 his3 leu2 trp1 can1 kanMX4-PMET3-SEC63 [pDN259; CEN TRP1 sec63-201] | this study |
| BYY12 | MATa kanMX4-PMET3-SEC63 kanMX4-PMET3-SEC62 ade2 his3 trp1 ura3 | this study |
| BWY501 | MATα ade2 his3 leu2 trp1 sec65-1 | this study |
Media and growth conditions
Yeast and Escherichia coli strains were grown on standard media as previously described (Wilkinson et al., 1997). Repression of the MET3 promoter in minimal medium was achieved by the addition of l-methionine to early log phase cultures to a final concentration of 0.1 or 2 mM. For experiments using strain PRY26 or its derivatives, the final concentration of methionine in all cultures was 0.1 mM, as higher concentrations substantially reduced the efficiency of pulse-labelling experiments of this strain (data not shown). In all other experiments, a final concentration of 2 mM was used, as previously described (Cherest et al., 1985). Varying the concentration of methionine within this range had no effect on the extent or kinetics of repression (data not shown).
Mutagenesis and mutant isolation
RCY3 cells containing pRC7 were grown to stationary phase in minimal media supplemented with uracil and histidine. Six OD600 equivalents of cells were harvested and mutagenized using the alkylating agent ethylmethane sulfonate (EMS) as described by Stirling et al. (1992). After exposure to EMS, cells were allowed to recover in YPD overnight at room temperature before plating on minimal medium supplemented with histidine to select for uracil prototrophs. After 7–10 days incubation at 30°C, Ura+ colonies were re-streaked on medium lacking uracil. Recessive mutants were identified after mating to wild-type strain RCY4. Eighty-two strains were chosen for subsequent analysis.
Radiolabelling and immunoprecipitation
For labelling with 14C, 5 OD600 equivalents of cells were pelleted, and washed once with 1 ml of YNB + 2% glucose. Pellets were resuspended in 150 µl of 1.6× YNB, 3.3% glucose, and incubated at the same temperature used for growth for 5 min. Ten microcuries of [14C]l-amino acid mix (NEN) were added, and the suspension was incubated for a further 5 min. For labelling with 35S, 2 OD600 equivalents of cells were pelleted, and resuspended in 500 µl of culture supernatant. After a 5 min pre-incubation, 40 µCi of [35S]methionine were added, and cells were incubated for a further 5 min. Labelling reactions were stopped by addition of an equal volume of ice-cold 20 mM NaN3, followed by incubation on ice for 5 min. Cells were pelleted in a microfuge, and resuspended in 1 ml of spheroplast buffer (1.4 M sorbitol, 50 mM Tris–HCl pH 7.4, 2 mM MgCl2, 10 mM NaN3). Yeast lytic enzyme (ICN) was added to a concentration of 1.5 U/OD600 and incubated at 30°C for 30 min. Spheroplasts were pelleted and resuspended in 250 µl of IP lysis buffer (50 mM Tris–HCl pH 7.4, 1 mM EDTA, 1% SDS). Lysis was induced by heating to 95°C for 5 min, followed by 5 min on ice. A 1 ml aliquot of IP buffer (187.5 mM NaCl, 62.5 mM Tris–HCl pH 8.0, 6.25 mM EDTA, 1.25% Triton X-100) was added to each sample, followed by 50 µl of insoluble protein A suspension (Sigma). Samples were cleared by rotation at 4°C for 30 min, after which insoluble protein A was pelleted by 5 min in a microfuge. Supernatants were transferred to a clean tube, and 1 µl/OD600 of appropriate antibody was added. Samples were rotated at room temperature for 1 h (CPY) or 2 h (DPAP B). Following this, 20 µl pf protein A–Sepharose (as a 20% w/v suspension in IP buffer) were added and rotated for a further 2 h at room temperature. Beads were pelleted in a microfuge and washed three times with 1 ml of IP buffer. Antigen was dissociated by addition of 50 µl of 2× SDS–PAGE sample buffer and heating to 95°C for 5 min.
Antibodies
Antibodies to Sec61p and DPAP B have been described previously (Stirling et al., 1992; Wilkinson et al., 2000). Antibodies to CPY, Sec62p and Sec63p were raised in sheep (Diagnostics Scotland, Lanarkshire, UK) with antigens described below. Gene fusions encoding glutathione S-transferase (GST) plus the C-terminal cytosolic domain of Sec63p (residues 240–663) or the N-terminal cytosolic domain of Sec62p (residues 1–158) were created by subcloning PCR-amplified products into pGEX-4T-3 (Amersham-Pharmacia Biotech, Amersham, UK). Fusion protein was purified on a pre-packed GSTrap™ column (Amersham-Pharmacia) according to the manufacturer’s instructions. Residues 155–525 of CPY were tagged with His10 in pET-16b (Novagen), and the fusion protein was extracted in 6 M guanidine and purified on Ni-NTA resin (QIAgen) according to the manufacturer’s instructions.
Acknowledgments
Acknowledgements
We thank R.Schekman, N.Kleckner, H.A.Mountain, M.D.Rose, J.L.Brodsky and J.D.Brown for providing various plasmids, yeast strains and antibodies. This work was supported by the Wellcome Trust. B.P.Y., R.A.C. and P.J.R. were supported by BBSRC Studentships.
References
- Alani E. and Kleckner,N. (1987) A new type of fusion analysis applicable to many organisms: protein fusions to the URA3 gene of yeast. Genetics, 117, 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J.L. and Schekman,R. (1993) A Sec63p–BiP complex from yeast is required for protein translocation in a reconstituted proteoliposome. J. Cell Biol., 123, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J.L., Goeckeler,J. and Schekman,R. (1995) BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast ER. Proc. Natl Acad. Sci. USA, 92, 9643–9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Nguyen,N.T. and Surdin-Kerjan,Y. (1985) Transcriptional regulation of the MET3 gene of Saccharomyces cerevisiae. Gene, 34, 269–281. [DOI] [PubMed] [Google Scholar]
- Corsi A.K. and Schekman,R. (1997) The lumenal domain of Sec63p stimulates the ATPase activity of BiP and mediates BiP recruitment to the translocon in S.cerevisiae. J. Cell Biol., 137, 1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven R.A., Egerton,M. and Stirling,C.J. (1996) A novel Hsp70 of the yeast ER lumen is required for the efficient translocation of a number of protein precursors. EMBO J., 15, 2640–2650. [PMC free article] [PubMed] [Google Scholar]
- Dierks T. et al. (1996) A microsomal ATP-binding protein involved in efficient protein transport into the mammalian endoplasmic reticulum. EMBO J., 15, 6931–6942. [PMC free article] [PubMed] [Google Scholar]
- Feldheim D., Rothblatt,J. and Schekman,R. (1992) Topology and functional domains of Sec63p, an ER membrane protein required for secretory protein translocation. Mol. Cell. Biol., 12, 3288–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlich D. and Rapoport,T.A. (1993) Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell, 75, 615–630. [DOI] [PubMed] [Google Scholar]
- Gorlich D., Prehn,S., Hartmann,E., Kalies,K.U. and Rapoport,T.A. (1992) A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell, 71, 489–503. [DOI] [PubMed] [Google Scholar]
- Hamman B.D., Hendershot,L.M. and Johnson,A.E. (1998) BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell, 92, 747–758. [DOI] [PubMed] [Google Scholar]
- Hansen W. and Walter,P. (1988) Prepro-carboxypeptidase Y and a truncated form of pre-invertase, but not full-length pre-invertase, can be posttranslationally translocated across microsomal vesicle membranes from S.cerevisiae. J. Cell Biol., 106, 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J.E., Myers,A.M., Koerner,T.J. and Tzagoloff,A. (1986) Yeast/E.coli shuttle vectors with multiple unique restriction sites. Yeast, 2, 163–168. [DOI] [PubMed] [Google Scholar]
- Johnson A.E. and van Waes,M.A. (1999) The translocon: a dynamic gateway at the ER membrane. Annu. Rev. Cell Dev. Biol., 15, 799–842. [DOI] [PubMed] [Google Scholar]
- Jungnickel B. and Rapoport,T.A. (1995) A posttargeting signal sequence recognition event in the ER membrane. Cell, 82, 261–270. [DOI] [PubMed] [Google Scholar]
- Liao S.R., Lin,J.L., Do,H. and Johnson,A.E. (1997) Both lumenal and cytosolic gating of the aqueous ER translocon pore are regulated from inside the ribosome during membrane protein integration. Cell, 90, 31–41. [DOI] [PubMed] [Google Scholar]
- Longtine M.S., McKenzie,A.,III, Demarini,D.J., Shah,N.G., Wach,A., Brachat,A., Philippsen,P. and Pringle,J.R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in S.cerevisiae. Yeast, 14, 953–961. [DOI] [PubMed] [Google Scholar]
- Lyman S.K. and Schekman,R. (1995) Interaction between BiP and Sec63p is required for the completion of protein translocation into the ER of S.cerevisiae. J. Cell Biol., 131, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman S.K. and Schekman,R. (1997) Binding of secretory precursor polypeptides to a translocon subcomplex is regulated by BiP. Cell, 88, 85–96. [DOI] [PubMed] [Google Scholar]
- Matlack K.E.S., Plath,K., Misselwitz,B. and Rapoport,T.A. (1997) Protein transport by purified yeast sec complex and Kar2p without membranes. Science, 277, 938–941. [DOI] [PubMed] [Google Scholar]
- Matlack K.E., Misselwitz,B., Plath,K. and Rapoport,T.A. (1999) BiP acts as a molecular ratchet during posttranslational transport of prepro-α factor across the ER membrane. Cell, 97, 553–564. [DOI] [PubMed] [Google Scholar]
- Meyer H.A., Grau,H., Kraft,R., Kostka,S., Prehn,S., Kalies,K.U. and Hartmann,E. (2000) Mammalian Sec61 is associated with Sec62 and Sec63. J. Biol. Chem., 275, 14550–14557. [DOI] [PubMed] [Google Scholar]
- Mountain H.A. and Korch,C. (1991) TDH2 is linked to MET3 on chromosome X of Saccharomyces cerevisiae. Yeast, 7, 873–880. [DOI] [PubMed] [Google Scholar]
- Ng D.T. and Walter,P. (1996) ER membrane protein complex required for nuclear fusion. J. Cell Biol., 132, 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D.T., Brown,J.D. and Walter,P. (1996) Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J. Cell Biol., 134, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta C.V. and Blobel,G. (1993) Lumenal proteins of the mammalian ER are required to complete protein translocation. Cell, 73, 989–998. [DOI] [PubMed] [Google Scholar]
- Panzner S., Dreier,L., Hartmann,E., Kostka,S. and Rapoport,T. (1995) Post-translational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell, 81, 561–570. [DOI] [PubMed] [Google Scholar]
- Parker R., Simmons,T., Shuster,J.W., Silicano,P.G. and Guthrie,C. (1988) Genetic analysis of small nuclear RNA in S.cerevisiae: viable sextuplet mutants. Mol. Cell. Biol., 8, 3150–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon M., Romisch,K., Quach,D. and Schekman,R. (1998) Sec61p serves multiple roles in secretory precursor binding and translocation into the endoplasmic reticulum membrane. Mol. Biol. Cell, 9, 3455–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath K., Mothes,W., Wilkinson,B.M., Stirling,C.J. and Rapoport,T.A. (1998) Signal sequence recognition in post-translational protein translocation across the yeast ER membrane. Cell, 94, 795–807. [DOI] [PubMed] [Google Scholar]
- Roberts C.J., Pohlig,G., Rothman,J.H. and Stevens,T.H. (1989) Structure, biosynthesis, and localization of DPAP B, an integral membrane glycoprotein of the yeast vacuole. J. Cell Biol., 108, 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblatt J.A., Deshaies,R.J., Sanders,S.L., Daum,G. and Schekman,R. (1989) Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J. Cell Biol., 109, 2541–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler I., Chiang,A., Kurihara,T., Rothblatt,J., Way,J. and Silver,P. (1989) A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, and E.coli heat shock protein. J. Cell Biol., 109, 2665–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S.L., Whitfield,K.M., Vogel,J.P., Rose,M.D. and Schekman, R.W. (1992) Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell, 69, 353–365. [DOI] [PubMed] [Google Scholar]
- Scidmore M.A., Okamura,H.H. and Rose,M.D. (1993) Genetic interactions between KAR2 and SEC63, encoding eukaryotic homologues of DnaK and DnaJ in the ER. Mol. Biol. Cell, 4, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Raden,D., Mandon,E.C. and Gilmore,R. (2000) Role of Sec61α in the regulated transfer of the ribosome–nascent chain complex from the signal recognition particle to the translocation channel. Cell, 100, 333–343. [DOI] [PubMed] [Google Scholar]
- Stirling C.J. and Hewitt,E.W. (1992) The S.cerevisiae SEC65 gene encodes a component of yeast signal recognition particle with homology to human SRP19. Nature, 356, 534–537. [DOI] [PubMed] [Google Scholar]
- Stirling C.J., Rothblatt,J., Hosobuchi,M., Deshaies,R. and Schekman,R. (1992) Protein translocation mutants defective in the insertion of integral membrane proteins into the ER. Mol. Biol. Cell, 3, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.J. and Rothstein,R. (1989) Elevated recombination rates in transcriptionally active DNA. Cell, 56, 619–630. [DOI] [PubMed] [Google Scholar]
- Tyedmers J. et al. (2000) Homologs of the yeast sec complex subunits sec62p and sec63p are abundant proteins in dog pancreas microsomes. Proc. Natl Acad. Sci. USA, 97, 7214–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson J.R. and Stirling,C.J. (2000) LHS1 and SIL1 provide a lumenal function that is essential for protein translocation into the endoplasmic reticulum. EMBO J., 19, 6440–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.P., Misra,L. and Rose,M.D. (1990) Loss of Bip/GRP78 function blocks translocation of secretory proteins in yeast. J. Cell Biol., 110, 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A., Brachat,A., Pohlmann,R. and Philippsen,P. (1994) New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast, 10, 1793–1808. [DOI] [PubMed] [Google Scholar]
- Walter P. and Johnson,A.E. (1994) Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu. Rev. Cell Biol., 10, 87–119. [DOI] [PubMed] [Google Scholar]
- Wilkinson B., Esnault,Y., Craven,R.A., Skiba,F., Fieschi,J., Kepes,F. and Stirling,C.J. (1997) Molecular architecture of the yeast ER translocase: mapping interacting domains between Sec61p and Sss1p. EMBO J., 16, 4549–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B.M., Tyson,J.R., Reid,P.J. and Stirling,C.J. (2000) Distinct domains within yeast sec61p involved in post-translational translocation and protein dislocation. J. Biol. Chem., 275, 521–529. [DOI] [PubMed] [Google Scholar]