Abstract
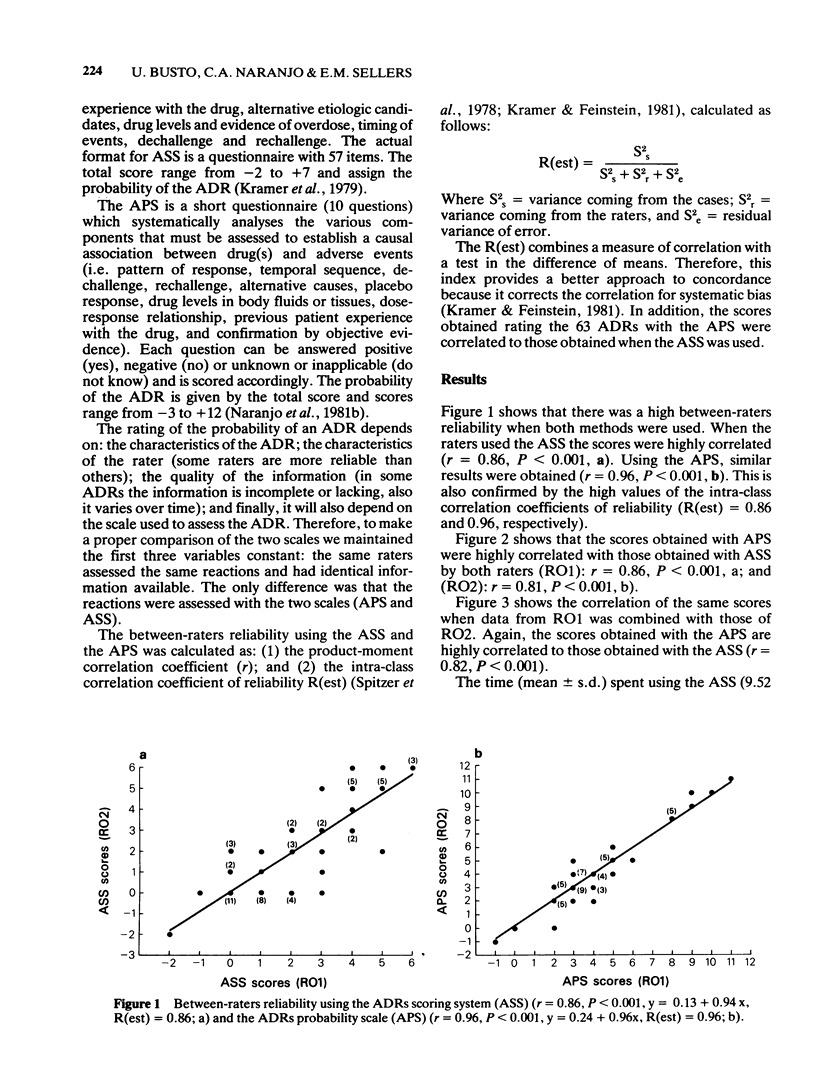
1 A simple valid and reliable method for estimating the probability of adverse drug reactions (adverse drug reactions probability scale, APS) has been recently described (Naranjo et al., 1981a). 2 The results using APS were compared to those obtained with another more detailed algorithm (adverse reactions scoring system, ASS) described by Kramer et al. (1979). 3 Sixty-three randomly selected adverse drug reactions (ADRs) were rated by two observers, using APS and ASS one year apart. The cases were ordered in a random sequence. Between-raters reliability using APS (R(est) = 0.96 and ASS (R(est) = 0.86), was very high. 4 ADR scores obtained with both methods were highly correlated (r = 0.82, P less than 0.001). However, time spent using ASS was significantly longer (paired t-test, t = 1.70, P less than 0.05). 5 These results suggest that while ASS is somewhat more complex than APS both are equally reliable and will give very similar conclusions regarding the probability of ADRs. Such algorithms must be used if the clinical assessment of ADRs is to become acceptably reliable.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hurwitz N., Wade O. L. Intensive hospital monitoring of adverse reactions to drugs. Br Med J. 1969 Mar 1;1(5643):531–536. doi: 10.1136/bmj.1.5643.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson T. A., Leventhal J. M., Kramer M. S., Karch F. E., Lipman A. G., Feinstein A. R. An algorithm for the operational assessment of adverse drug reactions. II. Demonstration of reproducibility and validity. JAMA. 1979 Aug 17;242(7):633–638. [PubMed] [Google Scholar]
- Karch F. E., Lasagna L. Adverse drug reactions. A critical review. JAMA. 1975 Dec 22;234(12):1236–1241. [PubMed] [Google Scholar]
- Karch F. E., Lasagna L. Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther. 1977 Mar;21(3):247–254. doi: 10.1002/cpt1977213247. [DOI] [PubMed] [Google Scholar]
- Karch F. E., Smith C. L., Kerzner B., Mazzullo J. M., Weintraub M., Lasagna L. Adverse drug reactions-a matter of opinion. Clin Pharmacol Ther. 1976 May;19(5 Pt 1):489–492. doi: 10.1002/cpt1976195part1489. [DOI] [PubMed] [Google Scholar]
- Koch-Weser J., Sellers E. M., Zacest R. The ambiguity of adverse drug reactions. Eur J Clin Pharmacol. 1977 Jan 3;11(2):75–78. doi: 10.1007/BF00562895. [DOI] [PubMed] [Google Scholar]
- Kramer M. S., Feinstein A. R. Clinical biostatistics. LIV. The biostatistics of concordance. Clin Pharmacol Ther. 1981 Jan;29(1):111–123. doi: 10.1038/clpt.1981.18. [DOI] [PubMed] [Google Scholar]
- Kramer M. S., Leventhal J. M., Hutchinson T. A., Feinstein A. R. An algorithm for the operational assessment of adverse drug reactions. I. Background, description, and instructions for use. JAMA. 1979 Aug 17;242(7):623–632. [PubMed] [Google Scholar]
- Miller R. R. Hospital admissions due to adverse drug reactions. A report from the Boston Collaborative Drug Surveillance Program. Arch Intern Med. 1974 Aug;134(2):219–223. [PubMed] [Google Scholar]
- Naranjo C. A., Busto U., Sellers E. M., Sandor P., Ruiz I., Roberts E. A., Janecek E., Domecq C., Greenblatt D. J. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


