Abstract
During development, extracellular signals often act at multiple thresholds to specify distinct transcriptional and cellular responses. For example, in the embryonic midgut of Drosophila, low Wingless levels stimulate the transcription of homeotic genes whereas high Wingless levels repress these genes. Wingless- mediated transcriptional activation is conferred by Drosophila T-cell factor (dTCF) and its co-activator Armadillo, but the nuclear factors mediating transcriptional repression are unknown. Here we show that teashirt is required for Wingless-mediated repression of Ultrabithorax in the midgut. Teashirt is also a repressor of the homeotic gene labial in this tissue. Furthermore, the target sequence for Tsh within the Ultrabithorax midgut enhancer coincides with the response sequence for Wingless-mediated repression. Finally, we demonstrate that the zinc finger protein Teashirt behaves as a transcriptional repressor in transfected mammalian cells. It thus appears that the response to high Wingless levels in the Drosophila midgut is indirect and based on transcriptional activation of the Teashirt repressor.
Keywords: Drosophila/teashirt/transcriptional repression/Ultrabithorax/wingless
Introduction
During Drosophila development, Wingless (Wg) specifies developmental decisions in multiple tissues during embryonic and larval development. These tissues include the embryonic and larval epidermis (e.g. Nüsslein-Volhard and Wieschaus, 1980; Baker, 1987; Struhl and Basler, 1993; Couso et al., 1994), the nervous system (e.g. Patel et al., 1989; Phillips and Whittle, 1993) and the midgut (Bienz, 1994). Interestingly, Wg does not only control binary decisions in a single tissue, but can act at multiple signalling thresholds to elicit distinct transcriptional and cellular responses (Hoppler and Bienz, 1995; Jiang and Struhl, 1996; Lawrence et al., 1996; Lecuit et al., 1996; Zecca et al., 1996; Neumann and Cohen, 1997).
How Wg elicits distinct responses in the same tissue is not known. The specificity of the cellular response is unlikely to be determined by the two redundant Wg receptors, Frizzled and Frizzled2 (Kennerdell and Carthew, 1998; Bhanot et al., 1999; Bhat, 1999; Chen and Struhl, 1999; Boutros et al., 2000). Indeed, genetic evidence indicates that most if not all Wg responses are mediated by the architectural protein Drosophila T-cell factor (dTCF) and its transcriptional co-activator Armadillo (Peifer et al., 1991; Noordermeer et al., 1994; Siegfried et al., 1994; Brunner et al., 1997; van de Wetering et al., 1997). However, there is strong biochemical and genetic evidence that this bi-partite factor activates transcription (Behrens et al., 1996; Molenaar et al., 1996; Riese et al., 1997; van de Wetering et al., 1997), yet several Wg target genes are repressed in response to Wg signalling. These genes include shavenbaby (svb) (Payre et al., 1999), rhomboid (Alexandre et al., 1999) and stripe (Piepenburg et al., 2000) in the embryonic epidermis, decapentaplegic (dpp) in the developing leg imaginal disc (Brook and Cohen, 1996; Jiang and Struhl, 1996), wg in the wing disc (Rulifson et al., 1996), and labial (lab), Ultrabithorax (Ubx) and wg in the embryonic midgut (Hoppler and Bienz, 1995; Yu et al., 1998). Although repression in many of these cases depends on dTCF and armadillo, this does not necessarily imply that dTCF/Armadillo may be directly modified by the signalling to become a transcriptional repressor (see below). Rather, their involvement may be indirect: dTCF/Armadillo may activate the localized expression of a transcriptional repressor, which in turn represses the Wg target gene. Whatever the case, no such repressors of Wg target genes have been identified as yet.
We searched for such a repressor with a role in endoderm induction, a well characterized inductive process which results in the patterning of the midgut and in which Wg plays a critical part (Bienz, 1994). The Drosophila midgut consists of two cells layers, the visceral mesoderm and the subjacent endoderm (Figure 1A). Wg is expressed in a narrow band of mesodermal cells in parasegment (ps) 8, from where it stimulates the adjacently expressed homeotic gene Ubx (in ps7) (Thüringer and Bienz, 1993). In the subjacent endoderm, Wg stimulates the homeotic gene labial (Immerglück et al., 1990; Hoppler and Bienz, 1995). Dpp, a transforming growth factor (TGF)-β-like protein expressed in ps7 of the visceral mesoderm, synergizes with Wg in the transcriptional stimulation of these homeotic target genes (Thüringer et al., 1993). However, in both cell layers, the two homeotic genes are repressed by high levels of Wg (Hoppler and Bienz, 1995; Yu et al., 1998).
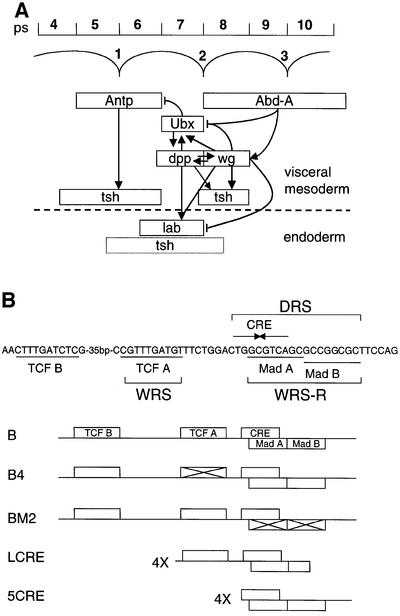
Fig. 1. The role of Ubx during endoderm induction and its signal-responsive enhancer. (A) The parasegments (ps) within the visceral mesoderm (above the dashed line) and the positions of the midgut constrictions are shown at the top. Underneath, the expression domains of homeotic genes and of their targets dpp and wg are outlined; Ubx initiates the process of endoderm induction in the visceral mesoderm, and its ultimate target in the endoderm is labial (for references see text and Bienz, 1996). Expression of tsh in both cell layers is also outlined; its expression in ps8 requires wg and dpp (Mathies et al., 1994; note that in the endoderm, neither the regulation nor the precise expression of tsh has been determined, as indicated by dotted lines). Known regulatory relationships between genes are indicated (arrowheads, activation; barred lines, repression). The stimulatory effects of Dpp and Wg on Ubx and labial are known to be direct. Autoregulatory loops are omitted, for clarity. (B) The signal-responsive sequences within the Ubx B midgut enhancer are outlined at the top, with the WRS, DRS and WRS-R indicated. These include binding sites for the Wg effector, dTCF, and for the Dpp effector, Mad, as well as a cAMP response element (CRE)-like sequence. The presence of these binding sites within the wild-type and mutant Ubx B enhancers, and within the synthetic LCRE and 5CRE enhancers, is shown underneath (cross indicates mutation of the site; for references see text).
Analysis of the Ubx midgut enhancer (called Ubx B) has shown that the stimulatory effect of Wg on this enhancer is mediated by the Wg response sequence (WRS), a TCF-binding site (Riese et al., 1997) (Figure 1B). However, repression of Ubx B by high Wg levels is mediated by a distinct sequence, called WRS-R, whereas the TCF-binding site is dispensable for Wg-mediated repression (Yu et al., 1998). Interestingly, the WRS-R coincides with the Dpp response sequence (DRS), which spans a tandem of binding sites for the Dpp effector Mad (Szüts et al., 1998). Furthermore, Wg-mediated repression also requires dpp (Yu et al., 1998). It was suggested that the high Wg levels in ps8 may activate localized expression of a transcriptional repressor, which in turn would bind to the WRS-R to repress Ubx (Yu et al., 1998). A candidate for this Wg-induced repressor could be a putative repressive Smad which would bind to the Mad-binding sites within the DRS and whose translocation into the nucleus would depend on Dpp signalling (Massagué, 1998), thus explaining the dpp-dependence of the Wg-mediated repression. Alternatively, this repressor could be an unknown WRS-R-binding protein whose localized expression in ps8 depends on high Wg levels as well as on Dpp signalling.
Recently, the zinc-finger protein Teashirt (Tsh) was found to modulate the Wg response in the ventral embryonic epidermis (Gallet et al., 1998, 1999). Furthermore, it was shown that Tsh acts genetically downstream of armadillo, and that it binds to the C-terminus of Armadillo protein (Gallet et al., 1998, 1999). Nevertheless, the mechanism by which Tsh modulates the Wg response is not understood. In addition to its role in the embryonic epidermis (Fasano et al., 1991; Röder et al., 1992; Gallet et al., 1998), tsh is also required in the embryonic midgut for the formation of the first and second midgut constrictions (Mathies et al., 1994). Interestingly, tsh is expressed in ps4–6 (under the control of the homeotic gene Antennapedia, Antp) and in ps8 of the visceral mesoderm (under the control of the homeotic gene abdominal-A, adb-A and of wg), in other words, in cells flanking those expressing Ubx (Figure 1A). Moreover, tsh is a target gene of Wg in ps8: its expression in this parasegment requires wg, and tsh is also responsive to ectopic Wg. Notably, tsh expression in ps8 also requires stimulation by dpp (Mathies et al., 1994). Finally, Mathies et al. (1994) noticed that, at late stages of endoderm induction, tsh begins to be expressed in the endoderm both throughout the second gut lobe and trailing towards either side of it (i.e. beyond the labial expression domain). The tsh expression pattern in the midgut prompted us to investigate whether this gene might control Ubx in the midgut.
Here, we show that Wg-mediated repression of the Ubx midgut enhancer depends on tsh. Furthermore, we identify the WRS-R as the target sequence for Tsh-mediated repression. We further demonstrate that Tsh is a potent repressor of Ubx and labial in the midgut. Finally, we show that Tsh is a transcriptional repressor when targeted to DNA by a heterologous DNA-binding domain. Thus, Tsh is a transcriptional repressor in many cell types, and mediates transcriptional repression in response to high Wg levels in the Drosophila midgut.
Results
teashirt is required for Wg-mediated repression of Ubx
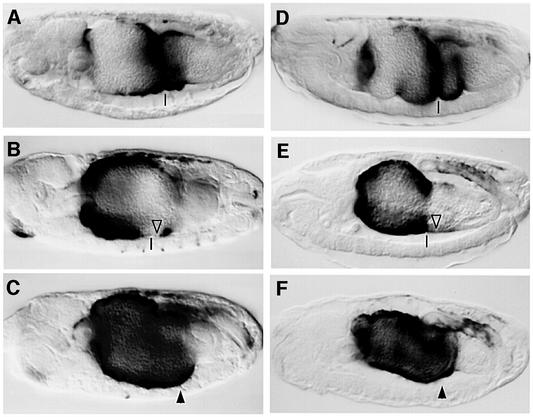
To test whether Tsh might be required for Wg-mediated repression of Ubx B, we asked whether high Wg levels would repress Ubx B in tsh null mutants. In wild-type embryos, this enhancer mediates lacZ expression in the middle midgut, between the first and third midgut constrictions (ps6–9) (Figure 2A and D). If Wg is overexpressed throughout the mesoderm with the Gal4 system (Brand and Perrimon, 1993), lacZ staining is evenly pronounced throughout the anterior midgut, but is largely undetectable posterior to the second midgut constriction (Figure 2B, open triangle). The anterior lacZ staining reflects synergistic activation of Ubx B by Wg and by the anteriorly derepressed Dpp, whereas the lack of staining posterior to ps7 reflects repression of Ubx B by Wg signalling, which reaches particularly high levels near the Wg source (in ps8) (Yu et al., 1998). However, if Wg is overexpressed in tsh mutant embryos, lacZ staining from Ubx B is strong in the anterior midgut as well as throughout ps8 and 9 (Figure 2C, arrowhead; note also the lack of the first and second gut constrictions in the mutant). Thus, tsh is necessary for Wg-induced repression of Ubx B posterior to ps7, but not for Wg-induced activation of this enhancer in the anterior midgut.
Fig. 2. tsh is required for Wg-mediated repression of the Ubx midgut enhancer. Side views of 12- to 14-h-old embryos bearing Ubx B, stained with antibody against lacZ to visualize enhancer activity. Anterior to the left, dorsal up. (A and D) wild type; (B) UAS.Wg/24B.Gal4; (C) tsh8/tsh8; UAS.Wg/24B.Gal4; (E) UAS.ArmS10; 24B.Gal4; (F) UAS.ArmS10, tsh8/tsh8; 24B.Gal4. Transcriptional repression due to high Wg signalling is indicated by open triangles, and transcriptional activation by arrowheads. Note the absence of repression in the tsh mutants. The positions of the second midgut constrictions (if present) is indicated by vertical lines.
We also tested whether Armadillo could repress Ubx B in the absence of tsh. A constitutively active form of Armadillo (called S10) (Pai et al., 1997) was overexpressed throughout the mesoderm. This revealed that, as in the case of Wg overexpression, Armadillo S10 could repress Ubx B in ps8 and 9 of wild-type embryos (Figure 2E) but not of tsh mutants (Figure 2F). This demonstrates that tsh is required downstream of Armadillo to mediate repression of Ubx B in response to high Wg levels in the middle midgut.
teashirt is required during midgut formation to repress Ubx and labial
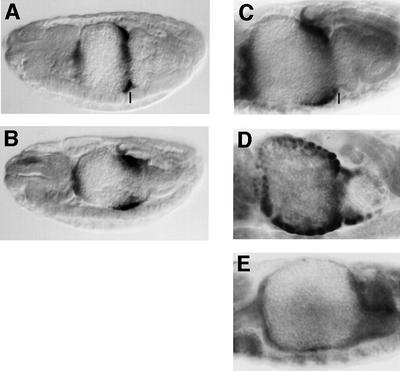
We asked whether tsh mutation also affects Ubx itself which is repressed by high Wg levels in ps8. We thus examined the activity of an extended Ubx enhancer fragment (called RP) which closely mimics Ubx expression in ps7 of the visceral mesoderm (Thüringer and Bienz, 1993) (Figure 3A). Transcription from RP is synergistically activated by Wg and Dpp in ps7, while it is repressed by high Wg levels in ps8 and 9. This is in contrast to Ubx B which mediates broader expression (see above) since this truncated minimal enhancer has lost much of its responsiveness to high Wg levels (Yu et al., 1998). As shown in Figure 3B, tsh mutants exhibit a striking expansion of RP-mediated lacZ staining, which is strong in ps8, and also trails into ps6 and ps9. Thus, lacZ staining in the mutants spans a domain that is nearly three times as wide as that in the wild type (compare Figure 3B with A). The ectopic expression of RP9 in ps8 and 9 in the tsh mutants provides further evidence that tsh is required for Ubx repression in these parasegments near the source of Wg. Since we also observe derepression of RP staining towards anterior, in ps6 in which tsh expression is under the control of Antp, this suggests that Tsh can repress Ubx in the absence of Wg signalling or of abd-A (see Discussion).
Fig. 3. tsh represses Ubx and labial. Side views of ∼14-h-old embryos bearing Ubx RP, stained with antibody against lacZ to visualize enhancer activity (A and B), or with antibody against Labial (C, D and E). (A and C) wild type; (B and D) tsh8/tsh8; (E) UAS.Tsh/ 48Y.Gal4. Note the increased width of Ubx RP and labial expression in tsh mutants, and the repression of labial by endodermally overexpressed Tsh.
Next, we tested whether tsh might control labial, a target for Wg-mediated repression in the endoderm. In wild-type embryos, labial is expressed in a graded fashion in midgut epithelial cells approximately vis-à-vis the Ubx-expressing visceral mesoderm cells (Figure 3C). However, in tsh mutants, this graded expression is lost, and labial expression is much expanded towards anterior and posterior (Figure 3D). This expansion of labial expression in tsh mutants seem to correspond approximately to the cells expressing tsh (Mathies et al., 1994). Thus, tsh is essential for the normal anterior and posterior limits of labial expression. We also noticed that additional cells within the normal labial domain express this gene in the tsh mutants (not shown), most likely the prospective interstitial cells, which in the wild type do not express labial (Hoppler et al., 1994). These results show that tsh is required for the repression of labial in multiple cells, including the cells that abut the labial domain posteriorly, indicating that Tsh may participate in the Wg-mediated repression of labial in this region vis-à-vis the Wg source.
Overexpressed Tsh represses Ubx and labial
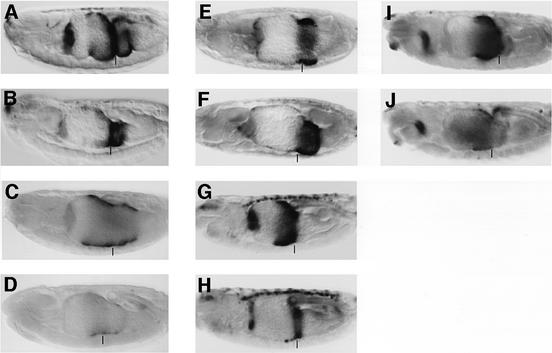
To examine whether Tsh is a position-independent repressor of Wg target genes, we overexpressed Tsh in the embryonic midgut with the Gal4 system. Overexpression of Tsh throughout the mesoderm caused repression of Ubx B (compare Figure 4B with A). In most cases, lacZ staining was virtually undetectable anteriorly to ps8/9, and the only remaining lacZ staining was seen in the third gut lobe. This repression of Ubx B by ectopic Tsh was observable from the onset of Ubx B activity (not shown). Interestingly, the gut morphology was also altered: the first constriction was absent (reminiscent of Wg overexpression; Yu et al., 1996) and the third gut lobe (between the 2nd and 3rd constriction) often appeared to be reduced in size (Figure 4B).
Fig. 4. The WRS-R is the target for Tsh-mediated repression of Ubx B. Side views of ∼14-h-old embryos bearing Ubx B (A and B), B4 (C and D), BM2 (E and F), L-CRE (G and H) or 5CRE (I and J), stained with antibody against lacZ. (A, C, E, G and I) wild type; (B, D and F) UAS.Tsh/24B.Gal4; (H and J) UAS.Tsh/48Y.Gal4. The second midgut constrictions are marked by vertical bars; the lacZ staining in (H) within this constriction reflects the activity of L-CRE in the visceral mesoderm, which is not repressed by endodermally expressed Tsh. Note that BM2 is the only enhancer which is not repressible by ectopic Tsh.
We also overexpressed Tsh throughout the endoderm and stained these embryos with an antibody against Labial. This condition caused dramatic repression of labial in the midgut. Only in rare embryos could we detect individual endoderm cells with residual labial staining while, in the vast majority of embryos, no staining was detectable whatsoever (Figure 3E). This was somewhat surprising, given that tsh appears to be co-expressed with labial in the wild type (see above). However, the Gal4 driver we used produces fairly high expression levels from an early stage of midgut development (Martin-Bermudo et al., 1997; our own observations), whereas normal tsh expression does not start until after labial expression is already induced and only reaches moderate levels (Mathies et al., 1994). This probably explains why we observe a strong repressive effect of Tsh on labial under conditions of precocious and substantive overexpression. Note also that this repressive effect was not observed if Tsh was overexpressed in the mesoderm (not shown), indicating that Tsh represses labial in a cell-autonomous fashion.
Labial specifies the development of copper cells in the larval midgut (Hoppler and Bienz, 1994). Thus we next asked whether ectopic Tsh expression also repressed copper cell differentiation. Copper cells can be recognized under UV light by a striking and highly specific orange fluorescence in the gut of larvae that have been fed on a copper-containing diet. No larvae hatched if Tsh was overexpressed in the endoderm at 25°C, however, we obtained a few escaper larvae if embryos overexpressing Tsh were collected and aged at 20°C. After feeding these escapers with a copper-containing diet, their guts showed no orange fluorescence whatsoever under UV light, whereas orange-fluorescing copper cells were readily observable in control larvae (data not shown). Furthermore, under Normarski optics, we only observed large flat cells, but no copper cells in the guts of Tsh-expressing escaper larvae, a phenotype typical for larval guts that have been stimulated by high levels of ubiquitous Wg (Hoppler and Bienz, 1995). These results confirm that ectopic Tsh, like high Wg levels, prevents copper cell differentiation in the larval gut.
WRS-R is the target sequence for Tsh repression of Ubx B
As mentioned above, the target sequence for Wg-mediated repression within Ubx B, the WRS-R, is distinct from the TCF-binding site (the target for Wg-mediated activation), but coincides with a tandem of Mad-binding sites within the DRS of this enhancer (Yu et al., 1998) (Figure 1B). If Tsh was the transcription factor that repressed Ubx B in response to high Wg levels, we would expect Tsh to act through the WRS-R. Thus, mutation of the WRS-R should abrogate Tsh-induced repression of Ubx B. However, since Tsh was shown to bind to Armadillo (see Introduction), one might also expect that Tsh would repress Ubx B via TCF/Armadillo and, thus, via the TCF-binding site.
We thus tested two mutant versions of Ubx B under conditions of Tsh overexpression throughout the mesoderm, namely B4 whose TCF-binding site was mutated, and BM2 whose WRS-R was mutated (Figure 1B). Interestingly, B4 was still repressed by ectopic Tsh (compare Figure 4D with C). Indeed, Tsh-mediated repression was more extensive in this case in that we even observed repression of B4 in the ps8/9 region, near the Wg source (to the right of the vertical bar in Figure 4D which marks the second midgut constriction). Clearly, Tsh can repress B4 efficiently, and can do so even in ps8/9, probably because B4 can no longer be stimulated in this region by Wg (Riese et al., 1997). This indicates that the WRS (and thus dTCF/Armadillo) is not only dispensable for Tsh-mediated repression, but that its presence even antagonizes the repressive function of Tsh to some extent. It also argues against a contribution of dCBP in this repressive event since the target for repression by this histone acetyltransferase is dTCF, and its response sequence in Ubx B coincides with the WRS (Waltzer and Bienz, 1998). In support of this, tsh is not detectably derepressed in dCBP mutant embryos (not shown).
In contrast, BM2 was no longer repressible by Tsh: the lacZ staining pattern appeared identical in embryos overexpressing Tsh and in control embryos (compare Figure 4F with E). These results indicate that the Tsh response sequence in Ubx B is defined by BM2 and thus coincides with the WRS-R.
To further support this conclusion, we tested Tsh-mediated repression of two synthetic reporter genes derived from Ubx B that contain multimers of signal-responsive sequences (called 5CRE and L-CRE; Figure 1B). 5CRE spans the DRS and is thus Dpp-responsive, albeit mainly in the endoderm (Eresh et al., 1997), whereas L-CRE spans the WRS as well as MadA, and is thus Wg- and Dpp-responsive in both cell layers of the midgut (Riese et al., 1997). We expressed Tsh throughout the endoderm, and found that this condition strongly reduced lacZ staining from L-CRE in this cell layer (compare Figure 4H with G), although the lacZ staining in the visceral mesoderm (visible in the second constriction and marked by vertical bar in Figure 4H) was unaffected. Furthermore, 5CRE was also efficiently repressed by endodermally overexpressed Tsh (compare Figure 4J with I). Evidently, both synthetic reporters are repressible by Tsh, demonstrating that the target sequence for Tsh repression is contained in both synthetic reporters. The overlap between the two thus identifies the MadA site as the minimal Tsh target sequence. This confirms that BM2 defines the target for Tsh repression, and further supports our earlier conclusion that Tsh mediates transcriptional repression in response to high Wg levels.
Tsh is a transcriptional repressor when targeted to DNA
While these and previous results (Alexandre et al., 1996) strongly suggest that Tsh is a transcriptional repressor, this has not been directly demonstrated so far. We thus sought to determine whether targeting of Tsh to a promoter would be sufficient for transcriptional repression of this promoter. In order to do so, we performed transfection experiments with human Saos-2 cells. We fused Tsh to the Gal4 DNA-binding domain and tested whether this fusion protein could repress the transcription of a luciferase reporter gene placed under the control of four tandem Gal4-binding sites and the cytomegalovirus (CMV) promoter (Figure 5A).
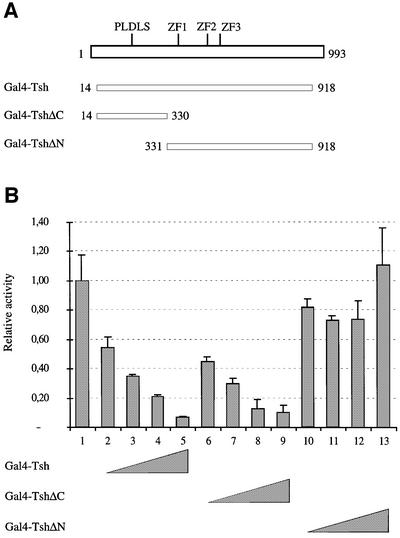
Fig. 5. Tsh functions as a transcriptional repressor when directly bound to DNA. (A) Expression constructs encoding the Gal4 DNA-binding domain fused to different parts of Tsh. The three zinc fingers (ZF) of Tsh and the putative CtBP-binding sequence (PLDLS) are indicated. (B) Relative luciferase activities of the Gal4 reporter in transfected Saos-2 cells in response to increasing doses of Tsh constructs, as indicated below the panel (100% activity corresponds to the activity of the luciferase reporter alone).
If we expressed increasing amounts of the Gal4–Tsh fusion protein in transfected cells, we observed a dose-dependent repression of the luciferase gene (Figure 5B, lanes 2–5). Furthermore, the N-terminal domain of Tsh fused to Gal4 (Gal4–TshΔC) was sufficient to repress the luciferase reporter up to 10-fold (Figure 5B, lanes 6–10). Conversely, a mutant form of Tsh in which the N-terminal region was deleted (Gal4–TshΔN) did not repress transcription of this reporter (Figure 5B, lanes 11–13). These results demonstrate that Tsh is a transcriptional repressor if targeted to DNA, and that this protein harbours a potent transcriptional repression domain in its N-terminal region.
Discussion
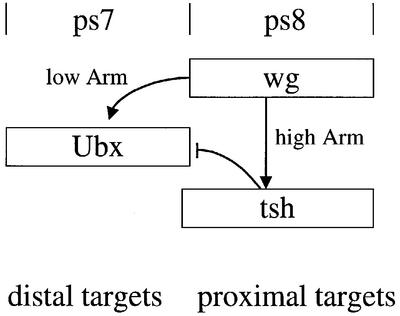
The identification of TCF as the nuclear end point of the Wnt signalling pathway has laid the basis for the dissection of the transactivation mechanisms in response to this signalling. The main open questions are how different levels of Wnt signalling are translated into distinct programs of gene activation, and how repression in response to Wnt signalling is achieved. The latter is particularly poignant since the available molecular evidence indicates that the TCF–β-catenin/Armadillo complex is a transcriptional activator. Our work provides an answer to both these questions regarding the Drosophila midgut, implicating Tsh as a transcriptional repressor in mediating the response of homeotic target genes to high Wg levels. In this tissue, transcriptional activation of target genes in response to low Wg levels is directly achieved by dTCF/Armadillo (Riese et al., 1997), while transcriptional repression of these genes in response to high Wg levels is indirect and achieved through localized transcriptional activation of the Tsh repressor (Figure 6).
Fig. 6. Repression of Ubx by high Wg levels is achieved through transcriptional coupling. Schematically depicted is the transcriptional response of Ubx to Wg signalling in the midgut. The distal stimulatory effect of low Wg levels is directly mediated by dTCF/Armadillo, whereas the proximal repressive effect of high Wg levels is indirect and requires localized transcriptional activation of the Tsh repressor.
Tsh mediates Wg-induced transcriptional repression in the midgut
We have shown previously that during endoderm induction, high Wg levels repress labial in the endoderm (Hoppler and Bienz, 1995), and Ubx and wg in the visceral mesoderm (Yu et al., 1998). Our present evidence implicates Tsh in this response as a transcriptional repressor of labial and Ubx. First, we show that these genes are derepressed in tsh mutant embryos in cells that experience high Wg levels (near the Wg source in ps8) and in which these genes are normally repressed by these high Wg levels. Secondly, overexpressed Wg or constitutively active Armadillo require tsh in order to repress Ubx B, the minimal signal-responsive midgut enhancer of Ubx. This indicates that tsh functions downstream of Armadillo in the repression of Ubx. Thirdly, the Tsh response sequence within the Ubx B enhancer coincides with the WRS-R, the sequence mediating transcriptional repression of this enhancer in response to high Wg levels. Finally, ectopic Tsh can repress Ubx and labial, and also behaves as a transcriptional repressor in transfected mammalian cells.
tsh is a Wg target gene in the midgut, and is expressed near the Wg source in ps8 in response to Wg stimulation (Mathies et al., 1994). This indicates that the transcriptional activation of tsh requires high Wg levels that are only found close to the signalling source. In contrast, transcriptional stimulation of Ubx and labial occurs more distal from this source and requires lower levels of Wg. It is reasonable to assume that the proximal, like the distal activation events (Riese et al., 1997), are mediated directly by the activating dTCF/Armadillo complex, except that the latter are expected to require less activated Armadillo than the former. Thus, the dependence of tsh expression on wg explains why the Wg-mediated transcriptional repression of Ubx B requires dTCF and armadillo (Yu et al., 1998). Evidently, this requirement is at least partly indirect and does not necessarily implicate the TCF/Armadillo complex directly in the process of transcriptional repression (see below). Similarly, other instances of Wnt-mediated repression of target genes could be based on indirect transcriptional activation of Wnt target genes that encode transcriptional repressors.
It is interesting that tsh expression in ps8 of the midgut also requires dpp signalling from the neighbouring ps7 (Mathies et al., 1994). This may account for the dpp-dependence of the Wg-mediated repression of Ubx B in ps8. Given this dpp requirement, and the fact that the WRS-R coincides with a tandem of Mad-binding sites, we suggested previously that the protein mediating transcriptional repression in response to high Wg levels may be a repressive Smad, whose translocation to the nucleus requires Dpp signalling (Yu et al., 1998). While this remains a possibility (see below), it is equally likely that the sole reason for the dpp-dependence of the Wg-mediated repression of Ubx B is that tsh expression depends on dpp.
It has been proposed that in the embryonic epidermis, Tsh modulates a late function of wg in the trunk region: loss of tsh function, like late loss of wg, was reported to result in loss of naked cuticle while ectopic Tsh blocked denticle formation (Gallet et al., 1998). In this tissue, svb specifies cell-autonomously the denticle cell fate while Wg signalling specifies the naked cell fate by repressing svb (Payre et al., 1999). It was thus a possibility that the repression of svb by high Wg levels would be mediated by Tsh, given the function of Tsh in Wg-mediated repression in the midgut. Indeed, ectopic Tsh repressed svb; however, we found unexpectedly that svb expression was reduced rather than derepressed in tsh mutants (not shown). This loss of svb expression in tsh mutants may explain why tsh mutants not only show loss of naked cuticle, but also loss of denticles (Fasano et al., 1991; our unpublished observations). The latter, however, is inconsistent with a wg phenotype that invariably results in extra denticles (Nüsslein-Volhard and Wieschaus, 1980; Baker, 1987). Therefore, the consequences of tsh loss in the embryonic epidermis will need further investigation, and perhaps re-interpretation, before any clear regulatory interactions between Tsh, Armadillo and svb can be established.
Mechanisms of repression by Tsh
We have shown that Tsh can mediate Wg-induced transcriptional repression through the WRS-R. Since it was reported that Tsh can bind DNA specifically (Alexandre et al., 1996), we asked whether Tsh may bind to the WRS-R directly to repress transcription. However, we were unable to detect direct DNA binding of Tsh (or of individual Zn finger-containing fragments of Tsh) to the WRS-R (unpublished results). Consistent with this, the WRS-R does not share any sequence similarities with either of the two putative Tsh-binding DNA sequences identified by Alexandre et al. (1996)—although it is worth noting that the latter two sequences are not related to each other either. It thus remains to be established whether Tsh binds DNA robustly in a sequence-specific manner.
Alternatively, Tsh could be recruited to the WRS-R by a DNA-binding partner. It was previously shown that Tsh can cooperate with other homeotic proteins (de Zulueta et al., 1994). Since Abd-A also represses Ubx in the posterior midgut (Bienz and Tremml, 1988), it is conceivable that Abd-A could be this Tsh-recruiting partner. However, this is unlikely since Tsh can repress Ubx B in cells that do not express any Abd-A. Another candidate for a Tsh-recruiting protein may be the dTCF/Armadillo complex bound to the neighbouring WRS, given that Tsh was reported to bind to the C-terminal domain of Armadillo (Gallet et al., 1998). However, this is also unlikely since Tsh can repress Ubx B in cells that are not stimulated by Wg, and which are thus not expected to contain any nuclear Armadillo. Also, we have been unable to observe consistent binding between Armadillo and Tsh in vitro (unpublished results), which suggests that the interaction between the two proteins would not be strong enough to account for Tsh recruitment. Finally, the Tsh-recruiting partner could be a repressive Smad, as previously proposed (Yu et al., 1998). This is a possibility since Mad itself binds to the WRS-R (Kim et al., 1997; Szüts et al., 1998). Furthermore, Smad proteins are known to interact with various transcription factors to elicit different transcriptional responses (Attisano and Wrana, 2000). For example, in the Drosophila midgut, Mad has been shown to act in combination with other transcription factors on a dpp target gene (Xu et al., 1998). If a Smad recruited Tsh to the WRS-R, this would result in a repressive Smad–Tsh complex that could compete with the activating dpp-responsive Mad complex, as previously proposed (Yu et al., 1998). However, for this possibility to apply, the putative Tsh-recruiting Smad would have to be expressed widely to account for the widespread Tsh-mediated repression of Ubx B.
Our results and those of others (Alexandre et al., 1996) strongly argue that Tsh represses transcription in vivo. Furthermore, our transfection experiments indicate that Tsh is a genuine transcriptional repressor that functions in a heterologous system. Its N-terminus is necessary and sufficient for its repressive function, while the zinc fingers are dispensable for this function. Interestingly, this N-terminus contains the motif P-L-X-L-S/T, which was discovered in the adenovirus protein E1A (Schaeper et al., 1995) and is required for recruitment of the transcriptional co-repressor CtBP (Turner and Crossley, 1998). A number of transcriptional repressors in Drosophila contain DNA-binding zinc fingers and repress transcription through interaction with CtBP (Nibu et al., 1998; Poortinga et al., 1998). It is thus possible that Tsh functions as a repressor by recruiting the conserved and widespread CtBP co-repressor. Intriguingly, a functional link between CtBP and Wnt signalling has been reported in Xenopus, where XTcf-3 has been shown to interact with XCtBP (Brannon et al., 1999).
Distinct responses to different Wg thresholds through transcriptional coupling
In the Drosophila midgut, transcriptional stimulation of distantly expressed homeotic target genes by low Wg levels is a direct response to Wg signalling, while transcriptional repression of these genes by high Wg levels near the Wg source appears to be an indirect response requiring transcriptional coupling (Figure 6). Indeed, it is possible that transcriptional coupling of Tsh expression to high levels of Wg signalling is the only event necessary to bring about the repression of Wg target genes. It seems unlikely that dTCF and Armadillo have a direct function in the repressive process, given that the WRS-R is not a TCF-binding site and that the WRS (the TCF A-binding site) is dispensable for Wg- and Tsh-mediated repression. However, we should emphasize that we cannot test this directly, given that Ubx B is barely active in dTCF and armadillo mutants, and that a mutant Ubx enhancer that lacks both TCF-binding sites is completely inactive (Yu et al., 1996, 1998). Nevertheless, our results indicate that the Wg-mediated repression of Ubx in the midgut is distinct from that of stripe in the embryonic epidermis, which requires the TCF-binding sites in the stripe enhancer (Piepenburg et al., 2000).
Indirect transcriptional coupling provides a versatile mechanism for triggering distinct threshold-dependent signal responses since it does not require multiple transcription factors to be activated directly by a single signalling effector (such as Armadillo). Furthermore, it may be a more reliable mechanism, allowing for sharper signal responses, than multiple direct readouts of different effector levels. To illustrate this with our example of the Drosophila midgut, the primary measurement of Armadillo effector levels appears to be achieved at the level of enhancer activation whereby we expect Ubx and labial to be stimulated by low Armadillo levels, while tsh would require high Armadillo levels for activation. This primary readout at the Ubx and labial midgut enhancers is then modified by the transcriptionally induced Tsh, which impinges on these enhancers, determining the ultimate readout (Figure 6). This read-out, namely the transcriptional repression of Ubx and labial, presumably coincides strictly with Tsh expression, which in turn reflects an integration event of multiple spatial inputs (including at least two signals, wg and dpp; Mathies et al., 1994). This may contribute to, if not account for, the sharpness of the posterior limit of Ubx and labial expression in the midgut. It thus would appear that transcriptional coupling may be a mechanism operating in other developmental contexts, in which different signalling thresholds elicit distinct cellular responses.
Materials and methods
Fly strains
The following mutant alleles and fly transformants were used: tsh8 (Fasano et al., 1991); nej3 (Akimaru et al., 1997); UAS.Tsh13 (Gallet et al., 1998); UAS.Wg (Lawrence et al., 1996); UAS.ArmS10 (Pai et al., 1997); UAS.Dpp (Staehling-Hampton and Hoffmann, 1994). Expression of UAS constructs was achieved using the following drivers: 24B.Gal4 (mesodermal expression; Brand and Perrimon, 1993), 48Y.Gal4 (endodermal expression; Martin-Bermudo et al., 1997). The following transgenic lines bearing β-galactosidase (lacZ) reporter constructs were used: RP (Thüringer and Bienz, 1993); Ubx B (Thüringer et al., 1993); B4, L-CRE (Riese et al., 1997); 5CRE (Eresh et al., 1997); BM2 (Szüts et al., 1998).
Phenotypic analysis
Standard crosses were set up and embryos were collected at 25°C. All mutants were identified using ‘blue’ balancer chromosomes or by their midgut phenotypes. Antibody staining of embryos with antibodies against lacZ or Labial was carried out as previously described (Waltzer and Bienz, 1998). tsh expression was monitored by in situ hybridization using standard protocols. For copper cell observation, young hatched larvae were placed in copper containing medium (0.3 mg/ml of CuSO4 dissolved in baker’s yeast paste) for at least 5 h before observation under UV light (using a 4′-6-diamidine-2-phenylindoline filter) as described (Hoppler and Bienz, 1994).
Reporter plasmids and expression vectors
The reporter plasmid pG4-Luc (gift from D.Trouche) containing four Gal4-binding sites upstream of a luciferase reporter gene was used. Standard cloning techniques were used in the construction of Gal4–Tsh chimeras, which were ultimately inserted into pCMV–Gal4 (gift from D.Trouche) for expression in transfected mammalian cells. These chimeras (Gal4–Tsh, Gal4–Tsh ΔN, Gal4–Tsh ΔC) contain the Gal4 DNA-binding domain and amino acids 14–918, 331–918 and 14–330 of Tsh, respectively. Details of the constructs are available upon request.
Transfection and luciferase assays
Plasmids used for transfections were prepared with the Qiagen Maxiprep plasmid purification kit. Saos-2 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal calf serum. Cells were seeded at 2 × 105 cells/6-well plates 8 h prior to transfection, and subsequently transfected by the calcium phosphate precipitation method. Fifty nanograms of the lacZ reporter pCMV–β-gal were used per transfection as an internal control (Magnaghi-Jaulin et al., 1998). Empty pCMV expression vector was added as required to keep the amount of CMV plasmid constant. pUC plasmid was added as carrier DNA to a total amount of 8 µg DNA per transfection. Transfected cells were washed and collected 48 h after transfection.
Luciferase assays were performed using a Lumat LB 9501 luminometer. β-galactosidase activity was measured with a Galacto-Light Plus kit (Tropix Inc.) according to the manufacturer’s instructions. Transfection experiments were carried out in duplicate and repeated at least three times.
Acknowledgments
Acknowledgements
We thank M.Haenlin, D.Trouche, F.Payre and S.Kerrigde for providing plasmids and fly strains, and Elisabeth Saller for critical comments on the manuscript. L.W. is grateful to M.Haenlin for his support while finishing this work in his laboratory. This work was supported by the Association de Recherche contre le Cancer.
REFERENCES
- Akimaru H., Chen,Y., Dai,P., Hou,D.X., Nonaka,M., Smolik,S.M., Armstrong,S., Goodman,R.H. and Ishii,S. (1997) Drosophila CBP is a co-activator of cubitus interruptus in hedgehog signaling. Nature, 386, 735–738. [DOI] [PubMed] [Google Scholar]
- Alexandre E., Graba,Y., Fasano,L., Gallet,A., Perrin,L., De Zulueta,P., Pradel,J., Kerridge,S. and Jacq,B. (1996) The Drosophila teashirt homeotic protein is a DNA-binding protein and modulo, a HOM-C regulated modifier of variegation, is a likely candidate for being a direct target gene. Mech. Dev., 59, 191–204. [DOI] [PubMed] [Google Scholar]
- Alexandre C., Lecourtois,M. and Vincent,J. (1999) Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Development, 126, 5689–5698. [DOI] [PubMed] [Google Scholar]
- Attisano L. and Wrana,J. (2000) Smads as transcriptional co-modulators. Curr. Opin. Cell Biol., 12, 235–243. [DOI] [PubMed] [Google Scholar]
- Baker N. (1987) Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J., 6, 1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., von Kries,J., Kühl,M., Bruhn,L., Wedlich,D., Grosschedl,R. and Birchmeier,W. (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature, 382, 638–642. [DOI] [PubMed] [Google Scholar]
- Bhanot P., Fish,M., Jemison,J.A., Nusse,R., Nathans,J. and Cadigan,K.M. (1999) Frizzled and DFrizzled-2 function as redundant receptors for wingless during Drosophila embryonic development. Development, 126, 4175–4186. [DOI] [PubMed] [Google Scholar]
- Bhat K. (1999) frizzled and frizzled 2 play a partially redundant role in wingless signaling and have similar requirements to wingless in neurogenesis. Cell, 95, 1027–1036. [DOI] [PubMed] [Google Scholar]
- Bienz M. (1994) Homeotic genes and positional signalling in the Drosophila viscera. Trends Genet., 10, 22–26. [DOI] [PubMed] [Google Scholar]
- Bienz M. (1996) Induction of the endoderm in Drosophila. Semin. Cell Dev. Biol., 7, 113–119. [Google Scholar]
- Bienz M. and Tremml,G. (1988) Domain of Ultrabithorax expression in Drosophila visceral mesoderm from autoregulation and exclusion. Nature, 333, 576–578. [DOI] [PubMed] [Google Scholar]
- Boutros M., Mihaly,J., Bouwmeester,T. and Mlodzik,M. (2000) Signaling specificity by Frizzled receptors in Drosophila. Science, 288, 1825–1828. [DOI] [PubMed] [Google Scholar]
- Brand A. and Perrimon,N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- Brannon M., Brown,J., Bates,R., Kimelman,D. and Moon,R. (1999) XCtBP is a XTcf-3 co-repressor with roles throughout Xenopus development. Development, 126, 3153–3170. [DOI] [PubMed] [Google Scholar]
- Brook W. and Cohen,S. (1996) Antagonistic interactions between wingless and decapentaplegic responsible for dorsal–ventral pattern in the Drosophila leg. Science, 273, 1373–1377. [DOI] [PubMed] [Google Scholar]
- Brunner E., Peter,O., Schweizer,L. and Basler,K. (1997) pangolin encodes a Lef-1 homolog that acts downstream of Armadillo to transduce the Wingless signal. Nature, 385, 829–833. [DOI] [PubMed] [Google Scholar]
- Chen C. and Struhl,G. (1999) Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development, 126, 5441–5452. [DOI] [PubMed] [Google Scholar]
- Couso J., Bishop,S. and Martinez-Arias,A. (1994) The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development, 120, 621–636. [DOI] [PubMed] [Google Scholar]
- de Zulueta P., Alexandre,E., Jacq,B. and Kerridge,S. (1994) Homeotic complex and teashirt genes co-operate to establish trunk segment identities in Drosophila. Development, 120, 2278–2296. [DOI] [PubMed] [Google Scholar]
- Eresh S., Riese,J., Jackson,D.B., Bohmann,D. and Bienz,M. (1997) A CREB-binding site as a target for decapentaplegic signalling during Drosophila endoderm induction. EMBO J., 16, 2014–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano L., Röder,L., Coré,N., Alexandre,E., Vola,C., Jacq,B. and Kerridge,S. (1991) The gene teashirt is required for the development of Drosophila embryonic trunk segments and encodes a protein with widely spaced zinc finger motifs. Cell, 64, 63–79. [DOI] [PubMed] [Google Scholar]
- Gallet A., Erkner,A., Charroux,B., Fasano,L. and Kerridge,S. (1998) Trunk-specific modulation of wingless signalling in Drosophila by teashirt binding to armadillo. Curr. Biol., 8, 893–902. [DOI] [PubMed] [Google Scholar]
- Gallet A., Angelats,C., Erkner,A., Charroux,B., Fasano,L. and Kerridge,S. (1999) The C-terminal domain of Armadillo binds to hypophosphorylated Teashirt to modulate Wingless signalling in Drosophila. EMBO J., 18, 2208–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppler S. and Bienz,M. (1994) Specification of a single cell type by a Drosophila homeotic gene. Cell, 76, 689–702. [DOI] [PubMed] [Google Scholar]
- Hoppler S. and Bienz,M. (1995) Two different thresholds of wingless signalling with distinct developmental consequences in the Drosophila midgut. EMBO J., 14, 5016–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immerglück K., Lawrence,P.A. and Bienz,M. (1990) Induction across germ layers in Drosophila mediated by a genetic cascade. Cell, 62, 261–268. [DOI] [PubMed] [Google Scholar]
- Jiang J. and Struhl,G. (1996) Complementary and mutually exclusive activities of decapentaplegic and wingless organise axial patterning during Drosophila leg development. Cell, 86, 401–409. [DOI] [PubMed] [Google Scholar]
- Kennerdell J.R. and Carthew,R.W. (1998) Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell, 95, 1017–1026. [DOI] [PubMed] [Google Scholar]
- Kim J., Johnson,K., Chen,H., Carroll,S. and Laughon,A. (1997) Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature, 388, 304–308. [DOI] [PubMed] [Google Scholar]
- Lawrence P., Sanson,B. and Vincent,J.-P. (1996) Compartments, wingless and engrailed: patterning the ventral epidermis of Drosophila embryos. Development, 122, 4095–4103. [DOI] [PubMed] [Google Scholar]
- Lecuit T., Brook,W., Ng,M., Calleja,M., Sun,H. and Cohen,S. (1996) Two distinct mechanisms for long-range patterning by decapentaplegic in the Drosophila wing. Nature, 381, 387–393. [DOI] [PubMed] [Google Scholar]
- Magnaghi-Jaulin L., Groisman,R., Naguibneva,I., Robin,P., Lorain,S., Le Villain,J., Troalen,F., Trouche,D. and Harel-Bellan,A. (1998) Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature, 391, 601–605. [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo M., Dunin-Borkowski,O. and Brown,N. (1997) Specificity of PS integrin function during embryogenesis resides in the alpha subunit extracellular domain. EMBO J., 16, 4184–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. (1998) TGF-β signal transduction. Annu. Rev. Biochem., 67, 753–791. [DOI] [PubMed] [Google Scholar]
- Mathies L., Kerridge,S. and Scott,M. (1994) Role of the teashirt gene in Drosophila midgut morphogenesis: secreted proteins mediate the action of homeotic genes. Development, 120, 2799–2809. [DOI] [PubMed] [Google Scholar]
- Molenaar M., van de Wetering,M., Oosterwegel,M., Peterson-Maduro,J., Godsave,S., Korinek,V., Roose,J., Destree,O. and Clevers,H. (1996) XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell, 86, 391–399. [DOI] [PubMed] [Google Scholar]
- Neumann C. and Cohen,S. (1997) Long-range action of Wingless organizes the dorsal–ventral axis of the Drosophila wing. Development, 124, 871–880. [DOI] [PubMed] [Google Scholar]
- Nibu Y., Zhang,H. and Levine,M. (1998) Interaction of short-range repressors with Drosophila CtBP in the embryo. Science, 280, 101–104. [DOI] [PubMed] [Google Scholar]
- Noordermeer J., Klinglensmith,J., Perrimon,N. and Nusse,R. (1994) dishevelled and armadillo act in the Wingless signalling pathway in Drosophila. Nature, 367, 80–82. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C. and Wieschaus,E. (1980) Mutations affecting segment number and polarity in Drosophila. Nature, 287, 795–801. [DOI] [PubMed] [Google Scholar]
- Pai L., Orsulic,S., Besjovec,A. and Peifer,M. (1997) Negative regulation of Armadillo, a Wingless effector in Drosophila. Development, 124, 2255–2266. [DOI] [PubMed] [Google Scholar]
- Patel N., Schafer,B., Goodman,C. and Holmgren,R. (1989) The role of segment polarity genes during Drosophila neurogenesis. Genes Dev., 3, 890–904. [DOI] [PubMed] [Google Scholar]
- Payre F., Vincent,A. and Carreno,S. (1999) ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature, 400, 271–275. [DOI] [PubMed] [Google Scholar]
- Peifer M., Rauskolb,C., Williams,M., Riggleman,B. and Wieschaus,E. (1991) The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development, 111, 1029–1043. [DOI] [PubMed] [Google Scholar]
- Phillips R.G. and Whittle,J.R. (1993) wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development, 118, 427–438. [DOI] [PubMed] [Google Scholar]
- Piepenburg O., Vorbrüggen,G. and Jäckle,H. (2000) Drosophila segment borders result from unilateral repression of hedgehog activity by wingless signaling. Mol. Cell, 6, 203–209. [PubMed] [Google Scholar]
- Poortinga G., Watanabe,M. and Parkhurst,S. (1998) Drosophila CtBP: a hairy-interacting protein required for embryonic segmentation and hairy-mediated transcriptional repression. EMBO J., 17, 2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese J., Yu,X., Munnerlyn,A., Eresh,S., Shu-Chi,H., Grosschedl,R. and Bienz,M. (1997) LEF-1, a nuclear factor coordinating wingles and decapentaplegic signalling. Cell, 88, 777–787. [DOI] [PubMed] [Google Scholar]
- Röder L., Vola,C. and Kerridge,S. (1992) The role of teashirt gene in trunk segmental identity in Drosophila. Development, 115, 1017–1033. [DOI] [PubMed] [Google Scholar]
- Rulifson E., Micchelli,C., Axelrod,J., Perrimon,N. and Blair,S. (1996) wingless refines its own expression domain on the Drosophila wing margin. Nature, 384, 72–74. [DOI] [PubMed] [Google Scholar]
- Schaeper U., Boyd,J., Verma,S., Uhlmann,E., Subramanian,T. and Chinnadurai,G. (1995) Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc. Natl Acad. Sci. USA, 92, 10467–10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried E., Wilder,E.L. and Perrimon,N. (1994) Components of wingless signalling in Drosophila. Nature, 367, 76–80. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K. and Hoffmann,F.M. (1994) Ectopic decapentaplegic in the Drosophila midgut alters the expression of five homeotic genes, dpp and wingless, causing specific morphological defects. Dev. Biol., 164, 502–512. [DOI] [PubMed] [Google Scholar]
- Struhl G. and Basler,K. (1993) Organizing activity of wingless protein in Drosophila. Cell, 72, 527–540. [DOI] [PubMed] [Google Scholar]
- Szüts D., Eresh,S. and Bienz,M. (1998) Functional intertwining of Dpp and EGFR signaling during Drosophila endoderm induction. Genes Dev., 12, 2022–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thüringer F. and Bienz,M. (1993) Indirect autoregulation of a homeotic Drosophila gene mediated by extracellular signaling. Proc. Natl Acad. Sci. USA., 90, 3899–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thüringer F., Cohen,S.M. and Bienz,M. (1993) Dissection of an indirect autoregulatory response of a homeotic Drosophila gene. EMBO J., 12, 2419–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. and Crossley,M. (1998) Cloning and characterization of mCtBP2, a co-repressor that associates with basic Kruppel-like factor and other mammalian transcriptional regulators. EMBO J., 17, 5129–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M. et al. (1997) Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell, 88, 789–799. [DOI] [PubMed] [Google Scholar]
- Waltzer L. and Bienz,M. (1998) Drosophila CBP represses the transcription factor TCF to antagonize Wingless signalling. Nature, 395, 521–525. [DOI] [PubMed] [Google Scholar]
- Xu X., Yin,Z., Hudson,J., Ferguson,E. and Frasch,M. (1998) Smads proteins act in combination with synergistic and antagonistic regulators to target Dpp responses to the Drosophila mesoderm. Genes Dev., 12, 2354–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Hoppler,S., Eresh,S. and Bienz,M. (1996) Decapentaplegic, a target gene of the wingless signalling pathway in the Drosophila midgut. Development, 122, 849–858. [DOI] [PubMed] [Google Scholar]
- Yu X., Riese,J., Eresh,S. and Bienz,M. (1998) Transcriptional repression due to high levels of Wingless signalling. EMBO J., 17, 7021–7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M., Basler,K. and Struhl,G. (1996) Direct and long-range action of a wingless morphogen gradient. Cell, 87, 833–844. [DOI] [PubMed] [Google Scholar]