Abstract
The Arabidopsis ABSCISIC ACID–INSENSITIVE3 (ABI3) protein has been identified previously as a crucial regulator of late seed development. Here, we show that dark-grown abi3 plants, or abi3 plants returned to the dark after germination in the light, developed and maintained an etioplast with a prominent prolamellar body at developmental stages in which the wild type did not. Overexpression of ABI3 led to the preservation of the plastid ultrastructure that was present at the onset of darkness. These observations suggest that ABI3 plays a role in plastid differentiation pathways in vegetative tissues. Furthermore, the analysis of deetiolated (det1) abi3 double mutants revealed that DET1 and ABI3 impinge on a multitude of common processes. During seed maturation, ABI3 required DET1 to achieve its full expression. Mature det1 abi3 seeds were found to be in a highly germinative state, indicating that germination is controlled by both DET1 and ABI3. During plastid differentiation in leaves of dark-grown plants, DET1 is required for the action of ABI3 as it is during seed development. Together, the results suggest that ABI3 is at least partly regulated by light.
INTRODUCTION
The abscisic acid–insensitive3 (abi3) mutant originally was isolated for its ability to germinate in the presence of inhibiting concentrations of abscisic acid (ABA; Koornneef et al., 1984). ABI3 has been assigned a role in ABA signaling largely based on its involvement in late seed development (Koornneef et al., 1989, 1998; Nambara et al., 1994, 1995; Parcy et al., 1994; Leung and Giraudat, 1998). ABI3 encodes a transcription factor that shares marked similarity with viviparous1 (vp1) of maize (Giraudat et al., 1992). Studies with transgenic plants overexpressing ABI3 indicated that ABI3 is able to direct the expression of seed-specific genes in response to ABA in tissues other than seeds (Parcy et al., 1994). These experiments suggested that ABI3 might modulate ABA responsiveness in vegetative tissues.
Aside from the defects in ABA-induced seed dormancy, severe abi3 mutant alleles display additional phenotypes, such as impaired chlorophyll breakdown, lack of reserve accumulation, and premature initiation of leaf formation (Ooms et al., 1993; Nambara et al., 1995). The presence of chloroplasts during seed maturation and the premature initiation of leaf development previously have been explained as pleiotropic consequences of aberrant seed maturation. However, we have shown recently that ABI3 is not exclusively expressed during seed development but is additionally expressed de novo in arrested apical meristems of dark-grown Arabidopsis seedlings (Rohde et al., 1999). We hypothesized based on this observation that ABI3 plays a role in vegetative quiescence and that the premature initiation of leaf development on dark-grown abi3 mutants could have been a consequence of impaired meristem quiescence. To further elaborate on a role for ABI3 during vegetative development, we have investigated whether the presence of the impaired de-greening of mature seeds could be attributed to a more general role of ABI3 in determining plastid identity, and if so, whether ABI3 interacts with light signal transduction pathways.
Here, we show that leaves from dark-germinated abi3 mutant plants or plants returned to darkness after germination in the light (hereafter termed redarkened; see Methods) contain etioplasts at developmental stages in which wild-type leaves contain undifferentiated or degenerated plastids, respectively. Considering the expression of ABI3 in the apical zone (Rohde et al., 1999), these data suggest that ABI3 indeed plays a role beyond seed development, namely, in determining plastid identity in the shoot apex.
Because impaired chloroplast development is a typical criterion for the identification of light signal transduction mutants (Chory et al., 1989; von Arnim and Deng, 1996), the question arose whether ABI3 plays a specific role in light signal transduction or whether cross-talk between light and ABA signal transduction pathways exists during seed and vegetative development. Therefore, we have characterized the phenotype of deetiolated (det1) abi3 double mutants and monitored the expression of a chimeric ABI3 promoter–β-glucuronidase (GUS) gene fusion (ABI3–GUS) in the det1 mutant. The DET1 gene belongs to the CONSTITUTIVE PHOTOMORPHOGENIC/DEETIOLATED/FUSCA (COP/DET/FUS) group of genes that are necessary for the repression of photomorphogenesis in the dark (Miséra et al., 1994; Chory et al., 1996; Kwok et al., 1996; von Arnim and Deng, 1996) and encodes a putative repressor (Pepper et al., 1994). In addition to chloroplast development, dark-grown det1 mutants accumulate anthocyanins and express several light-regulated genes in the dark. Based on the det1 abi3 double mutant phenotypes, our results indicate that ABI3 and DET1 simultaneously influence photomorphogenetic programs in the seed, during growth in the dark, and during flowering. DET1 plays an essential role during seed maturation because it is required for the full expression of ABI3. In addition, the Det1 phenotype is epistatic to the Abi3 phenotype with respect to plastid ultrastructure in dark-grown leaves, suggesting that DET1 is required for the action of ABI3, as it is in the seed.
RESULTS
ABI3 Plays a Role in Plastid Development after Germination
abi3 mutants remain responsive to light throughout the course of seed development, and ∼10% of the abi3 mutants develop leaf primordia at the end of seed maturation (Nambara et al., 1995). As a consequence, abi3 mutants immediately produce leaves when germinated in the dark. As shown in Table 1 for different abi3 alleles and wild-type plants germinated in the dark, leaf development was initially observed exclusively in a fraction of the abi3 mutant seedlings. However, from 14 days on, a fraction of the wild-type plants had also initiated leaf primordia, but at a lower frequency than the abi3 mutants (Table 1; Rohde et al., 1999). Figures 1H and 1I show that dark-grown abi3 mutants were etiolated, as were wild-type plants. Nevertheless, the cotyledons and the leaves of abi3 mutants turned green faster than did those of wild-type plants upon exposure to light (data not shown). This observation suggested that there are differences in plastid structure between abi3 mutants and the wild type, in both the cotyledons and the leaves. Because seeds of abi3 mutants are green at maturity (Ooms et al., 1993; Nambara et al., 1994), the presence of partially differentiated chloroplasts in the cotyledons of dark-grown abi3 plants was not unexpected (Nambara et al., 1995). However, the putative presence of partially developed chloroplasts in leaves of dark-grown abi3 mutants was more intriguing because it would suggest that ABI3 plays a role in plastid differentiation outside the seed.
Table 1.
Average Percentage of Shoot Development in the Dark of abi3 Compared with the Wild Type
| Percentage of Shoot Developmenta
|
||||||||
|---|---|---|---|---|---|---|---|---|
| 7 Days
|
14 Days
|
21 Days
|
28 Days
|
|||||
| Genotype | n | % | n | % | n | % | n | % |
| Lerb | 344 | 0.0 | 794 | 3.1 | 184 | 9.2 | 1042 | 29.9 |
| Colc | 337 | 0.0 | 793 | 1.0 | 188 | 2.7 | 1000 | 14.7 |
| abi3-1 Ler | 323 | 0.0 | 351 | 2.6 | 119 | 16.0 | 586 | 21.2 |
| abi3-4 Ler | 313 | 1.6 | 442 | 10.0 | 157 | 36.6 | 675 | 53.6 |
| abi3-5 Ler | 234 | 0.8 | 327 | 6.1 | 120 | 15.0 | 498 | 35.9 |
| abi3-6 Col | 289 | 3.1 | 256 | 10.9 | 130 | 11.5 | 352 | 43.8 |
The percentage of shoot development was calculated by dividing the number of individual plants with shoot development by the number of germinated plants (n). The appearance of at least the first leaf was scored as positive shoot development.
Ler, ecotype Landsberg erecta.
Col, ecotype Columbia.
Figure 1.

Phenotypic Comparison of abi3, det1, and det1 abi3 Mutants.
(A) det1 seeds.
(B) det1 abi3 seeds.
(C) abi3 seeds.
(D) Seven-day-old det1 dark-germinated plants.
(E) Seven-day-old det1 abi3 dark-germinated plants.
(F) det1 abi3 plant after 28 days in the dark.
(G) det1 plant after 28 days in the dark.
(H) Wild-type plant after 28 days in the dark.
(I) abi3 plants after 28 days in the dark.
(J) Greenhouse-grown det1 abi3 (left) and det1 plants (right), 8 weeks after transfer to soil.
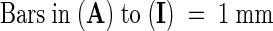 ;
; 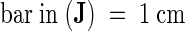 .
.
To reveal the plastid ultrastructure in abi3 mutants, we analyzed the leaves of 7- and 21-day-old dark-grown abi3 and wild-type plants by transmission electron microscopy, as summarized in Table 2 and shown in Figures 2 and 3. Leaf primordia of 7-day-old, dark-grown abi3 mutants contained pseudochloroplasts with prothylakoid membranes (Figure 2E). Pseudochloroplasts are characterized by the partial differentiation of prothylakoid membranes and the absence of a prolamellar body and are commonly observed in the photomorphogenic cop/det/fus mutants (reviewed in von Arnim and Deng, 1996). Wild-type seedlings had not formed leaves after 7 days in the dark. To determine the extent of plastid development inherent to any kind of leaf development in the dark, we examined the plastid ultrastructure of wild-type leaves after 21 days of growth in the dark, when etiolated leaves had developed in ∼3% of the Columbia wild-type plants (Table 1). These leaves carried plastids without signs of differentiation but some rudimentary membranes (Figure 3A). In contrast, the first two leaves of abi3 mutants grown for 21 days in the dark, which possibly were initiated during seed development, contained typical etioplasts with a prominent prolamellar body and some prothylakoid membranes (Figure 3C). Also any additionally formed leaves, the development of which presumably would have initiated during vegetative growth in the dark, contained etioplasts, although generally of smaller size than those in the first two leaves (Figure 3D).
Table 2.
Summary of the Plastid Ultrastructure Observed under Different Conditions in the Wild Type, abi3, det1, det1 abi3, and Plants Transformed with EN35S–ABI3
| Germination
|
|||
|---|---|---|---|
| Plant | 7 Days Dark | 21 Days Dark | 7 Days Light/21 Days Dark |
| Cotyledons | |||
| Wild type | Etioplast | ||
| abi3 | Partially differentiated chloroplast | ||
| det1 | Plastid with plastoglobuli | ||
| det1 abi3 | Partially differentiated chloroplast | ||
| Leaves | |||
| Wild type | N.O.a | Undifferentiated plastid | Arrested plastid |
| abi3 | Pseudochloroplast | Etioplast | Etioplast |
| det1 | Pseudochloroplast | Pseudochloroplast | Degenerating pseudochloroplast |
| det1 abi3 | Pseudochloroplast | Pseudochloroplast | Degenerating pseudochloroplast |
| EN35S–ABI3 | N.O. | Undifferentiated plastid | Chloroplast |
N.O., not observed, because the wild type and EN35S–ABI3 have no leaves at this stage.
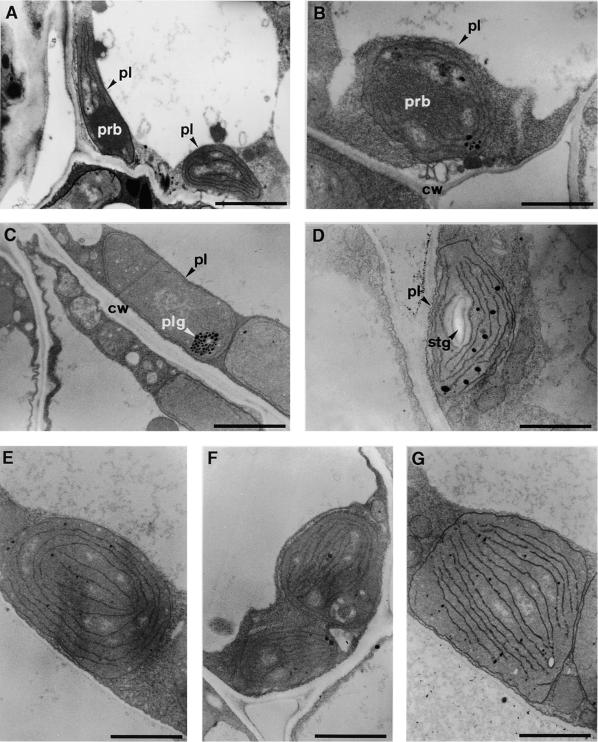
Figure 2.
Transmission Electron Microscopy of Plastid Ultrastructure in Cotyledons and Leaves of 7-Day-Old Dark-Germinated Seedlings.
(A) Etioplast of wild-type cotyledons.
(B) Partially differentiated chloroplast of abi3 cotyledons.
(C) Plastid of det1 cotyledons.
(D) Partially differentiated chloroplast of det1 abi3 cotyledons.
(E) Pseudochloroplast of abi3 leaf primordia.
(F) Pseudochloroplast of det1 leaves.
(G) Pseudochloroplast of det1 abi3 leaves.
cw, cell wall; pl, plastid; plg, plastoglobuli; prb, prolamellar body; stg, starch granule. 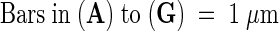 .
.
Figure 3.
Transmission Electron Microscopy of Plastid Ultrastructure in Leaves of 21-Day-Old Dark-Germinated Seedlings.
(A) Undifferentiated plastid of wild-type leaves (grown without close contact with sucrose).
(B) Amyloplast of wild-type leaves (grown in close contact with sucrose).
(C) Etioplast of abi3 leaves.
(D) Etioplast of abi3 dark-initiated leaves.
pl, plastid; prb, prolamellar body; stg, starch granule. 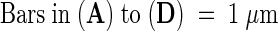 .
.
To further elaborate these data, we supplied the apices of dark-grown abi3 and wild-type plants with sucrose. Under these conditions, leaf development occurs in 100% of the seedlings after 21 days in the dark for both wild-type plants and abi3 mutants (Rohde et al., 1999). Wild-type plants also contained undifferentiated plastids, whereas abi3 mutants contained etioplasts (Figure 3B; data not shown). In both cases, the plastids contained starch granules. Therefore, the difference in the ultrastructure of abi3 and wild-type plastids was not due to a different timing of leaf development. The plastid ultrastructure of transgenic Arabidopsis plants overexpressing ABI3, EN35S–ABI3, was indistinguishable from the wild type 3 weeks after germination in darkness (data not shown).
To rule out the possibility that the presence of etioplasts in the leaves of abi3 mutants merely was due to the fact that the first leaf primordia had developed in “light conditions” during seed maturation, we germinated abi3 and wild-type plants, grew them in the light for 7 days, and subsequently transferred them to the dark for 21 days. As shown in Figure 4B, abi3 mutants contained typical etioplasts. In contrast, wild-type leaves contained arrested plastids (Figure 4A), indicating that light-initiated formation of leaf primordia in the seed is not the cause for the presence of etioplasts in abi3 mutants. Remarkably, the leaves of transgenic EN35S–ABI3 plants contained chloroplasts when transferred from light to darkness (Figure 4C). Together, these data indicate that ABI3 plays an active role in plastid differentiation after germination. In addition, the development of leaf primordia in the seed and the presence of pseudochloroplasts and etioplasts in dark-initiated leaves suggested possible interactions between ABI3-mediated signal transduction and light signal transduction pathways. Therefore, the phenotype of det1 abi3 double mutants was analyzed throughout their development.
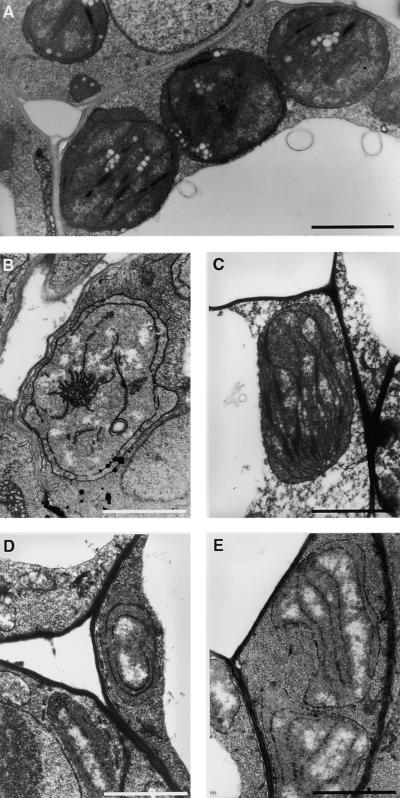
Figure 4.
Transmission Electron Microscopy of Plastid Ultrastructure in Leaves of Seedlings That Were Germinated for 7 Days in the Light and Subsequently Grown for 21 Days in the Dark.
(A) Arrested wild-type plastids.
(B) Etioplast of abi3 leaves.
(C) Chloroplast of EN35S–ABI3 leaves.
(D) Degenerating pseudochloroplasts of det1 leaves.
(E) Degenerating pseudochloroplasts of det1 abi3 leaves.
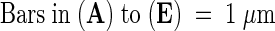 .
.
The Loss of ABI3 and DET1 Results in a Highly Nondormant Seed
Arabidopsis seed development normally concludes upon formation of a dormant, brown seed. Figure 1 shows that det1 mutant seeds resembled wild-type seeds phenotypically, whereas abi3 seeds remained green (Figures 1A and 1C). Seeds of det1 abi3 double mutants, in contrast, were bicolored (Figure 1B). The color on the hypocotyl side varied between brown and light green, whereas massive accumulation of chlorophyll and anthocyanins resulted in an almost black cotyledon side. The flat, elongated seed of det1 abi3 mutants suggested that the seeds were in a highly germinative state. Indeed, when freshly harvested det1 abi3 seeds were placed on wet filter paper, they germinated overnight. In contrast, freshly harvested abi3 seeds required 2 to 3 days to reach the same stage of germination. Both fresh det1 and wild-type seeds were unable to germinate on wet filter paper, unless the seeds had been stratified previously.
Figure 1 and Table 3 show that det1 abi3 plants tended to produce more leaves than did det1 plants. Most probably, the increased number of leaves is a consequence of the precocious development of det1 abi3 mutants at the seed stage relative to the det1 controls, because the trend was observed similarly under light and dark conditions (Table 3). In conclusion, det1 abi3 seeds appeared to be even less dormant than seeds of the nondormant abi3 parent.
Table 3.
Number of Leaves Formed during 4 Weeks of Growth
| Leaves
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant | Days | na | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
| Dark | |||||||||
| det1/det1 abi3/abi3b | 7 | 72 | 1.4 | 16.7 | 75.0 | 6.9 | |||
| 14 | 84 | 15.5 | 40.5 | 40.5 | 3.5 | ||||
| 21 | 70 | 5.7 | 15.7 | 25.7 | 44.3 | 5.7 | 2.9 | ||
| 28 | 78 | 3.8 | 9.0 | 21.8 | 24.4 | 38.5 | 2.5 | ||
| det1/det1 abi3/+b | 7 | 83 | 4.8 | 95.2 | |||||
| 14 | 116 | 8.6 | 42.2 | 49.1 | |||||
| 21 | 188 | 3.2 | 23.4 | 20.2 | 51.1 | 2.1 | |||
| 28 | 84 | 8.3 | 4.8 | 14.3 | 47.6 | 25.0 | |||
| det1/det1 | 7 | 54 | 3.7 | 96.3 | |||||
| 14 | 40 | 57.5 | 42.5 | ||||||
| 21 | 49 | 8.2 | 28.6 | 61.2 | 2.0 | ||||
| 28 | 37 | 18.9 | 32.4 | 43.2 | 5.4 | ||||
| abi3/abi3 | 7 | 74 | 4.0 | 96.0 | |||||
| 14 | 80 | 91.2 | 7.5 | 1.3 | |||||
| 21 | 65 | 69.2 | 20.0 | 10.8 | |||||
| 28 | 57 | 56.1 | 19.3 | 15.8 | 8.8 | ||||
| Wild type | 7 | 75 | 100 | ||||||
| 14 | 80 | 93.8 | 6.2 | ||||||
| 21 | 90 | 97.8 | 2.2 | ||||||
| 28 | 95 | 84.2 | 11.6 | 3.2 | 1.0 | ||||
| Light | |||||||||
| det1/det1 abi3/abi3b | 7 | 52 | 3.8 | 21.2 | 71.2 | 3.8 | |||
| 14 | 86 | 4.6 | 36.0 | 46.5 | 9.3 | 3.5 | |||
| det1/det1 abi3/+b | 7 | 55 | 100 | ||||||
| 14 | 113 | 40.7 | 53.1 | 6.2 | |||||
| det1/det1 | 7 | 53 | 9.4 | 88.7 | 1.9 | ||||
| 14 | 55 | 3.6 | 40.0 | 38.2 | 17.3 | 1.8 | |||
| abi3/abi3 | 7 | 53 | 100 | ||||||
| 14 | 50 | 2.0 | 58.0 | 40.0 | |||||
| Wild type | 7 | 67 | 3.0 | 97.0 | |||||
| 14 | 64 | 4.7 | 81.2 | 14.1 | |||||
n, number of individuals examined.
The double homozygous det1/det1 abi3/abi3 plants and the det1/det1 abi3/+ or det1/det1 +/+ plants originated from the same det1/det1 abi3/+ plant. The discrimination of the genotypes was based on the green seed color present only in seeds homozygous for abi3. The plants were examined individually under a binocular microscope to determine the number of leaves. The plants were grouped according to the number of leaves, and the percentage of plants in each group is given. This experiment has been repeated with similar results.
A detailed description of embryo morphology at seed maturity is provided in Figure 5. Similar to wild-type embryos, det1 embryos exhibited a normally developed, dormant embryo surrounded by the endosperm layer and seed coat (Figures 5A, 5B, 5E, 5F, 5I, and 5J). abi3 embryos exhibited an enlarged apical meristem and advanced vascular differentiation (Figures 5A to 5C and 5E to 5G), indicating that shoot development had been initiated. In contrast to these single mutants, the embryos of det1 abi3 seeds showed signs of true germination (Figures 5D, 5H, and 5L). The cotyledons were flattened, the hypocotyl was thickened, and root elongation had started (Figure 5D). Occasionally, the root even protruded through the seed coat of ripe seeds, an event that occurs in the wild type only 24 to 36 hr after imbibition (Mansfield and Briarty, 1996). In addition, the shoot apical meristem was more developed in det1 abi3 mutants than it was in abi3 mutants (Figure 5H), and new leaf primordia occasionally were observed at the apical meristem in the double mutants (data not shown). Both the cotyledons and the hypocotyl of det1 abi3 embryos showed precocious vascular differentiation, with the development of protoxylem elements in the hypocotyl (Figure 5H). Altogether, the phenotype of det1 abi3 was clearly distinct from that of the abi3 and det1 embryos, which never showed such an advanced germinative state.
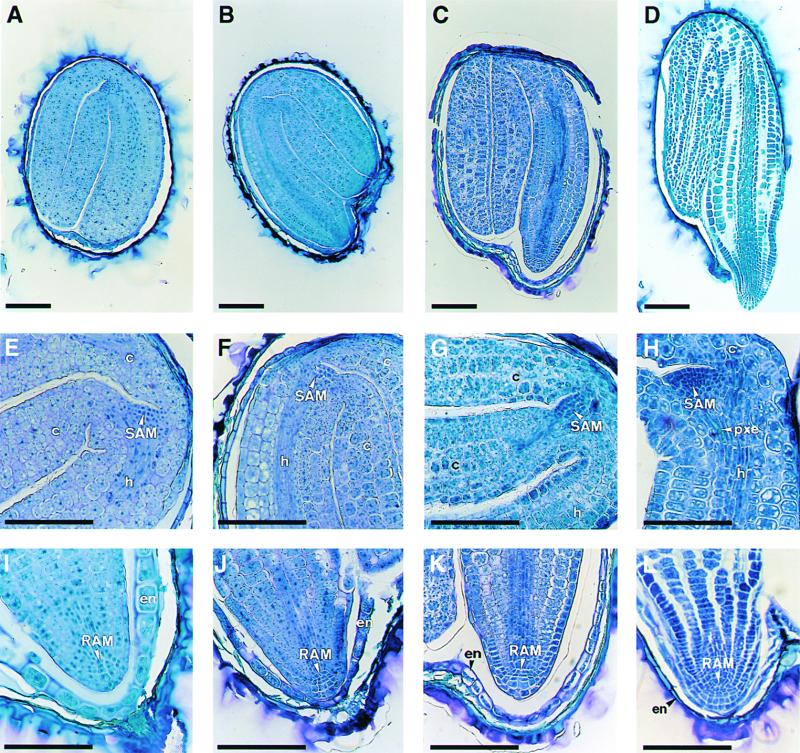
Figure 5.
Longitudinal Sections of Embryos of the Wild Type, abi3, and det1 abi3 at Seed Maturity.
(A) Whole embryo of the wild type.
(B) Whole embryo of det1.
(C) Whole embryo of abi3.
(D) Whole embryo of det1 abi3.
(E) Shoot apical meristem of the wild type.
(F) Shoot apical meristem of det1.
(G) Shoot apical meristem of abi3.
(H) Shoot apical meristem of det1 abi3.
(I) Root apical meristem of the wild type.
(J) Root apical meristem of det1.
(K) Root apical meristem of abi3.
(L) Root apical meristem of det1 abi3.
c, cotyledon; en, endosperm layer; h, hypocotyl; pxe, protoxylem elements; RAM, root apical meristem; SAM, shoot apical meristem. 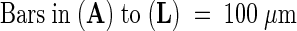 .
.
Transmission electron microscopy revealed additional differences in the ripe cotyledons of the mutants. Figure 6A shows that the cotyledons in ripe wild-type seeds possess cells with prominent lipid and protein bodies, which clearly is not the case for det1 and abi3 cotyledons (Figures 6A to 6C). In abi3, fewer protein and lipid bodies were visible than in det1 or the wild type (Figure 6B), which is consistent with the previously described defects in reserve accumulation in this mutant (Nambara et al., 1992; Ooms et al., 1993). det1 abi3 cotyledon cells often contained a large vacuole instead of the otherwise predominant protein bodies (Figure 6D). Such vacuoles are formed in the wild type from protein body fusion only 42 hr after imbibition, and the particulate material found dispersed throughout these vacuoles is derived from the storage proteins that remain after fusion (Mansfield and Briarty, 1996). Similarly, the endosperm of det1 abi3 seeds already was depleted of most of its reserves at seed maturity (data not shown). Indeed, a comparable endosperm ultrastructure was observed in the empty seed coat of the wild type, det1, and abi3, only 7 days after germination (data not shown). In both abi3 and det1 abi3 cotyledons, plastids with some degree of prothylakoid membrane differentiation were present. This observation is in agreement with the impaired de-greening of the abi3 mutant after the green-cotyledon stage (Figures 6E and 6F). Interestingly, the endosperm cells of abi3 mutants also contained partially differentiated chloroplasts (data not shown). Neither wild-type nor det1 cotyledon cells showed plastid differentiation (data not shown).
Figure 6.
Transmission Electron Microscopy of the Cotyledon at Seed Maturity in Wild-Type Plants and abi3, det1, and det1 abi3 Mutants.
(A) Cotyledon of the wild type.
(B) Cotyledon of abi3.
(C) Cotyledon of det1.
(D) Cotyledon of det1 abi3.
(E) Chloroplast of det1 abi3.
(F) Chloroplast of abi3.
chl, chloroplast; lb, lipid body; pb, protein body; stg, starch granule; vac, vacuole. 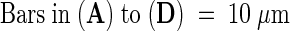 ; b
; b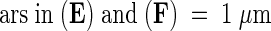 .
.
Taken together, the ultrastructural features of the cotyledon and the endosperm are compatible with the observation that the germination program is initiated prematurely in det1 abi3 seeds. Furthermore, the distinct ultrastructure and the germinative state of the det1 abi3 double mutant seeds suggest that both ABI3 and DET1 act at the transition from seed development to germination.
ABI3 and DET1 Interact during Seed Development
One explanation for the precocious germination of det1 abi3 seeds might be that DET1 and ABI3 impact on similar processes during seed development. To investigate this, we dissected embryos at various stages of seed development and analyzed them by light microscopy as shown in Figure 7.
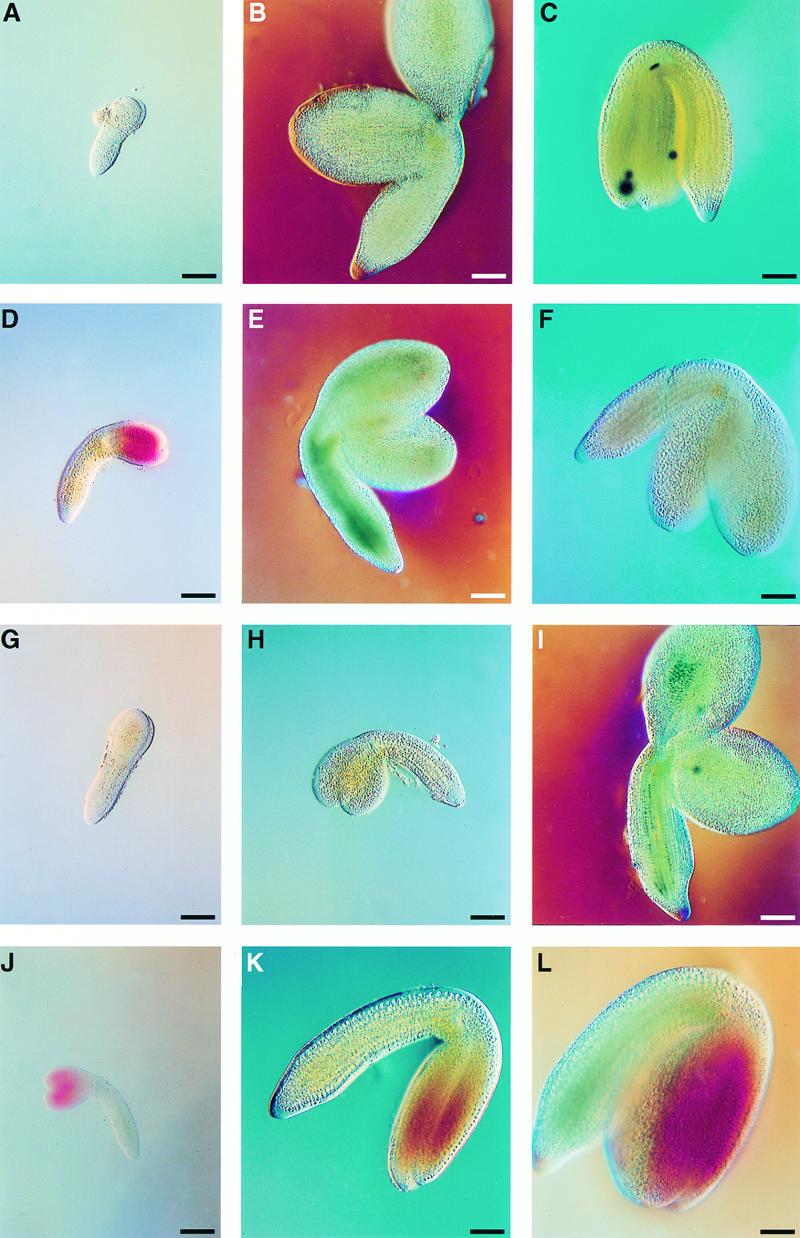
Figure 7.
Whole-Mount Preparations of det1, abi3, and det1 abi3 Embryos at Early- and Late-Torpedo Stage, Green-Cotyledon Stage, and Seed Maturity.
(A) Torpedo stage of the wild-type embryo.
(B) Green-cotyledon stage of the wild-type embryo.
(C) Mature embryo of the wild type.
(D) Torpedo stage of a det1 embryo.
(E) Green-cotyledon stage of a det1 embryo.
(F) Mature embryo of det1.
(G) Torpedo stage of an abi3 embryo.
(H) Green-cotyledon stage of an abi3 embryo.
(I) Mature embryo of abi3.
(J) Torpedo stage of a det1 abi3 embryo.
(K) Green-cotyledon stage of a det1 abi3 embryo.
(L) Mature embryo of det1 abi3.
As a result of the chlorallactophenol treatment, part of the chlorophyll content was lost, especially in torpedo-stage embryos. 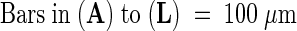 .
.
Wild-type embryos are green from the torpedo stage until the onset of maturation, which leads to de-greening and reserve accumulation (Mansfield and Briarty, 1991, 1992; Figures 7A to 7C). Interestingly, det1 embryos had red cotyledons and a green hypocotyl at the torpedo stage (Figure 7D). Similar to the wild type, det1 embryos were green at the early-cotyledon stage and did de-green during the maturation phase (Figures 7E and 7F). In contrast, abi3 embryos remained green throughout seed development (Figures 7G to 7I). det1 abi3 double mutants, however, had red cotyledons and a green hypocotyl from the torpedo stage until maturity (Figures 7J to 7L). The red coloration in the cotyledons of ripe det1 abi3 embryos was more prominent in the epidermal cell layers of the cotyledon and was absent in mesophyll cells (data not shown).
Mature wild-type and det1 embryos required 2 days to be cleared with chlorallactophenol, indicating a strong accumulation of reserves in the seed (Figures 7C and 7F). By comparison, mature abi3 and det1 abi3 embryos became translucent after only 5 min in chlorallactophenol, suggesting a diminished acquisition of reserves (Figures 7I and 7L). When total seed proteins were resolved by SDS-PAGE, the difference in reserve accumulation could be attributed to a reduced accumulation of the major 2S and 12S reserve proteins (data not shown).
To further investigate the putative interactions of DET1 and ABI3 during seed development, we analyzed transgenic det1 plants expressing a chimeric ABI3 promoter–GUS gene. Figure 8 shows quantitative assays that compare ABI3–GUS expression in det1 and wild-type seeds. Seeds in yellow det1 siliques contained ∼70% of the wild-type GUS activity. Most strikingly, det1 seeds contained only 22 to 25% of wild-type GUS activity at seed maturity (Figure 8). In contrast, GUS activity was upregulated to 134% in ripe abi3 seeds, which were included as a control. The latter observation is in agreement with the fact that the ABI3 protein is more abundant in abi3 mutant seeds (Parcy et al., 1997). Together, the novel seed phenotypes of the double mutant and the altered expression of ABI3–GUS in det1 mutants indicate that DET1 has, among others, a role in positively regulating the expression of ABI3 during seed maturation.
Figure 8.
Expression of a Chimeric ABI3–GUS Gene during Seed Development of det1 Mutants.
A plant transgenic for a chimeric ABI3–GUS gene was crossed with det1 mutants, and lines homozygous for det1 and ABI3–GUS were used for the experiment. Four independent det1 lines transgenic for ABI3–GUS were assayed in the experiment. The mean GUS activity of all lines is expressed relative to the GUS activity in wild-type seeds at the same developmental stage.
ABI3 and DET1 Impinge on Similar Processes during Cotyledon and Leaf Development in the Dark
When det1 abi3 double mutants were germinated in the dark, they resembled the det1 parent in that they had a short hypocotyl and large cotyledons and developed rosette leaves later on (Figures 1F and 1G; Chory et al., 1989). Notwithstanding the general Det1-like phenotype, new phenotypic aspects were observed in the double mutant. After 7 days of growth in the dark, for example, the cotyledons of det1 abi3 seedlings had an even larger surface area than did those of det1 (Figures 1D and 1E). Light-grown det1 abi3 plants also had slightly larger cotyledons than did det1, although the difference was less pronounced than it was in the dark (data not shown). The ultrastructure of the plastids in the cotyledons is shown in Figure 2 and summarized in Table 2. After 7 days of germination in the dark, typical etioplasts were observed in wild-type and abi3 cotyledons (Figures 2A and 2B). Unlike the typical etioplasts of the wild type, the etioplasts of abi3 mutants were not completely devoid of chlorophyll. This aspect is characteristic for a plastid in transition from chloroplast to etioplast (Kirk and Tilney-Bassett, 1978; Gunning and Steer, 1986). det1 mutant cotyledons contained plastids with plastoglobuli (Figure 2C). det1 abi3 cotyledons essentially resembled the abi3 parent in that they contained plastids with prothylakoid membrane differentiation (Figures 2B and 2D). However, the absence of the prolamellar body and a more frequent starch deposition in the plastids clearly distinguished the det1 abi3 mutant from abi3 mutants (Figures 2B and 2D). Such starch deposition is indicative of photosynthetic activity of the plastid (Mansfield and Briarty, 1991, 1992).
In addition to these differences in the cotyledons, the leaves of dark-grown det1 abi3 plants were morphologically distinct from those of either parent (Figures 1F to 1I). Leaves of det1 abi3 plants had a larger leaf blade and a shorter petiole (Figure 1F). This phenotype is different from the narrow leaves and the long petioles of det1 or the highly etiolated leaves of abi3 (Figures 1G and 1I). Furthermore, the leaves of dark-grown det1 abi3 mutants often contained anthocyanins, which were not observed under our growth conditions in either det1 or abi3 mutants and the production of which is generally induced by light (Figure 1F; Cone et al., 1993). Together, these novel phenotypes of the double mutant imply that ABI3 and DET1 impinge on similar processes during growth in the dark.
det1 Is Epistatic to abi3 with Respect to Plastid Ultrastructure in Dark-Grown Leaves
Both det1 and abi3 mutants develop leaves in the dark. Whereas abi3 mutants develop etiolated leaves in a percentage of the seedlings (Nambara et al., 1995; Table 1), det1 has a deetiolated phenotype in all individuals (Chory et al., 1989). A simultaneous light-independent initiation of chloroplast development in the roots and dark-grown leaves of det1 has been reported previously (Chory and Peto, 1990). To determine a possible interaction of DET1 and ABI3 during chloroplast/etioplast formation in the dark, we investigated the leaves of det1, abi3, and det1 abi3 mutants. The ultrastructure of the plastids in leaves is shown in Figures 2 and 4 and summarized in Table 2. After 7 days in the dark, the leaves of det1 plants were similar to those of abi3 in that they contained pseudochloroplasts with clear bithylakoid membrane structures, as previously described (Figures 2E and 2F; Chory et al., 1989). The double mutant also had plastids with differentiated bithylakoid membranes (Figure 2G). After 21 days in the dark, a similar pseudochloroplast structure was noticed in both det1 and det1 abi3 mutants (data not shown), whereas abi3 mutants contained etioplasts at this stage (Figures 3C and 3D). When det1 and det1 abi3 were transferred to darkness after 7 days of germination in the light, the pseudochloroplasts of both det1 and det1 abi3 contained fewer and less preserved membranes than they contained during germination in the dark (Figures 4D and 4E). Taken together, the det1 abi3 plastid ultrastructure was similar to that of det1 mutants and clearly different from the etioplast structure of abi3 mutants, that is, the Det1 plastid phenotype was epistatic to the Abi3 plastid phenotype in leaves of dark-grown and redarkened seedlings.
ABI3 and DET1 Affect Internode Length, Plant Height, and Flowering Time
The det1 abi3 mutants unexpectedly displayed additional abnormalities when grown to maturity in the greenhouse. Flowering and the formation of siliques of det1 abi3 plants started at least 2 weeks later than those of the det1 control plants, as shown in Figure 1J and Table 4. After 7 weeks, when almost all det1 control plants had formed siliques, half of the det1 abi3 plants still had not. After 9 weeks, 91% of the control plants had ripe siliques, whereas of the 38% of the det1 abi3 plants that had developed siliques, only 10% were ripe.
Table 4.
Parameters of Vegetative Growth and Silique Development
| Parameters | det1/det1 abi3/abi3a | det1/det1, abi3/+, or +/+a |
|---|---|---|
| After 7 weeks | ||
| Rosette paraclades (n) | 5.3 ± 1.4 | 7.1 ± 1.4 |
| Accessory and cauline paraclades (n) | 18.6 ± 5.1 | 29.0 ± 11.5 |
| Longest branch (cm) | 4.8 ± 1.3 | 9.5 ± 1.6 |
| Average internode (mm) | 3.2 ± 0.2 | 4.7 ± 1.0 |
| Plants without siliques (%) | 52.9 | 2.9 |
| With 1 to 10 siliques (%) | 44.1 | 11.8 |
| With 10 to 50 siliques (%) | 2.9 | 47.0 |
| With >50 siliques (%) | 0 | 38.2 |
| After 9 weeks | ||
| Average internode (mm) | 3.8 ± 0.8 | 5.0 ± 1.1 |
| Plants without siliques (%) | 14.7 | 0 |
| With 1 to 10 siliques (%) | 5.9 | 0 |
| With 10 to 50 siliques (%) | 61.8 | 2.9 |
The double homozygous det1/det1 abi3/abi3 plants were compared with det1/det1 abi3/+ or det1/det1 +/+ plants, which were harvested from the same det1/det1 abi3/+ plant. The discrimination of the genotypes was based on the green seed color present only in seeds homozygous for abi3. Plants were analyzed 7 and 9 weeks after the transfer of in vitro plants to the greenhouse. The averages with standard deviation are given. Data on abi3 and wild-type plants were omitted because their phenotypes are clearly distinct. The comparison of wild-type and det1 growth was described previously by Chory and Peto (1990).
Flowering det1 abi3 plants essentially resembled det1 plants: they were small and had lost apical dominance (Figure 1J). As shown in Table 4, det1 abi3 plants had fewer rosette paraclades, fewer accessory and cauline paraclades, and a reduced height and internode length compared with the det1/det1 abi3/+ or det1/det1 +/+ plants. The number of paraclades is indicative of apical dominance; approximately three rosette paraclades develop in the wild-type condition of apical dominance (Pepper and Chory, 1997). Therefore, the det1 mutant seemed to be partially reverted by abi3 in the double mutant. A similar reversion of the Det1 phenotype has been described for extragenic suppressors of det1 (Pepper and Chory, 1997). In conclusion, abi3 affected the morphology of the flowering stalk in the det1 mutant background, reinforcing again that ABI3 also plays a role beyond seed development.
DISCUSSION
ABI3 Plays a Role in the Determination of Plastid Identity
Multiple functions have been ascribed to ABI3 during seed maturation, but its precise mode of action in the various processes remains to be uncovered (Bonetta and McCourt, 1998; Leung and Giraudat, 1998). Whereas the activation of seed-specific genes has been unequivocally demonstrated, other effects, such as impaired chloroplast breakdown in abi3 seeds, have been explained in terms of pleiotropic consequences of aberrant seed maturation in the mutant (Parcy et al., 1994; Nambara et al., 1995). Here, we present arguments for a more direct role of ABI3 in plastid development, because plastids also develop aberrantly in leaves of dark-grown and redarkened plants. Together with the phenotypic alterations of det1 abi3 double mutants, our data revise the previous hypothesis that ABI3 is a seed-specific transcription factor and assert that ABI3 functions beyond seed development.
Plastid development was found to be perturbed in the leaves of abi3 mutants. Leaves of dark-grown as well as redarkened abi3 mutants carried etioplasts at stages in which wild-type leaves did not (Figures 2 to 4). When wild-type plants are subjected to prolonged darkness, the material contained in the etioplast is metabolized, and the plastid finally degenerates. By contrast, the etioplast of abi3 mutants was maintained over a prolonged period of time and not further converted into a degenerated plastid. Thus, the etioplasts of abi3 mutants were not prone to plastid degeneration, suggesting that ABI3 is required for the determination of the “normal” etioplast identity. Moreover, constitutive overexpression of ABI3 led to the preservation of the chloroplast (Figure 4C), suggesting that ABI3 acts negatively on the transition from chloroplast to etioplast. Although this result is more difficult to interpret because it derives from ectopic expression of ABI3, it again emphasizes that ABI3 plays a role in plastid identity.
The observation that abi3 mutants green faster when transferred from the dark to the light independently pointed toward either the preservation of plastid structure (etioplast) or the presence of a component that enabled a faster chloroplast differentiation and/or chlorophyll synthesis. The pronounced prolamellar body in abi3 mutant plastids could be one of the reasons for this faster greening (Figure 3), because the formation of chloroplasts from mature etioplasts is faster than from proplastids (Sundqvist and Dahlin, 1997). Furthermore, the presence of a prolamellar body correlates with the accumulation of photoactive forms of protochlorophyllide and the presence of protochlorophyllide oxidoreductase (POR; Apel et al., 1980; Ryberg and Sundqvist, 1982; Dehesh and Ryberg, 1985). These photoactive protochlorophyllide pigments not only are readily available as chlorophyll precursors but also protect against photoinduced damage during greening (Sundqvist and Dahlin, 1997).
Together, the presence of a preserved etioplast and the partial light-independent leaf development in dark-grown abi3 plants on the one hand (Tables 1 and 2, and Figures 2 to 4) and the presence of a partially differentiated chloroplast together with the expression of chlorophyll a/b binding protein and chalcone synthase in abi3 seeds on the other hand (Nambara et al., 1995) suggest an integration of ABI3 function with light signal transduction during and beyond seed development. In seeds and leaves of dark-grown plants, ABI3 might prevent a part of the developmental program that is triggered by light. Alternatively, ABI3 could render cells sensitive to an ABA signal that acts together with light. Significant increases in ABA concentration after the transfer from the light to the dark have been reported (Weatherwax et al., 1996). Such a function would be in agreement with the observed modifications of ABA sensitivity in ABI3-overexpressing plants (Parcy and Giraudat, 1997).
Regardless of whether the relationship of ABI3 to light signaling is direct or indirect, occurring via other components, loss of functional ABI3 prevents the cells from developing along the correct plastid differentiation pathway in seeds and vegetative tissues. At this point, the question arises whether these Abi3 phenotypes are correlated with ABI3 expression during vegetative development or are a mere consequence of an altered physiology in the mutant seed. We have shown previously that ABI3 is expressed in the apices of dark-grown and redarkened seedlings, suggesting that ABI3 is additionally present in vegetative tissues in the dark (Rohde et al., 1999). With ABI3 being expressed and most probably also functional at the apex, we hypothesize that ABI3 participates in determining the plastid differentiation trajectory of cells in the apex.
Further evidence for the integration of ABI3 with the light-dependent pathway for plastid formation comes from the epistasis with DET1 regarding the plastid morphology in leaves of dark-grown and redarkened det1 abi3 double mutants. The det1 mutant is epistatic to the abi3 mutant in the dark, because the double mutants display a similar plastid ultrastructure to that of the det1 mutants (Figures 2 to 4). In det1 mutants, the light-independent activation of part of the phytochrome signaling pathway leads to POR levels that are too low for detectable prolamellar body formation (Sperling et al., 1998). Consequently, leaves of dark-grown det1 and det1 abi3 mutants were found to be devoid of POR A (A. Rohde, unpublished result). This genetic relationship between the abi3 and det1 mutants implies that DET1 is prerequisite to the action of ABI3 in these responses. This explanation fits with the role of DET1 during seed development, where it appears to positively influence transcription from the ABI3 promoter (Figure 8).
det1 abi3 Double Mutants Identify Processes under Common Regulation of DET1 and ABI3
Given that mutations in abi3 appear to influence leaf development in the dark and to impair plastid differentiation in dark-grown plants, we investigated the putative interaction between ABI3-mediated signal transduction and light signal transduction through an analysis of det1 abi3 double mutants. Many of the observed characteristics of the det1 abi3 seeds at maturity correspond to developmental stages that wild-type seeds only attain ∼42 hr after imbibition (Figures 1, 5, and 6; Mansfield and Briarty, 1996). Accelerated germination on wet filter paper indicates that det1 abi3 mutants are even less dormant than the nondormant abi3 mutant. From this observation, we conclude that det1 abi3 mutants enter the germination program while they are still in the seed. The apparent absence of vivipary in det1 abi3 mutants in vivo is probably simply a consequence of the physical resistance of the seed coat and the limited water availability inside the silique. Integration of seed development with germination consequently is controlled by both DET1 and ABI3.
The det1 abi3 double mutant seed simultaneously accumulated chlorophyll and anthocyanins (Figures 7J to 7L), as do fus3 abi3 and leafy cotyledon1 (lec1) abi3 seeds (Keith et al., 1994; Meinke et al., 1994). Similar to det1 abi3 mutants, fus3 abi3 embryos were found to be in a highly germinative state and developed leaf primordia before the completion of desiccation (Figure 5; Keith et al., 1994). Based on the similarities of the phenotypes of det1 abi3 and fus3 abi3, DET1 should be integrated into the regulatory network controlling seed development that includes the FUS group, ABI3, and LEC1 (Castle and Meinke, 1994; Keith et al., 1994; Meinke et al., 1994; Miséra et al., 1994; West et al., 1994). It nevertheless should be noted that the loss of DET1, even though it plays a clear role in seed development, seems not to lead to aberrant chloroplast biogenesis in seeds, as it does in leaves and roots after germination. That DET1 definitely is part of the seed regulatory circuit is supported by the observation that DET1, either directly or indirectly, is required to achieve full expression from the ABI3 promoter. Only 25% of the wild-type ABI3–GUS expression was found in det1 mutants (Figure 8). This adds DET1 to FUS3 and LEC1 to the group of proteins that positively regulate ABI3 expression levels (Figure 8; Parcy et al., 1997).
The transition from the vegetative to the inflorescence meristem is known to be regulated by light and by gibberellins in Arabidopsis. In det1 abi3 double mutants, this transition is delayed in comparison to det1 single mutants, which flower relatively early after having initiated ∼10 leaves in both long and short days (Pepper and Chory, 1997). ABI3 therefore is required for the effect of the det1 mutation on earlier and day length–insensitive flowering. The delay in flowering time was surprising, because one would have expected an earlier flowering, taking into account the earlier germination and the advanced seedling development of the double mutants. In addition, the number and elongation of paraclades were altered in det1 abi3 double mutants (Table 4 and Figure 1J).
Because elongation processes are controlled primarily by gibberellin levels, which, in turn, are regulated by light (Chory and Li, 1997), this result supports the idea that in addition to the transduction of ABA signals, ABI3 is involved in light signal transduction or alternatively renders cells responsive to light. Defects in either of these processes (flowering time or elongation) have not been described for abi3 single mutants. However, ABI3–GUS expression has been detected at the branching points of the flowering stalk (Rohde et al., 1999), and abi3-1 mutants have been shown to produce more siliques and cauline leaves (Robinson and Hill, 1999).
Studies of double mutants only are limited and cannot provide any information on the nature of the interaction between DET1 and ABI3. Furthermore, the phenotype of the det1 abi3 double mutant and, hence, the nature of the genetic relationship between abi3 and det1 are highly dependent on the tissue and the developmental stage. This dimension of the interaction of ABI3 and DET1 is best illustrated with the synergism observed in the highly nondormant seed as opposed to the epistasis observed in plastid ultrastructure of dark-grown leaves. Additional molecular approaches will be required to refine our knowledge and to resolve remaining questions on the interactions that take place during signaling. Target genes of either ABI3 or DET1 that are known to be aberrantly expressed in the respective mutant could help to further reveal the shared and unique paths/nets used by ABI3 and DET1 (Sun et al., 1993; Parcy et al., 1994; Quaedvlieg et al., 1995; Mayer et al., 1996).
Conclusions
It is becoming increasingly obvious that light and ABA simultaneously influence gene expression (Williams et al., 1994; Weatherwax et al., 1996). Our results suggest that ABI3, although identified as an ABA signal transduction component, is a regulatory component that might function downstream or in cooperation with both light and ABA signals. In fact, our results lend support to earlier speculations that ABI3 is marginally integrated with ABA signal transduction (Bonetta and McCourt, 1998). Moreover, an ABI3 function downstream of light and ABA signals is compatible with its molecular nature. Mainly based on the molecular characterization of its maize ortholog VP1 (Hoecker et al., 1995; Hill et al., 1996; Suzuki et al., 1997; Schultz et al., 1998), ABI3 has been suggested to be a multifunctional transcription factor that “integrates” ABA and other regulatory signals of seed maturation (Leung and Giraudat, 1998). In summary, the epistasis with DET1 in plastid development and the requirement of DET1 for the full transcription from the ABI3 promoter in seeds suggest that ABI3 is controlled at least in part by light. In this rationale, ABI3 regulates part of the light program in seeds and vegetative tissues.
METHODS
Plant Material, Generation of the Double Mutants, and Growth Conditions
The det1-1 and abi3-6 mutations are in Arabidopsis thaliana ecotype Columbia. The transgenic plants carrying the ABI3–GUS and EN35S– ABI3 constructs are in the C24 ecotype. det1-1 was obtained from the Arabidopsis Biological Resource Center (Columbus, OH); abi3-1 was obtained from the Nottingham Arabidopsis Stock Centre (Nottingham, UK). Seeds of abi3-4 and abi3-5 were a gift of Drs. Karen Léon-Kloosterziel and Maarten Koornneef (Universiteit Wageningen, The Netherlands), and the abi3-6 seeds were generously provided by Dr. Eiji Nambara (Hokkaido University, Sapporo, Japan). Seeds of transgenic ABI3–GUS and EN35S–ABI3 plants were kindly provided by Drs. Jérôme Giraudat and François Parcy (CNRS, Institut des Sciences Végétales, Gif-sur-Yvette, France). The ABI3–GUS and EN35S–ABI3 constructs are described in detail by Parcy et al. (1994).
abi3-6 was crossed with det1-1 by using abi3-6 as a pollen donor. Green F2 seeds (indicative of abi3 homozygosity) were germinated in the dark and screened for the presence of phenotypes associated with the det1 mutation. The offspring of identified det1 abi3 double-homozygous plants was used for the experiments. Because abi3-homozygous seeds are intolerant to desiccation, the double mutants had to be continuously propagated.
The cross of det1-1 (Columbia) with a line carrying the ABI3–GUS construct (C24) was conducted without emasculation by using transgenic ABI3–GUS plants as a pollinator. F2 plants homozygous for det1-1 were grown on medium with 50 mg L−1 kanamycin. Lines homozygous for kanamycin resistance were identified in the F3 generation and were used for the experiments.
After the seeds were harvested, they were treated for 2 weeks at 28°C and stored at 4°C until use (except for green, desiccation-intolerant, homozygous abi3 seeds). For germination, seeds were surface-sterilized and placed on a Murashige and Skoog medium (Murashige and Skoog, 1962) supplemented with 10 g L−1 sucrose. After the seeds had undergone a cold treatment for homogenous germination (overnight and 4 days for fully stratified and freshly harvested seeds, respectively), they were exposed to 20°C, 50 μmol m−2 sec−1 light intensity, 70% relative humidity, under a 16-hr-light/8-hr-dark cycle. For the dark treatments, plates were wrapped twice with aluminum foil and placed in a dark container in the same culture room. The greenhouse conditions for plant growth and crosses were as follows: 40 μmol m−2 sec−1 light intensity at plant level (MBFR/U 400 W incandescent lamps; Philips, Eindhoven, The Netherlands), a 16-hr-light/8-hr-dark cycle, 40% relative humidity, 23°C, and without shielding from incident daylight.
Light Microscopy
For whole-mount embryo preparations, seeds were dissected under a binocular microscope by using an 18-gauge needle. The fresh embryos were immediately immersed in chlorallactophenol (2:1:1 mixture of chloral hydrate, lactic acid, and phenol; Beeckman and Engler, 1994) to clear the tissues and then examined with Nomarski optics (Jenalumar contrast microscope; Jenoptik, Jena, Germany).
For sectioning, fresh plant material was fixed in formaldehyde–acetic acid–alcohol and passed over a graded ethanol series. Ethanol subsequently was replaced by Technovit 7100 (Heraeus Kulzer, Wehrheim, Germany), and material in Technovit was polymerized at room temperature according to the manufacturer's instructions. Sections of 2 to 4 μm were cut on a rotary microtome with a glass knife. Morphological sections of the embryos were stained with 0.05% toluidine blue for 8 min before examination under a light microscope.
Transmission Electron Microscopy
Embryos, seeds, cotyledons, or leaves were cut into small pieces and fixed with a mixture of 4% paraformaldehyde and 3% glutaraldehyde and postfixed with 2% OsO4 and 1.5% K3Fe(CN)6 in 0.1 M Na-cacodylate buffer, pH 7.2. Samples were dehydrated through a graded ethanol series, including a bulk staining with 2% uranyl acetate at the 50% ethanol step. Ethanol subsequently was replaced by LR white embedding medium (London Resin, Basingstoke, UK), and samples in LR white were polymerized in flat embedding molds under UV light at 4°C for 16 hr, followed by 16 hr at 60°C. Ultrathin sections of gold interference color were cut using an ultramicrotome and collected on collodion-coated Cu grids of 200 to 300 mesh. The grids were post-stained with 2% uranyl acetate for 10 min, washed, and allowed to air dry. Sections were examined by using a transmission electron microscope (Elmiskop 101; Siemens, Karlsruhe, Germany).
Fluorometric GUS Assays
GUS was assayed as described by Jefferson (1987). Tissues were homogenized in extraction buffer (50 mM phosphate buffer, pH 7.0, 1 mM EDTA, 10 mM β-mercaptoethanol, and 0.1% Triton X-100). Seed protein extracts were adjusted to each other according to fresh weight because the protein accumulation is affected by abi3 (Parcy et al., 1997). Each fluorometric measurement with 1 mM 4-methylumbelliferyl-β-d-glucuronide as a substrate (Sigma) was performed in duplicate in a computer-directed microtiter plate reader (Labsystems Fluoreskan II, Helsinki, Finland). The activity was expressed as a percentage relative to the activity in wild-type plants carrying the ABI3–GUS gene at the same developmental stage; the average percentage of three to six independent measurements is given with standard deviation. A quantitative comparison of seeds in very young siliques (<3 mm) was not done, because the standardization to similar amounts of seeds is unreliable at that stage.
Protein Analysis
Mature seeds were ground and extracted in a buffer containing 100 mM Tris-HCl, pH 7.5, 0.5% SDS, 10% glycerol, and 2% β-mercaptoethanol. After adjusting the amount of protein to fresh weight, proteins were denatured and resolved by SDS-PAGE by using a 15% gel and stained with Coomassie Brilliant Blue R 250.
NOTE ADDED IN PROOF
Additional evidence for a function of ABI3 in flowering time of Arabidopsis recently has been provided by the analysis of constans abi3 double mutants (Kurup, S., Jones, H.D., and Holdsworth, M.J. [2000]. Interactions of the developmental regulator ABI3 with proteins identified from developing Arabidopsis seeds. Plant J., in press).
Acknowledgments
We thank Eiji Nambara, Jérôme Giraudat, and François Parcy for providing the abi3-6 mutant and the ABI3–GUS and EN35S–ABI3 transgenic plants, respectively. For the seeds of abi3-4 and abi3-5, Drs. Karen Léon-Kloosterziel and Maarten Koornneef are gratefully acknowledged. We further thank Gregory Armstrong for discussion and the POR polyclonal antibody, Dominique Van Der Straeten and Mark Davey for critical reading of the manuscript, Martine De Cock for help in preparing it, and Karel Spruyt and Rebecca Verbanck for photographs and artwork. A.R. was a Research Assistant of the Fund for Scientific Research (Flanders).
References
- Apel, K., Santel, J.-J., Redlinger, T.E., and Falk, H. (1980). The protochlorophyllide holochrome of barley (Hordeum vulgare L.): Isolation and characterization of the NADPH-protochlorophyllide oxidoreductase. Eur. J. Biochem. 111 251–258. [DOI] [PubMed] [Google Scholar]
- Beeckman, T., and Engler, G. (1994). An easy technique for the clearing of histochemically stained plant tissue. Plant Mol. Biol. Rep. 12 37–42. [Google Scholar]
- Bonetta, D., and McCourt, P. (1998). Genetic analysis of ABA signal transduction pathways. Trends Plant Sci. 3 231–235. [Google Scholar]
- Castle, L.A., and Meinke, D.W. (1994). A FUSCA gene of Arabidopsis encodes a novel protein essential for plant development. Plant Cell 6 25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory, J., and Li, J. (1997). Gibberellins, brassinosteroids and light-regulated development. Plant Cell Environ. 20 801–806. [Google Scholar]
- Chory, J., and Peto, C.A. (1990). Mutations in the DET1 gene affect cell-type-specific expression of light-regulated genes and chloroplast development in Arabidopsis. Proc. Natl. Acad. Sci. USA 87 8776–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory, J., Peto, C., Feinbaum, R., Pratt, L., and Ausubel, F. (1989). Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell 58 991–999. [DOI] [PubMed] [Google Scholar]
- Chory, J., Chatterjee, M., Cook, R.K., Elich, T., Fankhauser, C., Li, J., Nagpal, P., Neff, M., Pepper, A., Poole, D., Reed, J., and Vitart, V. (1996). From seed germination to flowering, light controls plant development via the pigment phytochrome. Proc. Natl. Acad. Sci. USA 93 12066–12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone, K.C., Cocciolone, S.M., Moehlenkamp, C.A., Weber, T., Drummond, B.J., Tagliani, L.A., Bowen, B.A., and Perrot, G.H. (1993). Role of the regulatory gene pl in the photocontrol of maize anthocyanin pigmentation. Plant Cell 5 1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehesh, K., and Ryberg, M. (1985). The NADPH-protochlorophyllide oxidoreductase is the major protein constituent of prolamellar bodies in wheat (Triticum aestivum L.). Planta 164 396–399. [DOI] [PubMed] [Google Scholar]
- Giraudat, J., Hauge, B.M., Valon, C., Smalle, J., Parcy, F., and Goodman, H.M. (1992). Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning, B.E.S., and Steer, M.W. (1986). Plant Cell Biology—An Ultrastructural Approach. (Dublin: M.W. Steer).
- Hill, A., Nantel, A., Rock, C.D., and Quatrano, R.S. (1996). A conserved domain of the viviparous-1 gene product enhances the DNA binding activity of the bZIP protein EmBP-1 and other transcription factors. J. Biol. Chem. 271 3366–3374. [DOI] [PubMed] [Google Scholar]
- Hoecker, U., Vasil, I.K., and McCarty, D.R. (1995). Integrated control of seed maturation and germination programs by activator and repressor functions of Viviparous-1 of maize. Genes Dev. 9 2459–2469. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A. (1987). Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Rep. 5 387–405. [Google Scholar]
- Keith, K., Kraml, M., Dengler, N.G., and McCourt, P. (1994). fusca3: A heterochronic mutation affecting late embryo development in Arabidopsis. Plant Cell 6 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk, J.T.O., and Tilney-Bassett, R.A.E. (1978). The Plastids. (Amsterdam: Elsevier).
- Koornneef, M., Reuling, G., and Karssen, C.M. (1984). The isolation and characterization of abscisic acid–insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 61 377–383. [Google Scholar]
- Koornneef, M., Hanhart, C.J., Hilhorst, H.W.M., and Karssen, C.M. (1989). In vivo inhibition of seed development and reserve protein accumulation in recombinants of abscisic acid biosynthesis and responsiveness mutants in Arabidopsis thaliana. Plant Physiol. 90 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., Léon-Kloosterziel, K.M., Schwartz, S.H., and Zeevaart, J.A.D. (1998). The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol. Biochem. 36 83–89. [Google Scholar]
- Kwok, S.F., Piekos, B., Miséra, S., and Deng, X.-W. (1996). A complement of ten essential and pleiotropic Arabidopsis COP/DET/FUS genes is necessary for repression of photomorphogenesis in darkness. Plant Physiol. 110 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J., and Giraudat, J. (1998). Abscisic acid signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 199–222. [DOI] [PubMed] [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1991). Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can. J. Bot. 69 461–476. [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1992). Cotyledon cell development in Arabidopsis thaliana during reserve deposition. Can. J. Bot. 70 151–164. [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1996). The dynamics of seedling and cotyledon cell development in Arabidopsis thaliana during reserve mobilization. Int. J. Plant Sci. 157 280–295. [Google Scholar]
- Mayer, R., Raventos, D., and Chua, N.-H. (1996). det1, cop1, and cop9 mutations cause inappropriate expression of several gene sets. Plant Cell 8 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke, D.W., Franzmann, L.H., Nickle, T.C., and Yeung, E.C. (1994). Leafy cotyledon mutants in Arabidopsis. Plant Cell 6 1049–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miséra, S., Müller, A.J., Weiland-Heidecker, U., and Jürgens, G. (1994). The FUSCA genes of Arabidopsis: Negative regulators of light responses. Mol. Gen. Genet. 244 242–252. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15 473–497. [Google Scholar]
- Nambara, E., Naito, S., and McCourt, P. (1992). A mutant of Arabidopsis which is defective in seed development and storage protein accumulation is a new abi3 allele. Plant J. 2 435–441. [Google Scholar]
- Nambara, E., Keith, K., McCourt, P., and Naito, S. (1994). Isolation of an internal deletion mutant of the Arabidopsis thaliana ABI3 gene. Plant Cell Physiol. 35 509–513. [PubMed] [Google Scholar]
- Nambara, E., Keith, K., McCourt, P., and Naito, S. (1995). A regulatory role for the ABI3 gene in the establishment of embryo maturation in Arabidopsis thaliana. Development 121 629–636. [Google Scholar]
- Ooms, J.J.J., Léon-Kloosterziel, K.M., Bartels, D., Koornneef, M., and Karssen, C.M. (1993). Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana. A comparative study using abscisic acid–insensitive abi3 mutants. Plant Physiol. 102 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy, F., and Giraudat, J. (1997). Interactions between ABI1 and the ectopically expressed ABI3 genes in controlling abscisic acid responses in Arabidopsis vegetative tissues. Plant J. 11 693–702. [DOI] [PubMed] [Google Scholar]
- Parcy, F., Valon, C., Raynal, M., Gaubier-Comella, P., Delseny, M., and Giraudat, J. (1994). Regulation of gene expression programs during Arabidopsis seed development: Roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6 1567–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy, F., Valon, C., Kohara, A., Miséra, S., and Giraudat, J. (1997). The ABSCISIC ACID–INSENSITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell 9 1265–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper, A.E., and Chory, J. (1997). Extragenic suppressors of the Arabidopsis det1 mutant identify elements of flowering-time and light-response regulatory pathways. Genetics 145 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper, A., Delaney, T., Washburn, T., Poole, D., and Chory, J. (1994). DET1, a negative regulator of light-mediated development and gene expression in Arabidopsis, encodes a novel nuclear-localized protein. Cell 78 109–116. [DOI] [PubMed] [Google Scholar]
- Quaedvlieg, N., Dockx, J., Rook, F., Weisbeek, P., and Smeekens, S. (1995). The homeobox gene ATH1 of Arabidopsis is derepressed in the photomorphogenic mutants cop1 and det1. Plant Cell 7 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, C.K., and Hill, S.A. (1999). Altered resource allocation during seed development in Arabidopsis caused by the abi3 mutation. Plant Cell Environ. 22 117–123. [Google Scholar]
- Rohde, A., Van Montagu, M., and Boerjan, W. (1999). The ABSCISIC ACID–INSENSITIVE 3 (ABI3) gene is expressed during vegetative quiescence processes in Arabidopsis. Plant Cell Environ. 22 261–270. [Google Scholar]
- Ryberg, M., and Sundqvist, C. (1982). Characterization of prolamellar bodies and prothylakoids fractionated from wheat etioplasts. Physiol. Plant. 56 125–132. [Google Scholar]
- Schultz, T.F., Medina, J., Hill, A., and Quatrano, R.S. (1998). 14-3-3 proteins are part of an abscisic acid–VIVIPAROUS1 (VP1) response complex on the Em promoter and interact with VP1 and EmBP1. Plant Cell 10 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling, U., Franck, F., van Cleve, B., Frick, G., Apel, K., and Armstrong, G.A. (1998). Etioplast differentiation in Arabidopsis: Both PORA and PORB restore the prolamellar body and photoactive protochlorophyllide-F655 to the cop1 photomorphogenetic mutant. Plant Cell 10 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L., Doxsee, R.A., Harel, E., and Tobin, E.M. (1993). CA-1, a novel phosphoprotein, interacts with the promoter of the cab140 gene in Arabidopsis and is undetectable in det1 mutant seedlings. Plant Cell 5 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist, C., and Dahlin, C. (1997). With chlorophyll pigments from prolamellar bodies to light-harvesting complexes. Plant Physiol. 100 748–759. [Google Scholar]
- Suzuki, M., Kao, C.Y., and McCarty, D.R. (1997). The conserved B3 domain of VIVIPAROUS1 has a cooperative DNA binding activity. Plant Cell 9 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnim, A., and Deng, X.-W. (1996). Light control of seedling development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 215–243. [DOI] [PubMed] [Google Scholar]
- Weatherwax, S.C., Ong, M.S., Degenhardt, J., Bray, E.A., and Tobin, E.M. (1996). The interaction of light and abscisic acid in the regulation of plant gene expression. Plant Physiol. 111 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, M.A.L., Yee, K.M., Danao, J., Zimmerman, J.L., Fischer, R.L., Goldberg, R.B., and Harada, J.J. (1994). LEAFY COTYLEDON1 is an essential regulator of late embryogenesis and cotyledon identity in Arabidopsis. Plant Cell 6 1731–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, S.A., Weatherwax, S.C., Bray, E.A., and Tobin, E.M. (1994). A gene which is negatively regulated by phytochrome action in Lemna gibba can also be positively regulated by abscisic acid. Plant Physiol. 105 949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]