Abstract
Flowering plants possess specialized extracellular matrices in the female organs of the flower that support pollen tube growth and sperm cell transfer along the transmitting tract of the gynoecium. Transport of the pollen tube cell and the sperm cells involves a cell adhesion and migration event in species such as lily that possess a transmitting tract epidermis in the stigma, style, and ovary. A bioassay for adhesion was used to isolate from the lily stigma/stylar exudate the components that are responsible for in vivo pollen tube adhesion. At least two stylar components are necessary for adhesion: a large molecule and a small (9 kD) protein. In combination, the two molecules induced adhesion of pollen tubes to an artificial stylar matrix in vitro. The 9-kD protein was purified, and its corresponding cDNA was cloned. This molecule shares some similarity with plant lipid transfer proteins. Immunolocalization data support its role in facilitating adhesion of pollen tubes to the stylar transmitting tract epidermis.
INTRODUCTION
There are a number of examples in plants in which cells produce highly modified walls across which cell to cell communication can occur without the benefit of cytoplasmic connections such as plasmodesmata. The best-described system is that of self-incompatibility in the angiosperms, in which glycoproteins localized to the extracellular matrices of the stigma and stylar transmitting tract cells are responsible for recognition and rejection of self-pollen. In compatible pollination, we have proposed that adhesion between the pollen tube and the stigma and/or stylar transmitting tract is an essential part of this interaction (Sanders and Lord, 1989). Our hypothesis has been that the adhesion event in vivo is partly responsible for the fast and guided progress of the tube cells to the ovary (Lord et al., 1996).
The extracellular matrix of the transmitting tract in the gynoecium includes various materials that induce adhesion, hydration, germination, and growth and guide the pollen tube (Cheung, 1996; Taylor and Hepler, 1997; Luu et al., 1999). In the style, this adhesion event could provide the physical connection between the pollen tube cell and the transmitting tract cells that enables signaling between the two different cell types. In lily, which has a hollow style, the transmitting tract is a secreting epidermis that lines the canal leading from the stigma to the ovary. The pollen tubes adhere to and are guided by these secreting cells in their passage to the ovules. In lily, the rate of pollen tube growth is much faster in vivo than in vitro (Jauh and Lord, 1995). To date, no in vitro germination medium allows for the tube growth rates, morphology, or behavior of the in vivo system for any species. We have proposed that the missing element is the extracellular matrix of the stylar transmitting tract, which is a matrix of bound substances that must be isolated for proper in vitro behavior of pollen tube cells (Jauh et al., 1997).
Much has been written about the ultrastructure and biochemistry of the style, but no mention had been made of the adhesive nature of the transmitting tract or its secretions until recently. This is probably due to the fact that chemical fixation can remove or disrupt the adhesive components, and the result is that pollen tubes appear to grow in a fluid matrix separated from the transmitting tract cells of hollow styles like those of lily. When we used fresh cryosections, fixation methods specifically designed to preserve adhesives (Jauh and Lord, 1995, 1996), or high-pressure frozen/freeze-substituted transmission electron microscopy preparations of lily and Arabidopsis (Roy et al., 1997; Lennon et al., 1998), we saw a fibrillar extracellular matrix component that appears to fuse the tube cell to the transmitting tract and to other pollen tubes. The in vivo–grown pollen tubes in lily form a mat of adhered cells, which is like a tissue (Jauh and Lord, 1996). Such adhesion between pollen tubes does not occur in vitro. When in vivo–grown pollen tubes are removed from the lily style and processed with rhodamine phalloidin to observe the filamentous actin (F-actin) microfilaments, star-shaped clusters of F-actin, similar to focal adhesions in moving animal cells (Burridge and Chrzanowska-Wodnicka, 1996), are observed (Jauh and Lord, 1995). These configurations were first described in lily by Pierson et al. (1986) and are not detectable in pollen tubes grown in vitro. The adhesion events, the faster growth rates of pollen tubes, and the F-actin configurations seen only in in vivo–grown lily pollen tubes all suggest a stylar influence on pollination in lily, perhaps mediated by cell–cell adhesion.
Recently, several laboratories have isolated molecules from pollen tubes and styles that either have adhesive properties, such as the transmitting tract–specific arabinogalactan protein (Cheung et al., 1995), or show some similarity to known adhesive compounds, as do the extensin-like PEX proteins in maize pollen (Rubinstein et al., 1995). Moreover, Wilhelmi and Preuss (1996) have described sterile mutants of Arabidopsis that show a lack of pollen tube adhesion to the ovule. In our model for cell adhesion and movement in pollination, adherence of the transmitting tract extracellular matrix to the pollen tube wall provides additional traction for tube cell movement. These adhesion sites could also allow for increased polymerization of F-actin in the tube cell, which might be involved in the motive force for tube cell movement. We developed an adhesion assay for plant cells by binding the stylar transmitting tract extracellular matrix from lily to nitrocellulose membranes (Jauh et al., 1997). This provided an in vitro artificial stylar matrix that can be used to perform functional adhesion assays when in vitro–germinated pollen tubes are applied. We have shown that lily pollen tubes not only attach to this artificial matrix but also grow on it and at faster rates than in previous in vitro culture systems.
Here, we describe the use of this adhesion assay to isolate two fractions from the stigma/stylar transmitting tract extracellular matrices that are necessary for adhesion: one contains a small protein, and the other contains a large molecule of unknown identity. Neither of these molecules alone is adhesive; the combination of the two is necessary to induce pollen tube adhesion to other pollen tubes and to the in vitro stylar matrix. We have purified the small protein and isolated its corresponding cDNA clone. Analysis of the partial protein sequence, its cDNA, and the deduced amino acid sequence indicate that this protein is similar to lipid transfer proteins (LTPs) in plants.
RESULTS
Lily Pollen Tubes Adhere to Epidermal Cells of the Transmitting Tract in the Style in Vivo and Can Adhere in Vitro to an Artificial Stylar Matrix
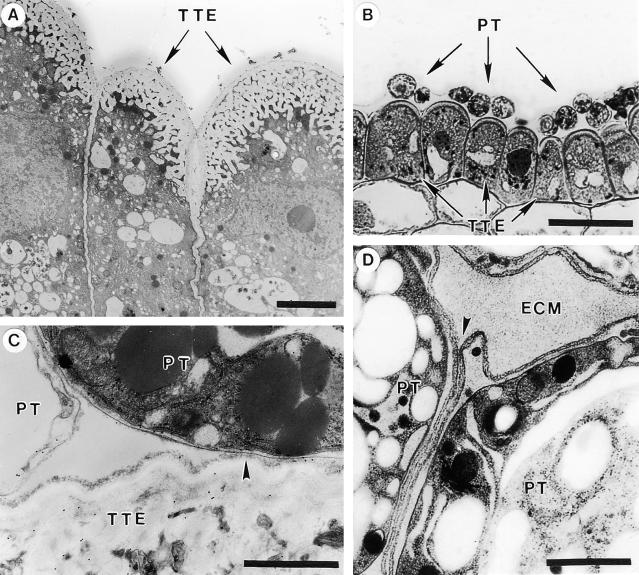
The stigma of lily is composed of multicellular papillae that are continuous with the secretory epidermis of the stylar canal in this open-style system. In vivo, pollen tubes germinate on the secretory epidermis of the stigma and enter the style by tracking the epidermal surface beneath the cuticle. Figure 1A shows the transmitting tract epidermal cells that line the canal of the open style in lily with their thick transfer cell–like outer walls overlaid by a thinner outermost wall layer. The pollen tubes closely adhered to this cell surface, as shown in Figure 1B. Figure 1C reveals that when ruthenium red was used in the fixative, a fibrous surface layer was detected on the outermost surface of the transmitting tract epidermal cells. The adhesion of the pollen tube to this layer was evident by using transmission electron microscopy. When tissue was prepared using high-pressure freezing followed by freeze substitution, as illustrated in Figure 1D, pollen tubes adhered tightly to each other, and an extensive stylar extracellular matrix or exudate was preserved surrounding them.
Figure 1.
Lily Transmitting Tract and Pollen Tube Adhesion in Vivo.
(A) and (C) show chemical fixation, (B) shows a cryofixed section, and (D) shows a section prepared by high-pressure frozen/freeze substitution.
(A) Cross-section of transmitting tract epidermal cells. Note the transfer cell–like outer walls.
(B) Cross-section of pollinated style stained with toluidine blue O. Note adhesion of pollen tubes to transmitting tract epidermal cells.
(C) Pollen tube adhesion to transmitting tract epidermal cell and another pollen tube. Ruthenium red was added to the fixative. Note fibrillar extracellular matrix retained at the juncture of the tube cell wall and transmitting tract epidermal cell wall (arrowhead).
(D) Stylar extracellular matrix embedded between adhering pollen tubes. Note the tube cell wall adhesion (arrowhead). This amount of stylar extracellular matrix was retained only in high-pressure frozen/freeze substituted preparations.
ECM, extracellular matrix; PT, pollen tube; TTE, transmitting tract epidermis.  ;
; 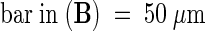 ;
; 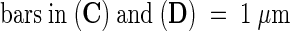 .
.
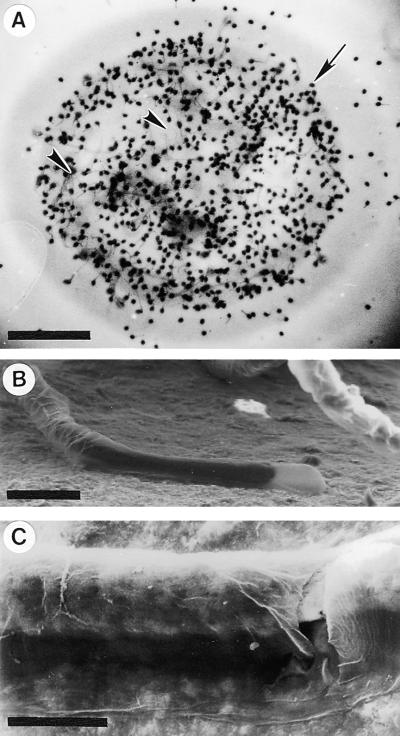
It is this stylar exudate that can be isolated from dissected styles and used in an adhesion assay. Figure 2 shows that when nitrocellulose membranes were coated with either the stylar exudate (Figure 2A) or petal homogenate (Figures 2B and 2C) in combination with a small molecule fraction from the stigma or stylar exudate, they became adhesive matrices when germinated lily pollen tubes were applied in liquid growth medium. Staining the adhered pollen grains was necessary to quantify the adhesion (Figure 2A). Pollen tubes adhered to the coated nitrocellulose membrane within an hour or two; adhesion occurred at the tube tip region (Figures 2B and 2C), with the rest of the tube detached from the membrane and floating in the medium. In vivo–grown pollen tubes also adhered tightly to the transmitting tract epidermis at their tips, and the remainder of the tube cell typically was released from the stylar matrix but remained bound to other tube cells surrounding it. Use of germinated tobacco pollen in this assay did not result in adhesion (data not shown).
Figure 2.
Views of the Adhesion Assay Showing Lily Pollen Tubes Adhered to an in Vitro Stylar Matrix Bound to Nitrocellulose Membrane.
(A) Toluidine blue O staining. The 2-hr in vitro–germinated pollen tubes (arrowheads) adhered to the in vitro matrix made from stylar exudate; the incubation time was 5 hr. Pollen grains (arrow) were stained with toluidine blue O to quantify the adhesion assay.
(B) and (C) Scanning electron microscope images of pollen tubes adhered to the in vitro matrix made from petal homogenate and filtered stigma exudate. (B) shows pollen tube tip adhesion. (C) shows the area of tube wall tightly adhered to the matrix.
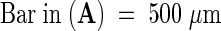 ;
; 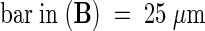 ;
;  .
.
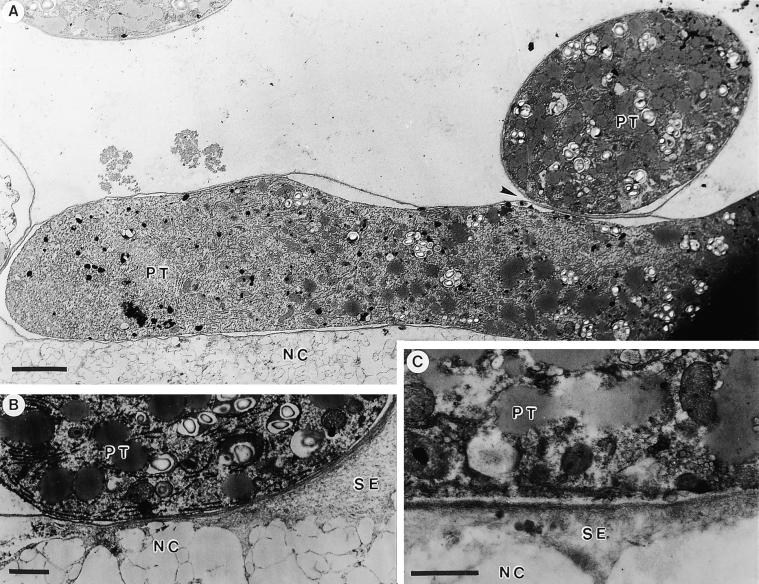
Figures 3A to 3C show transmission electron microscopy preparations of the pollen tube adhesion assay displaying the close adhesion of the tube cell wall to the nitrocellulose membrane impregnated with the stylar exudate. When pollen tubes came in contact with each other in this assay system, they adhered to each other as they do in vivo. A partial in vivo assay was also developed in which styles were dissected to expose a 4-cm-long area of transmitting tract epidermal cell surfaces, and ∼1800 hydrated pollen grains in germination medium were applied. In 5 hr, ∼550 pollen tubes had adhered to and grown on the stylar epidermal cells of the transmitting tract. This adhesion withstood centrifugation at 600g for 2 min (data not shown).
Figure 3.
Transmitting Electron Microscope Images of Adhesion Assay.
(A) Pollen tube adhesion to the stylar extracellular matrix and to other pollen tubes (arrowhead).
(B) and (C) Pollen tube adhesion to the nitrocellulose membrane surface impregnated with stylar exudate.
NC, nitrocellulose membrane; PT, pollen tube; SE, stylar exudate.  ;
;  .
.
At Least Two Stylar Molecules Are Required for in Vitro Pollen Tube Adhesion
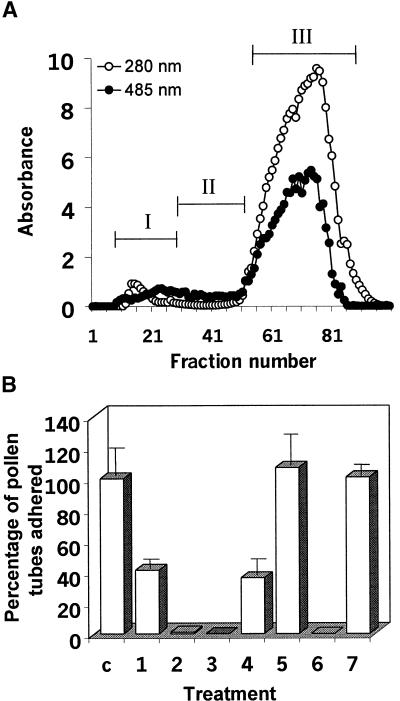
To identify the components of the stylar matrix that facilitate pollen tube adherence, we size-fractionated the stylar exudate, which contains ∼9.7% (w/w) protein, using a Sephadex G200 column. Figure 4A shows how we pooled fractions into three size groups based on the distribution of proteins and carbohydrates. Figure 4B shows the adhesion activity of these three groups and combined fractions. Group I (large molecules) showed adhesion activity by itself but only half that of the starting material. Groups II and III (small molecules) showed no adhesion on their own. A combination of groups I and III recovered the adhesion activity obtained with the initial material. SDS-PAGE analysis later showed that some group III molecules were present in the group I fraction (data not shown), thereby explaining the adhesion activity detected when group I was assayed on its own. Analysis of the fractions suggested that at least two molecules were involved in the adhesion event in the style, one a small molecule (<30 kD; group III) and the other a larger molecule (>100 kD; group I).
Figure 4.
Protein (280 nm) and Carbohydrate (485 nm) Profiles of the Stylar Exudate Fractions and Adhesion Assay Using Groups of Fractions.
(A) Fractionation. Stylar exudate was fractionated over a Sephadex G200 column, and the total fractions were combined into three groups: group I (fractions 8 to 30), group II (fractions 31 to 52), and group III (fractions 53 to 100).
(B) Adhesion assays. The groups were used for adhesion assays separately or in combinations of equivalent amounts. For each assay, 75 μg (dry weight) of total material was used. The adhesion percentage was obtained by comparison with the control stylar exudate, which contained 200 to 300 adhered pollen tubes. c, control; 1, group I; 2, group II; 3, group III; 4, groups I and II; 5, groups I and III; 6, groups II and III; 7, groups I to III.
Table 1 displays the steps taken to isolate the small molecule by use of the adhesion assay. The large molecule from stylar exudate may be somewhat generic because it could be replaced by homogenates of other tissues such as leaf or petal. The homogenates of leaf and petal showed no adhesion activity alone. Adhesion activity occurred only when the small molecules (group III) were combined with homogenate of leaf (data not shown) or petal. Petal homogenate was preferred to the leaf homogenate because it lacked the chlorophyll contamination that obscured the visualization of the pollen tubes when leaf extracts were used in the assay. Petal homogenate was used as a source of the large molecule for adhesion assays performed during the purification steps of the group III molecule. These assays required a larger amount of petal homogenate than stylar exudate (Table 1). Proteinase K treatment of group III molecules greatly decreased the adhesion activity in assays performed using petal homogenate (data not shown) or a citrate-soluble stylar extract, indicating the involvement of protein (Table 2).
Table 1.
Adhesion Assays Demonstrating Purification of the Group III Small Molecule (Lipid Transfer–like Protein)
| Fraction | No. of Pollen Tubes Adhereda |
|---|---|
| Stylar exudate | 246 ± 14 |
| PHb | 7 ± 1 |
| Stylar group III fraction + PH | 552 ± 6 |
| Stigma exudate | 3 ± 1 |
| Stigma exudate + PH | 271 ± 35 |
| Cation exchange–unbound stigma exudate + PH |
15 ± 3 |
| Cation exchange–bound stigma exudate + PH |
209 ± 13 |
| 9-kD proteinc eluted from SDS-PAGE + PH |
202 ± 48 |
Numbers of pollen tubes adhered are the mean of at least two experiments.
PH, petal homogenate (500 μg).
Cation exchange–bound molecules were separated by SDS-PAGE, and the 9-kD protein was recovered by electroelution.
Table 2.
Adhesion Assays Using a Combination of the Lily Lipid Transfer–like Protein (Group III, Small Molecule) and the Citrate-Soluble Stylar Extract (Group I, Large Molecule)
| Fraction | No. of Pollen Tubes Adhereda |
|---|---|
| CSEb + BSA | 26 ± 14 |
| CSE + lipid transfer–like proteinc | 283 ± 34 |
| CSE + lipid transfer–like protein treated with proteinase K |
17 ± 6 |
| CSE + lipid transfer–like protein eluted from SDS-PAGEd |
257 ± 11 |
Numbers of pollen tubes adhered are the mean of at least two experiments.
Citrate-soluble stylar extract.
Cation exchange–bound stigma exudate.
Cation exchange–bound molecules were separated by SDS-PAGE, and the lipid transfer–like protein was recovered by electroelution. Twenty micrograms of protein was used in combination with 75 μg (dry weight) of citrate-soluble stylar extract for the adhesion assays.
A Lipid Transfer–like Protein Is One of the Molecules Required for in Vitro Pollen Tube Adhesion
Filtered exudate collected from the stigma substituted in the adhesion assay for the stylar exudate group III molecules (Table 1). Because the stigma exudate had a simpler protein profile in the <30-kD size range relative to the stylar exudate, the group III molecule was purified from the stigma exudate by using a cation exchange column. The NaCl-eluted fraction from the cation exchange column showed adhesion activity when it was combined with petal homogenate, and the materials that did not bind to the exchange column were not adhesive (Table 1). The major protein of the NaCl-eluted fraction was a 9-kD protein, as revealed by SDS-PAGE separation (Figure 5A). Two-dimensional PAGE was performed using the cation exchanger–bound materials. The 9-kD protein was a major spot on the gel, with only a trace amount of small peptides observed on the front line of the gel when a large amount of material (10 μg) was loaded, as shown in Figure 5B. When this 9-kD protein was eluted from the SDS gel and combined with the petal homogenate or a citrate-soluble stylar extract, each fraction of which had no adhesion activity alone, the mixtures showed adhesion activity (Tables 1 and 2).
Figure 5.
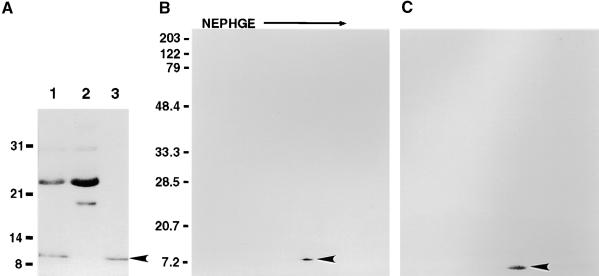
Cation Exchanger Purification of 9-kD Protein.
(A) SDS gel stained with Coomassie blue. The gel shows partial purification of a 9-kD protein from stigma exudate. Lane 1 contains 5 μg of stigma exudate protein; lane 2, CM–Sephadex cation exchange–unbound protein (5 μg of protein); and lane 3, 2 M NaCl eluate of cation exchanger (1 μg of protein). The arrowhead indicates the position of the 9-kD protein. Molecular mass standards (in kilodaltons) are shown at left.
(B) Two-dimensional gel of stigma exudate cation exchange–bound protein stained with Coomassie blue. Three micrograms of protein was loaded on the first-dimension gel, nonequilibrium pH gel electrophoresis (NEPHGE), and the second-dimensional separation (SDS-PAGE) was done on a 12 to 15% gradient gel. The arrowhead indicates the 9-kD protein. Molecular mass standards (in kilodaltons) are shown at left.
(C) Immunoblot of gel shown in (B), using polyclonal antibodies to lily lipid transfer–like protein (arrowhead).
We raised polyclonal antibodies against the lily 9-kD protein in chicken and used it for immunoblots. Figure 5C revealed that the 9-kD protein spot was recognized by our antibody. Microsequencing of the 9-kD protein demonstrated that the N-terminal amino acid sequence (35 residues) had a high degree of similarity to LTPs. In addition, the 9-kD protein was recognized by polyclonal antibodies raised against a lipid transfer–like protein of tomato (gift of E.M. Bray, University of California, Riverside; data not shown). The SDS-PAGE gel seen in Figure 6A shows that this 9-kD protein was present in the group III molecule fraction as well as in the stigma and stylar exudates. We demonstrate in Figure 6B by using our lily polyclonal antibodies that this 9-kD protein is a lily lipid transfer–like protein.
Figure 6.
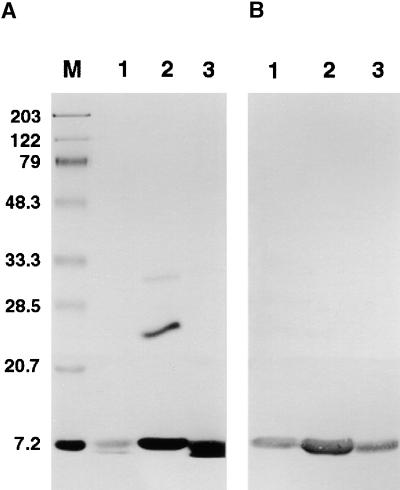
Immunoblot Analysis of Proteins from Stigma and Style Exudates.
(A) Proteins were separated on a 13% acrylamide gel and stained with Coomassie blue. Lane 1 contains stylar exudate group III protein of a Sephadex G200 column fraction (1 μg); lane 2, 100-kD centricon filtrate of stigma exudate (5 μg); and lane 3, stylar exudate (10 μg). Molecular mass standards (in kilodaltons) are shown at left and in lane M.
(B) Immunoblot of gel as given in (A) but with half the amount of protein in each lane. The 9-kD LTP was detected with polyclonal antibodies to lily lipid transfer–like protein.
A cDNA clone (650 bp) corresponding to the 9-kD protein was obtained by using a reverse transcription–polymerase chain reaction. The cDNA clone encoded a 9.45-kD polypeptide with an isoelectric point of 8.71. The deduced amino acid sequence matched that determined for the purified protein by microsequencing. The deduced sequence of the lily 9-kD protein had identity with LTPs from rice (47.2%), maize (54.3%), and Arabidopsis (55.3%) but less identity (22.9%) with a lily tapetum–specific LTP (Crossley et al., 1995). The sequence, as displayed in Figure 7, contained a typical hydrophobic signal peptide at the N terminus and eight cysteine residues that are conserved in known LTPs from plants.
Figure 7.
Amino Acid Sequence Alignments of Lily 9-kD Protein with Plant LTPs.
The deduced amino acid sequence (GenBank accession number AF171094) was aligned with rice (GenBank accession number AF017361), maize (GenBank accession number M57249), and Arabidopsis (Swiss-Prot accession number Q42589) LTPs. The amino acids determined by microsequencing of the lily 9-kD protein are underlined. The arrowhead indicates the N terminus of the mature lily 9-kD protein. Conserved cysteine residues are in boldface. Identical amino acids are indicated by colons, and spaces introduced to maximize alignment are indicated by dashes.
The Lipid Transfer–like Protein Localized to the Transmitting Tract Epidermis of the Style and to in Vivo but Not to in Vitro–Grown Pollen Tubes
Immunogold labeling of lily tissue, as shown in Figures 8A to 8C, demonstrated the presence of the lipid transfer–like protein in the stylar transmitting tract epidermis, to which pollen tubes adhere, in the stylar extracellular matrix, and in the in vivo–grown pollen tubes. The lily lipid transfer–like protein was not detected by protein gel blotting of in vitro–grown pollen tubes but was detected in 24-hr-old in vivo–grown pollen tubes removed from the style and in in vitro–grown pollen tubes exposed to the purified lily lipid transfer–like protein, as shown in Figure 8D.
Figure 8.
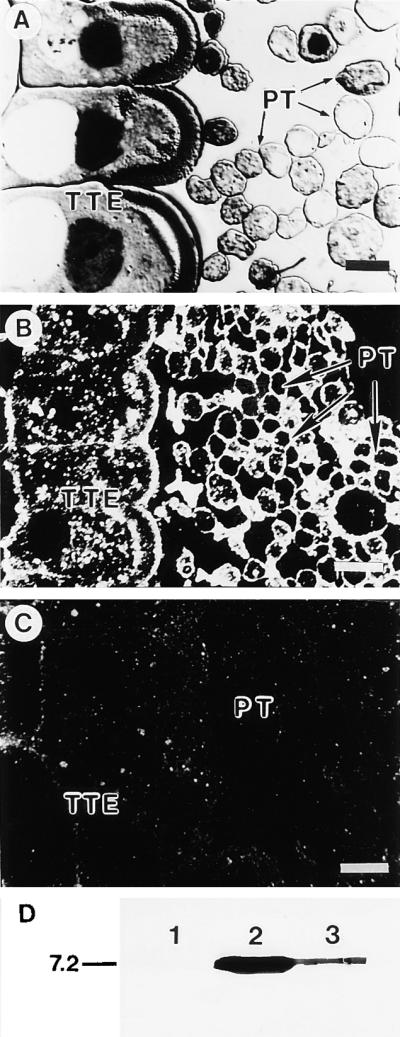
Immunogold Labeling of Pollinated Lily Style Tissue and Protein Gel Blot of Pollen Proteins.
(A) Section of lily pollinated style stained with toluidine blue O.
(B) Dark-field immunogold localization of protein in pollinated style using antibodies against lily lipid transfer–like protein.
(C) Control preimmune serum.
(D) Protein gel blot of protein extracted from in vivo and in vitro pollen tubes using antibodies to lily lipid transfer–like protein. All lanes contain 15 μg of protein. Lanes 1 and 2 show protein extracted from in vitro–grown pollen tubes. Pollen tubes were cultured for 2 hr in germination medium and continued to grow for another 5 hr without (lane 1) or with (lane 2) the addition of cation exchange–bound purified lily lipid transfer–like protein added to the germination medium. Lane 3 shows protein extracted from in vivo–grown pollen tubes removed from the style 24 hr after pollination. The molecular mass standard (in kilodaltons) is at left.
PT, pollen tube; TTE, transmitting tract epidermis. 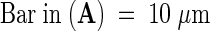 ;
; 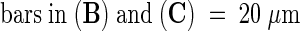 .
.
The Group I Fraction, Also Required for in Vitro Pollen Tube Adhesion, Is Enriched in Carbohydrates
The group I large molecule fraction involved in adhesion was detected in a citrate-soluble extract isolated from dissected and washed styles after a phenol–acetic acid–water treatment and acetone precipitation. The citrate-soluble stylar extract accounted for only 3% (w/w) of the total material precipitated by acetone. Table 2 shows that this fraction was not adhesive alone but was adhesive when used in combination with the lily lipid transfer–like protein. Although the phenol–acetic acid–water treatment normally extracts mostly noncovalently bound wall proteins, most of the protein remained in the citrate-insoluble material. The citrate-soluble extract was composed of 18% (w/w) protein, and analysis of monosaccharide composition revealed the presence of three major carbohydrates: glucose (29.9 mole %), galacturonic acid (20.3 mole %), and arabinose (17.6 mole %). The uronic acids galacturonic acid and glucuronic acid represented one-quarter of the total carbohydrate content.
DISCUSSION
Adhesion and Guidance in Pollination
Whether or not guidance mechanisms exist for pollen tubes in the gynoecium remains controversial (Malho, 1998; Lush, 1999). Our previous work on adhesion and guidance during pollination demonstrated a very restricted path of pollen tube growth on the secretory matrix that covers the epidermis in the open style and is localized between cells in closed styles (Lord et al., 1996; Lennon et al., 1998). Adhesion of the pollen tube to the transmitting tract epidermal cells may facilitate the effective movement of the pollen tube and sperm cells to the ovary, as shown in our model for lily pollen tube/stylar interaction in Figure 9. Instead of postulating a chemical ionic gradient such as that of Ca2+ guiding pollen tubes in the style, we suggest that there is a substrate adhesion molecule in the transmitting tract that can induce a contact-stimulated guidance, a haptotactic rather than a chemotactic phenomenon that need not involve a gradient (Lord and Sanders, 1992). Recent work has revealed that two extracellular matrix molecules, laminin and netrin, are involved in tracking neurons (Hedgecock and Norris, 1997). This is a two-component system involving a large, somewhat generic extracellular matrix molecule, laminin, and a small secreted protein, netrin, which together define the pathway that guides neuron outgrowth. Rather than the predicted gradient of a diffusible molecule being involved, the secreted soluble netrin is incorporated into a stable matrix and simply defines the path of neuron growth. Netrin is an adhesive molecule that interacts both with laminin in the extracellular matrix and with surface receptors on the neuron. The two-component adhesion system in lily pollination that we describe here may be operating in a similar fashion to guide pollen tubes to the ovules.
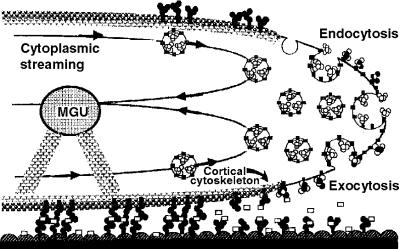
Figure 9.
Model for the Role of Cell Adhesion Molecules in Pollen Tube Growth in the Lily Style.
The pollen tube tip is illustrated as it progresses along the stylar transmitting tract epidermis. The cell wall of the pollen tube is thin at the tip, where vesicles fuse, contributing esterified pectins to the tube cell wall. The male germ unit (MGU) is composed of the tube cell nucleus and the generative cell (or two sperm). The putative adhesion molecules secreted from the style (open squares) may act between the pollen tube cell wall and the stylar transmitting tract epidermis. Solid squares denote membrane-bound arabinogalactan protein, solid circles indicate unesterified pectins, and open circles represent esterified pectins. (Reproduced with permission from Lord et al. [1996].)
Whether the lipid transfer–like protein we describe in this study is acting directly as an adhesin between a receptor in the pollen tube wall/plasma membrane and the stylar extracellular matrix (Figure 9) or is behaving as a signal molecule that induces adhesion indirectly has not been resolved. The experiment using in vitro–grown pollen tube cultures, to which the purified lipid transfer–like protein was added, showed that the pollen tubes could take up and/or bind the added lipid transfer–like molecule. These experiments also demonstrated that in vitro–grown pollen itself did not contain detectable levels of the lipid transfer–like protein. Recent work on the LTPs of Arabidopsis using RNA hybridization showed no signals in the pollen (Clark and Bohnert, 1999).
Plant LTPs Are Multifunctional Molecules
Plant LTPs have been studied for more than two decades in research on molecules that transfer lipids from one organellar membrane to another (Kader, 1997). They are often called nonspecific LTPs because they have a broad affinity for various lipids. Several LTPs have been shown to bind strongly to fatty acids in vitro, and the three-dimensional structure has been reported for maize (Shin et al., 1995), wheat (Gincel et al., 1994; Sodano et al., 1997), rice (Lee et al., 1998), and barley LTPs (Heineman et al., 1996). Nevertheless, there is no evidence that plant LTPs bind lipids in vivo.
It has been shown that plant LTPs are synthesized as precursor molecules with a signal peptide (Bernhard et al., 1991). Thus, LTPs are secreted proteins that reside in plant extracellular matrices (Molina et al., 1993; Thoma et al., 1994; Garcia-Carrido et al., 1998; Song et al., 1998). The expression pattern for LTPs in plant tissues displays a wide range in a variety of different organs and tissue types. Roles proposed for plant LTPs include participation in cutin biosynthesis, antimicrobial activity, symbiosis, embryogenesis, and adaptation to various stresses. These proposed roles are based on localization patterns and some experimental work (Kader, 1997).
LTPs are thought to belong to a group of plant antimicrobial peptides, including thionins and defensins, that act as a primitive defense system with analogs common to all multicellular organisms (Broekaert et al., 1997). There are theories that innate immunity in both plants and animals evolved from a common ancestor (Baker et al., 1997; Hoffmann et al., 1999). The presence of antimicrobial peptides in the stigma and style of lily is not surprising given the number of defense-related compounds that reside in these tissue types (Gasser, 1991). In addition, self-incompatible pollination and host–pathogen responses are thought to operate via parallel mechanisms (Hodgkin et al., 1988). Whether LTPs carry lipids in vivo is still debated, but the antimicrobial function is now well established for several species both in vitro and in vivo. For example, transgenic tobacco constitutively expressing barley LTP shows reduced disease symptoms (Broekaert et al., 1997). Perhaps antimicrobial LTP in lily was recruited evolutionarily as a pollen tube adhesion and guidance molecule. Adhesion activity could be a secondary function, the primary one being antimicrobial behavior. Recent work on defensins, which are antimicrobial compounds in animals and plants with features similar to LTPs, has demonstrated that these molecules can also act as chemotactic molecules with a role in signaling (Yang et al., 1999).
Wall Molecules Involved in Adhesion
No adhesion molecules in the plant extracellular matrix are currently known. It is assumed that pectins in the middle lamella are responsible for cell–cell attachment throughout the plant, but this assumption has not been tested experimentally (Fry, 1986; Knox, 1992). Typically, in plants, adhesion results when two daughter cells fail to separate, but there are cases in which two cells can actively come together and adhere. Pollen/stigma adhesion has been demonstrated in several cases, and arabinogalactan proteins have been implicated. Adhesion-defective mutants for pollen/stigma interaction have been isolated, but the adhesive molecules involved have not been identified (Zinkl et al., 1998; Luu et al., 1999). Carpel fusion requires adhesion (Walker, 1975), as do all postgenital organ fusions. Several mutants have been isolated that show adhesion where it normally does not occur, as in the epidermis (Becraft et al., 1996; Sinha and Lynch, 1998; Lolle and Pruitt, 1999). Thus, it appears that several kinds of adhesion molecules may occur in plants.
A large collection of molecules called adhesins in bacteria and fungi is responsible for adhesion of microbes to receptors in plant or animal tissues (Matthysse, 1996; Cormack et al., 1999). Some of these interactions are specific and under regulatory control (Foster and Hook, 1998). It is likely that similar interactions occur in the pollen tube/stigma/stylar interaction, but the necessary adhesion molecules have not been identified. The only reference to the presence of an LTP correlated with an adhesion event in plants is that of Stranger et al. (1999), who describe an angiosperm parasite that secretes an LTP when its haustorium binds to the host.
The lily lipid transfer–like protein induced adhesion of the pollen tube to an artificial stylar matrix in vitro and was localized to the pollen tube wall and transmitting tract in vivo. This small protein appears to act as an adhesive agent between the pollen tube wall and a larger molecule in the stylar transmitting tract epidermis much as does a recently described yeast adhesin, which is a protein possessing a carbohydrate ligand in the host (Cormack et al., 1999). If so, this lipid transfer–like protein in the lily style may be the first such adhesion molecule to be described for plants. Alternatively, the lipid transfer–like molecule may function in adhesion indirectly, even as a carrier of lipophilic compounds that act as signal molecules. Variation in epidermal cell wall lipid composition is correlated with adhesion in several Arabidopsis mutants, as is permeability (Lolle and Pruitt, 1999). Moreover, recent studies of the stigma of several species implicate certain lipids in directional pollen tube growth (Wolters-Arts et al., 1998). Purified lipid transfer–like protein is not adhesive by itself, but pollen tubes adhere to an artificial matrix in vitro when this protein is combined with a fraction containing a larger molecule (Table 2). Identification of the larger stylar molecule that is also required for adhesion will allow us to better understand the mode of action of the lily lipid transfer–like pollen tube adhesion protein.
METHODS
Plant Material
Lily (Lilium longiflorum cv Nellie White) was grown in the greenhouse, and flowers were collected 2 to 3 days after anthesis. Anthers were used directly for in vitro and/or semi–in vivo pollen tube germination or stored at −80°C. Stigmas and styles were separated from the gynoecium before collecting exudates.
Stigma and Stylar Exudate Preparation
The stylar exudate was extracted from longitudinally bisected styles with 84 mM citric acid, pH 3.0, and 2 mM Na2S2O5 for 2 to 3 hr on ice (1 mL per style) and filtered through nylon mesh to remove styles. The stylar exudate was centrifuged at 6000g for 10 min. The supernatant was concentrated, dialyzed using Spectra/Pore membrane (molecular weight cutoff of 3.5 kD; Spectrum Laboratories, Inc., Laguna Hills, CA) against distilled water, and lyophilized.
The stigma exudate was extracted from excised stigmas in 1 mM sodium phosphate buffer, pH 5.5, containing 1 mM DTT, and 1 mM Na2S2O5 in ice for 2 to 3 hr. Stigmas were removed by filtration on nylon mesh, and the filtrate was centrifuged at 12,000g for 15 min at 4°C. The supernatant was filtered through Centricon Plus columns (molecular weight cutoff of 100 kD; Millipore Corporation, Bedford, MA) to eliminate viscous and large molecules. The filtrate was then concentrated and dialyzed against distilled water before lyophilization.
Size Fractionation of the Stylar Exudate
The stylar exudate was resuspended in distilled water and applied to Sephadex G-200 chromatography column (120 × 1 cm). The eluted fractions (1.5 mL) were assayed for protein (280 nm) and carbohydrate (485 nm, using the phenol–sulfuric acid assay). The fractions were separated into three groups, I (large molecules) to III (small molecules).
Purification, Microsequencing, and Antiserum Preparation of Group III Molecules
The lyophilized stigma exudate was resuspended in 50 mM sodium phosphate buffer, pH 5.5, and mixed with a CM Sephadex C-25 cation exchanger (Sigma) prepared in the same buffer. The equilibrium slurry was washed with 5 volumes of 50 mM sodium phosphate buffer, pH 5.5, to remove the unbound materials. Bound materials were desorbed with 2 M NaCl, dialyzed exhaustively against distilled water, and freeze-dried. Purified samples were analyzed by SDS-PAGE and two-dimensional PAGE. In two-dimensional PAGE, isoelectric focusing was replaced with nonequilibrium pH gel electrophoresis for the first dimension (O'Farrell et al., 1977). The protein sample was prepared in a lysis buffer containing 2% ampholines, pH 3.0 to 10.0, applied to the acidic end of the gel, and electrophoresed for 100 min at 400 V. The size separation was performed by SDS-PAGE on a 12 to 15% gradient gel. Proteins were visualized by staining with Coomassie Brilliant Blue R 250.
For microsequencing, 3 μg of the cation exchange–bound sample was separated by SDS-PAGE (13%) and transferred to polyvinyl difluoride membrane (Millipore Corporation). After Coomassie Brilliant Blue R 250 staining, a total of three 9-kD protein bands were excised from the membrane and microsequenced at the University of California Riverside Micro-Sequencing Facility. For antiserum preparation, the cation exchange–bound stigma exudate was separated by using SDS-PAGE (13% gel). The protein band at 9 kD was excised, and the protein was collected by electroelution. Polyclonal antibodies were raised in chickens.
Protein Extraction from in Vitro– and in Vivo–Grown Pollen Tubes
Pollen grains from three anthers were germinated at room temperature in vitro in germination medium containing 1.27 mM CaCl2, 0.162 mM H3BO3, 0.99 mM KNO3, and 10% sucrose, pH 5.2, with agitation until the length of the majority of pollen tubes was five times the pollen grain diameter (∼2 hr). The germinated pollen was divided into two Petri dishes. Ten micrograms of cation exchange column–purified lipid transfer–like protein was added to 3 mL of germination medium containing the germinated pollen grains. The control consisted of germination medium only. After 5 hr of culture, pollen tubes were collected by filtration on nylon mesh and washed several times with germination medium to eliminate the free protein. In vivo pollen tubes were collected from the style after 24 hr of pollination, as described previously (Jauh and Lord, 1995). The in vivo– and in vitro–grown pollen tubes were homogenized in PBS, pH 7.2, and the supernatant was collected after centrifugation at 12,000g for 5 min at room temperature. After a protein assay (Bio-Rad, Richmond, CA), the sample was mixed with 2 × SDS buffer.
Immunoblot Analysis
Protein samples were separated on SDS–polyacrylamide gels and transferred to nitrocellulose membrane by electroblotting. The membranes were blocked with 5% nonfat dry milk and incubated with antiserum raised against the lily lipid transfer–like protein. The antibody was detected with an alkaline phosphatase–conjugated secondary antibody.
Isolation of the cDNA Encoding the Group III Molecule
Total RNA was prepared from styles of 5-cm-long flower buds (Maniatis et al., 1982), and 5 μg of total RNA was used in a 50-μL reverse transcription reaction with the Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). For polymerase chain reaction, 1 μL of the reverse transcription reaction was used as a template. The cDNA was amplified by using an oligo(dT) primer possessing an EcoRI site and a degenerate primer based on the N-terminal peptide determined by microsequencing. The primers used for reverse transcription–polymerase chain reaction were 5′-CGAATTCCTTTTTTTTTTTT-3′ and 5′-GGYGTNATHCCNCCRGGNTGYTG-3′, where Y is C or T; H is A, T, or C; R is A or G; and N is A, G, C, or T. This amplification generated a partial cDNA of 480 bp (pLInsLTP-3). To isolate the 5′ end of the lily lipid transfer–like protein cDNA, a poly (G)-tail was added to the 3′ end of the first-strand cDNA by using dGTP and terminal deoxynucleotide transferase (Promega). The N terminus of the cDNA was amplified from poly (G)–tailed cDNA by using an oligo(dC) primer (5′-CGGAATTCCCCCCCCCC-3′) and an internal primer (5′-GGAGATTTACCTCACCTTGTTGC-3′) designed from the lily lipid transfer–like clone pLInsLTP-3. Polymerase chain reaction products were cloned into pGEM-T Easy Vector (Promega) and sequenced by using an automatic sequencer (LI-COR, Lincoln, NE).
Isolation of Group I Molecules from Style Fragments
Longitudinally bisected styles previously washed with 1 mM sodium phosphate buffer, pH 5.5, containing 1 mM DTT and 1 mM Na2S2O5 were incubated with a phenol–acetic acid–water reagent composed of 2.5 parts 80% (w/w) phenol and 1 part acetic acid at 70°C for 30 min under agitation. The extract was cooled at room temperature and filtered through a GF/A glass fiber filter (Whatman, Maidstone, UK). Acetone (5 volumes) and 10% ammonium formate (1/20 volume) were added to the filtrate, which was stored overnight on ice. Materials precipitated from the extract were collected by centrifugation at 10,000g for 30 min. After washing three times with 80% acetone, the pellet was resuspended in 50 mM sodium citrate buffer, pH 5.5, for 4 hr on ice. After centrifugation (12,000g for 15 min), the citrate-soluble extract was filtered through a Centricon Plus column (molecular weight cutoff of 100 kD), and the retentate was washed three times with water and lyophilized.
Chemical Analysis of Citrate Buffer–Soluble Stylar Extract
Total carbohydrate, uronic acid, and protein contents were estimated by the phenol–sulfuric acid method (Dubois et al., 1956), the meta-hydroxybiphenyl method (Blumenkrantz and Asboe-Hansen, 1973), and the Bio-Rad protein assay, respectively.
Analysis of monosaccharide composition was performed by gas liquid chromatography of the trimethylsilyl derivatives, as in Komalavilas et al. (1991). Glycosides were separated with a gas chromatograph (model 5890; Hewlett Packard, Atlanta, GA) by using a fused silica capillary column with helium as carrier gas. The injector and detector were held at 250°C. The oven temperature started at 105°C, was increased immediately to 160°C at a rate of 10°C min−1, held for 2 min, and then was raised again at the rate of 2°C min−1 up to 220°C. Monosaccharides were identified by their retention times, with myoinositol as an internal standard. Response factors were determined using authentic monosaccharides.
Adhesion Assays
The in vitro assay was performed as previously described (Jauh et al., 1997), with several modifications. Materials were immobilized on a 28-mm2 region of nitrocellulose membrane by application of vacuum using a device modified from a dot blot system, and the membrane was air-dried. The dried nitrocellulose membranes with immobilized materials were submerged in a Petri dish containing 20 mL of germination medium with pollen tubes pregerminated in vitro (for 2 hr) from six anthers. Pollen tubes were evenly distributed over the membranes and incubated for 5 to 6 hr in the dark without any disturbance. The membranes were washed in germination medium to remove nonadhered pollen tubes and stained with Coomassie Brilliant Blue R 250. The pollen grains with tubes adhering to the membranes were counted under a dissecting microscope.
For the adhesion assay, 75 μg of style exudate, 20 μg of stigma exudates, and 10 or 20 μg of lily lipid transfer–like protein (group III, from style or stigma exudate) were used separately or in combination with 50, 75, or 100 μg (dry weight) of citrate-soluble stylar extract or with 500 μg (dry weight) of petal homogenate. In a previous study, we established the amount of exudate necessary to adhere the maximum number of pollen tubes (Jauh et al., 1997). The petal homogenate was obtained by grinding small fragments of petals in 1 mM sodium phosphate buffer, pH 5.5, containing 1 mM DTT and 1 mM Na2S2O5. Ground petals were filtered through nylon mesh (53 μm), and the filtrate was used for adhesion assays.
For proteinase K treatment, 100 μg of lipid transfer–like protein was treated with proteinase K (1% [w/v]) in 10 mM Tris-HCl, pH 7.5, at 32°C for 2 hr and then boiled for 15 min. This fraction was combined with the citrate-soluble stylar extract and applied to nitrocellulose membranes. For controls, we added BSA or inactivated proteinase K (previously boiled) instead of proteinase K to the fractions. In a separate experiment, we showed that the lipid transfer–like molecule is still active after boiling for 15 min, whereas proteinase K is denatured.
For the semi–in vivo adhesion assay, gynoecia were collected (1 to 2 days after anthesis), and the middle of the style was dissected to expose the transmitting tract epidermis. Pollen grains hydrated in germination medium were applied to the surface of the transmitting tract. After 5 hr of incubation, the dissected styles in germination medium were centrifuged at 600g to remove nonadhered pollen grains and tubes. The number of adhered pollen tubes on the transmitting tract was counted after staining the tissue with Coomassie Brilliant Blue R 250.
Light Microscopy and Transmission and Scanning Electron Microscopy
Gynoecial tissue was fixed and embedded in HistoResin glycol methacrylate (Leica, Inc., Heidelberg, Germany) for light microscopy or in Spurr's resin (Ted Pella, Inc., Redding, CA) for electron microscopy (Jauh and Lord, 1995). Sections stained with toluidine blue O were observed with the use of differential interference optics on a Nikon (Tokyo, Japan) Multiphoto microscope. Images were captured on Kodak (Rochester, NY) Technical Pan 2415 film. Immunogold labeling with silver enhancement was performed as previously described (Wang et al., 1996). PBS, with 1% globulin-free BSA and 0.1% Tween 20, was used as the initial blocking agent, as the washing agent, as diluent for the antibodies, and alone as a control in place of antibodies. The secondary antibody was donkey anti–chicken IgY conjugated to 18-nm gold (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). The primary and secondary antibodies were diluted 1:1000 and 1:40, respectively. Silver enhancement was performed with 1% gelatin. Sections were observed with the use of dark-field optics on a Zeiss Universal (Oberkochen, Germany) microscope. Images were captured on Kodak Tri-X film.
Preparation of adhesion assay samples for scanning electron microscopy observation was performed as in Jauh et al. (1997). Specimens were examined using a Philips (model XL30-FEG; FEI, Hillsboro, OR) microscope. Images were collected as digital files. Preparation of materials for transmission electron microscopy was as described by Jauh and Lord (1995), with or without ruthenium red in the fixative, or by high-pressure freezing methods as described by Roy et al. (1997).
Acknowledgments
Support for this work was provided by National Science Foundation Grant No. IBN-9603826 to E.M.L. The high-pressure freezing preparation was made in Peter Hepler's laboratory at the University of Massachusetts, Amherst, by Stephane Roy.
References
- Baker, B., Zambryski, P.L., Staskawicz, B., and Dinesh-Kumar, S.P. (1997). Signaling in plant–microbe interactions. Science 276 726–733. [DOI] [PubMed] [Google Scholar]
- Becraft, P.W., Stinard, P.S., and McCarty, D.R. (1996). CRINKLY4: A TNFR-like receptor kinase involved in maize epidermal differentiation. Science 273 1406–1409. [DOI] [PubMed] [Google Scholar]
- Bernhard, W.R., Thoma, S., Botella, J., and Somerville, C.R. (1991). Isolation of a cDNA clone for a spinach lipid transfer protein and evidence that the protein is synthesized by the secretory pathway. Plant Physiol. 95 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz, N., and Asboe-Hansen, G. (1973). New method for quantitative determination of uronic acids. Anal. Biochem. 54 484–489. [DOI] [PubMed] [Google Scholar]
- Broekaert, W.F., Cammue, B., De Bolle, M., Thevissen, K., and De Samblanx, G. (1997). Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 16 297–323. [Google Scholar]
- Burridge, K., and Chrzanowska-Wodnicka, M. (1996). Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12 463–519. [DOI] [PubMed] [Google Scholar]
- Cheung, A.Y. (1996). Pollen–pistil interactions during pollen-tube growth. Trends Plant Sci. 1 45–51. [Google Scholar]
- Cheung, A.Y., Wang, H., and Wu, H.-M. (1995). A floral transmitting tissue–specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82 383–393. [DOI] [PubMed] [Google Scholar]
- Clark, A.M., and Bohnert, H.J. (1999). Cell-specific expression of genes of the lipid transfer protein family from Arabidopsis thaliana. Plant Cell Physiol. 40 69–76. [DOI] [PubMed] [Google Scholar]
- Cormack, B.P., Ghori, N., and Falkow, S. (1999). An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285 578–582. [DOI] [PubMed] [Google Scholar]
- Crossley, S.J., Greenland, A.J., and Dickinson, H.G. (1995). The characterization of tapetum-specific cDNAs isolated from a Lilium henryi L. meiocyte subtractive cDNA library. Planta 196 523–529. [DOI] [PubMed] [Google Scholar]
- Dubois, M., Gilles, K.A., Hamilton, J.K., Robers, P.A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28 350–356. [Google Scholar]
- Foster, T.J., and Hook, M. (1998). Surface protein adhesions of Staphylococcus aureus. Trends Microbiol. 6 484–488. [DOI] [PubMed] [Google Scholar]
- Fry, S.C. (1986). Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu. Rev. Plant Physiol. 37 165–186. [Google Scholar]
- Garcia-Carrido, J.M., Menossi, M., Puigdomenech, P., Martinez-Izquierdo, J.A., and Delseny, M. (1998). Characterization of a gene encoding an abscisic acid–inducible type-2 lipid transfer protein from rice. FEBS Lett. 428 193–199. [DOI] [PubMed] [Google Scholar]
- Gasser, C.S. (1991). Molecular studies on the differentiation of floral organs. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42 621–649. [Google Scholar]
- Gincel, E., Sinmore, J.P., Caille, A., Marion, D., Ptak, M., and Vovelle, F. (1994). Three-dimensional structure of a wheat lipid-transfer protein from multidimensional H-NMR data. Eur. J. Biochem. 22 413–422. [DOI] [PubMed] [Google Scholar]
- Hedgecock, E.M., and Norris, C.R. (1997). Netrins evoke mixed reactions in motile cells. Trends Genet. 13 251–253. [DOI] [PubMed] [Google Scholar]
- Heineman, B., Anderson, K.V., Nielsen, P.R., Bech, L.M., and Poulsen, F.M. (1996). Structure in solution of a four-helix lipid binding protein. Protein Sci. 5 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, T., Lyon, G.D., and Dickinson, H.G. (1988). Recognition in flowering plants: A comparison of the Brassica self-incompatibility system and plant pathogen interactions. New Phytol. 110 557–569. [Google Scholar]
- Hoffmann, J.A., Kafatos, F.C., Janeway, C.A., Jr., and Ezekowitz, R.A.B. (1999). Phylogenetic perspectives in innate immunity. Science 284 1313–1317. [DOI] [PubMed] [Google Scholar]
- Jauh, G.Y., and Lord, E.M. (1995). Movement of the tube cell in the lily style in the absence of the pollen grain and the spent pollen tube. Sex. Plant Reprod. 8 168–172. [Google Scholar]
- Jauh, G.Y., and Lord, E.M. (1996). Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 199 251–261. [Google Scholar]
- Jauh, G.Y., Eckard, K.J., Nothnagel, E.A., and Lord, E.M. (1997). Adhesion of lily pollen tubes on an artificial matrix. Sex. Plant Reprod. 10 173–180. [Google Scholar]
- Kader, J.-C. (1997). Lipid-transfer proteins: A puzzling family of plant proteins. Trends Plant Sci. 2 66–70. [Google Scholar]
- Komalavilas, P., Zhu, J.-K., and Nothnagel, E.A. (1991). Arabinogalactan-proteins from the suspension culture medium and plasma membrane of rose cells. J. Biol. Chem. 266 15956–15965. [PubMed] [Google Scholar]
- Knox, J.P. (1992). Cell adhesion, cell separation and plant morphogenesis. Plant J. 2 137–141. [Google Scholar]
- Lee, J.Y., Min, K., Cha, H., Shin, D.H., Hwang, K.Y., and Suh, S.W. (1998). Rice non-specific lipid transfer protein: The 1.6 Å crystal structure in the unliganded state reveals a small hydrophobic cavity. J. Mol. Biol. 276 437–448. [DOI] [PubMed] [Google Scholar]
- Lennon, K.A., Roy, S., Hepler, P.K., and Lord, E.M. (1998). The structure of the transmitting tissue of Arabidopsis thaliana (L.) and the path of pollen tube growth. Sex. Plant Reprod. 11 49–59. [Google Scholar]
- Lolle, S.J., and Pruitt, R.E. (1999). Epidermal cell interactions: A case for local talk. Trends Plant Sci. 4 14–19. [DOI] [PubMed] [Google Scholar]
- Lord, E.M., and Sanders, L.C. (1992). Roles for the extracellular matrix in plant development and pollination: A special case of cell movement in plants. Dev. Biol. 153 16–28. [DOI] [PubMed] [Google Scholar]
- Lord, E.M., Walling, L.L., and Jauh, G.-Y. (1996). Cell adhesion in plants and its role in pollination. In Membranes: Specialized Functions in Plants, M. Smallwood, J.P. Knox, and D.J. Bowles, eds (Oxford, UK: Bios Scientific Publications), pp. 21–37.
- Lush, W.M. (1999). Whither chemotropism and pollen tube guidance? Plant Sci. 4 413–418. [DOI] [PubMed] [Google Scholar]
- Luu, D.-T., Marty-Mazars, D., Trick, M., Dumas, C., and Heizmann, P. (1999). Pollen–stigma adhesion in Brassica spp involves SLG and SLR1 glycoproteins. Plant Cell 11 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malho, R. (1998). Pollen tube guidance—The long and winding road. Sex. Plant Reprod. 11 242–244. [Google Scholar]
- Maniatis, T., Fritsch, E.F., and Sambrook, J. (1982). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Matthysse, A.G. (1996). Adhesion in the rhizosphere. In Bacterial Adhesion: Molecular and Ecological Diversity, M. Fletcher, ed (New York: Wiley-Liss), pp. 129–153.
- Molina, A., Segura, A., and Garcia-Olmedo, F. (1993). Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett. 316 119–122. [DOI] [PubMed] [Google Scholar]
- O'Farrell, P.Z., Goodman, H.M., and O'Farrell, P.H. (1977). High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell 12 1133–1142. [DOI] [PubMed] [Google Scholar]
- Pierson, E.S., Derksen, J., and Traas, J.A. (1986). Organization of microfilaments and microtubules in pollen tubes grown in vitro or in vivo in various angiosperms. Eur. J. Cell Biol. 41 14–18. [Google Scholar]
- Roy, S., Eckard, K.J., Lancelle, S., Hepler, P.K., and Lord, E.M. (1997). High-pressure freezing improves the ultrastructural preservation of in vivo–grown lily pollen tubes. Protoplasma 200 87–98. [Google Scholar]
- Rubinstein, A., Broadwater, A., Lowrey, K., and Bedinger, P. (1995). Pex1, a pollen-specific gene with an extensin-like domain. Proc. Natl. Acad. Sci. USA 92 3086–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, L.C., and Lord, E.M. (1989). Directed movement of latex particles in the gynoecia of three species of flowering plants. Science 243 1606–1608. [DOI] [PubMed] [Google Scholar]
- Shin, D.H., Lee, J.Y., Hwang, K.Y., Kim, K.K., and Suh, S.W. (1995). High-resolution crystal structure of the non-specific lipid-transfer protein from maize seedlings. Structure 3 189–199. [DOI] [PubMed] [Google Scholar]
- Sinha, N., and Lynch, M. (1998). Fused organs in the adherent1 mutation in maize show altered epidermal walls with no perturbations in tissue identities. Planta 206 184–195. [Google Scholar]
- Sodano, P., Caille, A., Sy, D., de Person, G., Marion, D., and Ptak, M. (1997). H-NMR and fluorescence studies of the complexation of DMPG by wheat non-specific lipid transfer protein. Global fold of the complex. FEBS Lett. 416 130–134. [DOI] [PubMed] [Google Scholar]
- Song, J.Y., Choi, D.-W., Lee, J.S., Kwon, Y.M., and Kim, S.-G. (1998). Cortical tissue–specific accumulation of the root-specific ns-LTP transcripts in the bean (Phaseolus vulgaris) seedlings. Plant Mol. Biol. 38 735–742. [DOI] [PubMed] [Google Scholar]
- Stranger, A., Corbett, J., Dunn, M., Totty, N., Sterling, A., and Bolwell, G.P. (1999). Identification of developmentally specific markers in germinating and haustorial stages of Striga hermonthica (Del.) Benth. seedlings. J. Exp. Bot. 50 269–275. [Google Scholar]
- Taylor, L.P., and Hepler, P.K. (1997). Pollen germination and tube growth. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 461–491. [DOI] [PubMed] [Google Scholar]
- Thoma, S., Hecht, U., Kippers, A., Botella, J., De Vries, S., and Sommerville, C. (1994). Tissue-specific expression of a gene encoding a cell wall–localized lipid transfer protein from Arabidopsis. Plant Physiol. 105 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, D.B. (1975). Postgenital carpel fusion in Catharanthus roseus. III. Fine structure of the epidermis during and after fusion. Protoplasma 86 43–63. [Google Scholar]
- Wang, J.-L., Walling, L.L., Jauh, G.Y., Gu, Y.-Q., and Lord, E.M. (1996). Lily cofactor-independent phosphoglycerate mutase: Purification, partial sequencing, and immunolocalization. Planta 200 343–352. [DOI] [PubMed] [Google Scholar]
- Wilhelmi, L.K., and Preuss, D. (1996). Self-sterility in Arabidopsis due to defective pollen tube guidance. Science 274 1535–1537. [DOI] [PubMed] [Google Scholar]
- Wolters-Arts, M., Lush, W.M., and Mariani, C. (1998). Lipids are required for directional pollen-tube growth. Nature 392 818–821. [DOI] [PubMed] [Google Scholar]
- Yang, D., Chertov, O., Bykovskaia, S.N., Chen, Q., Buffo, M.J., Shogan, J., Anderson, M., Schröder, J.M., Wang, J.M., Howard, O.Z., and Oppenheim, J.J. (1999). β-Defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286 525–530. [DOI] [PubMed] [Google Scholar]
- Zinkl, G.M., Wilhelmi, L.K., and Preuss, D. (1998). Sticking together: Cell adhesion interactions in Arabidopsis reproduction. J. Plant Res. 111 299–305. [Google Scholar]