Abstract
Glucocorticoid receptor (GR) and related steroid hormone receptors are ligand-dependent transcription factors whose regulation is critical for both homeostasis and diseases. The structural maturation of the GR has been shown to require the Hsp90 molecular chaperone complex. Evidence indicates that Hsp90-dependent maturation is critical for GR ligand binding capacity and activity. While the role for Hsp90 in GR function is well established, the regulation of this process is not well understood. Here we discuss a recent finding that identifies reversible protein acetylation controlled by the deacetylase HDAC6 as a novel mechanism that regulates Hsp90-dependent GR maturation. We will also speculate on the implications of this finding in steroid hormone signaling, oncogenic transformation and its potential therapeutic utility.
Introduction
Steroid hormone receptors mediate hormone signaling by acting as ligand dependent transcription factors. Upon ligand binding, these receptors undergo conformational changes that are coupled with specific interactions with transcriptional co-factors and/or nuclear translocation, culminating with induction of specific transcriptional programs that control diverse processes from homeostasis and growth, to reproduction, development and metabolism [Robinson-Rechavi et al., 2003; Gronemeyer et al., 2004] . Although ligand binding is the primary regulatory signal for receptor activation, selected members of the steroid hormone receptor family require additional cellular machinery to achieve a conformation capable of responding to ligand. Extensive biochemical reconstitution and functional analysis of glucocorticoid receptor (GR) have revealed an obligatory role for the molecular chaperone Hsp90 in GR structural maturation and function [Pratt et al., 1992; Pratt et al., 1992] . It has been proposed that GR is normally associated with the Hsp90 chaperone complex in the cytoplasm prior to ligand binding. This association allows GR to achieve a structural conformation that is competent for ligand binding, nuclear translocation and consequently, gene regulation. This view is supported by the observation that treating cells with the Hsp90 inhibitor geldanamycin can effectively suppress GR ligand binding and transcriptional activity [Tago et al., 2004] . However, the critical issue as to whether the Hsp90-GR functional interaction is regulated and what mechanisms are involved has not yet been addressed. Our recent characterization of Hsp90 acetylation may have provided an answer to this important question.
In a search for proteins that associate with the microtubule-associated cytoplasmic deacetylase HDAC6 [Hubbert et al., 2002] , we identified Hsp90 as a substrate for HDAC6. We found that HDAC6 can deacetylate Hsp90 both in vitro and in vivo. Inactivation of HDAC6 leads to the accumulation of acetylated Hsp90, which is accompanied by a failure of Hsp90 to form a stable complex with GR, or with the critical co-chaperone p23. This results in GR that is defective in ligand binding, nuclear translocation and transcriptional activity. These findings provide experimental evidence that the Hsp90-GR interaction and, consequently the structural maturation of GR are indeed subject to regulation by acetylation, and implicates HDAC6-mediated deacetylation of molecular chaperones as a potential novel mechanism that impinges on steroid hormone signaling [Kovacs et al., 2005] .
Hsp90 - more than a stress protein
For a number of years Hsp90 has been perceived as a stress-induced protein with a more general housekeeping function. A very different picture, however, has recently started to emerge. Hsp90 and its co-factors, termed co-chaperones, form molecular chaperone complexes that facilitate the structural maturation of its substrates, termed client proteins. The Hsp90-assisted maturation of client proteins often leads to an enhanced activity and stability for these client proteins [Pratt and Toft, 2003] . The characterization of Hsp90 client proteins led to a surprising realization that, instead of proteins with housekeeping functions, many Hsp90 client proteins are critical for cell signaling, including oncogenic kinases such as ErbB2 Akt, Raf and Bcr-Abl and the aforementioned glucocorticoid receptor [Pratt, 1997] . Supporting the importance of Hsp90 in cell signaling, Hsp90 inhibitors, such as geldanamycin, display potent biological effects, and can induce growth arrest and apoptosis in tumor cell lines [Blagosklonny et al., 2001] . The emerging significance of Hsp90 in both normal and oncogenic signaling highlights the need to understand how Hsp90 is regulated, The characterization of the HDAC6-Hsp90 interaction now identifies acetylation as one potential regulatory mechanism for Hsp90 activity.
Got acetylation? It’s not just for histones and chromatin anymore
Traditionally post-translational modification by acetylation has been linked to histone and chromatin-dependent processes. Recent evidence, however, strongly suggests that reversible acetylation can regulate important biological processes independent of chromatin [Cohen and Yao, 2004] . For example, the characterization of HDAC6 has implicated protein acetylation in the regulation of microtubule dynamics, growth factor-induced chemotaxis and the processing of misfolded protein aggregates [Hubbert et al., 2002; Haggarty et al., 2003] . Consistent with these apparently non-genomic functions, HDAC6 is mainly localized to the cytoplasm where it dynamically associates with cytoskeleton. Accordingly, identifying substrates for HDAC6 would be critical to decipher how HDACs might regulate non-chromatin related processes. In a search for such targets, we identified Hsp90 as a substrate for HDAC6 [Kovacs et al., 2005] . Although it remains unclear whether Hsp90 also serves as a critical mediator for the established functions of HDAC6 in cell motility and aggresome formation, acetylation of Hsp90, induced by inhibiting HDAC6 via specific siRNA or through the use of an inhibitor Trichostatin A (TSA), clearly reduces GR activity. This is particularly interesting as recent reports also found that TSA treatment inhibits glucocorticoid-mediated transcriptional activity [Ito et al., 2004] . This inhibition is, however, unexpected as TSA treatment often activates gene transcription nonspecifically by inducing histone hyperacetylation. Our results help to reconcile these observations and point to an effect of TSA on cytoplasmic HDAC6 rather than on histones or chromatin. In the presence of TSA, Hsp90 is unable to properly bind GR, thus affecting the ability of GR to illicit a transcriptional response. Importantly, these findings also indicate that Hsp90-mediated GR maturation is not simply a constitutive or housekeeping process; rather, it can be regulated through Hsp90 acetylation, providing another level of regulation in steroid hormone signaling.
Other nuclear receptors and client proteins?
The relationship that we have characterized between reversible acetylation and GR signaling begs a series of interesting questions. One prevalent question is whether or not other steroid hormone receptors are subjected to the same regulation. Evidence indicates that AR, ER, and PR [Fang et al., 1996; Knoblauch and Garabedian, 1999; Kosano et al., 1998] all form complexes with Hsp90. The Hsp90 inhibitor geldanamycin (GA) has been reported to inhibit the activity of AR, GR, and PR [Solit et al., 2002; Tago et al., 2004; Smith et al., 1995] . If these receptors indeed require Hsp90 for full activity, HDAC6 might turn out to be an important modulator for more than just one steroid hormone receptor. Similarly, given that several prominent Hsp90 client proteins are intimately linked to human cancer [Neckers and Neckers, 2005] , it would be of great interest to investigate whether Hsp90 acetylation is also important for the maturation and activity of these oncoproteins. The answer to these questions not only would determine whether Hsp90 acetylation plays a more general role in regulating chaperone activity toward a broad spectrum of client proteins, but also point to a potential cancer therapeutic approach through the suppression of Hsp90 deacetylation.
Regulation of Hsp90 acetylation and cell signaling
Hsp90 appears to become transiently acetylated upon GR activation after ligand stimulation. Although the exact nature of this ligand-induced acetylation remains to be characterized, it is tantalizing to speculate that acetylation might be coupled with the maturation of GR. For example, Hsp90 acetylation might allow the conversion of GR-Hsp90 from a stable complex into a dynamic one by dissociating p23 from the Hsp90 complex, thereby enabling GR to enter the nucleus for transcriptional activation. As acetylated Hsp90 appears to have reduced binding toward GR and p23, the subsequent deacetylation by HDAC6 would then allow Hsp90 to re-enter the productive chaperone complex (Figure 1). Interestingly, it appears that certain cellular stresses, such as those associated with the transformation from normal to oncogenic state, can also cause Hsp90 chaperone complex remodeling [Kamal et al., 2004] . We speculate that acetylation of molecular chaperones might play a role in the dynamic reorganization of chaperone complexes in response to such “oncogenic stress”. We suspect that oncogenic stress, which might be induced by high demand for “growth and proliferation”-associated signaling, may lead to a spike in the levels of acetylated Hsp90 due to an increase in Hsp90-client proteins during robust cellular growth. Under this scenario, HDAC6 may then be required for robust Hsp90 activity by regenerating deacetylated Hsp90 and thereby ensuring effective cell signaling. This model would also argue that HDAC6 might play a more important role for cells under specific stress conditions, such as oncogenic transformation induced by Hsp90 client proteins.
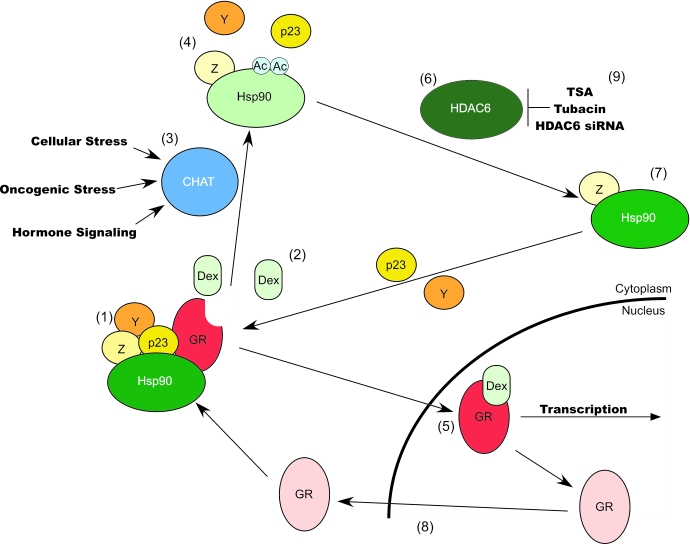
Figure 1. HDAC6-mediated reversible acetylation of Hsp90 and nuclear receptor signaling, a speculative model.
Reversible acetylation may regulate the recycling of Hsp90 in and out of functional chaperone complexes. The deacetylation of Hsp90 allows for the recruitment and binding of co-chaperones such as p23, and may allow for the recruitment of other co-chaperones (Y and Z) such as p50, Hop, immunophilins, etc (1). The formation of this chaperone complex facilitates folding of GR that is competent for ligand binding (red receptor), translocation into the nucleus and transcriptional activation. Ligand binding destabilizes the GR-Hsp90 chaperone complex interaction (2) and induces Hsp90 acetylation by the induction or activation of the Chaperone Acetyltransferase (CHAT; (3)). The CHAT may also be activated in response to other cellular stresses or “oncogenic stress”(3). Acetylated Hsp90, which has a reduced affinity for p23 and possibly for other co-chaperones (4), may further destabilize the GR-Hsp90 interaction, allowing for the nuclear translocation of GR and subsequent transcriptional activation (5). Note that binding of certain co-chaperones may be insensitive to the acetylation status of Hsp90 (Z). Acetylated Hsp90 is then deacetylated by HDAC6 (6), regenerating Hsp90 that is competent for co-chaperone(s) complex assembly (7), productive chaperone cycles, and associating with inactive GR (Pink) exported from the nucleus (8). Inactivation of HDAC6 by TSA, Tubacin or specific siRNA would inhibit the regeneration of deacetylated Hsp90 required for GR or selected client protein maturation (9).
Therapeutic implications
Steroid hormone receptors are critical for normal development and homeostasis. Over-expression or misregulation of receptors and their signaling pathways, however, can lead to a myriad of disease states. For example, aberrant glucocorticoid levels have been associated with muscle atrophy including disuse atrophy and cachexia, a disorder characterized by muscle wasting and malnutrition [Hasselgren, 1999] . Indeed, increased levels of GR signaling can induce muscle atrophy, possibly through activation of Foxo transcription factors and the atrophy-related ubiquitin ligases MaFbx and MuRF1, which are involved in the increase of cellular proteolysis associated with muscle atrophy [Bodine et al., 2001; Glass, 2003] . In the case of AR and ER, it is well documented that mutations and over-expression of AR are associated with prostate cancer [Rahman et al., 2004; Suzuki et al., 2003] while ER has been intimately linked to both the progression and metastasis of breast cancer [Osborne and Schiff, 2005] . If Hsp90 acetylation plays an important role in GR, AR and/or ER signaling, it is conceivable that pharmacological manipulation of Hsp90 acetylation could represent a critical therapeutic strategy to abrogate aberrant steroid receptor signaling. The development of selective high affinity inhibitors similar to tubacin [Haggarty et al., 2003] may allow for an attenuation of oncogenic signaling while allowing Hsp90 to manage house keeping cellular activities.
Conclusion
The dynamic Hsp90 acetylation in glucocorticoid receptor function has revealed another level of regulation in steroid hormone signaling. Many important questions are to be answered. For example, how does acetylation regulate Hsp90 function? Does acetylation affect the structure, conformation or ATPase activity of Hsp90? Recent evidence has come forth to suggest that acetylation of Hsp90 effects ATP binding [Murphy et al., 2005] , which could certainly affect Hsp90 activity. What is the identity of the Chaperone Acetyltransferase (which we have dubbed CHAT)? Is HDAC6 the only Hsp90 deacetylase? How is Hsp90 acetylation regulated by selective cell signaling? Is the entire population or only a selected pool of Hsp90 subject to the regulation by reversible acetylation? Are there other components of the chaperone complex regulated by reversible acetylation? By elucidating the functional relationship between acetylation and chaperone-mediated signaling in various cellular processes, we may go a long way towards understanding how we might use these mechanisms to our own advantage to battle everything from cancer to muscle atrophy. The dynamic Hsp90 acetylation in glucocorticoid receptor function has revealed another level of regulation in steroid hormone signaling. Many important questions are to be answered. For example, how does acetylation regulate Hsp90 function? Does acetylation affect the structure, conformation or ATPase activity of Hsp90? Recent evidence has come forth to suggest that acetylation of Hsp90 effects ATP binding [Murphy et al., 2005] , which could certainly affect Hsp90 activity. What is the identity of the Chaperone Acetyltransferase (which we have dubbed CHAT)? Is HDAC6 the only Hsp90 deacetylase? How is Hsp90 acetylation regulated by selective cell signaling? Is the entire population or only a selected pool of Hsp90 subject to the regulation by reversible acetylation? Are there other components of the chaperone complex regulated by reversible acetylation? By elucidating the functional relationship between acetylation and chaperone-mediated signaling in various cellular processes, we may go a long way towards understanding how we might use these mechanisms to our own advantage to battle everything from cancer to muscle atrophy.
Acknowledgments
This work is supported by the American Cancer Society to T.P.Y (RSG-03-147-01-CSM), who is a Leukemia and Lymphoma Society Scholar.
Abbreviations
- AR
Androgen Receptor
- ER
Estrogen Receptor
- GA
Geldanamycin
- GR
Glucocorticoid Receptor
- HDAC6
Histone Deacetylase 6
- Hsp90
Heat Shock Protein 90
- PR
Progesterone Receptor
- TSA
Trichostatin A
References
- Blagosklonny M. V., Fojo T., Bhalla K. N., Kim J. S., Trepel J. B., Figg W. D., Rivera Y., Neckers L. M. The Hsp90 inhibitor geldanamycin selectively sensitizes Bcr-Abl-expressing leukemia cells to cytotoxic chemotherapy. Leukemia. 2001;15:1537–43. doi: 10.1038/sj.leu.2402257. [DOI] [PubMed] [Google Scholar]
- Bodine S. C., Latres E., Baumhueter S., Lai V. K., Nunez L., Clarke B. A., Poueymirou W. T., Panaro F. J., Na E., Dharmarajan K., Pan Z. Q., Valenzuela D. M., DeChiara T. M., Stitt T. N., Yancopoulos G. D., Glass D. J. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Cohen T., Yao T. P. AcK-knowledge reversible acetylation. Sci STKE. 2004;2004 doi: 10.1126/stke.2452004pe42. [DOI] [PubMed] [Google Scholar]
- Fang Y., Fliss A. E., Robins D. M., Caplan A. J. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem. 1996;271:28697–702. doi: 10.1074/jbc.271.45.28697. [DOI] [PubMed] [Google Scholar]
- Glass D. J. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003;9:344–50. doi: 10.1016/s1471-4914(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Gustafsson J. A., Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–64. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- Haggarty S. J., Koeller K. M., Wong J. C., Grozinger C. M., Schreiber S. L. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003;100:4389–94. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselgren P. O. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care. 1999;2:201–5. doi: 10.1097/00075197-199905000-00002. [DOI] [PubMed] [Google Scholar]
- Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X. F., Yao T. P. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Ito K., Hanazawa T., Tomita K., Barnes P. J., Adcock I. M. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315:240–5. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Kamal A., Boehm M. F., Burrows F. J. Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol Med. 2004;10:283–90. doi: 10.1016/j.molmed.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch R., Garabedian M. J. Role for Hsp90-associated cochaperone p23 in estrogen receptor signal transduction. Mol Cell Biol. 1999;19:3748–59. doi: 10.1128/mcb.19.5.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosano H., Stensgard B., Charlesworth M. C., McMahon N., Toft D. The assembly of progesterone receptor-hsp90 complexes using purified proteins. J Biol Chem. 1998;273:32973–9. doi: 10.1074/jbc.273.49.32973. [DOI] [PubMed] [Google Scholar]
- Kovacs J. J., Murphy P. J., Gaillard S., Zhao X., Wu J. T., Nicchitta C. V., Yoshida M., Toft D. O., Pratt W. B., Yao T. P. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–7. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Murphy P. J., Morishima Y., Kovacs J. J., Yao T. P., Pratt W. B. Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J Biol Chem. 2005;280:33792–9. doi: 10.1074/jbc.M506997200. [DOI] [PubMed] [Google Scholar]
- Neckers L., Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics - an update. Expert Opin Emerg Drugs. 2005;10:137–49. doi: 10.1517/14728214.10.1.137. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol. 2005;23:1616–22. doi: 10.1200/JCO.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol Toxicol. 1997;37:297–326. doi: 10.1146/annurev.pharmtox.37.1.297. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Scherrer L. C., Hutchison K. A., Dalman F. C. A model of glucocorticoid receptor unfolding and stabilization by a heat shock protein complex. J Steroid Biochem Mol Biol. 1992;41:223–9. doi: 10.1016/0960-0760(92)90348-m. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Toft D. O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–33. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Rahman M., Miyamoto H., Chang C. Androgen receptor coregulators in prostate cancer: mechanisms and clinical implications. Clin Cancer Res. 2004;10:2208–19. doi: 10.1158/1078-0432.ccr-0746-3. [DOI] [PubMed] [Google Scholar]
- Robinson-Rechavi M., Escriva Garcia H., Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116:585–6. doi: 10.1242/jcs.00247. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Whitesell L., Nair S. C., Chen S., Prapapanich V., Rimerman R. A. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1995;15:6804–12. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solit D. B., Zheng F. F., Drobnjak M., Munster P. N., Higgins B., Verbel D., Heller G., Tong W., Cordon-Cardo C., Agus D. B., Scher H. I., Rosen N. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–93. [PubMed] [Google Scholar]
- Suzuki H., Ueda T., Ichikawa T., Ito H. Androgen receptor involvement in the progression of prostate cancer. Endocr Relat Cancer. 2003;10:209–16. doi: 10.1677/erc.0.0100209. [DOI] [PubMed] [Google Scholar]
- Tago K., Tsukahara F., Naruse M., Yoshioka T., Takano K. Hsp90 inhibitors attenuate effect of dexamethasone on activated NF-kappaB and AP-1. Life Sci. 2004;74:1981–92. doi: 10.1016/j.lfs.2003.07.056. [DOI] [PubMed] [Google Scholar]