Abstract
The involvement of Rho GTPases in signal transduction pathways leading to transcription activation is one of the major roles of this family of GTPases. Thus, the identification of transcription factors regulated by Rho GTPases and the understanding of the mechanisms of their activation and its biological outcome are of great interest. Here, we provide evidence that Rho GTPases modulate Stat5a, a transcription factor of the family of signal transducers and activators of transcription. RhoA triggers tyrosine phosphorylation (Y696) of Stat5a via a JAK2-dependent mechanism and promotes DNA-binding activity of Stat5a. Tyrosine phosphorylation of Stat5a is also stimulated physiologically by lysophosphatidic acid (LPA) in a Rho-dependent manner. Simultaneously, RhoA reduces serine phosphorylation of Stat5a at both serine residues S726 and S780, resulting in a further increase of activity as defined by mutagenesis experiments. Furthermore, serine dephosphorylation of Stat5a by RhoA does not take place by down-modulation of either JNK1, MEK1, or p38 MAP kinases, as determined by transfection experiments or chemical inhibition of both MEK1, p38, and JNK serine kinases. Thus, RhoA regulates Stat5a via tyrosine phosphorylation and via a yet to be determined novel down-modulating pathway that involves serine dephosphorylation. Finally, we provide evidence for a role of Stat5a in RhoA-induced epithelial-to-mesenchymal transition with concomitant increase in vimentin expression, E-cadherin down-regulation, and cell motility.
INTRODUCTION
Rho GTPases are a multimember family of molecular switches that belong to the Ras superfamily. Rho GTPases are involved in regulation of cellular functions such as cell cytoarchitecture and signal transduction, which relate to cell growth, development, apoptosis, tumorigenesis, and metastasis (Van Aelst and D'Souza-Schorey, 1997; Aznar and Lacal, 2001a,b; Bar-Sagi and Hall, 2000; Ridley, 2001). One of the most critical functions of Rho GTPases is the regulation of transcription through a variety of transcription factors (Van Aelst and D'Souza-Schorey, 1997; Aznar and Lacal, 2001a,b). Until recently, little has been known about the relationship between specific Rho-mediated regulation of transcription and cellular function. However, transcription factors modulated by Rho GTPases and the intracellular pathways that mediate their effects in the context of Rho GTPases are starting to be identified. Thus, some of the links between cell adhesion, motility, cell-cycle regulation, development, apoptosis, cytoskeletal rearrangements, transformation, and metastasis, with transcriptional regulation in the context of Rho, have been described (for review, see Aznar and Lacal, 2001b).
Rho GTPases activate nuclear factor (NF)-κB (Perona et al., 1997; Montaner et al., 1998, 1999), serum response factor (SRF) (Hill et al., 1995; Alberts et al., 1998; Montaner et al., 1999), and transcription factors (TFs) that depend on the JNK and p38 MAP kinase pathways. Substrates to these kinases include ELK, PEA3, ATF2, MEF2A (Marinissen et al., 2001), Max, and CHOP/GADD153 (Van Aelst and D'Souza-Schorey, 1997). However, the relationship of some of these TFs with Rho-induced cellular responses is complex. For instance, discrepancies have been described with respect to the activation of the SRF and its interdependence on Rho-mediated cytoskeletal rearrangements (Sahai et al., 1998; Zohar et al., 1998; Sotiropoulos et al., 1999).
The role of Rho GTPases in cellular transformation has attracted great attention in the past 5 years. The link between transcription with transformation, cell invasion, and metastasis is particularly interesting. Ever since it was shown that overexpression of either RhoA, Rac1, or Cdc42 transforms and promotes the metastatic phenotype of cultured 3T3 fibroblasts (Ballestero et al., 1991; Perona et al., 1993; del Peso et al., 1997), many studies have described the physiological relevance of Rho signaling and overexpression in human tumors (Clark et al., 2000; Mira et al., 2000; Van Golen et al., 2000a,b; Abraham et al., 2001; Kamai et al., 2001; Keely, 2001; Matsumoto et al., 2001; Takamura et al., 2001; Takemoto et al., 2001). Overexpression of Rho GTPases occurs in many types of human tumors, and RhoA and Rac2 constitute early markers for tumor progression of head and neck squamous cell cancer (Abraham et al., 2001). Accordingly, a role for RhoA in uPAR transcription and expression has been described (Muller et al., 2000). Activation of oncogenic RhoA by extracellular matrix signals such as laminin or fibronectin stimulates transcription of the uPAR gene promoter region and results in enhanced motility and invasiveness of cells (Bourdoulous et al., 1998). Which RhoA-modulated transcription factors are involved in this process is unknown, but the presence of AP-1– and NF-κB–responsive elements in the uPAR promoter points to a role of both TFs in this process (Dang et al., 1999; Wang et al., 2000). Furthermore, NF-κB has also been related to Rho GTPase–induced neoplastic transformation (Whitehead et al., 1999). Recently, Marinissen et al. (2001) described the role of two other transcription factors, ATF2 and MEF2A, in RhoA-mediated transformation via transcription of the c-jun gene. Furthermore, Rho GTPases promote the transcription and expression of cyclin D1, which is directly related to cell cycle entry and enables aberrant cell growth of tumoral cells (Westwick et al., 1997; Danen et al., 2000; Welsh et al., 2001). Additional transcriptional regulatory effects and transcription factors modulated by Rho GTPases have been described and thoroughly reviewed (Van Aelst and D'Souza-Schorey, 1997; Aznar and Lacal, 2001a,b; Charron et al., 2001; Delarue et al., 2001).
We have recently reported that oncogenic RhoA and Cdc42 activate the transcription factor Stat3 in human HEK cells and other cell lines (Aznar et al., 2001). RhoA induces both tyrosine and serine phosphorylation of Stat3 by JAK2- and JNK1-dependent pathways, respectively. Furthermore, Stat3 is essential for RhoA-mediated neoplastic transformation, because two dominant negative Stat3 mutants completely reverted Rho-induced anchorage–independent growth of human cells (Aznar et al., 2001). Other reports have related Stat3 with Rac1, and although the serine phosphorylation pathway appears to be SEK-1–dependent, the mechanism of Rac1-induced tyrosine phosphorylation is not yet fully understood (Schuringa et al., 2000; Simon et al., 2000; Faruqi et al., 2001; Schuringa et al., 2001).
Stat transcription factors constitute a large family of latent cytoplasmic transcription factors implicated in ligand-dependent growth stimulation or differentiation and in antiproliferative effects (Darnell, 1997). Seven mammalian Stat genes have been identified thus far. Here, we have investigated the effect of Rho GTPases on the regulation of Stat5. Two separately encoded Stat5 transcription factors, Stat5a and Stat5b, coexist (Grimley et al., 1999). Although Stat5 was initially discovered as a prolactin-stimulated ovine mammary gland factor (Gouilleux et al., 1994), it has become evident that a large number of different cytokines, growth factors, and oncogenes promote tyrosine phosphorylation and transcriptional activation of Stat5a and Stat5b (Grimley et al., 1999). Activation of Stat5 follows a paradigm common to all Stat proteins, in which phosphorylation of a single C-terminal tyrosine residue promotes Stat oligomerization via their SH2 domains, nuclear migration, and DNA binding to specific elements. These include primarily cytokines of class I and class II superfamilies, which couple to receptors that lack intrinsic tyrosine kinase activity and therefore utilize a cytoplasmic tyrosine kinase to phosphorylate Stat5a/b. The main family of cytoplasmic tyrosine kinases that mediate this process is the Janus kinase (JAK) family (Shuai et al., 1992; Stahl et al., 1995; Darnell, 1997). In addition, growth factor–stimulated receptor tyrosine kinases (RTKs) such as the insulin receptor, epidermal growth factor receptor, and platelet-derived growth factor receptor promote tyrosine phosphorylation of Stat5a on ligand binding, which might be JAK-independent (Davud et al., 1996; Chen et al., 1997; Valgeirsdottir et al., 1998). As with Stat3, Src family kinases have been also reported to directly phosphorylate and activate Stat5 (Yu et al., 1997).
In addition to tyrosine phosphorylation, serine phosphorylation is another frequent mechanism by which Stat activity is modulated (Wen et al., 1995; Wen and Darnell, 1997; Decker and Kovarik, 2000). Although serine phosphorylation of Stat1 and Stat3 positively modulates their transcriptional activity (Wen et al., 1995), the functional and biological implications of Stat5a/b serine phosphorylation appear to be more complex. Whereas two different Stat5a serine residues (S726 and 780) are susceptible to phosphorylation, only one has been found in Stat5b (S730) (Yamashita et al., 1998, 2002). Also, the impact of Stat5a/b serine phosphorylation on transcriptional activity might depend greatly on the cell type used as well as the DNA element chosen (Yamashita et al., 1998; Park et al., 2001). In this sense, phosphorylation of both Ser726 and Ser780 of Stat5a and Ser730 of Stat5b negatively regulate Stat5a/b transactivation in prolactin stimulation of the mammary gland (Yamashita et al., 1998). Subsequent bursts of glucocorticoids inhibit serine phosphorylation, eliminating its inhibitory effect, which translates into increased Stat5a/b activity and milk production (Beadling et al., 1996). Conversely, growth hormone (GH) stimulation of a GH-responsive luciferase reporter is positively dependent on Ser730 of Stat5b and Ser780 but not Ser726 of Stat5a (Yamashita et al., 2001). Finally, Stat5a/b transcriptional activity can be also regulated by interaction with other nuclear proteins (Grimley et al., 1999, Groner et al., 2000).
Here, we demonstrate that oncogenic RhoA induces activation of Stat5a by a mechanism that involves both tyrosine phosphorylation and reduction of serine phosphorylation. Also, we identify LPA as a physiological stimulus that induces tyrosine phosphorylation of Stat5a via RhoA. With respect to the biological effect of RhoA, we also show that Stat5a is necessary for the epithelial-to-mesenchymal transition (EMT) induced by oncogenic RhoA. Thus, Rho GTPases may modulate Stat5a in a manner that integrates other cell type–dependent signals and that differs mechanistically from that involved in Rho GTPase–induced Stat3 activation.
MATERIALS AND METHODS
Cell Culture, Transfections, and Chemical Inhibitors
Madin–Darby canine kidney (MDCK) epithelial cells, Chinese hamster ovary 4 cells (CHO4), and human mammary carcinoma cells (MCF-7) were cultured in DMEM supplemented with 10% fetal bovine serum and 1 mM glutamine. For transient expression assays, 2 × 105 cells were transfected in 33-mm dishes by the Lipofectamine Plus method as described by the manufacturer (Invitrogen, Life Technologies, San Diego, CA). The amount of plasmidic DNA was kept constant at 3–5 μg per 33-mm plate with the corresponding empty vector, and 0.5 μg of reporter was transfected in all experiments. Stable cell lines were generated by transfection as described above and selection with appropriate antibiotics. For MDCK-RhoAQL–expressing cells, eight independent clones were selected and maintained in 500 μg/ml G418 (Sigma, St. Louis, MO). For Stat5DN and Stat5awt stable cell lines, MDCKs-RhoAQL were transfected with 2.0 μg of Stat5 expression vectors together with 0.2 μg of pHygro, and selection was performed with 500 μg/ml G418 and 150 μg of Hygromycin (Sigma). Four independent clones were selected for each Stat5a vector. P38 inhibitor SB203580 (Calbiochem, La Jolla, CA) and MEK1 inhibitor PD98059 (Calbiochem) were used at a final concentration of 20 and 50 μM, respectively. LPA was purchased from Sigma and was used at a concentration of 50 μM at the indicated times.
Plasmids
PCDNAIIIB plasmid (Invitrogen) and derived expression vectors encoding for constitutively activated RhoA (QL), Rac1 (QL), and Cdc42Hs (QL) proteins have been described (Aznar et al., 2001). The 1× Sp1GLECAT contains the Stat5-responsive sequence of the human Sp2.1 promoter inserted into a pBLCAT5-derived plasmid. Expression vector for dominant negative JAK2 (pRk-JAK2-KE) and wild-type JAK2 were kind gifts from Dr. I. M. Kerr. Stat5a, Stat5b, Stat5a S726A, Stat5a S780A, and Stat5a S726/780A were generated as described (Yamashita et al., 1998,2001).
Gene Expression Analysis
MDCK, MCF-7, or CHO cells (n = 2 × 105) were transfected with the indicated plasmids. At 24–36 h after transfection, protein extracts were prepared by lysis with the commercially available Reporter lysis buffer (Promega, Madison, WI). The total amount of protein was determined with a commercial kit based on the Bradford method (Bio-Rad, Hercules, CA). Protein (2–4 μg) was assayed for chloramphenicol acetyl transferase (CAT) activity by use of a xylene-based method as described (Aznar et al., 2001). Total counts (cpm) were detected with a 1214 RackBeta Liquid scintillation counter (WALLAC, Turku, Finland) and normalized by micrograms of protein. Transfection efficiencies were corrected by detection of the expressed proteins by Western immunoblotting.
Western Blot Assays and Antibodies
For protein expression assays, cells were transfected with the corresponding plasmids and incubated in DMEM 0.5 or 10% FBS where indicated for the next 24–36 h. The lysis was performed in Reporter lysis buffer (Promega) containing 200 μM orthovanadate, 50 mM NaF, 20 μg/ml leupeptin, 20 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride. Thirty micrograms of total protein was analyzed by SDS electrophoresis on 10% polyacrylamide gels (Bio-Rad). After transfer of proteins to Immobilon-P PVDF membrane (Millipore, Bedford, MA), the blots were incubated with the corresponding antibodies. Immunocomplexes were visualized by enhanced chemiluminescence detection (Amersham Biosciences, Arlington Heights, IL) with either an anti-rabbit or anti-mouse antibody conjugated to peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). α-Stat5 monoclonal antibody was purchased from Transduction Laboratories (Lexington, KY), phospho-Stat5a/b (Tyr 694/699) and phospho-Stat5a/b (Ser 726/731) were purchased from Upstate Biotechnology (Lake Placid, NY), and all were used as indicated by the manufacturer. Rabbit antiserum against Stat5a-Ser780 was generated as described (Yamashita et al., 2001). For gel supershift analysis, anti-Stat5a was purchased from Santa Cruz Biotechnology. Mouse monoclonal anti-phospho-p44/42 MAP kinase (Thr202/Tyr204) and anti-phospho p38 were purchased from New England Biolabs (Beverly, MA). JAK2-, RhoA-, and vimentin-specific polyclonal antibodies were obtained from Santa Cruz Biotechnology.
Electrophoretic Mobility Shift Assays
For electrophoretic mobility shift assay (EMSA) assays, cells were transfected with the corresponding plasmids and incubated in DMEM–0.5% FBS for 36 h. Nuclear extracts were obtained as described (Perona et al., 1997). Nuclear protein was measured with a commercial kit based on the Bradford method (Bio-Rad). Two micrograms of nuclear protein was then incubated with 0.1 ng of hProGLE probe (5000 cpm) or with unlabeled probe and subjected to electrophoresis on a nondenaturing 4% acrylamide:bisacrylamide gel (29:1) (Bio-Rad). For gel supershift analysis, the nuclear extract was incubated for 10 min (at room temperature) with anti-Stat5a (Santa Cruz) before addition of the labeled probe. For nonspecific competition, an NF-κB binding element consisting of a single κB site was used.
Wound-Healing Assay
The indicated cell lines were seeded in 60-mm dishes and maintained in normal growth conditions to confluence. At this point, a wound was inflicted with a sterile tip, and cells were washed twice with complete DMEM. Cell motility was monitored at 1-hr intervals.
RESULTS
Oncogenic RhoA Induces Tyrosine Phosphorylation of Stat5a (Y696) but Not Stat5b (Y699) and Stat5a Transcriptional Activation in MDCK Epithelial Cells
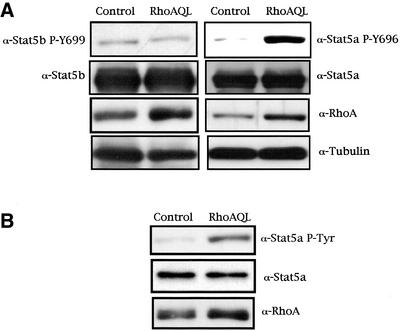
A phosphospecific antibody that recognizes the tyrosine phosphorylated form of Stat5a was used to verify whether oncogenic RhoA (QL) is capable of inducing tyrosine phosphorylation of this transcription factor. MDCK cells were transfected with 2 μg of Stat5a expression vector either with pcDNAIIIB or its derived vector encoding for a constitutively active mutant of RhoA. Cells were maintained in low serum content (0.5% FBS); 48 h after transfection they were lysed, and whole-cell extracts were assayed for Stat5a tyrosine phosphorylation by Western immunoblotting (Figure 1A, right). RhoA (QL) induces a readily detectable increase in Stat5a tyrosine phosphorylation. To verify whether these differences observed were not caused by differential transfection of either Stat5a or RhoA (QL), the same blot was tested for both Stat5a and RhoAQL expression. As seen in Figure 1A, the same levels of Stat5a were present in both lanes, indicating that this effect is specific to RhoA signaling. Furthermore, equal loading was verified by use of a tubulin-specific antibody.
Figure 1.
Oncogenic RhoA induces tyrosine phosphorylation (Y694) of Stat5a but not Stat5b. (A) MDCK cells transfected with either 2.0 μg of pcDNAIIIB or RhoA (QL), together with 2.0 μg of Stat5a, and harvested 48 h after transfection. Incubation of whole-cell extracts with a phosphospecific Stat5a-Y696 antibody revealed that RhoA induces tyrosine phosphorylation of Stat5a (right). Equal Stat5a transfection was verified with anti-Stat5a antibody. Expression of RhoA was detected with anti-RhoA antibody, and equal loading was verified by determining the levels of tubulin. The same type of experiment was performed with respect to Stat5b, and no tyrosine phosphorylation of residue 699 was observed under RhoA signaling (left). (B) RhoAQL induces tyrosine phosphorylation of endogenous Stat5a. RhoAQL or control vector was transfected in MDCK cells as in A, and tyrosine phosphorylation of Stat5a was determined by Western analysis. A and B show representative blots obtained in three independent experiments with similar results.
The same type of experiment was performed to verify whether RhoA stimulated tyrosine phosphorylation of Stat5b. As seen in Figure 1A, left, an antibody that recognizes Stat5b only when phosphorylated on tyrosine 699 did not detect any significant tyrosine phosphorylation of Stat5b in RhoAQL-expressing cells compared with parental MDCK cells. The observed effect on Stat5a was not a result of overexpression of the transcription factor along with RhoA, because a fivefold increase (determined by the relative intensities of the bands) in tyrosine phosphorylation of endogenous Stat5a in MDCK cells could be also observed (Figure 1B). Thus, RhoAQL induces tyrosine phosphorylation of Stat5a but not Stat5b.
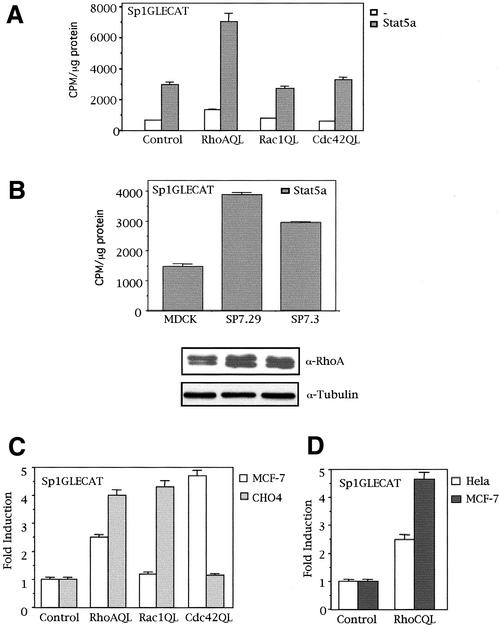
Tyrosine phosphorylation of Stat proteins enables their homodimerization or heterodimerization via their SH2 domains. Protein oligomerization, in turn, triggers a nuclear localization signal that carries the transcription factor to the nucleus, where it can interact with Stat-responsive DNA elements (Darnell, 1997). Thus, we next verified whether tyrosine phosphorylation of Stat5a by RhoA (QL) leads to an increase in Stat5a transcriptional activity in MDCK cells and other epithelial cell lines. A Stat5-responsive element from the Sp2.1 promoter was cloned upstream of a luciferase reporter gene, Sp1GLECAT, and Stat5 transcriptional activity was measured in RhoA (QL) transfectants. PcDNAIIIB control vector or RhoAQL, Rac1QL, and Cdc42QL were each cotransfected in MDCK cells with Stat5a expression vector, along with the Sp1GLECAT reporter, and CAT activity was measured 48 h after transfection (Figure 2A). Whereas RhoA induced a twofold to threefold increase in Stat5a transcriptional activity, both Rac1QL and Cdc42QL failed to promote Stat5a-dependent transcription. Furthermore, the same effect is observed with two different stable clones that express RhoA (QL), SP7.29 and SP7.3 (Figure 2B). According to the functional assay, we verified that Rac1 and Cdc42 do not induce tyrosine phosphorylation of Stat5a under the same conditions as with RhoA (our unpublished observations). A Western blot against RhoA is shown, and equal loading was verified with an anti-tubulin antibody. Interestingly, when other cell lines are verified for Stat5 activity in RhoA (QL), Rac1 (QL), or Cdc42 (QL) transfectants, a differential pattern of activation is observed. Thus, in MCF-7 cells, both RhoA and Cdc42 promote Stat5-dependent transcription, whereas Rac1 fails to do so (Figure 2C). Conversely, Cdc42 (QL) failed to activate Stat5 in CHO cells, but both RhoA (QL) and Rac1 (QL) do so (Figure 2C). Furthermore, RhoC promoted Stat5-dependent transcriptional activation in HeLa and MCF7 cells (Figure 2D). Thus, Stat5a activation by different members of the family of Rho GTPases appears to be cell type–specific.
Figure 2.
Rho GTPases differentially induce Stat5-dependent transcription of the Sp1GLECAT reporter in different cell lines. (A) RhoA but not Rac1 or Cdc42 (QL) activates Stat5a-dependent transcription of the Sp1GLECAT reporter via the endogenous and ectopically expressed Stat5a in MDCK cells. Cells transfected with Stat5a (2.0 μg) or not transfected, together with control vector or RhoA (QL), along with 0.5 μg of Sp1GLECAT reporter, were assayed for CAT activity 48 h after transfection. (B) RhoA (QL) activates Stat5a transactivation in two stable clones that constitutively express oncogenic RhoA (QL). MDCK or either clone was transfected with 0.5 μg of Sp1GLECAT reporter and 2.0 μg of Stat5a, and CAT activity was measured 48 h after transfection. The levels of expression of RhoA (QL) were determined with an anti-RhoA polyclonal antibody. Equal loading was verified with anti-tubulin antibody. (C) RhoA, Cdc42, and Rac1 differentially activate Stat5-dependent transcription in MCF-7 and CHO cells. Transfection of 1.0 μg of RhoA, Rac1, or Cdc42 (QL) along with the Sp1GLECAT reporter was performed in MCF-7 or CHO cells, and CAT activity was measured 24 h after transfection. (D) RhoC (QL) promotes Stat5-dependent transcription in HeLa and MCF-7 cells. Same experiment as in C was performed in both cell lines, and CAT activity was measured 24 h after transfection. All CAT experiments shown in this figure represent the mean of a single experiment performed in triplicate ±SD and are representative of at least three independent experiments with similar results.
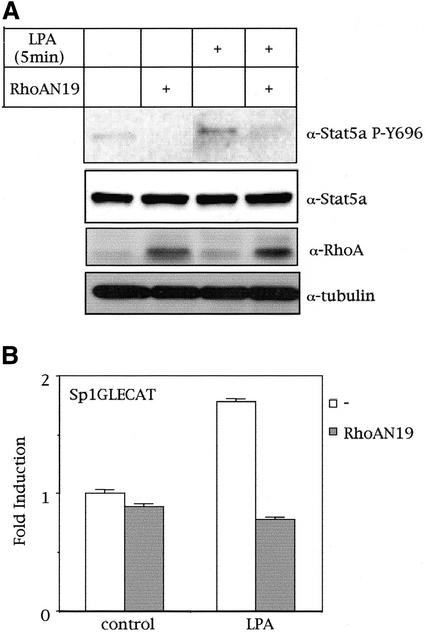
Finally, we verified whether a physiological activator of RhoA would induce tyrosine phosphorylation of Stat5a. To this end, we stimulated serum-starved MDCK cells with LPA for 5, 15, 30, and 60 min and verified tyrosine phosphorylation of Stat5a. In addition, dominant negative RhoA (N19) was expressed transiently in MDCK cells, which were then stimulated as mentioned with LPA. As observed in Figure 3A, exposure of MDCK cells to LPA for 5 min induced an approximately fourfold increase in the level of tyrosine phosphorylated Stat5a (as determined by relative band intensities) in a RhoA-dependent manner, because dominant negative RhoA (N19) completely abrogated this effect. LPA-induced tyrosine phosphorylation of Stat5 was observed as early as 5 min after stimulation and was sustained up to 30 min (data not shown). Steady-state levels of Stat5a and efficient expression of RhoAN19 were verified (Figure 3A). Furthermore, we verified whether LPA-induced tyrosine phosphorylation of Stat5a would translate into an increase in the transcriptional activity of the transcription factor. MDCK cells transfected with control vector or with dominant negative RhoAN19 along with the Sp1GLECAT reporter were left under low-serum conditions for 48 h and subsequently stimulated with LPA for 12 h. LPA-induced transcriptional activation of Stat5a was found to be weak but fully dependent on RhoA (Figure 3B). Thus, LPA activates Stat5 via a signaling pathway that depends on RhoA.
Figure 3.
LPA induces tyrosine phosphorylation and transcriptional activation of Stat5a via RhoA. (A) RhoA mediates LPA-induced Stat5a tyrosine phosphorylation. MDCK cells transfected with 2.0 μg of Stat5a and RhoAN19 expression vectors (where indicated) were treated with 50 μM LPA for 5 min, and tyrosine phosphorylation of Stat5a was determined by Western blot analysis. (B) LPA-induced Stat5a transcriptional activation is mediated by RhoA: 2.0 μg of control pcDNAIIIB or RhoAN19 (where indicated) expression vectors, along with the Sp1GLECAT reporter, were transfected in MDCK cells. Forty-eight hours after transfection, cells were treated with 50 μM LPA for 12 h, and CAT activity was determined. CAT experiment shown in this figure represents the mean of a single experiment performed in triplicate ±SD and is representative of at least three independent experiments with similar results. A shows a representative result obtained in three independent experiments.
Rho Promotes Binding of Stat5a to the Stat5-responsive Element of the Human Prolactin Promoter
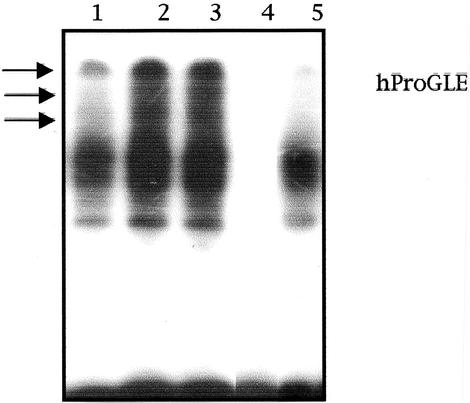
Tyrosine phosphorylation of Stat proteins promotes their homo-oligomerization or hetero-oligomerization and subsequent nuclear migration. Once in the nucleus, Stat5 oligomers may interact with other accessory nuclear factors and bind to their consensus sequences to modulate transcription (Grimley et al., 1999). Because RhoA triggers tyrosine phosphorylation of Stat5a, we next verified its DNA-binding potential under RhoA signaling. MDCK cells transfected with either empty vector or RhoA (QL) together with Stat5a were maintained in low serum for 36 h. Nuclear-enriched extracts were obtained, and equal amounts of protein were used to assess Stat5a DNA binding to the human prolactin gene promoter (hProGLE). Equal amounts of nuclear extracts from control and Rho-transfected cells were subjected to EMSA using radiolabeled hProGLE as a probe (Figure 4). RhoA (QL) transfectants showed a much more intense binding than control cells (lane 2 vs. lane 1). Moreover, whereas an NF-κB–binding element did not affect RhoA-dependent Stat5 DNA binding (lane 3), specific competition with excess amounts of unlabeled probe completely eliminated the signal (lane 4). In addition, incubation of nuclear extracts with anti-Stat5a antibody diminished the intensity of the two lower bands and eliminated the upper bands (lane 5), whereas Stat5b, Stat1, c-Jun, or ATF2 antibodies had no effect over nuclear complex migration (our unpublished observations). Thus, RhoA induces tyrosine phosphorylation of Stat5a, which promotes its translocation to the nucleus, in which it can interact with DNA elements responsive to this transcription factor.
Figure 4.
RhoA (QL) induces Stat5a DNA binding to the GAS sequence of the human prolactin promoter. Two micrograms of MDCK-Stat5a or MDCK-RhoAQL/Stat5a nuclear extracts was subjected to EMSA. Lanes 1 and 2 represent control cells and RhoAQL-transfected cells, respectively. Lanes 3 and 4 correspond to unspecific competition and specific competition with excess hProGLE probe, respectively. Lane 5 corresponds to competition with α-Stat5a antibody. Results shown are representative of four independent experiments.
RhoA (QL)-induced Tyrosine Phosphorylation of Stat5a Is Mediated by JAK2 Tyrosine Kinase
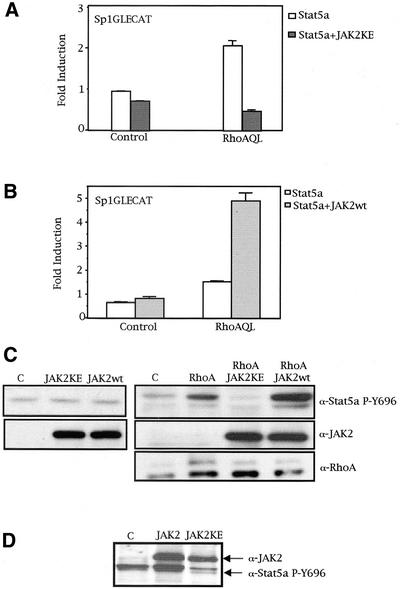
Tyrosine phosphorylation of Stat5 is induced by diverse cytokines and is performed by different tyrosine kinases (Grimley et al., 1999). We have recently found that RhoA induces tyrosine phosphorylation of Stat3 in human HEK cells via a JAK2-dependent pathway. Hence, we have studied the role of this tyrosine kinase in Stat5a tyrosine phosphorylation by oncogenic RhoA. Coexpression of a dominant negative mutant JAK2 (KE mutation) with RhoA, along with the Sp1GLECAT reporter, resulted in complete inhibition of RhoA-stimulated Stat5a transcriptional activity, with little effect over basal Stat5a transcriptional activity (Figure 5A). In addition, whereas expression of wild-type JAK2 produces no significant increase in Stat5a transactivation, cotransfection with RhoA (QL) leads to a synergism in Stat5a activity (Figure 5B).
Figure 5.
JAK2 mediates RhoA-induced Stat5a tyrosine phosphorylation. (A) Dominant negative JAK2 inhibits Stat5a transcriptional activation by RhoA (QL). One microgram of control pcDNAIIIB or RhoAQL was cotransfected with 1 μg of Stat5a, with either 3 μg of pRK-JAK2-KE or pRK control vector, along with 0.25 μg of Sp1GLECAT reporter. CAT activity was measured 48 h after transfection (0.5% FBS). (B) Wild-type JAK2 synergizes with RhoA (QL) to promote Stat5a-dependent transcription. Same amounts of control, RhoAQL, and Sp1GLECAT vectors were transfected as in A. Three micrograms of wild-type JAK2 was transfected where indicated. Data shown in A and B represent a single experiment performed in triplicate ±SD and are representative of three independent experiments. (C) JAK2 acts downstream of RhoA to promote Stat5a tyrosine phosphorylation, with no effect on basal tyrosine phosphorylation of Stat5a in control (C) cells. Thirty micrograms of the same extracts as in A and B were used for Western immunoblotting. Levels of JAK2 (KE and wt) and RhoA (QL) were verified with specific antibodies. (D) Same effect as in C is observed using a stable RhoA (QL) expressing MDCK cell line (clone SP7.29). Three micrograms of pRK-JAK2-KE or JAK2wt was transfected, and 48 h after transfection, protein expression was verified by Western blot. JAK2 (KE; wt) and Stat5a PY-694 were detected with specific antibodies. Immunoblot shown is representative of two independent experiments.
We also needed to verify whether the differences observed in Stat5a activity were caused by a modulatory effect of JAK2 on tyrosine phosphorylation of Stat5a in the context of RhoA (QL). The same extracts as used for CAT activity were tested for Stat5 tyrosine phosphorylation. As shown in Figure 5C, whereas modulation of JAK2 activity does not have any effect over Stat5 tyrosine phosphorylation in MDCK parental cells (left), it does have a profound effect over Stat5a tyrosine phosphorylation downstream of RhoA (QL). Thus, expression of a dominant negative mutant (JAK2KE) completely eliminated such phosphorylation, whereas wild-type JAK2 enhanced RhoA-induced tyrosine phosphorylation of Stat5a (Figure 5C, right). As a control, expression of both wild-type and dominant negative JAK2 was verified by use of an anti-JAK2 specific antibody. Also, expression of RhoA (QL) was verified and confirmed to be similar in all lanes. Accordingly, the same result was obtained when the SP7.29 MDCK clone that constitutively expresses oncogenic RhoA was transfected with either wild-type or dominant negative JAK2 (Figure 5D). Thus, JAK2 lies downstream of oncogenic RhoA and mediates tyrosine phosphorylation of Stat5a, thus enabling its dimerization, DNA binding, and transcriptional activation.
RhoA Inhibits Stat5a Serine Phosphorylation of Ser726 and Ser780, Preventing Its Inhibitory Effect on Stat5a Transcriptional Activity
A second regulatory mechanism independent of tyrosine phosphorylation has been described for several Stat proteins that involves serine phosphorylation. However, only recently has the effect of Stat5a/b serine phosphorylation on Stat5 activity begun to be unraveled. Two different serine residues are phosphorylated on prolactin or GH stimulation of Stat5a (serines 726 and 780), and only a single serine is phosphorylated in Stat5b (serine 730). Furthermore, serine phosphorylation of Stat5a/b appears to constitute a fine-tuning mechanism of Stat5 activity (Gouilleux et al., 1994; Yamashita et al., 1998, 2001; Park et al., 2001).
We therefore analyzed the pattern of serine phosphorylation downstream of oncogenic RhoA (QL). To this end, we used two phosphospecific antibodies that recognize either the serine-phosphorylated form of Stat5a on Ser726 or serine phosphorylated Stat5a on Ser780. In addition, three Stat5a serine mutants were used that contain a Ser726 or Ser780 mutated to alanine (Stat5a S726A and Stat5a S780A, respectively) and the double mutant (Stat5a S726/780A). Wild-type Stat5 or the serine mutants S726A and S780A were cotransfected into MDCK cells with either control vector or RhoA (QL), and whole-cell extracts were subjected to Western immunoblotting. Figure 6A shows that RhoA (QL) reduced the level of Stat5a serine phosphorylation of both Ser726 and Ser780. To confirm that this effect was not caused by differential expression of Stat5, the levels of total Stat5a were verified by use of an anti-Stat5 specific antibody. Furthermore, RhoA levels were detected to be equal in extracts derived from RhoA transfectants. Finally, equal loading was verified by determining the levels of intracellular tubulin. Thus, RhoA (QL) produces an inhibitory effect over Stat5a serine phosphorylation of both Ser726 and Ser780.
Figure 6.
RhoAQL reduces the level of Stat5a serine phosphorylation of serine residues 726 and 780, which leads to an increase in Stat5a activity. (A) RhoA inhibits Stat5a serine phosphorylation of serines 726 and 780. Cells were transfected with 2.0 μg of control vector, RhoA (QL), and wild-type Stat5a or serine mutants S726A and S780A as indicated. Forty-eight hours after transfection, cells were harvested and lysed. Western immunoblotting was performed with phospho-Stat5a S726 and phospho-Stat5a S780 antibodies where indicated. Equal expression of Stat5a RhoAQL was verified with anti-Stat5a and anti-RhoA antibodies, respectively. Equal loading was confirmed with anti-tubulin. The blots shown are representative of five independent experiments. (B) Stat5a serine phosphorylation of both serines 726 and 780 is inhibitory for Stat5a-dependent Sp1GLE transcription. Control vector or RhoAQL was cotransfected with the indicated Stat5a expression vectors, along with the Sp1GLECAT promoter, and CAT activity was measured. (C) Same extracts were used for Western immunoblotting, and the levels of Stat5a serine phosphorylation on serine 726 were confirmed to be lower in RhoA transfectants. Levels of Stat5a and RhoA were confirmed to be equal by anti-Stat5a and anti-RhoA antibodies. Experiments shown in B and C are representative of three independent results.
Next, the functional relevance of the inhibitory effect of RhoA over serine phosphorylation of Stat5a was investigated. Wild-type Stat5a or either single serine mutant (S726A, S780A) and the double serine mutant (S726/780) were coexpressed with RhoA or control vector, along with the Sp1GLECAT reporter, and CAT activity was measured (Figure 6B). Surprisingly, abrogation of either serine led to a twofold to threefold increase in Stat5a transcriptional activity compared with wild-type activity. Accordingly, Stat5A S726/780A that completely lacks serine phosphorylation showed a synergistic increase in activity with respect to wild-type Stat5a. With the same extracts, the level of serine phosphorylation was detected by Western blot (Figure 6C). As seen in Figure 6C, a significant reduction in Ser726 phosphorylation (approximately 50% reduction in intensity) of both wild-type Stat5a and Stat5a S780 was observed in RhoA-expressing cells with respect to control cells. We then verified the expression of both Stat5a and S780A with an anti-Stat5 antibody to confirm that these differences in both transcriptional activity and serine phosphorylation were not because of their differential expression. As expected, the levels of both wild-type and serine mutants in control cells versus RhoA-expressing cells were similar. Furthermore, this analysis established that the apparent lower levels of Ser726 phosphorylation of Stat5a S780A mutant with respect to wild-type Stat5a were a result of lower expression of the serine mutant. Also, RhoA (QL) was expressed equally in all RhoA transfectants. Similar results were obtained when the levels of phosphorylation of serine 780 were studied, although the effect of RhoA on dephosphorylation of this residue is milder than that obtained with serine 726, with a reduction of ∼30% in the content of phosphorylation (our unpublished observations ).
RhoA-induced Serine Dephosphorylation of Stat5a Is Not Mediated by MEK-1, p38, or JNK Kinases
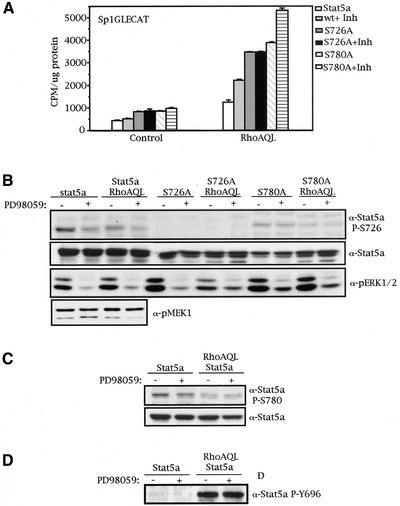
Because inhibition of Stat5a serine phosphorylation of both Ser726 and Ser780 by RhoA translates into an increase in Stat5a transcriptional activity, we next wanted to determine which serine kinase is down-modulated by RhoA under these circumstances that results in this effect. Constitutive serine phosphorylation of Stat5a in Nb2 lymphoma cells has been described to be MEK1-dependent, by use of the MEK1 inhibitor PD98059 (Kirken et al., 1997). Thus, we studied the effect of blocking MEK1 function on RhoA-induced Stat5a activation. Wild-type Stat5a, Stat5a S726A, or Stat5a S780A was cotransfected with control vector or RhoA (QL), together with the Sp1GLECAT reporter. Eight hours after transfection, cells were treated with PD98059 (50 μM) for 16 h, and cells were harvested for CAT assay and Western immunoblotting. Inhibition of MEK1 in both control cells and RhoA (QL)–expressing cells resulted in a twofold increase in Stat5a transcriptional activity (Figure 7A). We were able to determine Ser726 as the PD98059-sensitive Stat5a serine residue, because the S726A mutant was unaffected by PD98059, whereas the S780A mutant showed an increase in activity when treated with PD98059 (Figure 7A).
Figure 7.
MEK1 and RhoA (QL) modulate Stat5a serine phosphorylation of serine 726 by two independent mechanisms. (A) Treatment with MEK1 inhibitor PD98059 results in an increase of transcriptional activity of Stat5a and S780A mutant but not S726 mutant. MDCK cells transfected with 2.0 μg of the expression vectors indicated, together with 0.5 μg of Sp1GLECAT reporter, were maintained in 10% FBS for 24 h after transfection, and CAT activity was measured. (B) MEK-1 phosphorylates Stat5a serine 726 by a mechanism independent of RhoA. Same extracts as in A were used to verify the level of Stat5a serine phosphorylation on MEK-1 inhibition. As a control of inhibition, the amount of phospho-ERK1/2 was shown to decrease significantly on PD98059 treatment. The levels of transfected Stat5a or serine mutants were verified with specific anti-Stat5a antibody. (C) Treatment with PD98059 does not affect phosphorylation of serine 780. Same extracts as in A and B were used to determine the levels of Stat5a serine phosphorylation on serine 780. (D) PD98059 does not affect Stat5a tyrosine phosphorylation. Same extracts as in A were used to perform this experiment. Results shown here are representative of three independent experiments.
When Stat5a serine phosphorylation was determined by Western immunoblotting with the same extracts, this observation was confirmed (Figure 7, B and C). Whereas phosphorylation of serine 726 is sensitive to PD98059 treatment, with an approximate reduction in phosphorylation intensity of 40% (Figure 7B), phosphorylation of serine 780 remained unaffected (Figure 7C). The same effect over phosphorylation of serine 726 was observed with shorter exposures to PD98059, 6 and 8 h (our unpublished observations). Phospho-ERK1/2 levels were verified as a control of PD98059 functional inhibition. In addition, to study whether RhoA (QL) directly modulates the activation of MEK1, we verified the pattern of MEK1 phosphorylation in control cells versus RhoA transfectants (Figure 7B). The levels of MEK1 phosphorylation were the same in control and RhoA-transfected cells, indicating that the effects of both MEK1 and RhoA (QL) on Stat5a serine phosphorylation are independent events and constitute two parallel mechanisms of Stat5a transcriptional modulation. Finally, we verified that the differences in transcriptional activity observed after PD98059 treatment were not a result of changes in tyrosine phosphorylation of Stat5a, as shown in Figure 7D. Together, treatment with PD98059 and activation of RhoA (QL) led to an increase in Stat5a transcriptional activity, presumably as a consequence of the inhibition of phosphorylation of Ser726.
We also examined the possible effect of p38 and JNK on RhoA regulation of Stat5a activity using a type of analysis similar to that for MEK1. Thus, MDCK cells transfected with either Stat5a, Stat5a S726A, or Stat5a S780A, with control vector or RhoA (QL), along with the Sp1GLECAT reporter were treated with the p38-specific inhibitor SB203580. Treatment with SB203580 led to a 2.5-fold increase in Stat5a activity in RhoA transfectants (our unpublished observations). However, when either mutant was treated with the inhibitor, no change in activity was observed with respect to untreated cells. In addition, analysis of the phosphorylation of both Ser726 and Ser780 revealed that residues of both serines remain unaffected by treatment with SB203580. Thus, p38 negatively regulates Stat5a transcriptional activity by an unknown mechanism that does not involve direct modulation of serine phosphorylation of the transcription factor, which is independent of RhoA.
Finally, we verified whether the JNK pathway is involved in serine dephosphorylation of Stat5a induced by RhoA. To that end, we expressed in MDCK-RhoAQL–expressing cells the JNK-binding domain of the scaffold protein JIP1 (called JIP1Δ) and measured the transcriptional activity of Stat5a via the Sp1GLECAT reporter (our unpublished observations). We did not observe any effect on the transcription or serine phosphorylation of Stat5awt or the serine mutants S726A and S780A. Therefore, we concluded that the JNK1 pathway is not involved in RhoA-mediated activation of Stat5a.
Thus, RhoA (QL) down-modulates the activity of a yet to be identified Stat5a serine kinase, which in turn leads to activation of Stat5a transcription factor
Stat5a Is Necessary for RhoA-induced EMT in MDCK Cells
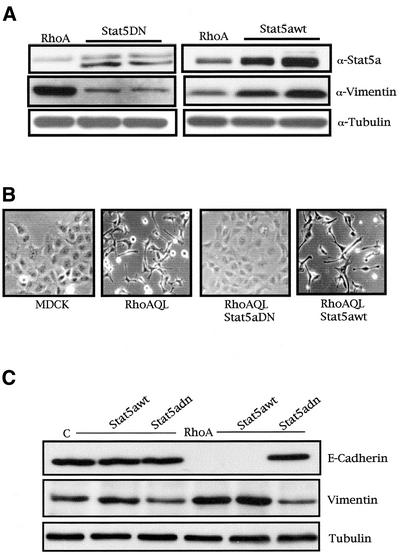
We wanted to study the effect(s) Stat5a could have on RhoA biological functions. To that end, we generated stable cell lines of MDCK-RhoAQL cells with Stat5awt or Stat5DN (Figure 8A). Interestingly, RhoAQL-expressing cells had undergone a readily visible EMT with respect to MDCK cells. However, whereas the morphology of Stat5DN stable cell lines resembled that of parental MDCK cells, stable expression of Stat5awt further potentiated a transition to a fibroblastic morphology (representative pictures are shown in Figure 8B). This effect was confirmed by analysis of the levels of expression of vimentin. In this sense, expression of dominant negative Stat5 significantly reduced the levels of vimentin with respect to MDCK-RhoAQL cells, whereas Stat5awt potentiated vimentin expression (Figure 8A). Furthermore, these effects were specific to RhoA signaling, because stable expression of dominant negative Stat5a or Stat5awt did not significantly change the morphology of the cells or vimentin expression of the parental MDCK cell line (Figure 8C).
Figure 8.
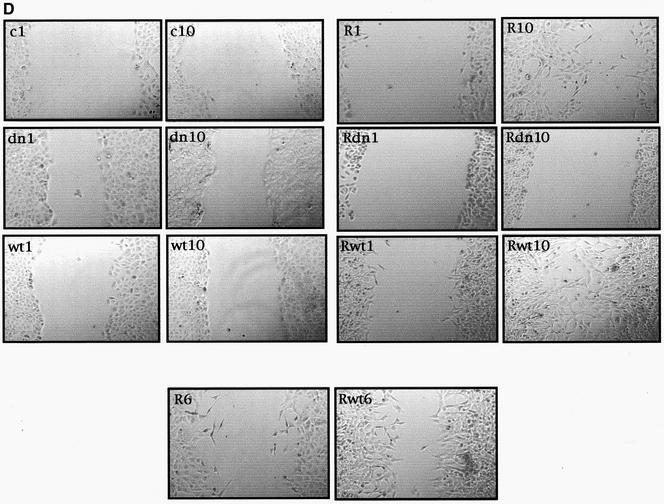
Stat5a is necessary for RhoA-induced EMT, vimentin expression, and loss of E-cadherin and motility in MDCK cells. (A) Stable expression of Stat5aDN or Stat5wt inhibits and enhances expression of vimentin induced by oncogenic RhoA, respectively. Stat5 stable cells lines were generated as described in MATERIALS AND METHODS and were verified for Stat5DN (left) and Stat5awt (right) stable expression with anti-Stat5a antibody and expression of endogenous vimentin with anti-vimentin. (B) Pictures of MDCK, MDCK-RhoAQL, RhoAQL/Stat5DN, and RhoAQL/Stat5awt stable cell lines with representative morphological changes. (C) Stat5a is necessary for loss of E-cadherin and vimentin expression accompanying RhoA-induced EM transition. Equal loading was verified with anti-tubulin. (D) Stat5a modulates cell motility induced by oncogenic RhoA. Cell motility was monitored at 1-h intervals after scratching, and representative pictures at 1 and 10 h are shown for each clone. An intermediate time point (6 h) is shown for RhoAQL- and RhoAQL/Stat5awt-expressing clones (c, MDCK-control vector; dn, MDCK-Stat5aDN; wt, MDCK-Stat5awt; R, MDCK-RhoAQL; Rwt, MDCK-RhoAQL/Stat5awt; Rdn, MDCK-RhoAQL/Stat5dn).
We next verified the effect Stat5 had over E-cadherin, an adhesion protein required for adherens junctions whose expression is lost after EMT. As observed in Figure 8C, whereas Stat5aDN or Stat5awt alone had no effect over E-cadherin, stable expression of RhoAQL led to complete down-regulation of E-cadherin. However, coexpression of dominant negative Stat5a with RhoAQL reverted E-cadherin expression back to MDCK levels (Figure 8C). Accordingly, vimentin expression was confirmed to reflect the morphology of the cells.
Finally, we studied the relative motility of MDCK and MDCK-RhoAQL cells and the effect Stat5a could have over such motility. To this end, we performed a wound-healing assay comparing the motility of the MDCK, MDCK-Stat5aDN, MDCK-Stat5awt, MDCK-RhoAQL, RhoAQL-Stat5aDN, and RhoAQL-Stat5awt stable cell lines. Figure 8D shows representative pictures taken at 1 and 10 h after the wound was inflicted. MDCK and the Stat5a transfectant cell lines had no visible motility. Conversely, RhoAQL-expressing cells were highly motile compared with the parental MDCK cell line. Moreover, whereas Stat5awt enhanced, Stat5aDN completely eliminated the motility of MDCK-RhoAQL cells (Figure 8D). The difference in relative motility between RhoAQL and RhoAQL-Stat5awt cells was visible as early as 6 h (Figure 8D). Thus, Stat5a is necessary for the EMT induced by RhoA.
DISCUSSION
We describe a novel signaling pathway that links RhoA to the transcription factor Stat5a. Specifically, RhoA stimulated activation of Stat5a, as demonstrated by inducible tyrosine phosphorylation of both endogenous and ectopically expressed protein, DNA binding, and Sp1GLE Stat5-dependent transcriptional activation. RhoA acts downstream of LPA to orchestrate the signaling proteins required for proper tyrosine phosphorylation of Stat5a that ultimately leads to its transcriptional activation. The mechanism of RhoA-induced tyrosine phosphorylation of Stat5a involved JAK2 tyrosine kinase, because a dominant negative JAK2 mutant completely inhibited, and wild-type JAK2 greatly enhanced, RhoA-mediated Stat5a tyrosine phosphorylation and transcriptional activation. A functional interaction between JAK2 and Rho GTPases has been described previously (Simon et al., 2000; Aznar et al., 2001). In this sense, both RhoA and Rac1 activate JAK2, which leads to tyrosine phosphorylation of Stat3. Thus, JAK2 may represent a convergence point between Rho signaling and Stat transcription factors.
Interestingly, we have not observed tyrosine phosphorylation of Stat5b using a phosphospecific antibody that recognizes only its tyrosine phosphorylated form. We do not know the functional implication of this finding, but several works have already described a discrimination of Stat5a and Stat5b activation after specific stimuli. In this sense, stimulation of the promonocytic U937 cells with IFN-α and IFN-γ leads to tyrosine phosphorylation and transactivation of Stat5a but not Stat5b (Meinke et al., 1996). The same study reported that IFN-γ did not activate either Stat5a or Stat5b in HeLa cells despite the expression of both Stat5 isoforms at similar levels (Meinke et al., 1996). Furthermore, granulocyte-monocyte colony–stimulating factor stimulates Stat5a but not Stat5b activation in human peripheral-blood monocytes (Rosen et al., 1996), whereas the same stimulus in human neutrophils activates Stat5b and not Stat5a (Al-Shami et al., 1998). Finally, selective gene disruption of Stat5a or Stat5b in mice has dissected individual functions, further pointing to the relevance of specific effects of each Stat5 isoform (Levy and Gilliland, 2000).
We have found that oncogenic RhoA lowers the serine phosphorylation content of Stat5a. Both Ser726 and Ser780 are constitutively phosphorylated under normal serum conditions, and on RhoA expression, a strong dephosphorylation of serine 726 and a milder dephosphorylation of serine 780 take place that lead to an enhancement of Stat5a transcriptional activity. The component that involves Stat5a serine dephosphorylation is intriguing. Thus, RhoA directly modulates the activity of yet to be identified Stat5a serine kinases that phosphorylate Ser726 and Ser780. This down-modulation might take place via direct inactivation of specific kinases responsible for Stat5a serine phosphorylation, modulation of either their half-life or expression, or activation of a specific phosphatase. In contrast to what would be expected from studies of Stat3 or Stat1, serine phosphorylation of Stat5a was found to be inhibitory in the cellular and molecular context of the present work. Two recent studies point to modulatory roles of Stat5a/b serine phosphorylation that may be positive or negative with regard to transcriptional regulation (Park et al., 2001; Yamashita et al., 2001). Thus, prolactin stimulation of murine mammary cells leads to both tyrosine and serine phosphorylation of Stat5a/b. However, Stat5 transcriptional activity in this context remains latent. Subsequent glucocorticoid stimulation does not affect Stat5 tyrosine phosphorylation but leads to the serine dephosphorylation of both Stat5 isoforms, which translates into an increase in Stat5 transcriptional activity (Groner et al., 2000). Conversely, Stat5a-dependent transcription of a GH-responsive ntcp-reporter gene positively depends on Ser731 of Stat5b and Ser780 of Stat5a (and not Ser726) (Park et al., 2001). Thus, mutation of these serine residues to alanine in the context of GH leads to a decrease in Stat5a/b transactivation. In contrast, mutants Stat5a-S726/780A and Stat5b-S731A in the same cell system display a twofold higher GH- or PRL-stimulated transcriptional activity compared with their wild-type Stat5a/b when assayed with a β-casein promoter reporter (Park et al., 2001). This clearly indicates that the promoter context used for measuring Stat5a/b transcriptional activity with respect to its serine phosphorylation is a key element to take into account. In the present study, we provide further evidence for a suppressive effect of serine phosphorylation on Stat5a transcription in the context of the Sp2.1 promoter and RhoA signaling. Attempts have been made to identify the mechanism for regulation of serine dephosphorylation. Our results demonstrate that treatment with the specific inhibitor PD98059 enhances the transcriptional activity of Stat5a. This effect is associated with dephosphorylation of S726 but not Ser780; PD98059 treatment related directly only to Ser726 dephosphorylation, because the S726A mutant was unaffected by PD98059, whereas the S780A mutant showed an increase in activity when treated with PD98059. However, RhoA does not modulate MEK1 activity in this cell line, indicating that both MEK1 and RhoA affect Stat5a serine phosphorylation by two independent mechanisms. These results are also consistent with a second, unidentified, serine kinase involved in the regulation of the S780 residue.
We have also established that a p38-dependent signal affects Stat5a regulation. Inhibition of p38 by SB203580 resulted in an increase in Stat5a activity. However, contrary to MEK1 blockage, inhibition of p38 had no effect on the phosphorylation state of either Ser726 or Ser780. These results are intriguing, because both serines are insensitive to p38 inhibition, and it would be expected that both Stat5a serine mutants show the same increase in transcriptional activity as the wild-type Stat5a does. Given that the activity of both mutants is the same in SB203580-treated and untreated cells, this implies an indirect mechanism; for instance, a protein interaction that depends on two intact serine residues of Stat5a, whereby mutation of either serine residue would eliminate this interaction and its inhibitory activity, thus eliminating the sensitivity to SB203580. Alternatively, p38 might modulate Stat5a activity via a novel serine residue. Furthermore, as for MEK1, no functional interaction between RhoA and p38 was found, indicating that both proteins modulate Stat5a activity by two independent mechanisms. To the best of our knowledge, this is the first evidence of a modulatory role for p38 on Stat5a. With respect to JNK, we have not found any modulatory action of this pathway over serine phosphorylation or transcriptional activity of Stat5a in the context of RhoA.
Although the present study demonstrated that RhoA activated Stat5a, the two other prototypes of Rho GTPases, Rac1 and Cdc42, were also capable of inducing Sp1GLE Stat5–dependent transcriptional activation in specific cell lines such as MCF-7 or CHO-4. However, the pattern of Sp1GLE transcription downstream of Rho GTPases differed depending on the cell line used. Thus, whereas Rho is capable of efficiently triggering Sp1GLE transcription in MDCK cells, Rac1 and Cdc42 fail to induce either tyrosine phosphorylation or transcriptional activation of Stat5a. Furthermore, we have found that oncogenic RhoC (V12) is also capable of triggering transcriptional activation of Stat5 in MCF7 and HeLa cells. Given that RhoC was recently found to be overexpressed in >90% of inflammatory metastatic breast cancer and that expression of RhoC in human mammary epithelium mimics the inflammatory metastatic breast cancer phenotype, the role of Stat5a in RhoC-induced neoplastic transformation is of direct interest. Furthermore, the specific mechanism whereby Rac1, Cdc42, or RhoC activates Stat5a or Stat5b remains to be determined.
Finally, we have determined that Stat5a is necessary for RhoA-induced EMT. Several works have pointed out a role of Rho GTPases in cell shape and motility (Evers et al., 2000; Ridley, 2001). For instance, RhoA is necessary for some of the morphological changes necessary for the EM transition of Ras-transformed mammary epithelial cells (Zhong et al., 1997). Also, RhoA mediates both transforming growth factor-β– (1 and 3) and Ras-induced transition to a fibroblastic phenotype of epithelial cells (Bakin et al., 2000; Zondag et al., 2000; Bhowmick et al., 2001; Kaartinen et al., 2002). In our system, modulation of Stat5a activity modulates the necessary elements essential for this cytoskeletal change induced by RhoA. This same effect was recently described for SRF, whose activity is modulated by changes in actin dynamics necessary for the epithelial to mesenchymal transition of tumor cells induced by RhoA (Sotiropoulos et al., 1999; Psichari et al., 2002). SRF-induced EMT takes place via expression of vinculin, actin, and SRF itself. In our particular system, we have observed that Stat5a modulates the expression of vimentin and E-cadherin. Whether SRF and Stat5a cooperate functionally to promote EMT is currently unknown. To the best of our knowledge, this is the first evidence of a role for Stat5 in the cytoskeletal changes that occur during EMT. Although Stat3 has been implicated in hepatocyte growth factor–induced tubulogenesis, we do not know whether both family members cooperate in this process (Boccaccio et al., 1998). With this in mind, we do not exclude the possibility that Stat5a might be involved in EM transition in a more general manner rather than only downstream of RhoA. Rho is capable of regulating cell adhesion both in a positive and in a negative manner depending on the stimulus and the intracellular pathways activated, namely ROCK and mDia, respectively (Sahai and Marshall, 2002). In our context, whether Stat5a is only positively implicated in RhoA-induced EMT via ROCK, or by contrast whether it constitutes a modulatory mechanism exerted by Rho on specific extracellular stimuli and matrix components to either disrupt or stabilize adherens junctions, is currently under study.
Thus, both these findings further potentiate the knowledge that regulation of transcription exerts profound effects in Rho GTPase–meditated functions that ultimately lead, in the context of tumoral cells, to a transformed phenotype. Together, these results point to a general role of Stat proteins in the biological functions of Rho GTPases.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–08–0454. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–08–0454.
REFERENCES
- Abraham MT, Kuriakose MA, Sacks PG, Yee H, Chiriboga L, Bearer EL, Delacure MD. Motility-related proteins as markers for head and neck squamous cell cancer. Laryngoscope. 2001;111:1285–1289. doi: 10.1097/00005537-200107000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts AS, Geneste O, Treisman R. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell. 1998;92:475–487. doi: 10.1016/s0092-8674(00)80941-1. [DOI] [PubMed] [Google Scholar]
- Al-Shami A, Mahanna W, Naccache PH. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils: selective activation of Jak2, Stat3, and Stat5b. J Biol Chem. 1998;273:1058–1063. doi: 10.1074/jbc.273.2.1058. [DOI] [PubMed] [Google Scholar]
- Aznar S, Lacal JC. Searching new targets for anticancer drug design: the families of Ras and RhoGTPases and their effectors. Prog Nucleic Acid Res Mol Biol. 2001a;67:193–234. doi: 10.1016/s0079-6603(01)67029-6. [DOI] [PubMed] [Google Scholar]
- Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001b;165:1–10. doi: 10.1016/s0304-3835(01)00412-8. [DOI] [PubMed] [Google Scholar]
- Aznar S, Valeron PF, del Rincon SV, Perez LF, Perona R, Lacal JC. Simultaneous tyrosine and serine phosphorylation of stat3 transcription factor is involved in rho a GTPase oncogenic transformation. Mol Biol Cell. 2001;12:3282–3294. doi: 10.1091/mbc.12.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition, and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- Ballestero RP, Esteve P, Perona R, Jiménez B, Lacal JC. Biological function of Aplysia californica rho gene: the superfamily of ras related genes. NATO Advanced Science Institute Series, 1991;A220:237–242. [Google Scholar]
- Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Beadling C, Ng J, Babbage JW, Cantrell DA. Interleukin-2 activation of STAT5 requires the convergent action of tyrosine kinases and a serine/threonine kinase pathway distinct from the Raf1/ERK2 MAP kinase pathway. EMBO J. 1996;15:1902–1913. [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- Bourdoulous S, Orend G, MacKenna DA, Pasqualini R, Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J Cell Biol. 1998;143:267–276. doi: 10.1083/jcb.143.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron F, Tsimiklis G, Arcand M, Robitaille L, Liang Q, Molkentin JD, Meloche S, Nemer M. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev. 2001;15:2702–2719. doi: 10.1101/gad.915701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sadowski HB, Kohanski RA, Wang LH. Stat5 is a physiological substrate of the insulin receptor. Proc Natl Acad Sci USA. 1997;94:2295–2300. doi: 10.1073/pnas.94.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- Danen EH, Sonneveld P, Sonnenberg A, Yamada KM. Dual stimulation of Ras/mitogen-activated protein kinase and RhoA by cell adhesion to fibronectin supports growth factor-stimulated cell cycle progression. J Cell Biol. 2000;151:1413–1422. doi: 10.1083/jcb.151.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, Boyd D, Wang H, Allgayer H, Doe WF, Wang Y. A region between −141 and −61 containing a proximal AP-1 is essential for constitutive expression of urokinase-type plasminogen activator receptor. Eur J Biochem. 1999;264:92–99. doi: 10.1046/j.1432-1327.1999.00583.x. [DOI] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Davud M, Wong L, Flavell R, Thompson SA, Wells A, Larner AC, Johnson GR. STAT activation by epidermal growth factor (EGF) and amphiregulin: requirement for the EGF receptor kinase but not for tyrosine phosphorylation sites or JAK1. J Biol Chem. 1996;271:9185–9188. doi: 10.1074/jbc.271.16.9185. [DOI] [PubMed] [Google Scholar]
- Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- del Peso L, Hernandez-Alcoceba R, Embade N, Carnero A, Esteve P, Paje C, Lacal JC. Rho proteins induce metastatic properties in vivo. Oncogene. 1997;15:3047–3057. doi: 10.1038/sj.onc.1201499. [DOI] [PubMed] [Google Scholar]
- Delarue FL, Taylor BS, Sebti SM. Ras and Rho suppress whereas RhoB enhances cytokine-induced transcription of nitric oxide synthase-2 in human normal liver AKN-1 cells and lung cancer A-549 cells. Oncogene. 2001;20:6531–6537. doi: 10.1038/sj.onc.1204801. [DOI] [PubMed] [Google Scholar]
- Evers EE, Zondag GC, Malliri A, Price LS, ten Klooster JP, van der Kammen RA, Collard JG. Rho family proteins in cell adhesion and cell migration. Eur J Cancer. 2000;36:1269–1274. doi: 10.1016/s0959-8049(00)00091-5. [DOI] [PubMed] [Google Scholar]
- Faruqi TR, Gomez D, Bustelo XR, Bar-Sagi D, Reich NC. Rac1 mediates Stat3 activation by autocrine IL-6. Proc Natl Acad Sci USA. 2001;98:9014–9019. doi: 10.1073/pnas.161281298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994;13:4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley PM, Dong F, Rui H. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 1999;10:131–157. doi: 10.1016/s1359-6101(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Groner B, Fritsche M, Stocklin E, Berchtold S, Merkle C, Moriggi R, Pfitzner E. Regulation of the trans-activation potential of STAT5 through its DNA-binding activity and interaction with heterologous transcription factors. Growth Horm IGF Res. 2000;10(suppl B):S15–S20. doi: 10.1016/s1096-6374(00)80004-0. [DOI] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Haataja L, Nagy A, Heisterkamp N, Groffen J. TGFbeta3-induced activation of RhoA/Rho-kinase pathway is necessary but not sufficient for epithelio-mesenchymal transdifferentiation: implications for palatogenesis. Int J Mol Med. 2002;9:563–570. [PubMed] [Google Scholar]
- Kamai T, Arai K, Tsujii T, Honda M, Yoshida K. Overexpression of RhoA mRNA is associated with advanced stage in testicular germ cell tumor. BJU Int. 2001;87:227–231. doi: 10.1046/j.1464-410x.2001.02030.x. [DOI] [PubMed] [Google Scholar]
- Keely PJ. Rho GTPases as early markers for tumor progression. Lancet. 2001;358:1744–1745. doi: 10.1016/S0140-6736(01)06840-4. [DOI] [PubMed] [Google Scholar]
- Kirken RA, Malabarba MG, Xu J, DaSilva L, Erwin RA, Liu X, Hennighausen L, Rui H, Farrar WL. Two discrete regions of interleukin-2 (IL-2) receptor beta independently mediate IL2 activation of a PD98059/rapamycin/wortmannin-insensitive Stat5a/b serine kinase. J Biol Chem. 1997;272:15459–15465. doi: 10.1074/jbc.272.24.15459. [DOI] [PubMed] [Google Scholar]
- Levy DE, Gilliland DG. Divergent roles of STAT1 and STAT5 in malignancy as revealed by gene disruptions in mice. Oncogene. 2000;19:2505–2510. doi: 10.1038/sj.onc.1203480. [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Chiariello M, Gutkind S. Regulation of gene expression by the small GTPase Rho through the ERK6 (p38γ) MAP kinase pathway. Genes Dev. 2001;15:535–553. doi: 10.1101/gad.855801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Tanaka K, Harimaya K, Nakatani F, Matsuda S, Iwamoto Y. Small GTP-binding protein Rho both increased and decreased cellular motility, activation of matrix metalloproteinase 2, and invasion of human osteosarcoma cells. Jpn J Cancer Res. 2001;92:429–438. doi: 10.1111/j.1349-7006.2001.tb01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke A, Barahmand-Pour F, Wohrl S, Stoiber D, Decker T. Activation of different Stat5 isoforms contributes to cell-type-restricted signaling in response to interferons. Mol Cell Biol. 1996;16:6937–6944. doi: 10.1128/mcb.16.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira JP, Benard V, Groffen J, Sanders LC, Kanus UG. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc Natl Acad Sci USA. 2000;97:185–189. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner S, Perona R, Saniger L, Lacal JC. Multiple signaling pathways lead to the activation of the nuclear factor kB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. doi: 10.1074/jbc.273.21.12779. [DOI] [PubMed] [Google Scholar]
- Montaner S, Perona R, Saniger L, Lacal JC. Activation of serum response factor by RhoA is mediated by the nuclear factor-kB and C/EBP transcription factors. J Biol Chem. 1999;274:8506–8515. doi: 10.1074/jbc.274.13.8506. [DOI] [PubMed] [Google Scholar]
- Muller SM, Okan E, Jones P. Regulation of urokinase receptor transcription by Ras- and Rho-family GTPases. Biochem Biophys Res Commun. 2000;270:892–898. doi: 10.1006/bbrc.2000.2531. [DOI] [PubMed] [Google Scholar]
- Park SH, Yanashita H, Rui H, Waxman DJ. Serine phosphorylation of GH-activated signal transducer, and activator of transcription 5a (STAT5a), and Stat5b: impact on Stat5 transcriptional activity. Mol Endocrinol. 2001;15:2157–2171. doi: 10.1210/mend.15.12.0746. [DOI] [PubMed] [Google Scholar]
- Perona R, Esteve P, Jimenez B, Ballestero RP, Ramon y Cajal S, Lacal JC. Tumorigenic activity of rho genes from Aplysia californica. Oncogene. 1993;8:1285–1292. [PubMed] [Google Scholar]
- Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal JC. Activation of nuclear factor-κB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- Psichari E, Balmain A, Plows D, Zoumpourlis V, Pintzas A. High activity of serum response factor in the mesenchymal transition of epithelial tumor cells is regulated by RhoA signaling. J Biol Chem. 2002;277:29490–29495. doi: 10.1074/jbc.M112368200. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Rosen RL, Winestock KD, Chen G, Liu X, Hennighausen L, Finbloom DS. Granulocyte-macrophage colony-stimulating factor preferentially activates the 94-kD STAT5A and an 80-kD STAT5A isoform in human peripheral blood monocytes. Blood. 1996;88:1206–1214. [PubMed] [Google Scholar]
- Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. Rock and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- Schuringa JJ, Dekker LV, Vellenga E, Kruijer W. Sequential activation of Rac1, SEK-1/MKK4, and protein kinase C delta is required for interleukin-6-induced STAT3 Ser-727 phosphorylation and transactivation. J Biol Chem. 2001;276:27709–27715. doi: 10.1074/jbc.M009821200. [DOI] [PubMed] [Google Scholar]
- Schuringa JJ, Jonk LJ, Dokter WH, Vellenga E, Kruijer W. Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1, and the kinase SEK-1/MKK-4 as signal transduction components. Biochem J. 2000;347:89–96. [PMC free article] [PubMed] [Google Scholar]
- Shuai K, Schindler C, Prezioso VR, Darnell JE., Jr Activation of transcription by IFN gamma: tyrosine phosphorylation of a 91 kDa DNA binding protein. Science. 1992;259:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Simon AR, Vikis HG, Stewart S, Fanburg BL, Cochran BH, Guan KL. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science. 2000;290:144–147. doi: 10.1126/science.290.5489.144. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamic. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Stahl N, Farruggella T, Boulton TG, Zhong Z, Darnell JE, Jr, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1352. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Takamura M, Sakamoto M, Genda T, Ichida T, Asakura H, Hirohashi S. Inhibition of intrahepatic metastasis of human hepatocellular carcinoma by Rho-associated protein kinase inhibitor Y-27632. Hepatology. 2001;33:577–581. doi: 10.1053/jhep.2001.22652. [DOI] [PubMed] [Google Scholar]
- Takemoto H, Doki Y, Shiozaki H, Imamura H, Utsunomiya T, Miyata H, Yano M, Inoue M, Fujiwara Y, Minden M. Localization of IQGAP1 is inversely correlated with intracellular adhesion mediated by E-cadherin in gastric cancers. Int J Cancer. 2001;91:783–788. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1121>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Valgeirsdottir S, Oaukku K, Silvennoinen O, Heldin CH, Claesson-Welsh L. Activation of Stat5 by platelet-derived growth factor (PDGF) is dependent on phosphorylation sites in PDGF beta-receptor juxtamembrane and kinase insert domains. Oncogene. 1998;16:505–515. doi: 10.1038/sj.onc.1201555. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. RhoGTPases and signaling network. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Van Golen KL, Wu ZF, Qiao XT, Bao L, Merajver SD. RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells. Neoplasia. 2000a;2:418–425. doi: 10.1038/sj.neo.7900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Golen KL, Wu ZF, Qiao XT, Bao L, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000b;60:5832–5838. [PubMed] [Google Scholar]
- Wang Y, Dang J, Wang H, Allgayer H, Murrell GA, Boyd D. Identification of a novel factor-kappa B sequence involved in expression of urokinase-type plasminogen activator receptor. Eur J Biochem. 2000;267:3248–3254. doi: 10.1046/j.1432-1327.2000.01350.x. [DOI] [PubMed] [Google Scholar]
- Welsh CF, Roovers K, Villanueva J, Liu Y, Schwartz MA, Assoian RK. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol. 2001;3:950–957. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- Wen Z, Darnell JE., Jr Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res. 1997;24:2062–2067. doi: 10.1093/nar/25.11.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Lambert QT, Clarck GJ, Symons M, Van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead IP, Lambert QT, Glaven JA, Abe K, Rossman KL, Mahon GM, Trzaskos JM, Kay R, Campbell SL, Der CJ. Dependence of Dbl and Dbs transformation on MEK and NF-kB activation. Mol Cell Biol. 1999;19:7759–7770. doi: 10.1128/mcb.19.11.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Nevalainen MT, Xu J, LeBaron MJ, Wagner KU, Erwin RA, Harmon JM, Hennighausen L, Kirken RA, Rui H. Role of serine phosphorylation of Stat5a in prolactin-stimulated beta-casein gene expression. Mol Cell Endocrinol. 2001;183:151–163. doi: 10.1016/s0303-7207(01)00546-9. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Xu J, Erwin RA, Rarrar WL, Kirken RA, Rui H. Differential control of the phosphorylation state of proline-juxtaposed serine residues Ser725 of Stat5a and Ser730 of Stat5b in prolactin-sensitive cells. J Biol Chem. 1998;273:3021–3024. doi: 10.1074/jbc.273.46.30218. [DOI] [PubMed] [Google Scholar]
- Yu CL, Jove R, Burakoff SJ. Constitutive activation of the Janus kinase-STAT pathway in T lymphoma overexpressing the Lck protein tyrosine kinase. J Immunol. 1997;159:5206–5210. [PubMed] [Google Scholar]
- Zhong C, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar M, Teramoto H, Katz BZ, Yamada KM, Gutkind JS. Effector domain mutants of Rho dissociated cytoskeletal changes from nuclear signaling and cellular transformation. Oncogene. 1998;17:991–998. doi: 10.1038/sj.onc.1202022. [DOI] [PubMed] [Google Scholar]
- Zondag GC, Evers EE, ten Klooster JP, van der Krammen RA, Collard JG. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–782. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]