Abstract
The adapter protein FADD consists of two protein interaction domains: a death domain and a death effector domain. The death domain binds to activated death receptors such as Fas, whereas the death effector domain binds to procaspase 8. An FADD mutant, which consists of only the death domain (FADD-DD), inhibits death receptor–induced apoptosis. FADD-DD can also activate a mechanistically distinct, cell type–specific apoptotic pathway that kills normal but not cancerous prostate epithelial cells. Here, we show that this apoptosis occurs through activation of caspases 9, 3, 6, and 7 and a serine protease. Simultaneous inhibition of caspases and serine proteases prevents FADD-DD–induced death. Inhibition of either pathway alone does not prevent cell death but does affect the morphology of the dying cells. Normal prostate epithelial cells require both the caspase and serine protease inhibitors to efficiently prevent apoptosis in response to TRAIL. In contrast, the serine protease inhibitor does not affect TRAIL-induced death in prostate tumor cells suggesting that the FADD-DD–dependent pathway can be activated by TRAIL. This apoptosis pathway is activated in a cell type–specific manner that is defective in cancer cells, suggesting that this pathway may be targeted during cancer development.
INTRODUCTION
Apoptotic caspases can be separated into “initiator caspases” such as caspase 8 and 9 that start an apoptotic cascade and “effector caspases” such as caspase 3, 6 and 7 that disassemble the cell (Nicholson, 1999). Two main pathways leading to caspase activation have been characterized (Hengartner, 2000). The extrinsic pathway is activated by ligand-bound death receptors of the tumor necrosis factor (TNF) receptor family (Ashkenazi and Dixit, 1998). The six identified death receptors contain an intracellular protein interaction domain called a death domain and induce apoptosis by forming a complex (called the DISC) at the death domain. The adapter FADD is an essential component of the DISC (Chinnaiyan et al., 1995). FADD consists of two protein interaction domains: a death domain and a death effector domain that interacts with a death effector domain on procaspase 8. FADD binds to the receptor or other adapters such as TRADD (Hsu et al., 1995) through interactions between death domains to recruit caspase 8 to the DISC. Aggregation of initiator caspases at the DISC leads to their autoactivation (Salvesen and Dixit, 1999), and they in turn activate effector caspases causing the cell to undergo apoptosis. Because FADD is an essential component of the DISC, a mutant (FADD-DD, also called FADD-DN) that consists of the death domain, but no death effector domain has been widely used to determine whether FADD signaling is required for apoptosis. FADD-DD acts as an inhibitor because it competes with the wild-type protein and binds to activated receptors but cannot recruit and activate caspase 8. FADD-DD inhibits apoptosis by all death ligands (Wajant et al., 1998) and several other stimuli.
Diverse stress pathways cause release of mitochondrial proteins into the cytosol to activate the other apoptosis pathway—the intrinsic pathway. Protein release occurs after binding of proapoptotic Bcl-2 family members and other proteins, e.g., the transcription factor TR3 (Li et al., 2000), to mitochondria. Antiapoptotic members of the Bcl-2 family such as Bcl-2 and Bcl-xL inhibit mitochondrial protein release to prevent apoptosis. Cytoplasmic cytochrome c (cyt c) interacts with Apaf-1, procaspase 9, and dATP to form a complex called the apoptosome (Li et al., 1997). This complex activates caspase 9, which then activates effector caspases to induce apoptosis. Other proapoptotic mitochondrial proteins include apoptosis-inducing factor (AIF; Susin et al., 1999), Smac/Diablo (Du et al., 2000; Verhagen et al., 2000), endonuclease G (Li et al., 2001), and Omi/HtrA2 (Suzuki et al., 2001; Hegde et al., 2002; Martins et al., 2002; Verhagen et al., 2002). Death receptors can activate the intrinsic pathway through cleavage of Bid (Luo et al., 1998).
Other proteases in addition to caspases are also involved in apoptosis (Johnson, 2000; Leist and Jaattela, 2001a). For example, several studies implicate lysosomal proteases (cathepsins) in apoptosis (Jones et al., 1998; Guicciardi et al., 2000; Foghsgaard et al., 2001; Leist and Jaattela, 2001b). The spectrum of proteases that are activated in response to a stimulus affects the commitment to and the phenotype of cell death (Leist and Jaattela, 2001a).
Recently, we identified an unusual proapoptotic activity for the FADD death domain (Morgan et al., 2001). Expression of FADD-DD from microinjected expression plasmids activated caspases and induced apoptosis in normal but not cancerous prostate epithelial cells. FADD-DD–induced apoptosis did not occur in normal prostate fibroblasts or smooth muscle cells, indicating that the effect is cell type specific. Despite the increased caspase activity in FADD-DD–expressing normal prostate cells, caspase inhibitors did not prevent cell death (Morgan et al., 2001). These results raise several questions. First, which caspases are activated by FADD-DD? Second, which caspase activation pathway (intrinsic or extrinsic) is used? Third, do the activated caspases actually have a role in this apoptosis response? Fourth, what is the nature of the signal that kills cells when the caspases are inhibited? In this article, we answer these questions.
MATERIALS AND METHODS
Cell Culture, Microinjection, and Adenovirus Infection
Normal human prostate epithelial cells were isolated from tissue samples or obtained from Clonetics (La Jolla, CA) and cultured as previously described (Morgan et al., 2001). Prostate cell lines were obtained from ATCC (Rockville, MD). Microinjection experiments were performed as previously described (Morgan et al., 2001); cells were injected with expression plasmids using an Eppendorf microinjector (Newport, RI). Quantitative cell survival experiments were performed by identifying injected fluorescent cells 3 h after injection and then determining the fate of each cell (i.e., whether it lived or died) after an additional 18-h incubation. Data presented in the histograms represents the mean ± SEM from between 3 and 10 separate injection experiments with different preparations of cells and plasmids. Each experiment involved 50–200 injected cells per sample. Recombinant doxycycline (Dox)-regulated YFP and YFP-FADD-DD adenoviruses were made using the AdenoX Tet-off kit from Clontech (Palo Alto, CA). Viruses were produced according to the manufacturer's instructions and coinfection with a Tet repressor virus was performed into prostate epithelial cells. Greater than 90% infection was achieved in both tumor cell lines and normal primary prostate epithelial cells. Repression was achieved by maintaining the cells in 1 μg/ml doxycycline, and gene expression was stimulated by removal of Dox. Where indicated, cells were treated with the general caspase inhibitor zVAD.fmk (0.1 mM; Alexis, San Diego, CA), the serine protease inhibitor AEBSF (0.3 mM; Sigma, St. Louis, MO) or the caspase 8 inhibitor zIETD.fmk (0.1 mM; Calbiochem, La Jolla, CA).
Western Blotting
For Western blot analysis of caspase cleavage, cells were harvested 24–48 h after adenovirus infection. Protein samples were separated by SDS-PAGE and probed with the following antibodies: anti-YFP (Clontech); anticaspase-cleaved cytokeratin 18 (Roche, Indianapolis, IN); anti-PARP, anticaspase 8, antiactive caspase 9, anticaspase 3, antiactive caspase 7, and anticaspase 6 (Cell Signaling, Beverly, MA); and antiactin (Sigma).
Reverse Two-hybrid Screen
Reverse two-hybrid screening was performed as previously described (Thomas et al., 2002). A library of >500,000 random FADD mutants was generated by mutagenic PCR and screened to identify point mutants that cannot bind to caspase 8 (a catalytically inactive mutant with cysteine 360 in the active site mutated to alanine was used) but retain the ability to bind to Fas as well as the wild-type protein. Between 1 and 23 separate isolates of the following single-point mutants were identified in the screen. Leu 7 to Pro, Leu 8 to Pro, Ser 10 to Pro, Ser 12 to Leu, Ser 12 to Pro, Ser 13 to Pro, Leu 15 to Pro, Leu 23 to Pro, Leu 23 to Arg, Leu 26 to Pro, Leu 49 to Pro, Leu 55 to Pro. The mutations to proline are likely to disrupt alpha helices in the DED. Mutants L8P, S12L, and L15P were chosen for further analysis, expressed as GFP-tagged fusion proteins and used for cell injection experiments.
Fluorescence Resonance Energy Transfer Assays of Caspase Activity
FRET assays for caspase activation were performed as previously described (Morgan and Thorburn, 2001) except that the blue fluorescent protein-yellow fluorescent protein described previously was replaced with a cyan-yellow fusion. The caspase-cleavable linker peptide was identical to the previous fusion protein. Cells were injected with the FRET construct along with FADD-DD expression plasmid (without a fluorescent tag) and Bcl-xL expression plasmid or empty vector then maintained in an environmental chamber at 37°C and 5% CO2 on a Zeiss Axiovert 100 microscope (Thornwood, NY). The following images were captured at 30-min intervals: phase, FRET (excite cyan at 440 nm, detect yellow emission at 575 nm), cyan (excite cyan at 440 nm, detect cyan emission at 485 nm). For each cell the ratio of yellow/cyan fluorescence per unit area was calculated for each time point after subtraction of the background fluorescence as previously described (Morgan and Thorburn, 2001). Quantitation was halted when the injected cells began to contract as determined by the total area of the cell being reduced by half. There was no consistent difference in the time that control and Bcl-xL–expressing cells began to contract. The increase in caspase activity was calculated as the inverse of the percent change in yellow/blue fluorescence ratio for each cell. Quantitation for 120 min before cell rounding is shown to provide a measure of the temporal changes in caspase activation that occur in individual cells.
Time-lapse Microscopy
YFP-FADD-DD–injected cells were maintained in the environmental chamber in the presence of zVAD.fmk (0.1 mM) and/or AEBSF (0.3 mM) where indicated. Fluorescent cells were identified and fluorescent and phase images of the same fields were captured using a Hamamatsu CCD (Malvern, PA) camera run by Openlab (Improvision, Warwick, UK) software. Images were captured at 30-min intervals for up to 24 h. Fluorescence images for each time point were overlayed on the corresponding phase image, and the resulting movie was saved in Quicktime format.
TRAIL-induced Apoptosis
Normal prostate epithelial cells or DU145 prostate tumor cells were pretreated for 30 min with 0.8 μg/ml cycloheximide and then treated with 100 ng/ml recombinant human TRAIL (Calbiochem) in the presence zVAD.fmk or AEBSF as indicated. Cells were monitored by microscopy after incubation with TRAIL for 24 h.
RESULTS
Adenoviral Expression of FADD-DD Causes Apoptosis of Normal Prostate Epithelial Cells
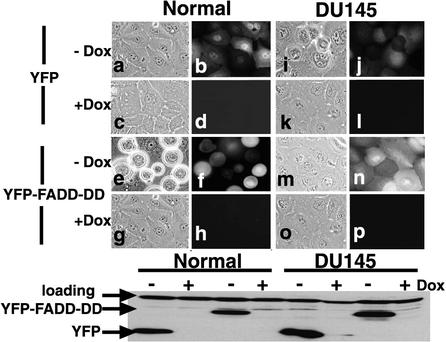
Our previous experiments showing that FADD-DD could induce prostate epithelial cell apoptosis were performed by microinjection of FADD-DD expression plasmids (Morgan et al., 2001). To exclude the remote possibility that apoptosis was dependent on the FADD-DD delivery method and to allow the use of biochemical assays, we constructed Dox-regulated adenoviruses expressing YFP-tagged FADD-DD or a YFP control. Coinfection of these viruses with a Tet repressor virus results in efficient repression in the presence of Dox and expression when Dox is removed. Figure 1 shows YFP and YFP-FADD-DD expression in normal primary prostate epithelial cells or the prostate epithelial tumor cell line DU145. Expression was tightly regulated by Dox as demonstrated by the fluorescence images and Western blot. FADD-DD but not the YFP control caused normal cells to die (compare panels a and b with e and f). FADD-DD did not kill the tumor cells (compare panels i and j with m and n). The Western blot shows that both normal and tumor cells expressed similar amounts of YFP and YFP-FADD-DD. The FADD-DD–expressing adenovirus did not kill normal primary prostate fibroblasts or other prostate cancer cell lines (LNCaP, PC3, CA-HPV7, unpublished data). These data extend our previous experiments (Morgan et al., 2001) and demonstrate that the difference in response between normal prostate epithelial cells and tumor cells is not due to differences in the expression levels of FADD-DD in the different cell types.
Figure 1.
FADD-DD selectively kills normal prostate epithelial cells. Normal primary prostate epithelial cells or DU145 tumor cells were infected with Dox-regulated adenoviruses expressing YFP (a,b,c,d,i,j,k,l) or YFP-tagged FADD-DD (e,f,g,h,m,n,o,p) and a TetR virus. Adjacent panels show phase and fluorescence images of the same field. Expression was induced by removing Dox. FADD-DD caused normal cells to die (e and f) but did not affect DU145 cells (m and n). YFP had no effect in either cell type. The lower panel shows total protein probed with anti-YFP. Equal amounts of protein were expressed in each case. The top band is a cross-reacting protein serving as a loading control.
FADD-DD Inhibits Fas-induced Apoptosis in Prostate Tumor Cells
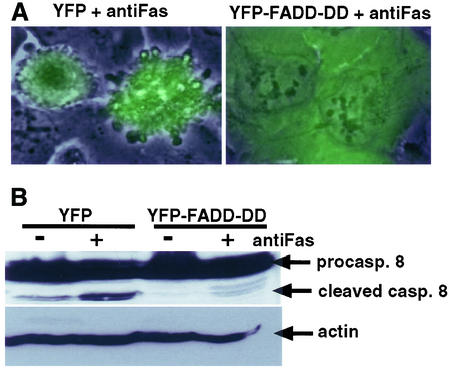
Induction of apoptosis by FADD-DD was an unexpected finding because this molecule has been widely used as an inhibitor of death receptor–induced apoptosis. We therefore tested whether our FADD-DD molecule could inhibit Fas-induced apoptosis in prostate tumor cells. DU145 cells activate caspase 8 and undergo apoptosis when stimulated with agonistic Fas antibodies in the presence of cycloheximide (Rokhlin et al., 1998). We expressed YFP or YFP-FADD-DD from the regulated adenovirus and then treated DU145 cells with anti-Fas in the presence of cycloheximide. Phase and fluorescence images of the same field were overlayed to allow examination of the morphology of YFP or YFP-FADD-DD–expressing cells after activation of Fas signaling. Figure 2A shows that FADD-DD prevented Fas-induced cell death, whereas YFP did not. The dying cells showed typical characteristics of apoptosis with multiple membrane blebs. Western blotting for the appearance of the cleaved form of caspase 8 showed that FADD-DD expression prevented activation of caspase 8 in response to Fas (Figure 2B). These data indicate that our FADD-DD molecule inhibits apoptosis signaling by activated death receptors. Therefore, the novel proapoptotic ability of FADD-DD in normal prostate epithelial cells occurs in addition to the established antiapoptotic functions of this molecule, which occur in prostate tumor cells.
Figure 2.
FADD-DD inhibits Fas-induced apoptosis in prostate tumor cells. DU145 cells were infected with the YFP or YFP-FADD-DD adenoviruses, expression was induced by removing Dox, and then cells were treated with an anti-Fas agonistic antibody. (A) Cellular morphology indicating Fas-induced apoptosis that is inhibited by YFP-FADD-DD but not by YFP. (B) Total protein samples Western blotted with anticaspase 8. Fas stimulates caspase 8 as indicated by the appearance of the processed caspase 8 band that is inhibited by YFP-FADD-DD.
FADD-DD Activates Caspases in Normal Epithelial Cells
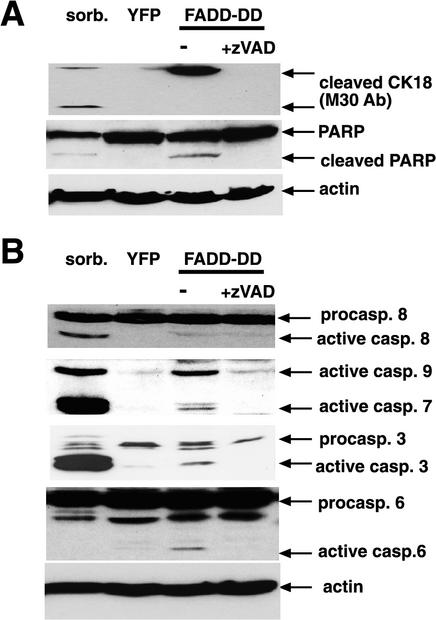
We used adenovirus-infected cells and Western blotting to test whether caspases are activated by FADD-DD. First, we tested whether FADD-DD could cause the appearance of caspase-dependent epitopes in endogenous proteins. Cytokeratin 18 is cleaved by effector caspases (Caulin et al., 1997) during epithelial cell apoptosis. A caspase-dependent neo-epitope revealed by cleavage of cytokeratin 18 at Asp398 is recognized by the M30 antibody (Bantel et al., 2000). The antibody recognizes two fragments of ∼45 and 21 kDa, depending on whether a second caspase site at Asp 237 is also cleaved (Bantel et al., 2000). To test whether FADD-DD expression led to cytokeratin 18 cleavage, we expressed YFP or YFP-FADD-DD from the regulated adenoviruses, harvested the cells, and probed a Western blot with the M30 antibody. Figure 3A shows that FADD-DD and the positive control (sorbitol treatment to induce hyperosmolar stress-induced apoptosis) caused appearance of the 45-kDa neo-epitope, whereas YFP expression did not. Sorbitol also caused the appearance of the 21-kDa band. We also detected caspase-cleaved PARP in response to FADD-DD and sorbitol. The cleaved proteins were not present when FADD-DD–expressing cells were treated with the caspase inhibitor zVAD.fmk. These data indicate that active effector caspases are induced by FADD-DD in normal prostate cells.
Figure 3.
Caspase activation by FADD-DD. Normal prostate epithelial cells were infected with YFP or YFP-FADD-DD adenoviruses or treated with 300 mM sorbitol as a control. Cells were harvested and total protein extracts separated on gels and Western blotted with the indicated antibodies. Panel A shows that FADD-DD causes the appearance of cleaved forms of cytokeratin 18 and PARP that are blocked by treatment with zVAD.fmk. Panel B shows that active forms of caspase 9, 7, 3, and 6 but not caspase 8 are induced by FADD-DD.
We next asked which caspases were activated after expression of YFP or YFP-FADD-DD (Figure 3B). As a positive control to ensure that the antibodies worked, we used sorbitol-treated cells. Unlike the other caspases, where cleavage is required and sufficient for activation, caspase 9 does not require processing for activation (Renatus et al., 2001). However, active caspase 9 digests itself when it is activated by dimerization (Renatus et al., 2001). Thus, the presence of cleaved caspase 9 indicates caspase 9 activity. Sorbitol treatment and FADD-DD expression caused the appearance of active forms of caspase 9, caspase 7, and caspase 3. Active caspase 6 was detected in FADD-DD–expressing cells but not in the cells treated with sorbitol. Sorbitol and FADD-DD led to similar levels of caspase 9 cleavage but sorbitol was more effective at activating caspase 3 and caspase 7 than FADD-DD. We could not detect activation of caspase 8 by FADD-DD, however, sorbitol was effective at activating caspase 8. Activation of caspase 8 by sorbitol likely contributes to the increased activity of caspase 3 and 7 compared with FADD-DD. zVAD.fmk inhibited the appearance of cleaved forms of the caspases in FADD-DD–expressing cells, suggesting that the effector caspases are activated as a result of activation of initiator caspases (i.e., caspase 9) and that the activated caspase 9 digests itself. These data suggest that FADD-DD stimulates the intrinsic pathway in normal prostate cells to activate caspase 9 and downstream effector caspases. FADD-DD does not activate caspase 8, which is usually activated by death receptors. This is not surprising because FADD-DD lacks the death effector domain that is required for caspase 8 activation.
Caspases and Serine Proteases Contribute to FADD-DD–induced Apoptosis
Despite the fact that caspases are activated by FADD-DD, we previously found that caspase inhibitors could not prevent FADD-DD–induced prostate epithelial cell death (Morgan et al., 2001). We therefore tested whether other proteases might be involved in this response. Several studies indicate a role for serine proteases in apoptosis (Johnson, 2000; Leist and Jaattela, 2001a). Furthermore, some proteins such as the tumor suppressor Bin1 induce apoptosis that can be blocked by serine protease inhibitors like AEBSF but not by caspase inhibitors (Elliott et al., 2000). To test whether a serine protease inhibitor could prevent FADD-DD–induced apoptosis, we injected normal prostate epithelial cells with FADD-DD expression plasmids then treated cells with zVAD.fmk or AEBSF and monitored cell survival by determining the fate of each FADD-DD–expressing cell.
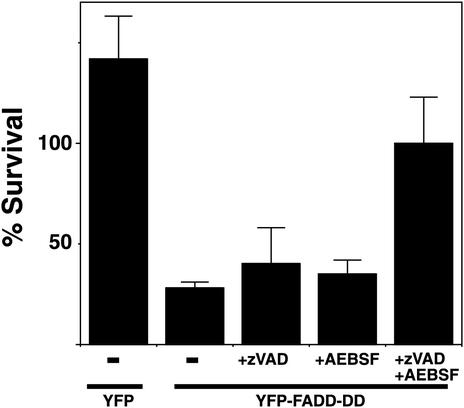
Figure 4 shows that neither zVAD.fmk nor AEBSF could prevent cell death when added on their own. However, when we treated FADD-DD–expressing cells with both inhibitors simultaneously, they survived. Some of the control YFP cells underwent cell division, resulting in an apparent survival of >100%, whereas the FADD-DD–expressing cells did not rise above 100% even in the presence of both inhibitors. This may reflect a separate effect of the protease inhibitors or FADD-DD inhibiting cell growth. Because both the caspase inhibitor and serine protease inhibitor are required to prevent cell death, these data suggest that separate caspase and serine protease signals are activated by FADD-DD and that one enzyme is not upstream of the other. Furthermore, if one signal is blocked, the other protease is sufficient to kill the cells. Because it has been reported that zVAD.fmk can inhibit cathepsin B (Schotte et al., 1999), we tested whether a cathepsin B–specific inhibitor (CA-074-ME) could cooperate with AEBSF to prevent FADD-DD–induced cell death. The cathepsin B inhibitor did not mimic the effect of zVAD.fmk (unpublished data) indicating that caspases themselves are important in the response.
Figure 4.
Caspases and serine proteases contribute to FADD-DD–induced apoptosis. Normal prostate cells were injected with the YFP control of YFP-FADD-DD expression plasmids as indicated then treated with the caspase inhibitor (zVAD.fmk, 0.1 mM) or serine protease inhibitor (AEBSF, 0.3 mM) as indicated. The percentage of injected cells that survive were determined after 24-h incubation, indicating that neither zVAD.fmk or AEBSF alone could prevent FADD-DD–induced cell death but the combination of both inhibitors did prevent death. Data shown are means ± SEM from five separate experiments.
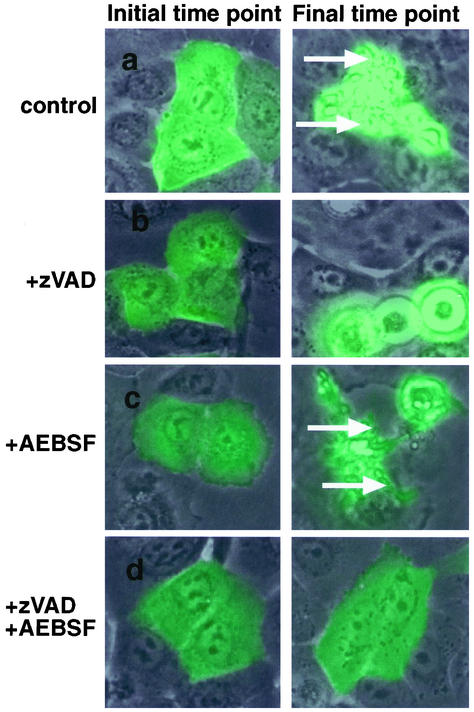
Caspases and Serine Proteases Have Different Effects on the Morphology of Dying Cells
There are examples in the literature where other proteases and caspases induce similar apoptotic phenotypes presumably by cleaving the same substrates (Foghsgaard et al., 2001). There are also examples where the phenotypes of cells dying in response to stimuli that can activate both caspases and other death signals is quite different (McCarthy et al., 1997). To determine whether normal prostate epithelial cells dying in response to FADD-DD utilize caspase- and serine protease-dependent signals to kill cells in different ways, we used time-lapse microscopy. Figure 5 shows frames from time-lapse series of FADD-DD–injected normal prostate cells in the absence or presence of zVAD.fmk and AEBSF. Fluorescence images are overlayed on the phase image. The figure shows the same cells at the initial time point and the final time point (6–7 h for the control, zVAD.fmk, and AEBSF-treated cells and 15 h for the zVAD.fmk +AEBSF–treated cells). The green cells express FADD-DD. Quicktime movies of this experiment are included in the supplementary material. With no inhibitors, FADD-DD–expressing cells contract and display numerous membrane blebs (arrows) that retain the fluorescent protein as is typical of caspase-dependent apoptosis. In the presence of zVAD.fmk, the dying cells contract, round up, and detach from the dish but do not display membrane blebs. Conversely, FADD-DD–injected cells dying in the presence of AEBSF with no zVAD.fmk display typical hallmarks of caspase activity such as membrane blebs. In agreement with the cell survival data shown in Figure 4, most of the cells that express FADD-DD in the presence of both zVAD.fmk and AEBSF remain flat and attached to the dish. Thus, the caspases that are activated by FADD-DD in normal epithelial cells cause the distinct membrane blebbing that is characteristic of the dying cells.
Figure 5.
Caspases and serine proteases regulate different aspects of FADD-DD–induced apoptosis. Normal prostate cells were injected with the YFP-FADD-DD expression plasmid then incubated with zVAD.fmk or AEBSF as indicated. Time-lapse fluorescence microscopy was performed to monitor the response of each injected cell. The images show overlayed fluorescence and phase images of the same cells at the beginning and end of the experiment indicating that FADD-DD causes membrane blebbing and cell fragmentation that is blocked by zVAD.fmk but not by AEBSF. Quicktime movies of these experiments are contained in the supplementary material.
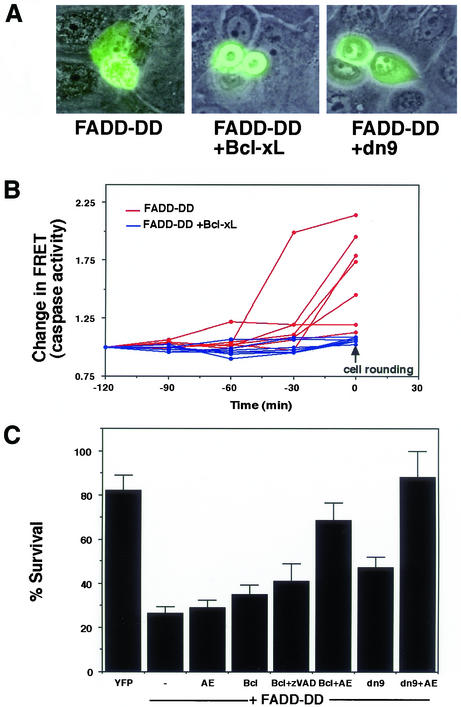
Bcl-xL Inhibits Caspase-dependent Phenotypes in FADD-DD–induced Apoptosis
Bcl-xL blocks release of cytochrome c and other mitochondrial proteins. We examined the morphology of cells that coexpressed FADD-DD and Bcl-xL. Bcl-xL prevented membrane blebbing but did not prevent cell rounding (Figure 6A). This suggests that Bcl-xL inhibited caspase activation but not the serine protease that is inhibited by AEBSF. Similar inhibition of blebbing was observed when we coexpressed FADD-DD with a dominant-negative version of caspase 9 (dn9), which has a cysteine-serine mutation at the active site. These data suggest that FADD-DD activates caspase 9 through the mitochondrial pathway and Bcl-xL should inhibit FADD-DD–induced caspases. We tested this hypothesis by directly measuring caspase activity in cells that were injected with FADD-DD using a FRET-based method (Morgan and Thorburn, 2001). The method allows the continual measurement of caspase activity in individual cells. We monitored changes in caspase activity for 120 min before the beginning of cell contraction and rounding in FADD-DD–expressing cells in the presence or absence of Bcl-xL. This is achieved by monitoring changes in the ratio of yellow (FRET) fluorescence/cyan fluorescence emitted from a CFP-YFP fusion that can be cleaved by caspase 3 and other effector caspases. Figure 6B shows caspase activity in seven FADD-DD– and seven FADD-DD+Bcl-xL–expressing cells. FADD-DD causes activation of a caspase that can cleave the FRET probe. This is shown by a rise in caspase activity as measured by a loss of FRET and increase in cyan fluorescence resulting in a change in the yellow/cyan ratio.
Figure 6.
Bcl-xL inhibits activation of caspases by FADD-DD. FADD-DD–expressing cells were coinjected with control, Bcl-xL, and dominant-negative caspase 9 (dn9) expression plasmids. Panel A shows the morphology of injected cells. Bcl-xL and dn9 inhibit the membrane blebbing that is typically observed with FADD-DD alone. The images are overlayed fluorescence and phase images, green cells express the YFP-tagged FADD-DD molecule. Panel B shows caspase activation in seven cells injected with FADD-DD (red lines) and seven cells injected with FADD-DD plus Bcl-xL (blue lines) along with the expression plasmid encoding the cyan-yellow FRET probe. Fluorescence images were captured for yellow and cyan fluorescence, and the ratio of yellow/cyan fluorescence per unit area was calculated for each time point. The inverse change in FRET ratio indicative of caspase activation was plotted after normalization for each cell. Quantitation was for 120 min before cell contraction and rounding began (designated time 0). FADD-DD causes an abrupt increase in caspase activity that is prevented by Bcl-xL. Panel C shows cell survival of FADD-DD injected cells, cells injected with FADD-DD plus Bcl-xL or cells injected with FADD-DD plus dominant-negative caspase 9 (dn9) in the presence or absence of AEBSF (AE) or zVAD.fmk (zVAD) where indicated. FADD-DD induces cell death that cannot be blocked by AEBSF, zVAD, Bcl-xL, or dn9 alone. Bcl-xL and zVAD.fmk do not cooperate to prevent FADD-DD–induced death. Both Bcl-xL and dominant-negative caspase 9 cooperate with AEBSF to inhibit FADD-DD–induced cell death, resulting in cell survival that is similar to the YFP control. Data are means ± SEM from between 4 and 12 experiments for each sample.
The increase in caspase activity begins abruptly 30–60 min before each cell began to contract, which was designated as time 0. Bcl-xL inhibited this caspase activation and there was no detectable increase in caspase activity in any of the Bcl-xL–expressing cells. Note, however, that like the FADD-DD alone cells, each of the cells expressing Bcl-xL did undergo cell contraction and rounding, beginning at the time point designated as 0 min. Caspases presumably become active shortly after the release of cytochrome c, which Green and colleagues have demonstrated to be rapid and coordinated within each cell (Goldstein et al., 2000). Together, these data suggest that the caspase activation occurs through the mitochondrial pathway and that the serine protease that is inhibited by AEBSF is activated through a different mechanism. If this is correct, Bcl-xL or dominant-negative caspase 9 should both fail to inhibit FADD-DD–induced cell death on their own but instead should cooperate with AEBSF to prevent cell death. Conversely, Bcl-xL should not cooperate with zVAD.fmk to inhibit FADD-DD–induced death. Figure 6C shows that this is indeed the case. Cell death was efficiently blocked only by the combination of AEBSF with either Bcl-xL or dominant-negative caspase 9.
Full-length FADD Induces Apoptosis via a Caspase 8–independent Mechanism in Normal but not Cancerous Prostate Cells
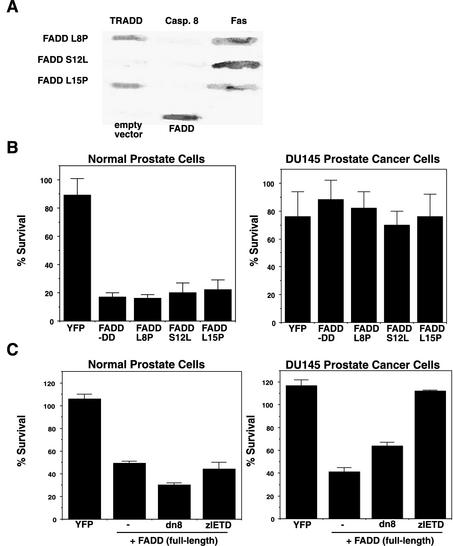
The previous experiments were performed using the truncated FADD-DD molecule. Expression of full-length wild-type FADD induces apoptosis in all cells by virtue of its ability to activate caspase 8. To test if the same pathway could be activated by full-length FADD rather than just the truncated molecule, we first identified FADD point mutants that are unable to bind caspase 8 using a reverse two-hybrid screen (Thomas et al., 2002). Reverse two-hybrid screens identify mutants that have lost the ability to interact with a particular protein. Our modified method requires that these mutants retain the ability to interact with a different protein and thus selects for mutants that lose specific binding interactions without affecting overall protein structure or stability. We screened >500,000 random FADD mutants and identified mutants that retained the ability to interact with Fas but could not interact with caspase 8. All the mutations were in the death effector domain of the protein. Three mutants (L8P, S12L, and L15P) were chosen for further analysis. All three mutants bound to Fas but not caspase 8. The L8P and L15P mutants also displayed wild-type binding to TRADD (Figure 7A). The mutants were made as GFP fusions and injected into normal prostate cells or DU145 tumor cells. If the FADD-dependent apoptosis in normal cells is distinct from FADD's established mechanism of action through caspase 8, these mutants should behave like FADD-DD and kill normal prostate cells but not prostate tumor cells. Figure 7B shows that this was the case.
Figure 7.
Full-length FADD kills normal prostate cells via a caspase 8–independent mechanism. (A) Yeast β-galactosidase filter assays of directed two-hybrid interactions of full length FADD mutants that were selected by reverse two-hybrid screening for failure to interact with caspase 8 while retaining the ability to interact with Fas. FADD L8P and L15P mutants bound both TRADD and Fas but not caspase 8. The S12L mutant did not bind caspase 8 or TRADD. Controls show β-Gal assays of yeast expressing the empty vector and wild-type FADD binding to caspase 8. (B) Cell survival assays after microinjection of YFP, FADD-DD, or the L8P, S12L, and L15P full-length FADD mutants into normal prostate cells and DU145 prostate cancer cells. FADD-DD and the three full-length FADD mutants killed normal cells but not DU145 cells. (C) Cell survival after microinjection of YFP or wild-type FADD into normal prostate cells or DU145 cells. Wild-type FADD killed both normal cells and cancer cells. Coexpression of dominant-negative caspase 8 (dn8) or pretreatment with a caspase 8 inhibitor (zIETD) inhibited FADD-induced death in DU145 cells but not in normal prostate cells. These data indicate that FADD can kill normal prostate cells in a caspase 8–independent mechanism that is not active in DU145 cells.
Although the FADD point mutants and the isolated FADD death domain are only able to kill normal epithelial cells, the wild-type protein can induce apoptosis in both normal and cancerous cells. We next tested if this death was inhibited by caspase 8 inhibitors. Coexpression of full-length, wild-type GFP-FADD with dominant-negative caspase 8 or treatment with a selective caspase 8 inhibitor (zIETD.fmk) inhibited apoptosis of prostate cancer cells but did not inhibit apoptosis of normal prostate cells (Figure 7C). These data indicate that FADD can activate two apoptosis pathways in prostate epithelial cells. The first pathway involves caspase 8 recruitment through the death effector domain and functions in both prostate cancer cells and normal prostate cells. The second pathway works through the FADD death domain, does not involve caspase 8 and only functions in normal cells.
TRAIL-induced Apoptosis of Normal Prostate Cells Occurs through Caspase- and Serine Protease-dependent Pathways
The previous experiments were performed using exogenously expressed FADD molecules. However, there is no reason to think that FADD signaling in response to physiological signals is mediated through regulation of FADD expression levels. Rather, FADD is activated by death receptors such as Fas, TNFR1, or the TRAIL receptors, and overexpressed FADD mimics the effects that occur in response to receptor signaling, e.g., by activating caspase 8. In most cases, apoptosis after activation of these receptors is inhibited by caspase inhibitors such as zVAD.fmk. There are, however, examples where these receptors induce caspase-independent apoptosis (Foghsgaard et al., 2001) and necrosis (Vercammen et al., 1998a, 1998b; Denecker et al., 2001).
If death receptors activate the FADD-DD–dependent apoptosis pathway, we should find that normal prostate cell apoptosis induced by the relevant ligand would not be completely inhibited by zVAD.fmk alone but would show increased inhibition by zVAD.fmk plus AEBSF. Conversely, prostate tumor cells should be unable to activate the caspase 8–independent pathway and should therefore be maximally protected by zVAD.fmk alone. TNF-α did not efficiently kill normal prostate cells (unpublished data) but both Fas ligand– and TRAIL-induced apoptosis of normal prostate cells. TRAIL has been reported to be unable to kill normal human cells, including normal prostate epithelial cells (Ashkenazi et al., 1999; Walczak et al., 1999). However, other investigators have found that TRAIL can induce apoptosis of normal prostate epithelial cells (Nesterov et al., 2002), thus supporting our observations.
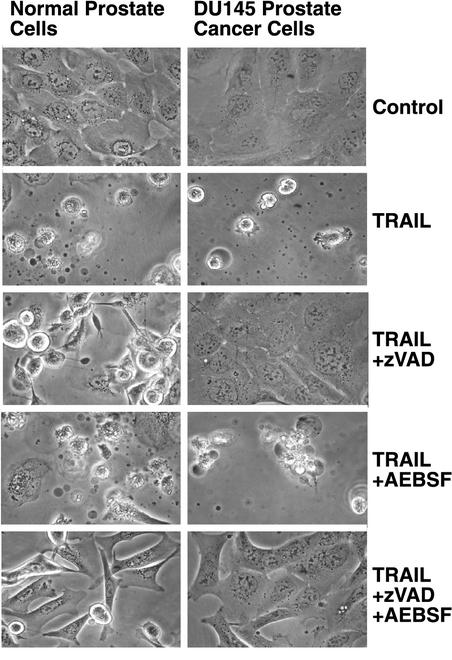
TRAIL-induced death of normal prostate cells was only partially inhibited by zVAD.fmk as shown by the presence of many rounded and detached cells but was blocked by zVAD.fmk plus AEBSF (Figure 8). AEBSF alone did not prevent TRAIL-induced cell death. The caspase inhibitor did prevent membrane blebbing because TRAIL treatment in the presence of zVAD.fmk resulted in many rounded cells without noticeable blebs. TRAIL alone and TRAIL plus AEBSF treatments resulted in many cells with noticeable membrane blebs. The involvement of an activity that is inhibited by AEBSF was specific to the normal cells because TRAIL-induced death of DU145 cells was inhibited completely by zVAD.fmk. The addition of AEBSF did not confer added protection to DU145 cells. These data suggest that TRAIL can activate the conventional, caspase 8–dependent apoptosis pathway and the pathway that involves both caspase and AEBSF-sensitive signals in normal prostate cells but can only activate the caspase 8 pathway in cancer cells.
Figure 8.
TRAIL induces apoptosis of normal but not cancerous prostate cells through an AEBSF-sensitive activity. Normal prostate epithelial cells and DU145 prostate cancer cells were treated with cycloheximide alone (control) or cycloheximide plus recombinant TRAIL in the presence of zVAD.fmk or AEBSF as indicated. Cell survival was determined by microscopy after 24 h. TRAIL killed both normal and cancerous prostate cells. The caspase inhibitor zVAD.fmk efficiently prevented cell death in the DU145 cells but did not prevent death in normal prostate cells. The addition of AEBSF and zVAD.fmk did prevent death of normal cells. These data indicate that TRAIL activates cell death pathways in normal prostate cells that have the same requirement for both caspase- and serine protease-dependent signals as FADD-DD.
DISCUSSION
In this article, we show that the isolated death domain of FADD, which inhibits death receptor–induced apoptosis in prostate tumor cells (Figure 2), can induce apoptosis in normal prostate cells. This response occurs only in normal epithelial cells, whereas epithelial cancer cells are resistant. Normal prostate fibroblasts and smooth muscle cells do not undergo FADD-DD–induced apoptosis (Morgan et al., 2001). These data, along with our previous identification of point mutants that do not induce apoptosis (Morgan et al., 2001), indicate that FADD-DD–induced apoptosis is not a nonspecific event. Rather, we suggest that the FADD death domain can activate a cell type–specific apoptotic pathway that functions in normal epithelial cells but is defective in tumor cells. Expression of exogenous wild-type FADD can activate caspase 8 to induce apoptosis of both normal cells and cancer cells. The apoptosis that occurs only in normal cells was therefore only apparent when we used a truncated protein that contains just the death domain, full-length FADD point mutants that cannot bind caspase 8 or when we inhibited caspase 8 in other ways.
FADD-DD activates the mitochondrial caspase-activation pathway by a Bcl-xL–sensitive mechanism to stimulate caspase 9 and then 7, 6, and 3 in normal prostate cells. In addition, at least one serine protease that can be inhibited by AEBSF is activated in normal cells by FADD-DD. There are several examples where noncaspase proteases are upstream of caspases. This can be achieved by digestion of the same signaling proteins (e.g., Bid) that are targeted by initiator caspases (Pinkoski et al., 2001; Stoka et al., 2001). In this case, an inhibitor of the upstream protease should prevent caspase activation. There are also examples where other proteases appear to be downstream of caspases (Jones et al., 1998; van Eijk and de Groot, 1999; Foghsgaard et al., 2001). In this case, a caspase inhibitor should prevent activation of the downstream protease. Activation of noncaspase proteases and caspases may also be mechanistically unrelated, and one class of protease may not be required for the activation of the other. FADD-DD activation of caspases and the AEBSF-sensitive serine protease in normal prostate cells is an example of the latter situation, because caspase and serine protease inhibitors must be combined to prevent cell death (Figure 4). Moreover, the morphology of the dying cells is different when caspase inhibitors or serine protease inhibitors are used, suggesting that the two types of protease target different substrates. If caspases are blocked, the dying cells do not display membrane blebbing and cell fragmentation but do round up and detach from the dish. Conversely, membrane blebbing is active in FADD-DD–expressing cells that are treated with the serine protease inhibitor (Figure 5). The requirement for caspase activity for membrane blebbing is well established and can be achieved by caspase cleavage of ROCK1 (Coleman et al., 2001; Sebbagh et al., 2001). These data suggest the model shown in Figure 9.
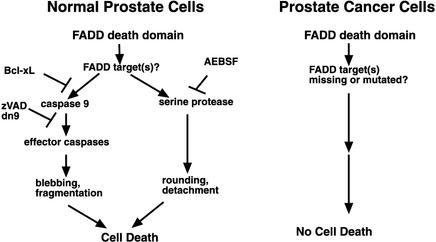
Figure 9.
Model of FADD-DD–induced apoptosis signaling in normal prostate epithelial cells. FADD-DD works through unidentified effectors to activate caspase 9 through a mitochondrial caspase activation pathway that is Bcl-xL sensitive. This leads to the activation of effector caspases, which cause cell death that is associated with membrane blebbing and cellular fragmentation. In addition, a serine protease pathway is activated that can be inhibited by AEBSF but not by Bcl-xL, which causes cell death that is associated with cell rounding and detachment. This pathway is not activated in prostate cancer cells because these cells possess defects in effector molecules that mediate the death domain signal.
There are other proteins that activate both caspase and serine protease-dependent pathways to kill cells. For example, the mitochondrial serine protease, Omi/HtrA2, is released into the cytoplasm where it can bind to and inhibit Inhibitor of Apoptosis proteins (Suzuki et al., 2001; Hegde et al., 2002; Martins et al., 2002; van Loo et al., 2002; Verhagen et al., 2002). This leads to increased caspase activity. In addition, Omi/HtrA2's serine protease activity can induce an atypical form of apoptosis (Suzuki et al., 2001; Verhagen et al., 2002). Although it is attractive to suggest that activation of Omi/HtrA2 might be responsible for all the effects that we observe with FADD-DD, we think this is unlikely for two reasons. First, caspase-independent death by Omi/HtrA2 has been reported to occur only when the serine protease is highly expressed (Martins et al., 2002). This suggests that physiological levels of this enzyme such as would be released in our cells may not kill by a serine protease-dependent mechanism. Second, Bcl-xL, which would presumably block the release of Omi/HtrA2, did not prevent FADD-DD–induced death but instead only prevented caspase activation and the caspase-dependent morphological phenotypes (Figure 6). This suggests that release of mitochondrial proteins is responsible for caspase activation but not for activation of the serine protease.
We previously found that the ability of FADD-DD mutants to interact with Fas or TRADD did not completely correlate with the mutants' ability to induce normal prostate cell apoptosis (Morgan et al., 2001). This implies that the truncated FADD is not functioning as an inhibitor of, for example, a Fas-induced survival signal. Instead, our data are more consistent with an active death pathway that is stimulated by the FADD death domain. TRAIL-induced death in normal prostate epithelial cells shows the same requirement for zVAD.fmk- and AEBSF-sensitive signals as FADD-DD. Therefore, TRAIL receptors may stimulate this FADD-dependent pathway under normal circumstances. We are further analyzing TRAIL- and FADD-DD–induced apoptosis in normal and cancerous prostate cells to test this hypothesis.
Apoptosis is a primary defense against cancer development (Hanahan and Weinberg, 2000; Green and Evan, 2002); however, apoptosis signaling pathways that perform this function have not been well characterized. Apoptosis pathways that serve to protect against cancer development should function in normal cells but not in cancer cells and may be cell type specific. Because FADD-DD has these characteristics, we suggest that the signaling pathway that is activated by FADD's death domain (perhaps in response to TRAIL) in normal epithelia suppresses carcinoma development. Further analysis of the mechanism of FADD-DD–induced apoptosis in normal epithelial cells and the mechanism of resistance in cancer cells may therefore provide new insights into the development of epithelial cancers and could identify new targets for cancer therapeutics. To this end, we are using genetically defined human epithelial cells to determine at which stage during the immortalization and transformation process epithelial cancer cells become resistant to FADD-DD–induced apoptosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Virshup for useful discussions and comments on the manuscript. We are grateful to Guy Salveson for providing the dominant-negative caspase 8 and 9 cDNAs. This work was supported by grants to A.T. from the Department of Defense, North Carolina Biotechnology Center and Wake Forest University. M.J.M. was supported by an National Cancer Institute training grant in Signal Transduction.
Abbreviations used:
- AEBSF
4-(2-aminoethyl)-benzenesulfonyl fluoride
- AIF
apoptosis-inducing factor
- CFP
cyan fluorescent protein
- DISC
death-inducing signaling complex
- Dox
doxycycline
- dn8
dominant-negative caspase 8
- dn9
dominant-negative caspase 9
- FADD
Fas-associated death domain protein
- FADD-DD
FADD-death domain
- FRET
fluorescence resonance energy transfer
- PARP
polyADP ribose polymerase
- TNF
tumor necrosis factor
- TRADD
TNF receptor–associated death domain protein
- TRAIL
TNF-related apoptosis inducing ligand
- YFP
yellow fluorescent protein
- zIETD.fmk
benzoylcarbonyl-Ile-Glu-Thr-Asp-fluoromethylketone
- zVAD.fmk
benzoylcarbonyl-Val-Ala-Asp-fluoromethylketone
Footnotes
Online version of this article contains video material. Online version is available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0207. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0207.
REFERENCES
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantel H, Ruck P, Schulze-Osthoff K. In situ monitoring of caspase activation in hepatobiliary diseases. Cell Death Differ. 2000;7:504–505. doi: 10.1038/sj.cdd.4400669. [DOI] [PubMed] [Google Scholar]
- Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Denecker G, et al. Death receptor-induced apoptotic and necrotic cell death: differential role of caspases and mitochondria. Cell Death Differ. 2001;8:829–840. doi: 10.1038/sj.cdd.4400883. [DOI] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Elliott K, Ge K, Du W, Prendergast GC. The c-Myc-interacting adaptor protein Bin1 activates a caspase-independent cell death program. Oncogene. 2000;19:4669–4684. doi: 10.1038/sj.onc.1203681. [DOI] [PubMed] [Google Scholar]
- Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M, Jaattela M. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol. 2001;153:999–1010. doi: 10.1083/jcb.153.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hegde R, et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts IAP-caspase interaction. J Biol Chem. 2002;277:432–438. doi: 10.1074/jbc.M109721200. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Johnson DE. Noncaspase proteases in apoptosis. Leukemia. 2000;14:1695–1703. doi: 10.1038/sj.leu.2401879. [DOI] [PubMed] [Google Scholar]
- Jones B, Roberts PJ, Faubion WA, Kominami E, Gores GJ. Cystatin A expression reduces bile salt-induced apoptosis in a rat hepatoma cell line. Am J Physiol. 1998;275:G723–G730. doi: 10.1152/ajpgi.1998.275.4.G723. [DOI] [PubMed] [Google Scholar]
- Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001a;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- Leist M, Jaattela M. Triggering of apoptosis by cathepsins. Cell Death Differ. 2001b;8:324–326. doi: 10.1038/sj.cdd.4400859. [DOI] [PubMed] [Google Scholar]
- Li H, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Martins LM, et al. The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a Reaper-like motif. J Biol Chem. 2002;277:439–444. doi: 10.1074/jbc.M109784200. [DOI] [PubMed] [Google Scholar]
- McCarthy NJ, Whyte MK, Gilbert CS, Evan GI. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Thorburn A. Measurement of caspase activity in individual cells reveals differences in the kinetics of caspase activation between cells. Cell Death Differ. 2001;8:38–43. doi: 10.1038/sj.cdd.4400800. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Thorburn J, Thomas L, Maxwell T, Brothman AR, Thorburn A. An apoptosis signaling pathway induced by the death domain of FADD selectively kills normal but not cancerous prostate epithelial cells. Cell Death Differ. 2001;8:696–705. doi: 10.1038/sj.cdd.4400866. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Ivashchenko Y, Kraft AS. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) triggers apoptosis in normal prostate epithelial cells. Oncogene. 2002;21:1135–1140. doi: 10.1038/sj.onc.1205151. [DOI] [PubMed] [Google Scholar]
- Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- Pinkoski MJ, Waterhouse NJ, Heibein JA, Wolf BB, Kuwana T, Goldstein JC, Newmeyer DD, Bleackley RC, Green DR. Granzyme B-mediated apoptosis proceeds predominantly through a Bcl-2-inhibitable mitochondrial pathway. J Biol Chem. 2001;276:12060–12067. doi: 10.1074/jbc.M009038200. [DOI] [PubMed] [Google Scholar]
- Renatus M, Stennicke HR, Scott FL, Liddington RC, Salvesen GS. Dimer formation drives the activation of the cell death protease caspase 9. Proc Natl Acad Sci USA. 2001;98:14250–14255. doi: 10.1073/pnas.231465798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokhlin OW, Glover RA, Cohen MB. Fas-mediated apoptosis in human prostatic carcinoma cell lines occurs via activation of caspase-8 and caspase-7. Cancer Res. 1998;58:5870–5875. [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci USA. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte P, Declercq W, Van Huffel S, Vandenabeele P, Beyaert R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 1999;442:117–121. doi: 10.1016/s0014-5793(98)01640-8. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Stoka V, et al. Lysosomal protease pathways to apoptosis: cleavage of bid, not Pro-caspases, is the most likely route. J Biol Chem. 2001;276:3149–3157. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- Susin SA, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, htra2, is released from the mitochondria and interacts with xiap, inducing cell death. Mol Cell. 2001;8:613–621. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- Thomas L, Stillman D, Thorburn A. Regulation of FADD death domain interactions by the death effector domain identified by a modified reverse two hybrid screen. J Biol Chem. 2002;277:34343–34348. doi: 10.1074/jbc.M204169200. [DOI] [PubMed] [Google Scholar]
- van Eijk M, de Groot C. Germinal center B cell apoptosis requires both caspase and cathepsin activity. J Immunol. 1999;163:2478–2482. [PubMed] [Google Scholar]
- van Loo G, et al. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ. 2002;9:20–26. doi: 10.1038/sj.cdd.4400970. [DOI] [PubMed] [Google Scholar]
- Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998a;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998b;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Verhagen AM, et al. HtrA2 promotes cell death through its serine protease activity and its ability to antagonise inhibitor of apoptosis proteins. J Biol Chem. 2002;277:445–454. doi: 10.1074/jbc.M109891200. [DOI] [PubMed] [Google Scholar]
- Wajant H, Johannes F-J, Haas E, Siemienski K, Schwenzer R, Schubert G, Weiss T, Grell M, Scheurich P. Dominant-negative FADD inhibits TNFR60-, Fas/Apo1- and TRAIL-R/Apo2-mediated cell death but not gene induction. Curr Biol. 1998;8:113–116. doi: 10.1016/s0960-9822(98)70042-9. [DOI] [PubMed] [Google Scholar]
- Walczak H, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.