Abstract
Background
Smear negative pulmonary tuberculosis (SNPT) accounts for 30% of pulmonary tuberculosis cases reported yearly in Brazil. This study aimed to develop a prediction model for SNPT for outpatients in areas with scarce resources.
Methods
The study enrolled 551 patients with clinical-radiological suspicion of SNPT, in Rio de Janeiro, Brazil. The original data was divided into two equivalent samples for generation and validation of the prediction models. Symptoms, physical signs and chest X-rays were used for constructing logistic regression and classification and regression tree models. From the logistic regression, we generated a clinical and radiological prediction score. The area under the receiver operator characteristic curve, sensitivity, and specificity were used to evaluate the model's performance in both generation and validation samples.
Results
It was possible to generate predictive models for SNPT with sensitivity ranging from 64% to 71% and specificity ranging from 58% to 76%.
Conclusion
The results suggest that those models might be useful as screening tools for estimating the risk of SNPT, optimizing the utilization of more expensive tests, and avoiding costs of unnecessary anti-tuberculosis treatment. Those models might be cost-effective tools in a health care network with hierarchical distribution of scarce resources.
Background
Tuberculosis is one of the most important health problems in the world, with more than 8 million new cases and almost 2 million deaths each year [1,2]. The detection and management of pulmonary tuberculosis (PT) is a principal aim of tuberculosis control programs. However, smear-negative pulmonary tuberculosis (SNPT) is an increasing clinical and epidemiological problem, particularly in areas that are affected by the dual tuberculosis/human immunodeficiency virus infection (TB/HIV) [3]. A recent DNA fingerprinting study from San Francisco attributed 17% of TB transmission in this low prevalence setting to patients with SNPT [4]. HIV infection has been associated with an increased incidence of SNPT [5] and a higher mortality rate among patients with SNPT [6]. In Brazil, almost 30% of PT cases among adults are SNPT [7,8].
Diagnosis of SNPT is a difficult task, and in developing countries, the majority of these cases has been treated only on the basis of clinical and chest radiographic findings. Without a standardized clinical work up, the misdiagnosis rates have been estimated as high as 35% to 52% [9-11]. Sputum cultures increase the sensitivity of diagnosis substantially, but at increased expenses and complexity. New diagnostic approaches that could identify patients with SNPT, such as nucleic acid amplification assays, are expensive and have not been validated in developing countries under field conditions [3]. Much effort has been employed in the development of predictive models for PT in developed nations, but the main focus has been to evaluate models for optimizing the use of expensive hospital respiratory isolation rooms for PT patients where AIDS cases are more prevalent [12-22].
The present study used both logistic regression and classification trees to develop a predictive model for SNPT among outpatients seen at the public health care system in Rio de Janeiro city.
Methods
Setting and patient selection
Adults with symptoms and/or signs suggestive of SNPT referred to the Chest Service of Clementino Fraga Filho University Hospital (HUCFF) at the Federal University of Rio de Janeiro, from April 1st, 1996 to December 30th, 1999, were invited to participate in this cross-sectional study. Patients, who reported more than 3 weeks of cough, and who had two consecutive samples of spontaneous sputum that were acid fast bacilli smear-negative, or who had an absence of expectorated sputum, were eligible. Patients receiving anti-TB treatment were not included. Patients were referred from seven community health care units (CHCU) and from the outpatient unit of HUCFF.
Procedures
Patients underwent a standardized interview, with questions regarding demographic variables and clinical history (e.g.: smoking, alcohol abuse [23], HIV infection/AIDS [24]). A physical examination was performed and one chest physician using a standardized form evaluated chest radiographs. Evaluation of chest radiographs was not blinded to clinical predictors, but was for the results of TB cultures. Patients underwent sputum induction or bronchoscopy with bronchoalveolar lavage based on clinical criteria. All 551 patients underwent sputum induction, but bronchoscopy was performed in 331 patients (60%). Cultures were kept to assess Mycobacterium tuberculosis growth for 60 days. All patients were followed up for six months. When requested by the attending physician, HIV testing by ELISA was offered, with Western blot confirmation of reactive ELISAs. Patients who did not complete the established protocol or who were lost during clinical follow up were excluded. The Institutional Review Board of HUCFF granted approval for this study. Written informed consent was obtained from all study subjects.
Radiographic analysis
Chest radiographs were classified as typical, compatible, atypical and normal. Typical were those considered as having any parenchymal infiltrate or cavity localized in the upper zone (defined as the area above the posterior third rib); compatible were those presenting a miliary pattern, pleural effusion or thoracic adenopathy, and atypical those showing any other abnormality.
Bacteriology tests
All respiratory specimens were stained by the Ziehl-Neelsen method and cultured in Löwestein-Jensen media. Species identification was performed according to Kubica's method [25].
Case definition
Confirmed SNPT cases were defined as those with a positive culture for Mycobacterium tuberculosis in respiratory specimen; presumptive SNPT as clinical improvement after three months of anti-TB treatment as judged by three different chest physicians in a blinded review. Non-PT was considered among patients whose acid-fast smears and culture for M. tuberculosis were negative and who had no chest radiographic changes after six months of follow-up.
Statistical analysis
We used statistical softwares S-Plus 4.5 (Statsci. Seattle, WA) for constructing regression and classification trees and STATA 6.0 (Stata Corporation, College Station, TX) for performing multiple logistic regression.
First, we used both techniques to identify the most significant independent factors for SNPT diagnosis. Then the predicted probability of SNPT for each patient was estimated from each model, and compared to the actual outcomes. The area under the receiver operator characteristic (ROC) curve, sensitivity, and specificity were used to evaluate the performance of the models.
In logistic regression, backward elimination was used to select variables to be maintained in the final model, using 10% as criterion for statistical significance of associations. Associations between predictive factors and the outcome were expressed as odds ratios (OR) and respective ninety-five percent confidence interval (95%CI). We tested for two-way interactions, but none was significant (data not shown). We also generated a score system using the logistic regression coefficients, as follows: we divided all coefficients by the lowest one and rounded the estimated value to the next integer, as described elsewhere [26].
Classification and regression trees (or CART) build a binary classification system (tree) through recursive partitioning, so the data set is successfully split into increasingly homogenous subgroups. At each stage (node) the CART algorithm selects the explanatory variable and splitting value that gives the best discrimination between two outcome classes. A full CART algorithm adds nodes until they are homogenous or contains few observations (≥ 5 is the standard cut off in S-Plus). The problem of creating a useful tree is to find suitable guidelines to prune the tree. The general principle of pruning is that the tree of best size would have the lowest misclassification rate for an individual not included in the original data [27].
Results
Between April 1st, 1996, and December 30th, 1998, 551 patients were eligible for enrollment. Five per cent of patients were excluded because of incomplete data regarding follow up and/or bacteriological results. Forty nine percent (270/551) of the patients were diagnosed as SNPT. Among the cases, 78% (210/270) were bacteriologically confirmed and 22% (60/270) were diagnosed based on clinical and radiological improvement with anti-TB drug therapy.
We divided the original sample with random allocation of patients in two equivalent groups, one for building the models and the other one for validating them. Table 1 shows the characteristics of study subjects. The mean age of patients with SNPT was 40 years old and 91% of those patients were ≤ 60 years old. No statistically significant association with SNPT was observed regarding sex, previous PT, close contact with active PT cases, diabetes mellitus, smoking, alcoholism, HIV infection or AIDS. Patients originating at primary health centers were more likely to have SNPT than those initially seen at the hospital, but this finding was not maintained in the logistic regression analysis.
Table 1.
Clinical and demographic characteristics of study subjects
| Pulmonary Tuberculosis | Non Pulmonary Tuberculosis | ||||||
| Clinical and Demographic Characteristics* | N | % | N | % | OR | 95%CI | p value |
| Age | |||||||
| ≤ 25 | 50 | 19.2 | 28 | 10.0 | 4.17 | 2.14–8.10 | <0.01 |
| 26–60 | 186 | 71.5 | 195 | 69.9 | 2.23 | 1.32–3.74 | <0.01 |
| > 60 | 24 | 9.2 | 56 | 20.1 | 1.00 | - | - |
| Sex | |||||||
| Male | 171 | 63.3 | 183 | 65.1 | 1.00 | - | - |
| Female | 99 | 36.7 | 98 | 34.9 | 1.08 | 0.76–1.53 | 0.66 |
| Origin | |||||||
| Primary Health Care Unit | 192 | 71. 6 | 151 | 53.7 | 1.00 | - | - |
| Tertiary Health Care Unit | 76 | 28.4 | 130 | 46.3 | 0.46 | 0.32–0.66 | <0.01 |
| Previous PT | |||||||
| Yes | 65 | 24.3 | 78 | 27.8 | 0.84 | 0.57–1.23 | 0.36 |
| No | 202 | 75.7 | 203 | 72.3 | 1.00 | - | - |
| Diabetes Mellitus | |||||||
| Yes | 18 | 6.9 | 20 | 7.2 | 0.96 | 0.49–1.85 | 0.89 |
| No | 243 | 93.1 | 258 | 92.8 | 1.00 | - | - |
| Alcohol abuse | |||||||
| Yes | 51 | 19.0 | 62 | 22.1 | 0.83 | 0.55–1.25 | 0.37 |
| No | 217 | 81.0 | 218 | 77.9 | 1.00 | - | - |
| Close Contact with PT | |||||||
| Yes | 121 | 45.2 | 112 | 40.0 | 1.24 | 0.88–1.73 | 0.22 |
| No | 147 | 54.9 | 168 | 60.0 | 1.00 | - | - |
| Smoking | |||||||
| Yes | 114 | 42.2 | 152 | 54.1 | 0.62 | 0.44–0.87 | <0.01 |
| No | 156 | 57.8 | 129 | 45.9 | 1.00 | - | - |
| HIV + | |||||||
| Yes | 57 | 40.7 | 70 | 41.4 | 0.97 | 0.62–1.53 | 0.90 |
| No | 83 | 59.3 | 99 | 58.6 | 1.00 | - | - |
| AIDS (Caracas Criteria) | |||||||
| Yes | 32 | 15.3 | 29 | 12.8 | 1.32 | 0.77–2.27 | 0.31 |
| No | 176 | 84.6 | 211 | 87.9 | 1.00 | - | - |
* The number of subjects may vary because of missing values
Table 2 shows the variables significantly associated with SNPT diagnostics in logistic regression. Age ≤ 60 years, presence of weight loss (more than 10%) and typical chest radiograph were positively associated with SNPT, while the presence of spontaneous sputum was negatively associated with SNPT. Non-specific clinical symptoms such as fever, headache, pain in the face and throat pain were not significantly associated with SNPT diagnosis, as was not dyspnea, thoracic pain and sweatiness (data not shown). Neither the length of symptoms nor previous use of antibiotics was significantly predictive of SNPT (data not shown). The score system generated by using the logistic regression coefficients attributed two points for a typical X-ray, minus 1 point for the presence of sputum, one point for weight loss and two points for age < 60 years.
Table 2.
Variables significantly associated with smear negative pulmonary tuberculosis (multiple logistic regression – final model)
| OR | 95% CI | p value | |
| Typical X-ray | |||
| No | 1.00 | ||
| Yes | 2.63 | 1.55 – 4.45 | <0.01 |
| Presence of sputum | |||
| No | 1.00 | ||
| Yes | 0.53 | 0.27 – 1.01 | 0.05 |
| Weight loss | |||
| No | 1.00 | ||
| Yes | 1.59 | 0.92 – 2.73 | 0.09 |
| Age | |||
| >60 years | 1.00 | ||
| 26–60 | 2.70 | 1.21 – 6.01 | 0.02 |
| ≤25 | 3.07 | 1.09 – 8.61 | 0.03 |
Figures 1 and 2 show the best classification trees, separated by the more powerful prediction variable: chest radiograph pattern. Therefore, two trees were constructed, simulating clinical practice. Other variables included using the classification and regression trees methodology, besides those identified by the logistic regression were chest pain, with positive prediction, and dyspnea, with negative prediction ability. As in logistic regression, age was also identified as an explanatory variable, and used for creating probabilities strata in both trees.
Figure 1.
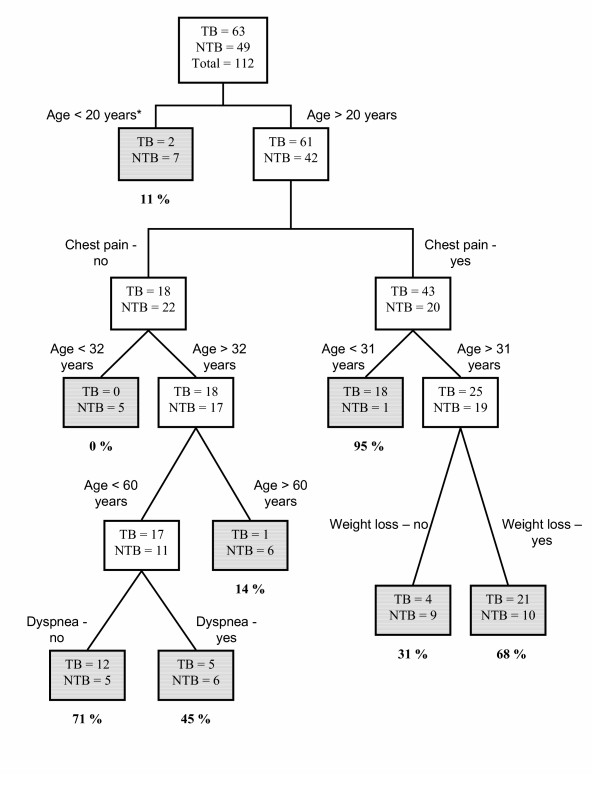
Classification tree for predicting smear negative pulmonary tuberculosis among patients with a typical chest radiograph.
Figure 2.
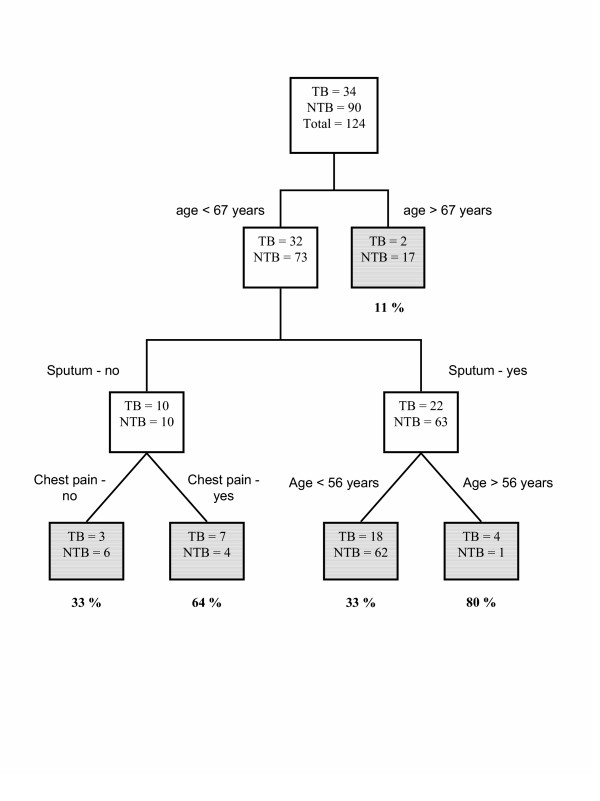
Classification tree for predicting smear negative pulmonary tuberculosis among patients with a atypical chest radiograph.
Table 3 shows the comparison of the predictive power of the different approaches in terms of the area under the ROC curve, sensitivity, and specificity. We looked for the cut off with best sensitivity and specificity relation (data not shown) for each model. Logistic regression and CART models had similar performance, considering both the original and the validation data sets.
Table 3.
Predictive performance of different multivariate models
| Sensitivity* (95% CI) | Specificity* (95% CI) | Area under ROC Curve* (95% CI) | |
| Multiple logistic regression (original sample) | 71.3 (62.4–79.1) | 59.4 (50.7–67.7) | 68.2 (61.9–74.6) |
| Multiple logistic regression (validation sample) | 67.4 (58.6–75.4) | 59.7 (50.9–68.1) | 67.0 (60.6–73.4) |
| Multiple logistic regression using scores (original sample) | 63.9 (54.8–72.4) | 63.8 (55.2–71.8) | 69.4 (62.8–76.0) |
| Multiple logistic regression using scores (validation sample) | 65.9 (57.0–74.0) | 60.5 (51.6–68.8) | 68.1 (61.6–74.7) |
| CART (original sample) | 63.7 (54.6–72.2) | 75.5 (67.5–82.4) | 74.9 (69.0–80.8) |
| CART (validation sample) | 64.2 (55.6–72.2) | 60.6 (51.9–68.8) | 66.4 (60.0–72.8) |
* Expressed in percents (%)
Discussion
The aim of this study was to evaluate the usefulness of logistic regression and CART models for the diagnosis of SNPT among outpatients in a high TB prevalence setting. Using clinical standardized interviews and chest radiographs it was possible to generate predictive models for SNPT with sensitivity ranging from 64% to 71% and specificity ranging from 58% to 76%. Evaluation of predictive models for PT respiratory isolation optimization has shown sensitivity ranging from 49% to 100%, and specificity from 46% to 86% [12-22]. It should be emphasized, however, that those models were applied to all PT suspect cases, including both smear negative and smear positive PT cases. Inclusion of smear positive cases potentially produces better results than obtained when analysis is restricted to smear negative patients. As smear negative patients present with a smaller mycobacterial burden, their clinical and radiographic findings may vary. Recently, a clinical radiological score for inpatients SNPT suspects was obtained through a retrospective chart review [28]. Its prediction ability was evaluated through likelihood ratios for each potential score and showed a potential utility if applied in settings with high prevalence of SNPT cases.
For SNPT, no single test provides sufficient accuracy for widespread use [3]. However, the techniques described here generated models for SNPT that were equivalent and were based on clinical variables and radiographic findings described elsewhere as useful for PT diagnosis [12,13,18-20]. In our study, the presence of expectoration was negatively associated with SNPT, as recently reported by Kanaya et al. [28].
The tree graphical probabilities results display or a score system may be easier to be performed under field conditions than the logistic regression formula, despite their equivalent performance. El-Sohl et al. [18] have already identified the utility of regression trees for PT diagnosis, with a slightly better result than logistic regression. The advantage of the models presented in this study is related to the variables identified in their development. They are readily available by conducting a routine clinical interview and by ordering a chest radiograph, so they could be applied in resource-limited settings. A possible example for using the score system, which might also be applied to CART models, using the probabilities of disease identified in our study, is the following:
• Score ≤0 – low probability of SNPT – indication of follow up at the primary health care unit, with observation and re-investigation over the next three months, time usually sufficient for clinical radiological evolution of markers of PT [29];
• Score from 1 to 4 – intermediary probability of SNPT – cases demanding further diagnostic investigation at a tertiary health care unit;
• Score ≥5 – high probability of SNPT – beginning empirical treatment for PT, with follow up at the primary health care unit.
Those models could also be useful as screenings tools, providing probabilities of PT, for evaluation of new diagnostics tests, as nucleic acid assays, for paucibacillary PT under clinical practice [30].
One limitation of the present evaluation was the absence of the tuberculin skin test. However, some authors had already described that in developing countries, tuberculin skin test is confounded by the high coverage of BCG vaccination, latent TB infection, the presence of nontuberculous mycobacteria, and anergy due to HIV infection or malnutrition, limiting its role in diagnostic algorithms [31]. Another limitation to be considered was the fact that only 56% of our patients underwent HIV testing; but the rate of TB/HIV co-infection in the present sample (21%) was close to the rate of TB/HIV co-infection rate in Rio de Janeiro's CHCUs at the same time (15%) [8].
Conclusion
Predictive models should be validated in the populations where they will be applied. Based on the dynamic nature of biologic systems, any predicted outcome is vulnerable to change in response to new diseases, or a shift in the pattern of natural disease processes or progression. Such models must therefore be validated and optimized in each setting.
The current proposed models, if prospectively confirmed, might be useful in guiding health care workers in estimating the risk of SNPT, optimizing the use of more expensive tests, such as bronchoscopy and nucleic acid assays, and of unnecessary anti-PT treatment. The models could be useful as a cost-effective tool in a health care system with limited resources.
List of abbreviations
SNPT Smear negative pulmonary tuberculosisPT pulmonary tuberculosis
TB Tuberculosis
HIV Human Immunodeficiency virus
HUCFF Clementino Fraga Filho University Hospital
CHCU Community health care units
ROC Receiver operator characteristic
CART Classification and regression tree
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
FCQM coordinated the writing of the manuscript, was the principal investigator, had the concept, and design of the study, performed the data analysis, participated in data acquisition, LGVB drafted the paper, participated in data acquisition, SLMS drafted the paper, participated in data acquisition, VMCR drafted the paper, participated in data acquisition, MBC drafted the paper, participated in data acquisition, REC drafted the paper, ALK drafted the paper, was the principal investigator, and had the concept, and design of the study, ARN drafted the paper, helped design the study, performed the data analysis and GLW drafted the paper, helped design the study, performed the data analysis. All authors contributed to the interpretation of results, have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors thank Dr Guida Vasconcelos and Dr. Solange Cavalcante from Rio de Janeiro City Tuberculosis Control Program for assistance with patient recruitment. This study was supported by CNPq: 52 14 59/96-6 and 52 11 30/95-6.
Contributor Information
Fernanda Carvalho de Queiroz Mello, Email: fcqmello@hucff.ufrj.br.
Luiz Gustavo do Valle Bastos, Email: gvbastos@hotmail.com.
Sérgio Luiz Machado Soares, Email: scoperao@bol.com.br.
Valéria MC Rezende, Email: vmrezende@hotmail.com.
Marcus Barreto Conde, Email: marcusconde@hucff.ufrj.br.
Richard E Chaisson, Email: rchaiss@jhmi.edu.
Afrânio Lineu Kritski, Email: kritskia@gmail.com.
Antonio Ruffino-Netto, Email: aruffino@fmrp.usp.br.
Guilherme Loureiro Werneck, Email: gwerneck@nesc.ufrj.br.
References
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- World Health Organization Global tuberculosis control: surveillance, planning, financing WHO Report 2000 Geneva. 2000.
- Colebunders R, Bastian I. A review of diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:97–107. [PubMed] [Google Scholar]
- Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, Small PM. Transmission of Mycobacterium tuberculosis from patient smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/S0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- Elliot AM, Namaambo K, Allen BW, Luo N, Hayes RJ, Pobee JO, McAdam KP. Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuber Lung Dis. 1993;74:191–194. doi: 10.1016/0962-8479(93)90010-U. [DOI] [PubMed] [Google Scholar]
- Harries AD, Nyangulu DS, Kang'ombe C, Salaniponi FM, Liomba G, Maher D, Nunn P. Treatment outcome of an unselected cohort of tuberculosis patients in relation to human immunodeficiency virus serostatus in Zomba Hospital, Malawi. Trans R Soc Trop Med Hyg. 1998;92:343–347. doi: 10.1016/S0035-9203(98)91036-7. [DOI] [PubMed] [Google Scholar]
- Ministério da Saúde do Brasil Boletim Eletrônico Epidemiológico do Ministério da Saúde Brasília. 2000.
- Secretaria Municipal de Saúde do Rio de Janeiro Boletim Eletrônico Secretaria Municipal de Saúde do Rio de Janeiro Rio de Janeiro. 2000.
- Gerhardt G, Natal SR, Pereira A, Lima SF, Penna MLF, Campos HS, Wanke B, Werneck A, Manceau JN. Tuberculose pulmonar sem confirmação bacteriológica [abstract] J Pneumol. 1988;14:137S. [Google Scholar]
- Mello FCQ, Soares SLM, Rezende VMC, Conde MB, Kritski AL, Empirically Treated Tuberculosis – TB Clinical Profile and Results of Treatment, in AIDS Reference Center – ARC, Rio de Janeiro City [abstract] Tuber Lung Dis. 1996;77:A95. [Google Scholar]
- Gordin FM, Slutkin G, Schecter G, Goodman PC, Hopewell PC. Presumptive diagnosis in treatment of pulmonary tuberculosis based on radiographic findings. Am Rev Respir Dis. 1989;139:1090–1093. doi: 10.1164/ajrccm/139.5.1090. [DOI] [PubMed] [Google Scholar]
- Tytle TL, Johnson TH. Changing patterns in pulmonary tuberculosis. South Med J. 1984;77:1223–1227. doi: 10.1097/00007611-198410000-00004. [DOI] [PubMed] [Google Scholar]
- Tattevin P, Casalino E, Fleury L, Egmann G, Ruel M, Bouvet E. The validity of medical history, classic symptoms and chest radiographs in predicting pulmonary tuberculosis – Derivation of a pulmonary tuberculosis prediction model. Chest. 1999;115:1248–1253. doi: 10.1378/chest.115.5.1248. [DOI] [PubMed] [Google Scholar]
- Selvyn PA, Pulmerantz AS, Durante A, Alcabes PG, Gourevitch MN, Boiselle PM, Elmore JG. Clinical predictors of Pneumocystis carinii pneumonia, bacterial pneumonia and tuberculosis in HIV-infected patients. AIDS. 1998;12:885–893. doi: 10.1097/00002030-199808000-00011. [DOI] [PubMed] [Google Scholar]
- Samb B, Henzel D, Daley CL, Mugusi F, Niyongabo T, Milka-Cabanne N, Kamanfu G, Dubry P, Mgaba I, Larouze B, Murray JF. Methods for diagnosing tuberculosis among in-patiens in Easten Africa whose sputum smears are negative. Int J Tuberc Lung Dis. 1997;1:25–30. [PubMed] [Google Scholar]
- Samb B, Sow PS, Kony S, Maynart-Badiane M, Diouf G, Cissokho S, Ba D, Sane N, Koltz F, Faye-Niang MA, Mboup S, Ndoye I, Delaporte E, Hane AA, Samb A, Coulaud JP, Coll-Seck AM, Larouze D, Murray JF. Risk factors for negative sputum acid-fast bacilli smears in pulmonary tuberculosis: results from Dakar, Senegal, a city with low HIV seroprevalence. Int J Tuberc Lung Dis. 1999;3:330–336. [PubMed] [Google Scholar]
- Wisnivesky JP, Kaplan J, Henschke C, McGinn TG, Crystal RG. Evaluation of clinical parameters predicts Mycobacterium tuberculosis in inpatients. Arch Intern Med. 2000;160:2471–2476. doi: 10.1001/archinte.160.16.2471. [DOI] [PubMed] [Google Scholar]
- El-Solh A, Mylotte J, Sherif S, Serghani J, Grant BJB. Validity of a decision tree for predicting active pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;155:1711–1716. doi: 10.1164/ajrccm.155.5.9154881. [DOI] [PubMed] [Google Scholar]
- El-Solh AA, Hsiao C-B, Goodnough S, Serghani J, Grant BJB. Predicting active pulmonary tuberculosis using an artificial neural network. Chest. 1999;116:968–973. doi: 10.1378/chest.116.4.968. [DOI] [PubMed] [Google Scholar]
- Cohen R, Muzaffar S, Capellan J, Azar H, Chinikamwala M. The validity of classic symptoms and chest radiographic configuration in predicting pulmonary tuberculosis. Chest. 1996;109:420–423. doi: 10.1378/chest.109.2.420. [DOI] [PubMed] [Google Scholar]
- Bock NN, McGowan JE, Jr, Ahn J, Tapia J, Blumberg HM. Clinical predictors of tuberculosis as a guide for a respiratory isolation policy. Am J Respir Crit Care Med. 1996;154:1468–1472. doi: 10.1164/ajrccm.154.5.8912766. [DOI] [PubMed] [Google Scholar]
- Mylotte JM, Rodgers J, Fassl M, Seibel K, Vacanti A. Derivation and validation of a pulmonary tuberculosis prediction model. Infect Control Hosp Epidemiol. 1997;18:554–560. doi: 10.1086/647671. [DOI] [PubMed] [Google Scholar]
- Masur J, Monteiro MG. Validation of the CAGE alcoholism screening test in a brazilian psychiatric inpatient hospital. Brazilian J Med Res. 1983;16:215–218. [PubMed] [Google Scholar]
- Weniger BG, Quinhoes EP, Sereno AB, De Perez MA, Krebs JW, Ismael C, Sion FS, Ramos-Filho CF, Morais de Sá CA, Byers RH, Rayfield MA, Rodrigues LGM, Zacarias F, Heyward WL. A simplified surveillance case definition of AIDS derived from empirical clinical data. The Clinical AIDS Study Group and the Working Group on AIDS case definition. J Acquir Immune Defic Syndr. 1992;5:1212–1223. [PubMed] [Google Scholar]
- Kent PT, Kubica GP. Public Health Mycobacteriology – a guide for level III laboratory Atlanta. 1985.
- Barquet N, Domingo P, Caylà JÁ, González J, Rodrigo C, Fernández-Viladrich P, Moraga-Liop FA, Marco F, Vázquez J, Sáez-Nieto JA, Casal J, Canela J, Foz M. Prognostic factors in meningococcal disease: development of a bedside predictive model and scoring system. JAMA. 1997;278:491–496. doi: 10.1001/jama.278.6.491. [DOI] [PubMed] [Google Scholar]
- SPLUS SPLUS Guide for Statistical and Mathematical Analysis Seattle. 1998.
- Kanaya AM, Gliden DV, Chambers HF. Identifying pulmonary tuberculosis in patients with negative sputum smear results. Chest. 2001;120:349–355. doi: 10.1378/chest.120.2.349. [DOI] [PubMed] [Google Scholar]
- Harries AD, Banda HT, Boeree MJ, Welby S, Wirima JJ, Subramanyam VR, Maher D, Nunn P. Management of pulmonary tuberculosis suspects with negative sputum smears and normal or minimally abnormal chest radiographs in resource-poor settings. Int J Tuberc Lung Dis. 1998;2:999–1004. [PubMed] [Google Scholar]
- Catanzaro A, Perry S, Clarridge JE, Dunbar S, Goodnight-White S, LoBue PA, Peter C, Pfyffer GE, Sierra MF, Weber R, Woods G, Mathews G, Jonas V, Smith K, Della-Latta P. The role of clinical suspicion in evaluating a new diagnostic test for active tuberculosis. Results of a multicenter prospective trial. JAMA. 2000;283:639–645. doi: 10.1001/jama.283.5.639. [DOI] [PubMed] [Google Scholar]
- Raviglione MC, Narain JP, Kochi A. HIV associated tuberculosis in developing countries: clinical features, diagnosis and treatment. Bull World Health Organ. 1992;70:515–526. [PMC free article] [PubMed] [Google Scholar]


