Abstract
We previously found that a microdisruption of the plasma membrane evokes Ca2+-regulated exocytosis near the wound site, which is essential for membrane resealing. We demonstrate herein that repeated membrane disruption reveals long-term potentiation of Ca2+-regulated exocytosis in 3T3 fibroblasts, which is closely correlated with faster membrane resealing rates. This potentiation of exocytosis is cAMP-dependent protein kinase A dependent in the early stages (minutes), in the intermediate term (hours) requires protein synthesis, and for long term (24 h) depends on the activation of cAMP response element-binding protein (CREB). We were able to demonstrate that wounding cells activated CREB within 3.5 h. In all three phases, the increase in the amount of exocytosis was correlated with an increase in the rate of membrane resealing. However, a brief treatment with forskolin, which is effective for short-term potentiation and which could also activate CREB, was not sufficient to induce long-term potentiation of resealing. These results imply that long-term potentiation by CREB required activation by another, cAMP-independent pathway.
INTRODUCTION
Ca2+-regulated exocytosis is apparently universally present in cells (Dan and Poo, 1992; Steinhardt et al., 1994; Bi et al., 1995; Girod et al., 1995; Coorssen et al., 1996; Ninomiya et al., 1996; Togo et al., 1999; Andrews, 2000) and plays an essential role in maintaining the integrity of the cell membrane (McNeil and Steinhardt, 1997). If a cell experiences a membrane disruption in the micrometer diameter range, Ca2+ influx at the wound site triggers exocytosis that is essential for successful cell membrane repair (Steinhardt et al., 1994; Bi et al., 1995, 1997; Miyake and McNeil, 1995; Togo et al., 1999; Reddy et al., 2001). Both membrane repair and neurotransmission depend on Ca2+-regulated exocytosis that is inhibited by clostridial neurotoxins and tetanus toxin (Steinhardt et al., 1994; Bi et al., 1995; Togo et al., 1999). The specificity of these proteolytic toxins for specific cleavage sites on soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) and similar Ca2+ sensitivity suggest conservation of the vesicle docking/fusion proteins in 3T3 cells (Hajduch et al., 1997; Foran et al., 1999) and sea urchin eggs and embryos (Avery et al., 1997; Conner et al., 1997). The recruitment of vesicles to docking sites near the disruption is dependent on the motor proteins kinesin and myosin and the activity of Ca2+/calmodulin-dependent (CaM) kinase (Steinhardt et al., 1994; Bi et al., 1997). Exocytosis is accompanied by a decrease in membrane tension, which has been shown to be necessary for successful membrane resealing (Togo et al., 2000). Artificial agents that decrease membrane tension can substitute for exocytosis when exocytosis is inhibited (Togo et al., 1999, 2000). If cells are wounded again at the same site within a few minutes, the second wound reseals more quickly as a consequence of a more rapid decrease to the low permissive levels of membrane tension (Togo et al., 2000). Evidence suggests that the facilitated response depends on the generation of new vesicles in a protein kinase C (PKC)-dependent manner (Togo et al., 2000). However, an increased exocytotic response to a second wound could not be observed because the first wound locally depleted the labeled endocytotic compartment.
In this study, we wounded at a different site the second time and could directly observe a globally increased exocytotic response to second wounds. We also explored whether the exocytosis associated with cell membrane repair exhibits the property of long-term potentiation. We found that a previous event of Ca2+ influx rapidly increases the pool able to undergo exocytosis at a subsequent Ca2+ influx. In the early stages, this increase is dependent on cAMP and protein kinase A (PKA). In the intermediate term of several hours, protein synthesis is also required. In the long term (24 h), the increase in exocytosis depends on the activity of cAMP response element-binding protein (CREB). In all cases, the increase in exocytosis was correlated with faster resealing rates.
MATERIALS AND METHODS
Cell Culture
Swiss 3T3 fibroblasts were cultured in DMEM (Invitrogen, Carlsbad, CA) containing 8% fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA) and 50 μg/ml gentamicin (Invitrogen).
Cells for wounding and imaging experiments were plated on cover glass-inserts in plastic dishes (35 × 10 mm) and were grown for 1–2 d before use. During experiments, the cells were maintained in 1.8 mM Ca2+ Ringer's solution. Ca2+-free Ringer's solution contained 138 mM NaCl, 2.7 mM KCl, 1.06 mM MgCl2, 5.6 mM d-glucose, and 12.4 mM HEPES (pH 7.25). A stock solution of 100 mM CaCl2 was used to adjust the concentration of Ca2+. Normal Ringer's solution contained 1.8 mM Ca2+.
Stable Transfection
CREB vectors pCMV-CREB, pCMV-KCREB, and pCMV-CREB133 were from BD Biosciences Clontech (Palo Alto, CA). These vectors were transfected into Swiss 3T3 fibroblasts by using LipofectAMINE PLUS (Invitrogen) in accordance with the manufacturer's protocol. After the transfected cells were grown in DMEM containing 8% FBS without antibiotics for 48 h, the selective antibiotic G418 (BD Biosciences Clontech) was added at 600 μg of active reagent/ml. Culture medium containing G418 was changed every 3rd d. After 2 wk, transfected clones were isolated and maintained with 400 μg/ml G418. Transfected clones with similar growth rates were used for the experiments.
Transient Transfection
A destabilized enhanced green fluorescent protein (GFP) with a fluorescence half-life of ∼2 h (pCRE-dEGFP; BD Biosciences Clontech) was used to monitor CREB activation. Swiss 3T3 fibroblasts were plated at low density in cover glass-insert Petri dishes. Twenty-four hours later, the cells in the well portion of the glass-insert dishes were transfected with 0.4 μg of pCRE-dEGFP DNA by using LipofectAMINE PLUS Reagent as recommended by the supplier for a 24-well culture vessel. Forty-eight hours after transfection the wounding and forskolin experiments were initiated. To wound a large number of cells (Swanson and McNeil, 1987), a Pasteur pipette was dragged along a line on a cover glass containing transfected Swiss 3T3 fibroblasts. Cells remaining along the edge of the cleared area had a high probability of being wounded. Morphologically similar cells were compared in edge and nonedge regions. Excitation light from a 75-W xenon lamp was passed through an optical filter 485DF22 and reflected toward the cells by using the dichroic filter 505DRLP. Emission light from single cells encompassed by a circular aperture passed through a 510ALP filter and was collected by a photomultiplier. All optical filters were from Omega Optical (Brattleboro, VT).
Long-Term Double-Wounding Experiments
The dye fluorescein-dextran (10,000 mol. wt.) or fura-dextran (10,000 mol. wt.) (Molecular Probes, Eugene, OR) was injected into the cells as a marker of wounded cells. The marking injection also served as the initial wound. The dye at 5 mg/ml in 100 mM potassium aspartate, 20 mM HEPES (pH 7.2) was spun through a 0.45-μm filter and back-filled into borosilicate glass micropipettes made from 1-mm-diameter tubing with filament (WPI, Sarasota, FL). Microinjection was performed in 1.8 mM Ca2+ Ringer's solution by using an Eppendorf 5242 microinjector and 5170 micromanipulator mounted on an IM-35 inverted microscope (Carl Zeiss, Thornwood, NY). Injection time was 0.3–0.5 s. After the injection, the Ringer's solution was replaced with DMEM plus 8% FBS, and the dishes were incubated at 36°C until membrane resealing or exocytosis was analyzed (see below).
Assay of Exocytosis by N-(3-Triethylammoniumpropyl)-4-(4-(dibutylamino)styryl)pyridinium Dibromide (FM1-43) Destaining
For double-wounding experiments, microinjection with fura-dextran was used to label cells as described above. These and noninjected cells were incubated with fresh culture medium containing 4 μM FM1-43 (Molecular Probes) overnight. Overnight staining with FM1-43 likely labels many compartments in a cell in addition to the initial labeling of newly endocytosed vesicles. For example, in hippocampal cell dendrites, 1.5 μM FM1-43 for 16 h predominately labels SER/trans-Golgi–derived organelles, and calcium-evoked exocytosis significantly destains this label in a neuronal toxin-sensitive manner (Maletic-Savatic and Malinow, 1998). Each dish was washed with 1.8 mM Ca2+ Ringer's just before the experiment. Fluorescent images were acquired using a SIT68 video camera (MTI, Michigan City, IN) linked to an IM-35 inverted microscope (Carl Zeiss). FM1-43 fluorescence was excited at 495 nm. Images were digitized by a Digidata 2000 AD board (Axon Instruments, Foster City, CA) and were acquired at 4-s intervals by averaging four frames for each image (Axon Imaging Workbench; Axon Instruments). Cells were wounded by the same system as for the microinjection. Fluorescent intensity change was localized to the area around the wound. A 5-μm-diameter circle centered on the wound site was analyzed for wound-induced changes in FM1-43 fluorescence. The average intensities of this circle for the 4 s before and the 4 s after the wound were compared to quantify destaining. A change in cell shape on wounding or cell movement led to discarding data from that cell. Wound-induced destaining reflected exocytosis and not a leakage of labeled vesicles, because it was dependent on external calcium concentration and was blocked by injection of tetanus toxin (Togo et al., 1999). Tests of significant differences used the Student's t test.
Assay of Membrane Resealing
Membrane resealing was monitored by measuring emission fluorescence of the calcium sensitive dye fura-2 (fura-2 acetoxymethyl ester [AM]; Molecular Probes) as described previously (Steinhardt et al., 1994; Togo et al., 1999). Fura-2 was introduced into the cells by AM-ester loading. Fluorescein-dextran–injected or noninjected cells were loaded with fura-2 AM at 25°C for 1 h, and washed with Ringer's solution containing 1.8 mM Ca2+. For tests of membrane resealing, Fura-2–loaded cells were wounded with a glass needle by using an Eppendorf 5242 microinjector and 5170 micromanipulator mounted on an IM-35 inverted microscope (Carl Zeiss), and fura-2 fluorescence was monitored. The wounding time was 0.3 s. All wounding experiments were performed at 25°C. Gentle handing of culture dishes and solution changes prevented premature wounding. A persistent decrease of the calcium-insensitive 357-nm excited fluorescent intensity (as an indicator of dye loss) together with a persistent increase of the ratio of fluorescent intensity excited by 357/385-nm light (an indicator of increasing intracellular Ca2+ concentration) indicated resealing failure. The interval between wounding and when the fluorescent intensity stopped declining was defined as resealing time. The resealing rate was defined as the inverse of the resealing time in seconds. For cells that failed to reseal, the rate was defined as zero.
RESULTS
A Previous Wound Increases Rate of Exocytosis and Membrane Resealing
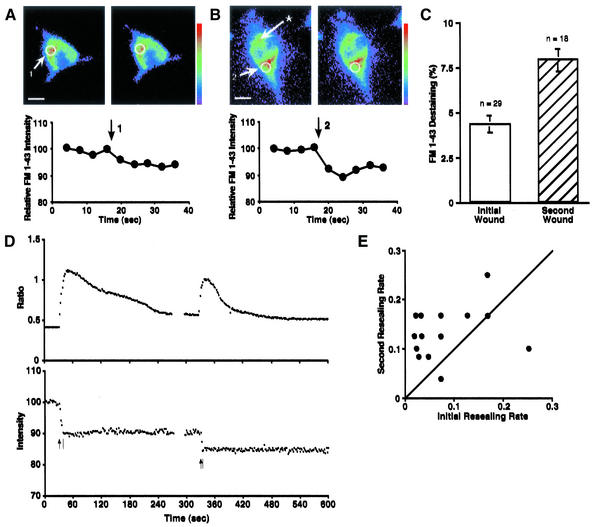
To determine whether 3T3 cells can increase the exocytotic response to membrane disruption and accelerate membrane resealing, we first wounded 3T3 cells within 5 min at two different sites 10–20 μm apart on the cell surface with a microglass needle. To observe exocytosis accompanying microdisruptions, the lipophilic fluorescent dye FM1-43 was preloaded by endocytosis overnight. FM1-43 intercalates into the outer leaflet of lipid bilayers and is much more fluorescent in hydrophobic than in hydrophilic environments (Cochilla et al., 1999). When cells are incubated with the dye and later washed, dye remaining in the plasma membrane rapidly diffuses away, leaving only dye that is trapped in the endocytosed membrane. Subsequent delivery of the FM1-43 into the plasma membrane by exocytosis allows diffusion of the dye into the external medium and results in a loss of cellular fluorescence near the wound site (Figure 1A). Exocytosis was increased to a second microdisruption at any other site globally throughout the cell (Figure 1B). Average destaining increased from 4.3 ± 0.5% (n = 29) for initial wounds to 7.9 ± 0.6% (n = 18) for second wounds at different sites (Figure 1C).
Figure 1.
Short-term facilitation of exocytosis and membrane resealing in 3T3 fibroblasts. (A) An example of FM1-43 destaining at an initial wound. 3T3 cells were loaded with FM1-43 overnight and washed with 1.8 mM Ca2+ Ringer's solution. Images were acquired at 4-s intervals, and the cell was wounded (arrow) by a glass needle between fourth (left) and fifth (right) image acquisition. Then the change of local fluorescent intensity around the disruption site (5-μm diameter) was analyzed. Bar, 10 μm. (B) An example of FM1-43 destaining at a second wound. The cell had a previous initial wound (at the site marked with an asterisk) before image acquisition was started. After the initial wound, images were acquired 4-s intervals, and the cell was wounded again at a different site (2) between the fourth (left) and fifth (right) image acquisitions by a glass needle 3–4 min after the initial wound. Then the change of local fluorescent intensity around the disruption site (5-μm diameter) was analyzed. Bar, 10 μm. (C) Comparison of FM1-43 destaining at initial and second wounds. Values are mean ± SE (p < 0.0001). (D) Changes in intracellular free Ca2+ monitored by the ratio of fura-2 emission intensities excited by 357 nm/380 nm (top trace). These data are simultaneously acquired with the data for the bottom trace, which show changes in 357-nm excited (calcium-insensitive) fura-2 fluorescence intensity during double wounding. 3T3 cells were loaded with fura-2 AM and wounded twice (arrows) at different sites. Bars indicate the completion time of membrane resealing. Units of fluorescence intensity were normalized to 100% before first wounding. (E) Comparison of membrane resealing rates at initial and second wounds. The resealing rate was defined as the inverse of the resealing time in seconds. For cells that failed to reseal, the rate was defined as zero. Each point represents one experiment. Points above diagonal show that membrane resealing at a second wound is facilitated.
Membrane resealing was monitored as described previously (Steinhardt et al., 1994; Togo et al., 1999). Briefly, a cell membrane puncture resulted in a sharp rise in the calcium-dependent fura-2 ratio (357/385 nm). Loss of dye from the cell was indicated by a decreasing intensity of the fura-2 calcium-insensitive excitation at 357 nm (Figure 1D). When the cell membrane resealed, this decline in fluorescent intensity stopped. To compare the timing of membrane resealing at initial and second wounds, the resealing rate was defined as the inverse of the resealing time in seconds. For cells that failed to reseal, the rate was defined as zero. The data were plotted as the rate of resealing of the first wound vs. the rate of the second wound (Figure 1E). Points above the diagonal indicate a more rapid response to the second wound. Our results indicated that a second wound made at a different site resealed more rapidly than the wound at the initial site. The average ratio of the second to the first resealing rate (2nd/1st) was 2.82 ± 0.59 (n = 16). The difference between the rates of first and second resealing was highly significant (p = 0.01).
Calcium entry was concurrently monitored by the calcium-dependent fura-2 ratio during wounding and membrane resealing. Calcium entry was not greater at the second wound and could not account for the increase in exocytosis and acceleration in resealing rates. The peak values of the calcium-sensitive ratio after initial and second wounds were 0.98 ± 0.03 (n = 16) and 0.92 ± 0.03 (n = 16), respectively. The duration of elevated Ca2+ was shorter at second wounds [46.3 ± 4.1 s (n = 16)] than at initial wounds [87.3 ± 11.9 s (n = 16)] due to the faster resealing rate at the second wounds.
Long-Term Potentiation of Exocytosis and Membrane Resealing
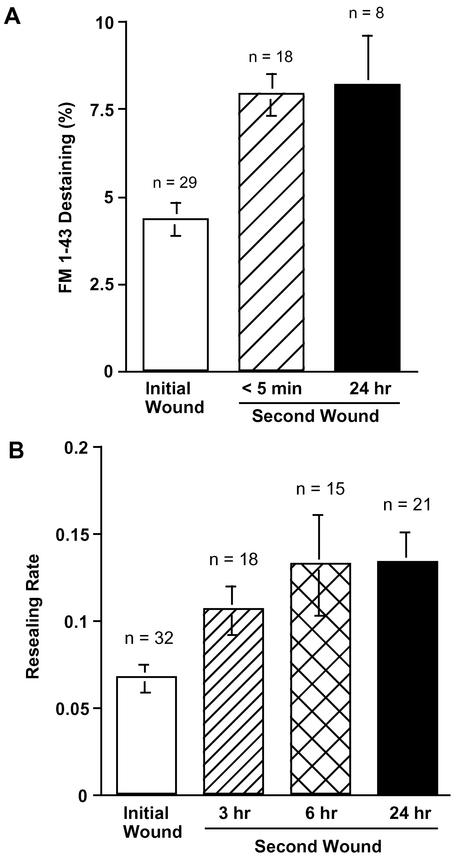
To investigate whether an initial wound results in long-term potentiation of exocytosis, 3T3 cells were wounded by the injection of fura-dextran as a marker before loading with FM1-43 overnight. Twenty-four hours after the first injection, changes in the fluorescent intensity of FM1-43 were measured for a second wound. As shown in Figure 2A, a previous wound increased the amount of exocytosis even when measured 24 h later. The amount of local FM1-43 destaining at the second wound at 24 h increased to 8.2 ± 1.4% (n = 8) from 4.3 ± 0.5% (n = 29) for initial wounds. For comparison, the data on destaining to initial and short-term second wounds are shown again in Figure 2A.
Figure 2.
Long-term facilitation of membrane resealing in 3T3 Fibroblasts. (A) FM1-43 destaining at initial and second wounds. For short-term experiments, cells were loaded with 4 μM FM1-43 overnight before wounding. For long-term experiments, cells were initially wounded by injection of fura-dextran as a marker and then loaded with 4 μM FM1-43 overnight. Each dish was washed with 1.8 mM Ca2+ Ringer's just before the measurement of FM1-43 destaining. Values are mean ± SE (p = 0.0021) (control vs. 24 h). (B) Resealing rate at initial and second wounds. 3T3 cells were wounded by injection of fluorescein-dextran as a marker. The resealing rates were analyzed 3, 6, or 24 h after the injection. Values are mean ± SE, p = 0.0143 (control vs. 3 h), p = 0.0052 (control vs. 6 h), and p = 0.003 (control vs. 24 h).
To investigate whether wounded 3T3 fibroblasts have a long-term increase in the rate of membrane resealing, cells were initially wounded and marked by the injection of the dye fluorescein-dextran. Then resealing rates for second wounds were measured 3, 6, or 24 h after the initial injection. After the various intervals, marked cells were loaded with fura-2 by the nondisruptive AM-ester method and the resealing rate for a second wound was measured. The resealing rate increased to 0.11 ± 0.014 (n = 18), 0.13 ± 0.029 (n = 15), or 0.13 ± 0.02 (n = 13) when previously wounded (dextran-injected) cells were wounded again at 3, 6, or 24 h after the initial injection (Figure 2B). In the same dishes, when unmarked cells were initially wounded the resealing rate was 0.068 ± 0.001 (n = 32) (Figure 2B).
Early Potentiation of Exocytosis and Membrane Resealing Are Dependent on PKA
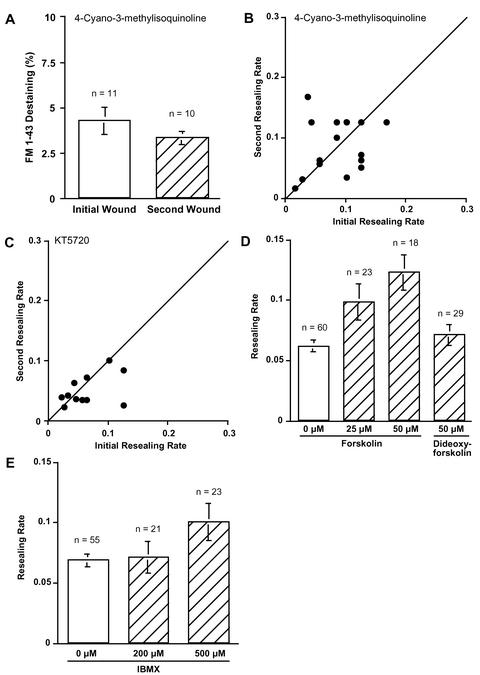
To test the effect of PKA inhibition on exocytosis at initial and second wounds, cells were loaded with FM1-43 overnight and wounded twice at different sites 10 min after adding 1 μM 4-cyano-3-methylisoquinoline (CMIQ), a specific PKA inhibitor (Lu et al., 1996). Although FM1-43 destaining in treated cells at the initial wound (4.3 ± 0.7%, n = 11) was statistically identical to the control value (Figure 3A vs. 1C), exocytosis at a second wound was not potentiated (3.3 ± 0.4%, n = 10) in PKA inhibitor-treated cells. These results suggest that the early increase of exocytosis to a second wound requires PKA activity.
Figure 3.
PKA dependence of potentiation of exocytosis and resealing. (A) FM1-43 destaining at initial and second wounds. 3T3 cells were incubated with fresh culture medium containing 4 μM FM1-43 overnight. Each dish was washed with 1.8 mM Ca2+ Ringer's solution just before the experiment and treated with 1 μM CMIQ. The local fluorescent intensity around the disruption site (5 μm diameter) was acquired at 4-s intervals. Values are mean ± SE. (B) Comparison of membrane resealing rates at initial and second wounds. Fura-2–loaded cells were incubated with a PKA inhibitor, CMIQ (1 μM), and wounded twice within 5 min at different sites. Each point represents one experiment. Points above diagonal show that membrane resealing at second wound is facilitated. (C) Comparison of membrane-resealing rates at initial and second wounds. Fura-2–loaded cells were incubated with a PKA inhibitor, KT5720 (10 μM), and wounded twice within 5 min at different sites. Each point represents one experiment. Points above diagonal show that membrane resealing at second wound is facilitated. (D) Average resealing rate of forskolin-treated cells. Fura-2–loaded 3T3 cells were treated with 25 or 50 μM forskolin for 15 min, and resealing rates were analyzed. Values are mean ± SE, p = 0.0031 (0 vs. 25 μM), and p < 0.0001 (0 vs. 50 μM). (E) Average resealing rate of IBMX-treated cells. Fura-2–loaded 3T3 cells were treated with IBMX for 15 min, and resealing rates were analyzed. Values are mean ± SE, p = 0.0138 (0 vs. 500 μM).
To investigate whether PKA is involved in the more rapid membrane resealing of a second wound, the cells were loaded with fura-2 by the nondisruptive AM-ester method, treated with CMIQ, and wounded twice. Figure 3B summarizes the results of the double-wounding experiments in the presence of 1 μM CMIQ. When cells were wounded twice at different sites within 5 min, the more rapid resealing at a repeated wound was inhibited. Average ratios of the initial and second resealing rates (2nd/1st) were 1.26 ± 0.23 (n = 19). Comparison with control-enhanced second rates (Figure 1E) was highly significantly different (p = 0.01). Treatment with CMIQ had no inhibitory effect on the resealing rate of initial wounds (Figure 3B). These results suggest that an initial wound activates a PKA-dependent pathway to accelerate membrane resealing. Similar results were obtained when a different PKA inhibitor, 10 μM KT5720, was used (Figure 3C).
The involvement of PKA in facilitation of membrane resealing was confirmed by treating cells with forskolin (an activator of adenylate cyclase) for 15 min before wounding (Figure 3D). Resealing rates in 25 or 50 μM forskolin-treated cells were facilitated (0.098 ± 0.015, n = 23 and 0.12 ± 0.015, n = 18) compared with control cell rates (0.061 ± 0.005, n = 60). Dideoxy-forskolin, which does not stimulate adenylate cyclase, had no effect on facilitation of membrane resealing (0.071 ± 0.009, n = 21) (Figure 3D).
To increase cytosolic cAMP levels by a different method, 3T3 cells were incubated for 15 min with 3-isobutyl-1-methylxanthine (IBMX), the phosphodiesterase inhibitor, before wounding (Figure 3E). The average resealing rate of control cells was 0.068 ± 0.005 (n = 55). Membrane resealing was facilitated by IBMX treatment in a dose-dependent manner. Average resealing rates were 0.071 ± 0.013 (n = 21) and 0.10 ± 0.015 (n = 23), when cells were treated for 15 min with IBMX at the concentration of 200 and 500 μM, respectively.
Intermediate-Term Potentiation of Exocytosis and Membrane Resealing Require Protein Synthesis
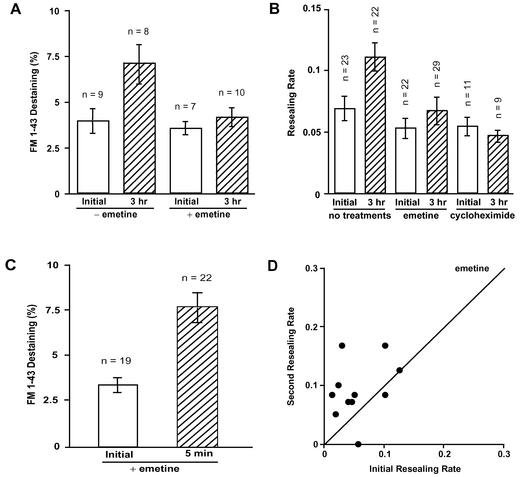
To investigate whether exocytosis is potentiated in the intermediate term of 3 h after an initial wound, 3T3 cells, which were loaded with 4 μM FM1-43 overnight, were wounded by the injection of fura-dextran as a marker. These cells were wounded again 3 h after the injection, while changes of fluorescent intensity of FM1-43 were measured. As shown in Figure 4A, a previous wound increased the amount of exocytosis at a second wound. The amount of FM1-43 destaining was increased from 3.9 ± 0.7% (n = 9) to 7.1 ± 1.1% (n = 8) at 3 h (Figure 4A).
Figure 4.
Intermediate-term potentiation of exocytosis and membrane resealing, but not short-term potentiation require protein synthesis. (A) Effect of emetine treatment on FM1-43 destaining at initial and second wounds. 3T3 cells were incubated with culture medium containing 4 μM FM1-43 overnight. Cells were washed with 1.8 mM Ca2+ Ringer's solution and pretreated with 15 μg/ml emetine for 30 min. These and untreated cells were then wounded by injection of fura-dextran as a marker and returned to culture medium with or without emetine for 3 h. FM1-43 destaining was analyzed for a second wound 3 h after the initial wound. Values are mean ± SE, p = 0.0243 (initial vs. 3 h in control cells). (B) Inhibition of protein synthesis affects the resealing rate at the second wound at 3 h. 3T3 cells were pretreated with 15 μg/ml emetine or 10 μg/ml cycloheximide for 30 min and wounded by injecting fluorescein-dextran. After three more hours in the inhibitors the cells were wounded again, and the resealing rates were analyzed. Values are mean ± SE. (C) Inhibition of protein synthesis with 15 μM emetine does not prevent short-term facilitation of FM1-43 destaining in 3T3 cells wounded twice (<5-min interval). Values are mean ± SE, p = 0.0019. FM1-43 was loaded as in A. (D) Short-term potentiation of membrane resealing in emetine-treated cells. 3T3 cells were treated with 15 μg/ml emetine for 3 h, loaded with fura-2, and then wounded twice (<5-min interval). The emission intensity of fura-2 after excitation with the calcium-insensitive 357 ± 2.5-nm light was used to monitor resealing.
To investigate whether protein synthesis is required for intermediate-term potentiation of exocytosis, 3T3 cells were loaded with FM1-43 overnight, and then preincubated with 15 μM emetine for 30 min before injection with fura-dextran as a marker of wounded cells. After three more hours in emetine, these cells were wounded again while changes of fluorescent intensity of FM1-43 were measured. Emetine for 3.5 h had no inhibitory effect on exocytosis at an initial wound (3.5 ± 0.4%, n = 8). On the other hand, potentiation of exocytosis at 3 h was inhibited in emetine-treated cells (4.1 ± 0.5%, n = 10) (Figure 4A).
To investigate whether protein synthesis is required for the intermediate-term potentiation of membrane resealing, 3T3 cells in 15 μg/ml emetine for 30 min were injected with fluorescein-dextran to both mark and wound the cells. After three more hours in emetine, the injected cells were wounded a second time, and resealing rates were compared with control cell rates. As shown in Figure 4B, emetine for 3.5 h had no significant inhibitory effect on the membrane resealing of an initial wound. In contrast, intermediate-term potentiation at 3 h was inhibited in emetine-treated cells. Similar results were obtained with cycloheximide (Figure 4B). These results indicate that intermediate-term potentiation of membrane resealing requires protein synthesis.
In short-term controls, 3T3 cells treated with emetine for >3 h were wounded twice within 5 min at different sites (Figure 4, C and D). Inhibition of protein synthesis with 15 μM emetine does not prevent short-term potentiation of exocytosis (FM1-43 destaining) in these cells. Values are mean ± SE (p = 0.0019) (Figure 4C). We also tested short-term potentiation of membrane resealing in cells treated with emetine. In Figure 4D, fura-2–loaded 3T3 cells were treated with 15 μg/ml emetine for 3 h and then wounded twice (<5-min interval). Each point represents one experiment. Points above diagonal show that membrane resealing was faster at second wounds in a majority of the cells. The average ratio of the initial and second resealing rate (2nd/1st) was 2.58 ± 0.65 (n = 11) in emetine-treated cells (Figure 4D). This value was not significantly different from the control value (2.89 ± 0.62, n = 15) (p = 0.78). This result indicates that the short-term potentiation of membrane resealing is not dependent on protein synthesis, and that this treatment does not have detectable nonspecific inhibitory effects.
A CREB-dependent Pathway Is Essential for Long-Term Potentiation of Exocytosis and Membrane Resealing
We were able to demonstrate activation of CREB-dependent transcription in wounded cells by transfecting 3T3 cells with a destabilized enhanced green fluorescent protein vector pCRE-d2EGFP (Table 1). At 3.5 h after mechanical wounding, the fluorescence of individual unwounded cells was compared with that of wounded cells or with cells in which CREB had been activated by 50 μM forskolin. All cells sampled were morphologically similar. Mean GFP fluorescence in wounded or forskolin-treated cells was significantly greater than for unwounded untreated cells (p < 0.0001).
Table 1.
Wounding of fibroblast cells activates CREB
| Mean dEGFP fluorescent emission (counts/s) | n | |
|---|---|---|
| Unwounded cells | 924 ± 71* | 45 |
| Wounded cells | 2974 ± 226 | 73 |
| Forskolin-treated cells | 2657 ± 190 | 45 |
Activation of CREB was monitored by excitation of destabilized enhanced green fluorescent protein in cells transfected with pCRE-d2EGFP. After mechanical wounding (3.5 h), the fluorescence of individual unwounded cells was compared with that of wounded cells. In another dish of transfected fibroblasts, 50 μM forskolin was added to DMEM medium containing 8% FBS 3.5 h before the destabilized enhanced green fluorescent protein fluorescence of individual cells was measured.
The mean fluorescence of unwounded cells was significantly different from both that of wounded cells and of forskolin-treated cells. p < 0.0001 by using the Mann–Whitney test.
To explore whether long-term potentiation of exocytosis and membrane resealing are mediated by a CREB-dependent pathway, 3T3 cells were transfected either with the vector pCMV-KCREB or the vector pCMV-CREB133. pCMV-KCREB constitutively expresses a mutant variant of the human CREB that contains mutations in its DNA-binding domain. pCMV-CREB133 constitutively expresses a mutant variant of the human CREB that contains a serine-to-alanine mutation corresponding to amino acid 133 in the mutant mouse CREB. Both KCREB and CREB133 have been shown to act as dominant negatives (BD Biosciences Clontech). Transfected clones were selected after 2 wk of growth in medium containing 600 μg/ml G418.
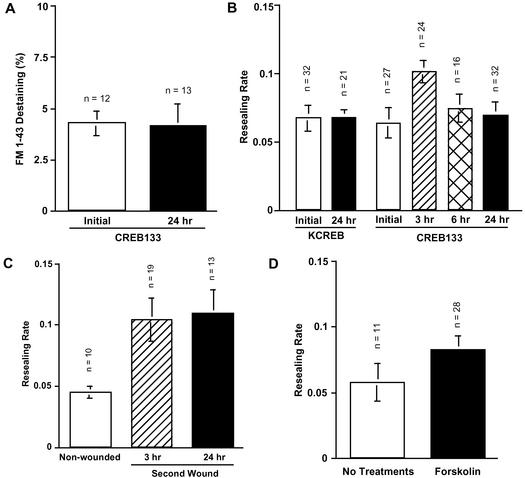
To investigate whether a previous wound results in long-term potentiation of exocytosis in CREB-dependent manner, nontransfected cells and stable transfected CREB133 cells were wounded and marked by injection of fura-dextran and then loaded with FM1-43 overnight. These and noninjected cells were wounded 24 h after the injection, and exocytosis was compared by FM1-43 destaining (Figure 5A). As shown in Figures 2A and 4A, an initial wound potentiated exocytosis in the long-term and intermediate-term in nontransfected 3T3 cells; the FM1-43 destaining value was 7.1 ± 1.1% (n = 8) at 3 h and 8.2 ± 1.4% (n = 8) at 24 h. However, long-term potentiation of exocytosis was blocked in CREB133 cells, and destaining (4.1 ± 1.1%, n = 13) was close to unpotentiated controls (Figure 5A).
Figure 5.
Long-term potentiation of exocytosis and membrane resealing are dependent on CREB, but independent of PKA activation. (A) FM1-43 destaining at initial and second wounds in CREB133 cells. 3T3 cells were transfected with pCMV-CREB133 and selected at 600 μg/ml G418. These cells were initially wounded by injection of fura-dextran as a marker, and loaded with 4 μM FM1-43 overnight. Each dish was washed with 1.8 mM Ca2+ Ringer's just before the experiment. The destaining was compared with those of cells that had not been previously wounded and marked by the injection of dextran. CREB133 destaining at a second wound was significantly different from controls. Values are mean ± SE, p = 0.0075. (B) Resealing rate of initial and second wounds in KCREB and CREB133 cells. 3T3 cells were transfected either with pCMV-KCREB or pCMV-CREB133, and stable transfectants were selected at 600 μg/ml G418. The cells were initially wounded by injection of fluorescein-dextran, and resealing rates of second wounds were compared with those of cells that had not been previously wounded. Resealing rates ± SE. Potentiation is still significant at 3 h (p = 0.0133 [control vs. 3 h] in CREB133 cells). (C) Inhibition of PKA during initial wounding does not affect long-term potentiation of membrane resealing. Cells were initially wounded by injection of fluorescein-dextran in the presence of 1 μM CMIQ and washed 15 min after the injection. Resealing rates were compared between noninjected and injected cells 24 h later (p = 0.0236 [control vs. 3 h] and p = 0.0113 [control vs. 24 h]). (D) Forskolin treatment does not induce long-term potentiation of membrane resealing. Cells were treated with 50 μM forskolin for 15 min and wounded 24 h later. Resealing rates were compared with those of nontreated cells. Resealing was not significantly different from controls (p = 0.2071).
We next investigated the effect of dominant-negative CREB expression on the long-term facilitation of membrane resealing. Cells stably transfected with either KCREB or CREB133 were wounded by injection of fluorescein-dextran, and the resealing rates were examined 3, 6, and 24 h after the injection (Figure 5B). Membrane resealing at initial wounds was not affected by expression of dominant-negative CREBs. Resealing rates of initial wounds were 0.064 ± 0.011 (n = 27) and 0.067 ± 0.009 (n = 32), when KCREB and CREB133 cells were wounded, respectively. Percentages of resealed cells were 100 and 81%, respectively. When KCREB cells were wounded again 24 h after the initial wound, facilitation was completely inhibited (0.067 ± 0.006, n = 21), although 100% of the cells resealed after the second wound. CREB133 cells also showed no facilitation 6 and 24 h after the initial wounds. Resealing rates were 0.074 ± 0.011 (n = 16) and 0.069 ± 0.01 (n = 32), respectively. For second wounds at 24 h, 94% of the CREB133 cells resealed. However, membrane resealing was facilitated at 3 h (0.1 ± 0.008, n = 24). These results indicate that CREB mutants did not affect membrane resealing itself but affected facilitation of membrane resealing, and that the long-term increases in the rates of membrane resealing has two phases; the early phase (up to 3 h) is CREB independent, whereas the late phase (>6 h) requires CREB-mediated gene expression.
In contrast to CREB, inhibition of PKA during the initial wound does not affect long-term potentiation of membrane resealing. Cells were initially wounded by injection of fluorescein-dextran in the presence of 1 μM CMIQ and washed 15 min after the injection. Three and 24 h later, resealing rates were compared between nonwounded controls and previously wounded cells (p = 0.0236 [control vs. 3 h] and p = 0.0113 [control vs. 24 h]) (Figure 5C). Furthermore, by itself forskolin treatment does not induce long-term potentiation of membrane resealing. Cells were treated with 50 μM forskolin for 15 min and wounded 24 h later. Resealing rates were compared with those of nontreated cells. Resealing was not significantly different from controls (p = 0.2071) (Figure 5D).
CREB Pathway Is Specifically Required for Long-Term Potentiation of Exocytosis and Membrane Resealing
To investigate whether the block of the CREB-dependent pathway was having nonspecific deleterious effects that would preclude the appearance of long-term potentiation, we examined the short-term potentiation of exocytosis and membrane resealing rates of CREB133 cells.
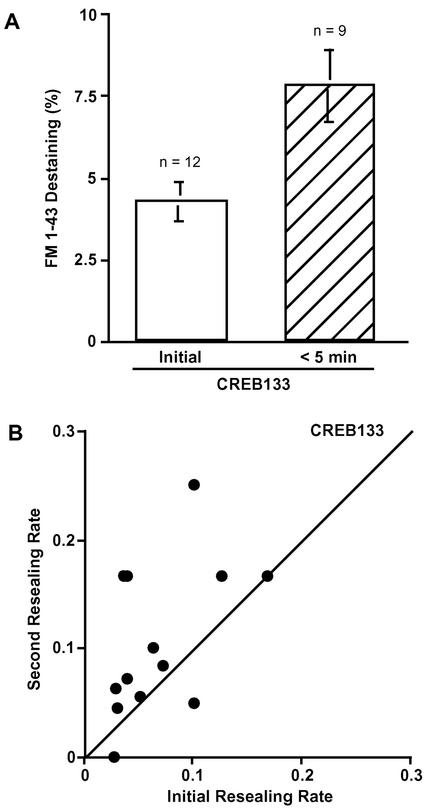
FM1-43 loaded CREB133 cells were wounded twice at different sites within 5 min and still showed the short-term potentiation of exocytosis at the second wound. FM1-43 destaining was 7.8 ± 1.1% (n = 9) (Figure 6A). The short-term potentiation of exocytosis was unaffected by block of the CREB-dependent pathway. These results indicate that the initial wound potentiates exocytosis long term, specifically via a CREB-mediated signaling pathway.
Figure 6.
Short-term facilitation is independent of protein synthesis and the CREB pathway. (A) Comparison of FM1-43 destaining at initial and second wounds. CREB133 cells were loaded with 4 μM FM1-43 overnight. Each dish was washed with 1.8 mM Ca2+ Ringer's just before the experiment. The local fluorescent intensity of FM1-43 around the disruption site (5 μm in diameter) was acquired at 4-s intervals. Values are mean ± SE (p = 0.0075). (B) Short-term facilitation of membrane resealing in CREB133 cells. CREB133 cells were loaded with fura-AM and wounded twice within 5 min.
Fura-2 ester-loaded CREB133 cells were wounded twice within 5 min at different sites (Figure 6B). Membrane resealing was faster at second wounds in a majority of the cells. The average ratio of the initial and second resealing rates (2nd/1st) was 1.84 ± 0.38 (n = 13) in CREB133 cells. These values were not significantly different from control values (2.89 ± 0.62, n = 15) (p = 0.19). These results indicate that the short-term potentiation of membrane resealing is not dependent on CREB-mediated gene expression and that the expression of CREB133 protein did not have detectable, nonspecific inhibitory effects.
PKC-dependent and Brefeldin A (BFA)-sensitive Short-Term Facilitation of Membrane Resealing Is a Local Polarized Response
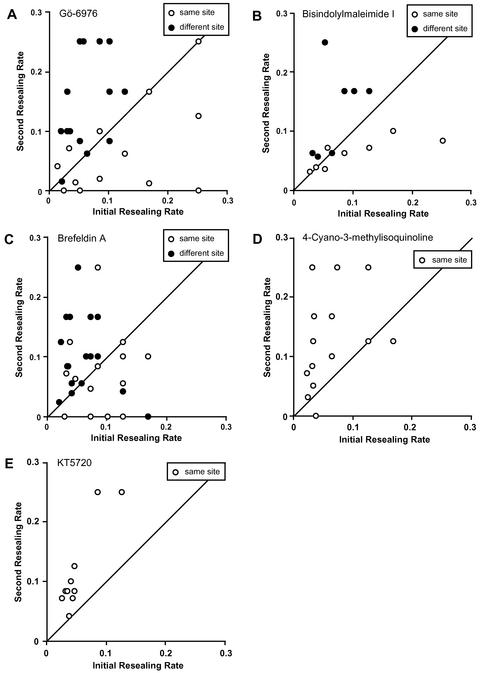
In a previous study, we demonstrated a short-term acceleration of membrane resealing if the same site was wounded twice (Togo et al., 1999). We used the term “facilitation” for acceleration of resealing when cells were wounded a second time at the same site. We could not determine directly whether exocytosis was also facilitated when the same site was wounded because the FM1-43 label was depleted locally by the initial wound (Togo et al., 1999). We also could not determine whether exocytosis or resealing showed long-term potentiation because it would be impossible to identify the same site due to cell movement, cell growth, and shape changes. The short-term facilitation we did uncover at same sites required a new vesicle/organelle pool generated via a PKC-dependent and BFA-sensitive process (Togo et al., 1999). Apparently, the new pool more than made up for the local depletion that occurred at the initial wound because resealing rates increased for second wounds. In the case of same site wounding, we could only infer an increase in exocytosis because by wounding we had depleted the FM1-43–labeled pool at the initial wound site. To determine whether the potentiation of resealing to second wounds at different sites also used the same pathway, we treated the cells either with Gö-6976, a specific inhibitor of Ca2+-dependent PKC isozymes, or BFA, an inhibitor of the Golgi apparatus. Then we wounded cells twice at the same or different sites. Figure 7 summarizes the results of the double-wounding experiments. When the same site of a cell membrane was wounded twice within 5 min in the presence of 1 μM Gö-6976, facilitation was inhibited as described previously (Figure 7A, open circles) (Togo et al., 1999). However, the inhibition of PKC had no effect on facilitation of resealing seen when different sites were wounded (Figure 7A, closed circles). The average ratio of the initial resealing rates (2nd/1st) was 0.82 ± 0.26 (n = 14) when wounding occurred at the same site in the presence of PKC inhibitor and was 2.93 ± 0.46 (n = 16) when different sites were wounded. The difference was highly significant (p = 0.001). The PKC inhibitor bisindolylmaleimide gave a result similar to Gö-6976 (Figure 7B). Similar results were also obtained when the cells were pretreated with 50 μM BFA (Figure 7C). Average ratios of the initial and second resealing rates (2nd/1st) were 1.13 ± 0.28 (n = 16) or 2.34 ± 0.47 (n = 16) when cells were wounded twice at the same site or at different sites, respectively, These values were significantly different (p = 0.02). Again there was no effect of BFA on the facilitation of resealing at a second wound seen at a different site. Membrane resealing at the initial wound was not affected by treatments with Gö-6976 or BFA (Togo et al., 1999). In confirmation of separate mechanisms, the PKA inhibitor CMIQ did not inhibit the facilitation when cells were wounded twice at same site. The average ratio of the initial and second resealing rates (2nd/1st) was 2.72 ± 0.58 (n = 14) (Figure 7D). The PKA inhibitor KT5720 gave a result similar to CMIQ (Figure 7E). These results indicate that PKC-dependent and BFA-sensitive short-term facilitation of membrane resealing is a separate polarized reaction to the site previously disrupted and locally depleted of vesicles and organelles able to undergo Ca2+-regulated exocytosis.
Figure 7.
PKC-dependent and BFA-sensitive short-term facilitation is a polarized reaction. Fura-AM–loaded 3T3 cells were incubated either with 1 μM Gö-6976 (A), 1 μM bisindolylmaleimide I (B), 50 μM BFA (C), 1 μM CMIQ (D), or 10 μM KT5720 (E) and wounded twice within 5 min at the same site (open circles) or at a different site (closed circles).
DISCUSSION
Ca2+-regulated exocytosis, which requires vesicle docking/fusion SNARE proteins, has been shown to be essential for resealing of micrometer-sized membrane disruptions in mammalian cells and invertebrate embryos (Steinhardt et al., 1994; Bi et al., 1995, 1997; Miyake and McNeil, 1995; Togo et al., 1999; Reddy et al., 2001).
In the present study, we observed that a second membrane disruption at a different site was resealed more rapidly than the wound at the initial site. Also, these second wounds resulted in approximately twice as much exocytosis as an initial wound even though more rapid resealing of the second disruption reduced Ca2+ entry (Figure 1). We demonstrated that PKA activity was involved in the facilitation of both membrane resealing and Ca2+-regulated exocytosis at repeated wounds (Figure 3). We also found intermediate- and long-term potentiation of membrane resealing and Ca2+-regulated exocytosis and showed that these processes required protein synthesis and a CREB-mediated signaling pathway in 3T3 fibroblasts (Figures 2, 4, and 5; Table 2). These results show for the first time that nonneuronal cells can have intermediate- and long-term potentiation of Ca2+-regulated exocytosis. Because exocytosis increased at any second membrane disruption site tested, the potentiation of exocytosis is a global response in the 3T3 cell. These adaptive responses leading to faster cell membrane resealing would work to minimize the toxic effects of excess of Ca2+ entry after membrane disruption (Trump and Berezesky, 1995) and the loss of crucial cellular constituents with repeated wounding.
Table 2.
Potentiation of exocytosis and membrane resealing for wounds at different sites
| Short-term | Intermediate-term | Long-term | |
|---|---|---|---|
| PKA | Dependent | Independenta | |
| cAMP | Dependent | Not sufficientb | |
| Protein synthesis | Independent | Dependent | |
| CREB | Independent | Independent | Dependent |
| Calcium ion | Dependent | Dependent | Dependent |
During the initial wound.
Raising cAMP for 15 min does not substitute for an initial wound.
Early Potentiation of Exocytosis and Facilitation of Membrane Resealing
Short-term potentiation of Ca2+-regulated exocytosis by PKA has been reported previously in several types of secretory cells such as chromaffin cells, pancreatic β cells, and neurons (O'Sullivan and Jamieson, 1992; Morgan et al., 1993; Ämmäläet al., 1994; Trudeau et al., 1996; Kuromi and Kidokoro, 2000). In addition to these types of cells, it has been recently shown that PKA also potentiates Ca2+-regulated exocytosis of endosomes with lysosomal markers in normal rat kidney fibroblasts (Rodríguez et al., 1999). In the present study, we found that the phosphorylative activity of PKA increased the amount of exocytosis at a second wound in 3T3 fibroblasts (Figures 1 and 3) and increased the rate of successful membrane repair.
Activation of Protein Kinases after Membrane Disruption
It is still not resolved how PKA is activated after a membrane disruption. Consistent with previous results with reverse transcription-polymerase chain reaction (Smit et al., 1998), Western blotting failed to detect known Ca2+-stimulated adenylate cyclases in 3T3 fibroblasts (our unpublished data). Therefore, membrane disruption might activate PKA via an indirect pathway instead of directly by Ca2+-stimulated adenylate cyclases. Prolonged activation of PKA by a Ca2+-stimulated nitric oxide/cGMP pathway has been described in the formation of long-term memory in honey bees (Müller, 2000). However, the failure of forskolin to induce long-term potentiation of resealing implies that another pathway is used to activate CREB. In addition to a PKA-dependent pathway, several different Ca2+-dependent pathways can be triggered by massive Ca2+ influx through the site of membrane disruption. One such pathway is the activation of CaM kinases. In fact, it has been shown that CaM kinase II is required for wound-induced exocytosis and membrane resealing in 3T3 fibroblasts and sea urchin embryos (Steinhardt et al., 1994; Bi et al., 1997). CaM kinases have a broad range of substrates and have been shown to activate CREB by phosphorylation of serine 133 (Shaywitz and Greenberg, 1999)
We have previously shown that 3T3 fibroblasts have another short-term mechanism for facilitation of membrane resealing in addition to the PKA pathway described herein. In the previous study, we found that the rate of membrane resealing with repeated wounds at the same site is facilitated and that this response is dependent on a PKC activity and is sensitive to BFA (Togo et al., 1999). The inhibition of same-site facilitation by PKC inhibitors and BFA and the results of our previous FM1-43 destaining measurements suggest that the PKC-dependent short-term facilitation reflects the availability of a newly generated vesicle/organelle pool from the trans-Golgi network. In the present study, we found that the same-site mechanism for facilitation was insensitive to the PKA inhibition that blocks the global facilitation seen when different sites are wounded. Same-site facilitation was found to be a polarized reaction only to the site where the calcium-sensitive vesicle/organelle pool had been depleted by a previous membrane disruption (Figure 7). These results suggest that the direction of vesicle/organelle transport from trans-Golgi network to the plasma membrane is actively regulated, although the mechanism of this polarized response has not yet been explored.
A Specific Requirement for Protein Synthesis and CREB for Intermediate- and Long-Term Potentiation
A specific requirement for protein synthesis for the development of intermediate-term potentiation has been demonstrated in studies of facilitation of sensory motor synapses in Aplysia (Sutton and Carew, 2000). At 3 h, we also demonstrate that protein synthesis is required for potentiation (Figure 4). At this intermediate stage CREB is not essential, but by 6 h or at 24 h, CREB is required (Figure 5). Neuronal long-term potentiation is known to require both protein synthesis and CREB-mediated transcription (Bourtchuladze et al., 1994; Martin et al., 1997; Bartsch et al., 1998; Casadio et al., 1999).
These requirements for long-term potentiation are not the result of nonspecific, deleterious effects of emetine treatment or CREB133 transfection, because short-term potentiation of exocytosis and the facilitation of membrane resealing rates of emetine-treated cells and CREB133 cells were normal (Figures 4 and 6).
Compartments Used in Membrane Repair
Depending on cell type and degree of membrane rupture, different pools of vesicles or organelles can undergo calcium-regulated exocytosis or fusion in response to plasma membrane wounding. The massive calcium influx that follows disruption of membrane would obviously trigger any compartment with sensitivity to calcium for exocytosis or fusion. Several calcium-sensitive exocytotic compartments used in membrane repair have been identified, including cortical granules (Steinhardt et al., 1994; Bi et al., 1995) and yolk granules (McNeil et al., 2000) in the sea urchin egg and embryo. In 3T3 fibroblasts, botulinum neurotoxin B and tetanus toxin, which target a specific cleavage site on cellubrevin and vesicle-associated membrane protein-2 SNARE proteins, have been shown to inhibit membrane resealing and block FM1-43 measured exocytosis at the site of membrane disruption (Steinhardt et al., 1994; Togo et al., 1999). In 3T3 cells, lysosomes have also been shown to be required for the repair of plasma membrane disruptions because both lysosomal exocytosis and membrane resealing are inhibited by the recombinant Synaptotagin VII C2A domain or anti-Synaptotagin VII C2A antibodies, or by antibodies against the cytosolic domain of lysosomal associated membrane protein-1, which specifically aggregate lysosomes (Reddy et al., 2001). The accumulated evidence indicates that subtraction of different individual vesicle populations can result in the failure of membrane repair. This implies that a successful, stable repair will use all substantial pools of calcium-sensitive organelles. It has already been shown that just the subtraction of the smaller, late-arriving, kinesin-dependent pool of exocytotic vesicles will eventually result in total failure to reseal (Steinhardt et al., 1994; Bi et al., 1997). Therefore, blocking one organelle pool and getting failure to reseal does not establish that it is the only organelle used for membrane repair.
In our experiments herein and elsewhere, the amount of exocytosis evoked at a wound is closely correlated with the rate of membrane resealing (Steinhardt et al., 1994; Bi et al., 1995, 1997; Miyake and McNeil, 1995; Togo et al., 1999). In every instance so far, we could have used the rate of resealing as a reliable proxy for exocytosis. Indeed, resealing has been helpful in quickly sorting out different possibilities, because the rate of resealing is far easier to measure.
Another mechanism is known to operate in special cases where cells have the ability to survive much larger membrane disruptions. In much larger disruptions no plasma membrane remains over large areas. There is literally no plasma membrane and therefore no target membrane for exocytosis. For example, neurons and muscle cells can survive complete transections (Casademont et al., 1988; Krause et al., 1994), and oocytes can survive the removal of large patches of surface plasma membrane (Terasaki et al., 1997; McNeil et al., 2000). Membrane repair on this scale requires the entire replacement of the plasma membrane. There is good evidence that the new membrane is formed by a massive endosomal fusion and that the newly formed membrane is then used to form a patch (Terasaki et al., 1997; McNeil et al., 2000). This fusion reaction has a higher calcium threshold than for the exocytosis-dependent repair of smaller lesions (Steinhardt et al., 1994).
CONCLUSION
Our present study revealed the existence of long-term potentiation of Ca2+-regulated exocytosis in nonneuronal cells. The increase in exocytosis is directly correlated with the persistent potentiation of membrane resealing that follows an initial wound. Because disruptions of plasma membranes are frequently observed in mechanically challenged animal tissues (McNeil and Steinhardt, 1997) and because membrane resealing and its facilitation have been observed in all cell types tested, our results imply the existence of a ubiquitous form of long-term potentiation of regulated exocytosis. We have demonstrated that long-term potentiation of exocytosis can be evoked anywhere on the surface of a simple generic cell, the 3T3 fibroblast, and that a multistage process is used to increase exocytosis for each different time period. In our work, we show that the requirement for CREB-dependent transcription is specific. Eliminating the CREB pathway only blocks long-term potentiation, leaving near-term potentiation intact. We speculate that the complex up-regulation of trafficking for membrane repair may share common origins with neuronal long-term potentiation, although details such as the end products affected must obviously differ. Membrane repair is accessible both in time and place and has special advantages for the study of underlying signaling pathways in the long-term up-regulation of vesicle trafficking. The exocytosis required for membrane resealing can now be used to investigate conserved mechanisms of up-regulation of Ca2+-regulated exocytosis by previous episodes of Ca2+ influx.
ACKNOWLEDGMENTS
We thank Dr. Mu-Ming Poo and Corey Goodman (University of California, Berkeley, CA) for a critical reading of the manuscript. This study was supported by the National Institutes of Health.
Abbreviations used:
- BFA
brefeldin A
- CaM
Ca2+/calmodulin
- CMIQ
4-cyano-3-methylisoquinoline
- CREB
cAMP response element-binding protein
- FBS
fetal bovine serum
- FM1-43
N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl)pyridinium dibromide)
- IBMX
3-isobutyl-1-methylxanthine
- PKA
protein kinase A
- PKC
protein kinase C
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0056. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0056.
REFERENCES
- Ämmälä C, Eliasson L, Bokvist K, Berggren P, Honkanen RE, Sjöholm Å. Activation of protein kinases and inhibition of protein phosphatases play a central role in the regulation of exocytosis in mouse pancreatic β cells. Proc Natl Acad Sci USA. 1994;91:4343–4347. doi: 10.1073/pnas.91.10.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews NW. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000;10:316–321. doi: 10.1016/s0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- Avery J, Hodel A, Whitaker M. In vitro exocytosis in sea urchin eggs requires a synaptobrevin-related protein. J Cell Sci. 1997;110:1555–1561. doi: 10.1242/jcs.110.14.1555. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- Bi G-Q, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G-Q, Morris RL, Liao G, Alderton JM, Scholey JM, Steinhardt RA. Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis. J Cell Biol. 1997;138:999–1008. doi: 10.1083/jcb.138.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Casademont J, Carpenter S, Karpati G. Vacuolation of muscle fibers near sarcolemmal breaks represents T tubule dilatation secondary to enhanced sodium pump activity. J Neuropathol Exp Neurol. 1988;47:618–628. doi: 10.1097/00005072-198811000-00005. [DOI] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1–43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- Conner S, Leaf D, Wessel G. Members of the SNARE hypothesis are associated with cortical granule exocytosis in the sea urchin egg. Mol Reprod Dev. 1997;48:106–118. doi: 10.1002/(SICI)1098-2795(199709)48:1<106::AID-MRD13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Coorssen JR, Schmitt H, Almers W. Ca2+-triggers massive exocytosis in Chinese hamster ovary cells. EMBO J. 1996;15:3787–3791. [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Quantal transmitter secretion from myocytes loaded with acetylcholine. Nature. 1992;359:733–736. doi: 10.1038/359733a0. [DOI] [PubMed] [Google Scholar]
- Foran PG, Fletcher LM, Oatey PB, Mohammed N, Dolly JO, Tavare JM. Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3–L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. J Biol Chem. 1999;274:28087–28095. doi: 10.1074/jbc.274.40.28087. [DOI] [PubMed] [Google Scholar]
- Girod R, Popov S, Alder J, Zheng JQ, Lohof A, Poo M. Spontaneous quantal transmitter secretion from myocytes and fibroblasts: comparison with neuronal secretion. J Neurosci. 1995;15:2826–2838. doi: 10.1523/JNEUROSCI.15-04-02826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduch E, Aledo JC, Watts C, Hundal HS. Proteolytic cleavage of cellubrevin and vesicle-associated membrane protein (VAMP) by tetanus toxin does not impair insulin-stimulated glucose transport or GLUT4 translocation in rat adipocytes. Biochem J. 1997;132:233–238. doi: 10.1042/bj3210233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause TL, Fishman HM, Ballinger ML, Bittner GD. Extent and mechanism of sealing in transected giant axons of squid and earthworms. J Neurosci. 1994;14:6638–6651. doi: 10.1523/JNEUROSCI.14-11-06638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron. 2000;27:133–143. doi: 10.1016/s0896-6273(00)00015-5. [DOI] [PubMed] [Google Scholar]
- Lu ZX, Quazi NH, Deady LW, Polya GM. Selective inhibition of cyclic AMP-dependent protein kinase by isoquinoline derivatives. Biol Chem Hoppe-Seyler. 1996;377:373–384. doi: 10.1515/bchm3.1996.377.6.373. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part 1. trans-Golgi network-dervived organelles undergo regulated exocytosis. J Neurosci. 1998;18:6803–6813. doi: 10.1523/JNEUROSCI.18-17-06803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Vogel SS, Miyake K, Terasaki M. Patching plasma membrane disruptions with cytoplasmic membrane. J Cell Sci. 2000;113:1891–1902. doi: 10.1242/jcs.113.11.1891. [DOI] [PubMed] [Google Scholar]
- Miyake K, McNeil PL. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol. 1995;131:1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A, Wilkinson M, Burgoyne RD. Identification of Exo2 as the catalytic subunit of protein kinase A reveals a role for cyclic AMP in Ca2+-dependent exocytosis in chromaffin cells. EMBO J. 1993;12:3747–3752. doi: 10.1002/j.1460-2075.1993.tb06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron. 2000;27:159–168. doi: 10.1016/s0896-6273(00)00017-9. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kishimoto T, Miyashita Y, Kasai H. Ca2+-dependent exocytotic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+ compound. J Biol Chem. 1996;271:17751–17754. doi: 10.1074/jbc.271.30.17751. [DOI] [PubMed] [Google Scholar]
- O'Sullivan AJ, Jamieson JD. Protein kinase A modulates Ca2+- and protein kinase C-dependent amylase release in permeabilized rat pancreatic acini. Biochem J. 1992;287:403–406. doi: 10.1042/bj2870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Rodríguez A, Martinez I, Chung A, Berlot CH, Andrews NW. cAMP regulates Ca2+-dependent exocytosis of lysosomes and lysosome-mediated cell invasion by trypanosomes. J Biol Chem. 1999;274:16754–16759. doi: 10.1074/jbc.274.24.16754. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Verzijl D, Iyengar R. Indentity of adenylate cyclase isoform determines the rate of cell cycle progression in NIH 3T3 cells. Proc Natl Acad Sci USA. 1998;95:15084–15089. doi: 10.1073/pnas.95.25.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Bi G-Q, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Carew TJ. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron. 2000;26:219–231. doi: 10.1016/s0896-6273(00)81152-6. [DOI] [PubMed] [Google Scholar]
- Swanson JA, McNeil PL. Nuclear reassembly excludes large macromolecules. Science. 1987;238:548–550. doi: 10.1126/science.2443981. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J Cell Biol. 1997;139:63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T, Alderton JM, Bi G-Q, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci. 1999;112:719–731. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]
- Togo T, Krasieva TB, Steinhardt RA. A decrease in membrane tension precedes successful cell membrane repair. Mol Biol Cell. 2000;11:4339–4346. doi: 10.1091/mbc.11.12.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau L-E, Emery DG, Haydon PG. Direct modulation of the secretory machinery underlies PKA-dependent synaptic facilitation in hippocampal neurons. Neuron. 1996;17:789–797. doi: 10.1016/s0896-6273(00)80210-x. [DOI] [PubMed] [Google Scholar]
- Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB J. 1995;9:219–228. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]