Abstract
There has been extensive research in the recent past looking into the molecular basis and mechanisms of the biological clock, situated in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus. Neurotransmitters are a very important component of SCN function. Thorough knowledge of neurotransmitters is not only essential for the understanding of the clock but also for the successful manipulation of the clock with experimental chemicals and therapeutical drugs. This article reviews the current knowledge about neurotransmitters in the SCN, including neurotransmitters that have been identified only recently. An attempt was made to describe the neurotransmitters and hormonal/diffusible signals of the SCN efference, which are necessary for the master clock to exert its overt function. The expression of robust circadian rhythms depends on the integrity of the biological clock and on the integration of thousands of individual cellular clocks found in the clock. Neurotransmitters are required at all levels, at the input, in the clock itself, and in its efferent output for the normal function of the clock. The relationship between neurotransmitter function and gene expression is also discussed because clock gene transcription forms the molecular basis of the clock and its working.
Introduction
Great advances have been made in the study of mechanisms of the circadian clock in the past decade. Since the identification of a master circadian clock in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus of mammals, researchers sought to identify the nature of the clock and characterize its components. The SCN, acting as circadian pacemakers, have the function of orchestrating the timing in physiology and behaviour. They control circadian rhythms in other parts of the brain, such as the cerebral cortex, in the pineal gland, and in peripheral tissues such as liver, kidney and heart [1]. The circadian clock not only can generate its own rhythms but can also be entrained by the environmental light-dark (LD) cycle. Multiple single cell circadian oscillators that are present in the clock can, when synchronized, generate coordinated circadian outputs which ultimately regulate the overt rhythms.
Studies pertaining to the molecular mechanisms of the clock have yielded valuable results with the identification of a protein responsible for the setting of the length of periods of activity and inactivity within cells. Many years of research by a dedicated team of scientists culminated in the discovery of this protein [2]. It is believed that the identification of this protein will have far reaching implications not only in the understanding of the working of the clock but also in clinical applications, such as the treatment of jet lag and the design of optimal times for the administration of anti-cancer drugs.
The master clock, as it is often called, is reset by light or photic stimuli [3] as well as by arousal-inducing or non-photic stimuli [4]. Whether the input is photic or non-photic, it reaches the clock through neurotransmitters in nerve terminals. Neurotransmitters are released at the inputs for entrainment, in the clock itself for integration and consolidated output, and in efferent projections for the control of overt rhythms. Several reviews on the neurotransmitters of the SCN have been previously published [5-10]. The present review concentrates on studies conducted in the last decade and gives particular attention to neurotransmitters whose involvement in the circadian clock have not been traditionally recognized.
About neurotransmitters in general
Studies have indicated the presence of a large number of neurotransmitters in the SCN [11-15]. However, information about their role individually as well as in combination in the functioning of the clock has been slow to come. It is observed that the presence of neurotransmitters in the afferent and efferent projections of the SCN is equally important for the entrainment of the clock and for the control of overt rhythms. Thus, we have neurotransmitters released at the inputs for entrainment, in the clock itself for the integration and consolidated output, and in the efferent projections for the control of overt rhythms.
There have been attempts to categorize the putative neurotransmitters of the SCN on the basis of their origin and function [16] and there have been reports indicating subdivisions of the SCN with relation to neurotransmitter function [17]. Further, it has been reported [18] that the human SCN also have well defined subdivisions with chemically defined neuronal groups comparable to the well defined subdivisions reported in the case of experimental animals, mainly rodents. There are many excellent reviews [19-22] highlighting various aspects of the neurotransmitters. From a functional point of view, two important aspects emerge. One is the fact that one particular neurotransmitter may have more than one function and thereby make the prediction of the function more difficult and complex. Another aspect is that the neurotransmitter input from various pathways and their influence may vary (1) by itself and (2) by way of modification of SCN function. Neurotransmitters like acetylcholine, glutamate, neuropeptide Y (NPY), serotonin, vasoactive intestinal peptide (VIP), peptide histidine isoleucine (PHI), and arginine vasopressin (AVP) have been implicated in the functioning of the SCN. Glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) are indicated as principal neurotransmitters of the retinohypothalamic tract (RHT), although excitatory amino acids like L-aspartate and N-acetyl-aspartylglutamate may also function as neurotransmitters in RHT. Substance P also might be a candidate as a neurotransmitter in RHT. Functional studies over the years have given evidence that PACAP alone or in concert with glutamate may be responsible for the light signalling to the clock.
The role of AVP in circadian time keeping has been well established. Its role in the control of circadian rhythm of food and water intake has been reported and well documented. Another intrinsic neuropeptide, VIP, acting through VPAC2 receptor (a type of receptor for VIP), participates in both resetting to light and maintenance of ongoing rhythmicity of the SCN. NPY and GABA seem to be the neurotransmitters in the projection from the intergeniculate leaflet to the SCN. Raphe nuclei projections to the SCN contain serotonin. AVP and prokineticin 2 are seen in the outputs from the SCN.
The neurotransmitter-dependent molecular basis of the working of the clock is yet to be understood completely. The specific roles of the various neurotransmitters may be based on the response of the neurons of the SCN on application of a neurotransmitter, capacity to phase shift a particular rhythm on application of neurotransmitter, effect of lesions on the entrained and free running rhythms, and disruptions seen in the rhythms after blockade by the antagonist/inhibitors. Possible targets for some of the neurotransmitters are the clock genes per1 and per2, which are induced in the SCN by light or by neurotransmitters, at night.
Evidence indicating co-localization of some of the neurotransmitters in the SCN has further complicated the investigations into the role of neurotransmitters in the working of the clock. It is likely that the functioning of the clock may depend on the presence of a particular neurotransmitter on a mechanism in which co-localized neurotransmitters interact in a functionally significant manner.
With more information available on the role of neurotransmitters in the working of the clock, which is involved in so many functions of the body, better opportunity for neurotransmitter-based manipulation of the clock has also been reported. Problems of shift-work insomnia and ill effects of jet lag are among the clock-related functions for which much attention has been given in recent years. Melatonin has been in use with some success in reducing the above effects. However, search for other chronobiotic agents is continuing and it is likely that there may be some new dimensions given to the problem and its solution in future. A report of a close relationship between circadian clock and cell proliferation makes things even more interesting. Investigations into the role of neurotransmitters in the SCN as well as in the afferent and efferent inputs have come a long way with the advent of newer techniques of positron emission transaxial tomography (PET) scan and polymerase chain reaction (PCR).
In both plants and animals, circannual rhythms are widely distributed. Endogenous circannual rhythms form the basis for many seasonal rhythms. A number of neuroendocrine mechanisms have been implicated in the regulation of seasonal changes in the physiology and behaviour of animals. Some of these neuroendocrine pathways are necessary for the regulation of particular overt seasonal responses, though they may not be directly linked to the circadian time-keeping system. Photoperiodic input and circannual function may have profound influence on many of the functions of body. Naturally, neurotransmitters are involved not only in circadian function but also in seasonal processes. It has been postulated that heterogeneity of the clocks which are seen within the SCN may be one of the factors that form the basis of seasonal adaptations [23]. A typical example of the close linkage between seasonal rhythms and affective disorders can be seen in a seasonal form of mood disorder, seasonal affective disorder (SAD). Treatment for SAD based on circadian principles includes not only light therapy but also the use of certain drugs, again based on circadian principles involving neurotransmitters.
The SCN has been subdivided into a dorsomedial shell and a ventrolateral core. This is based on retinal innervation patterns as well as the observation that these regions are defined by phenotypically distinct cell types [24]. Each nucleus contains about 10,000 neurons, thereby making 20,000 neurons in total. These neurons are characterized by small size and high density [25]. Isolated individual neurons are reported to produce circadian oscillations with periods ranging from 20–28 h [26,27]. Circadian oscillations are generated in the individual neurons of the SCN by a molecular regulatory network. Though individual cells oscillate with periods ranging from 20–28 h, at the tissue level SCN neurons display synchrony indicative of a robust inter-cellular coupling, and neurotransmitters appear to have an important role in the inter-cellular coupling. Gondze and co-workers [28] have introduced a molecular model for the regulatory network underlying the circadian oscillators in the SCN and stated that effective synchronization is achieved when the average neurotransmitter concentration damps the individual oscillators. Cells are effectively synchronized due to global neurotransmitter oscillation. Neither spiking in the neurons of the SCN nor chemically mediated transmission is needed for the pacemaking activity seen in individual cells. However, synchronization of circadian rhythmicity across neurons in the SCN does require neurotransmitters [25,29] and development of action potentials [30].
Before attempting a discussion of the neurotransmitters, it is necessary to identify the afferent projections to the SCN, and neurotransmitters present in it, neurotransmitters intrinsic to the SCN, and efferent projection with its neurotransmitters. The SCN is composed of different neuronal elements, each having its own specific function. Intensive interconnection and interaction among the heterogeneous neuronal elements is responsible for the functional output of the SCN. Different neurons of the SCN contain different neuropeptides, with several neurons having co-localization of neurotransmitters. Thus, we have, for example, gamma amino butyric acid (GABA) and glutamate, GABA and AVP, AVP and corticotrophin releasing hormone (CRH), AVP and its carrier protein neurophysin, VIP and peptide histidine isoleucine (PHI), and VIP and somatostatin (SS). Co-localization of different neuropeptides is seen not only in rat SCN but also in human SCN [31-33]. The combination of a variety of peptides with or without amino acid neurotransmitters within a single nucleus gives the SCN a variety of signalling properties as well. A set of SCN neurons and their neurotransmitters has the function of conveying the daily light-dark signal to hypothalamic target structures [34-36].
Three major incoming pathways have been identified for the SCN. These have been defined as the retinohypothalamic tract (RHT), geniculohypothalamic tract (GHT), and the projection from the raphe nuclei (Figure 1). Photic information is relayed directly from the retina to the SCN by way of the monosynaptic retinohypothalamic tract [37,38]. It is seen that transection of all visual pathways leaving the optic chiasm makes animals blind with no visual reflexes but with perfect normal entrainment of circadian rhythms. This has indicated that RHT is sufficient for entrainment. Also it has been demonstrated that sectioning of RHT abolishes entrainment without affecting visual functions [39]. Although the exact mechanism by which a monophasic stimulus, light, produces either no response or a biphasic response in SCN neurons is unclear at present, Myers and co-workers [40] provided a potential molecular explanation for the phenomenon. Using electron microscopy and immunohistochemistry, they identified the excitatory amino acid glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) as the main neurotransmitters of the RHT [41-43]. Although substance P (SP) was thought of as a neurotransmitter of the RHT, there is now substantial evidence against this possibility [44-46].
Figure 1.
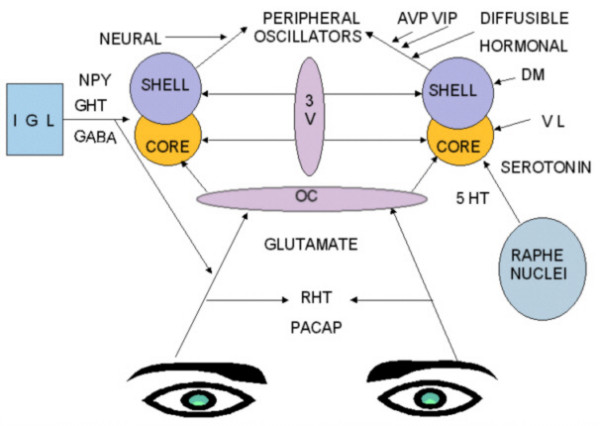
Afferent inputs and efferent pathways of the SCN. RHT: Retinohypothalamic tract, GHT: Geniculohypothalamic tract, OC: Optic chiasm, 3V: Third ventricle, IGL: Intergeniculate leaflet, DM: Dorsomedial SCN, VL: Ventrolateral SCN, NPY: Neuropeptide Y, GABA: Gamma amino butyric acid, PACAP: Pituitary adenylate cyclase-activating polypeptide.
Retinohypothalamic tract (RHT) and its neurotransmitters
The principal neurotransmitters involved in conveying photic information to the SCN have been identified as glutamate and PACAP. Light stimulation of the retina results in direct secretion of glutamate from the RHT into the ventral VIP-containing part of the SCN [47-49]. Glutamate as a transmitter at RHT/SCN synaptic connections plays an important and critical role in mediating photic regulation of circadian rhythmicity. RHT terminals innervating the SCN show glutamate immunoreactivity associated with synaptic vesicles [41,50], which confirms the role of glutamate as a neurotransmitter. Different types of glutamate receptors were identified and localized in the SCN using in situ hybridization and immunocytochemistry [51]. PACAP, which is co-localized in a subpopulation of glutamate-containing retinal ganglion cells and also involved in relaying light information, may potentiate the action of glutamate on the SCN [52,53]. Both glutamate and PACAP fulfil the criteria of being located in the RHT, being released on stimulation, affecting the cells of the SCN in a manner similar to light, and having their effects blocked by specific antagonists [20]. Exogenous application of glutamate receptor (GluR) agonists is found to excite SCN neurons [54,55] and cause phase shifts. On the other hand, GluR antagonists block light-induced phase shifts and Fos-induction in the SCN in vivo [56,57].
Nitric oxide (NO)
NO appears to be a crucial neuroactive substance for the function of the SCN. Presence of neurons showing nitric oxide synthase (nNOS) immunoreactivity in the SCN of dwarf hamster and rat [58,59] were further confirmed by the studies of Chen and co-workers [60] and Caillol and co-workers [61]. Nitric oxide production in the SCN has been linked to N-methyl D-aspartate (NMDA)-induced cyclic guanosine monophospahate (cGMP) production, and administration of cGMP produces phase shifts of circadian rhythms in vitro [62]. It has also been reported that NOS inhibitors prevent NMDA-induced phase shifts of circadian rhythms in vitro and in vivo [48]. There is also a possibility for an additional source of NO in the SCN from the astrocytes, as a group of cells positive for endothelial NOS (eNOS) was found in rat and hamster [61].
In terms of the functional impact of NO in the working of the SCN, blocking of NO production disrupts light transmission to the SCN [63], thus indicating the possibility of the role of NO in the light-input pathway. NO synthesis is required for phase changes of electrical activity [64]. Intracerebroventricular application of L-NAME (a drug that blocks NOS in hamsters) produces attenuation of light-induced phase-advances of activity rhythms [65]. Reports also indicate interruption in the light-triggered cascade of glutamate release from retinal terminals in the SCN by blockade of NO action in intact animals, which leads to subsequent interruption of NMDA receptor activation [66,67]. Interruption of intracellular increase of calcium, activation of nNOS, augmented production of cyclic guanosine monophosphate (cGMP), activation of protein kinase C, and phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), as well as interruption of the expression of immediate early genes, are other effects of blockade of NO action.
Starkey and co-workers [68] provided evidence for the presence of functional type II NOS within the SCN of guinea pig. All isotypes of NO synthase have also been identified in the normal adult mammalian SCN. Contribution by more than one NOS isotype to the regulation of circadian rhythms cannot be ruled out. In this context, it is interesting to note the demonstration by Kriegfeld and co-workers [69] that mice lacking the gene for type I NOS experience no change in the ability to phase-shift or entrain circadian rhythm of locomotor activity. Yet another study, also by Kreigfeld and co-workers [70], has suggested that endothelial isoform of NOS among the three known isoforms may not be necessary for photic entrainment in mice. However, considering the three different forms of NOS identified, until the isoforms of NOS involved in regulating the clock phase by modulating inputs are completely established it is difficult to speculate the exact role of NO.
There has been much speculation as to the photopigment mediating light information to the SCN. However, this is not yet known with certainty [71]. A novel opsin, melanopsin, was identified [72] and found to be exclusively expressed in the ganglion cells of RHT [20,73,74]. Melanopsin-containing RHT ganglion cells also use PACAP, another well known neurotransmitter of the RHT [20]. Melanopsin, however may not be the only circadian photoreceptor since melanopsin knock-out mice showed typical, although reduced, light responses such as entrainment and phase shifting.
Possibilities of other neuroactive substances serving as neurotransmitters in the RHT in addition to glutamate and PACAP, which are the most important candidates, have been indicated. Projections of substance P (SP)-containing ganglion cells to the ventrolateral part of the SCN have been demonstrated in lesion experiments in the rat [75]. Electrophysiological investigations also further support the role of substance P as an excitatory neuromodulator [76] responsible for the expression of both NMDA and non-NMDA receptor-mediated components of RHT transmission. Moreover, it is also reported that SP and glutamate work as agonists upstream of glutamate [77].
Histamine
Despite substantial evidence [17,78,79] suggesting a role for histamine as a neurotransmitter in circadian entrainment [17,78,79], its role has been underplayed. With more information available and even a suggestion that histamine may be acting as a final neurotransmitter on which photic and non-photic entrainment converge [80], there has been more attention in this direction. Histamine can induce phase shifts in circadian rhythms in a manner similar to that of light pulses. Intracerebroventricular injection of histamine is also found to alter circadian function [81]. Direct effects of histamine on SCN neurons have been shown in vitro either as inhibitory or excitatory depending on experimental conditions [82,83]. It is also reported that at the level of the SCN the direct excitatory effects of histamine on neuronal firing is mediated via H1 receptors and the inhibitory effects via H2 receptors [82,83]. However, in vivo studies, it has been shown that the effects of histamine on circadian rhythms may be mediated through receptors other than histamine receptors [84,85]. The foregoing discussion supports the view that histamine may exert modifying effects on circadian rhythmicity as well as neuronal excitability. There is a clear circadian rhythm in the histaminergic activity, with high levels during the active period and low levels during the sleep period. Maintenance of circadian rhythmicity of sleep-wakefulness cycles, food intake, motility and adrenocortical hormone release seems to depend on histaminergic activity. Thus, although evidence is accumulating for a role of histamine in circadian function, it is difficult to assign it a specific role in circadian activity at this time.
Neurotensin (NT)
Cell bodies of the rat SCN contain the neuropeptide neurotensin (NT) and two NT receptor types, namely NTS1 and NTS 2 [86-88]. In humans there is a larger population of NT neurons as compared to monkeys and other animals. Although involved in many physiological processes, the role of NT in circadian rhythm is not completely known at present. Meyer-Spasche and co-workers [89] reported that NT can phase shift the firing rate rhythm of SCN neurons. They also provided evidence that NT may play a role in regulating the circadian pacemaker through NTS1 and NTS2 receptors. NT-binding sites found in the ventral region of the SCN, which receives photic and non-photic information, is indicative of the involvement of NT in the synchronization of clock to these environmental stimuli [90]. Studies using NTS1 and NTS2 agonists, neurotransmitter receptor antagonists, as well as the exogenous application of NT, have yielded some valuable results. An increase in discharge rate of SCN neurons was observed on NT application [90]. NT-mediated effects on SCN neurons seem to result from activation of NTS1 and NTS2 receptors rather than involve glutamate or GABA receptors or modulation of the synaptic release of glutamate or GABA [90]. NPY, which is an established neurotransmitter of the geniculohypothalamic tract (GHT), was found to regulate SCN neuronal activity [91-93] and to produce long lasting suppression of firing rate of SCN neurons. When co-applied with NPY, NT was found to damp the profound inhibitory effect of NPY [90,92,93]. This is interesting since there are studies showing that NPY immunoreactive terminals overlap with NT-binding sites in the ventral part of the SCN. This was considered as evidence of an interaction of NPY and NT to regulate neural activity. From a developmental point of view, NT-expressing neurons developed earlier than the other 3 types of peptidergic neurons, NPY, VIP and AVP [94]. It remains to be seen whether NT-expressing neurons contribute significantly to the generation of circadian rhythms in early human life.
Neuromedin S (NMS)
A recent addition to the ever increasing list of neurotransmitters of the SCN is neuromedin S (NMS), a 36 amino acid neuropeptide. It is a potent brain-gut neuropeptide whose presence in the SCN was reported by Nakahara and co-workers [95] as a neurotransmitter of the circadian oscillator system. NMS expression is found to be restricted to the core part of the SCN and has a diurnal peak under light-dark cycle [96]. Intracerebroventricular administration of neuromedin S in rats activates SCN neurons and has the capability to induce non-photic type of phase shifts in the circadian rhythm of locomotor activity. It is also possible that NMS along with VIP may have a role in maintenance of circadian rhythmicity. Recently, it has been shown that neuromedin M (NMU) is regulated in a circadian manner with peak expression in the light phase of LD cycle [97]. Further studies are required to understand the specific role of NMS in the SCN. At present, it is implicated in the regulation of circadian rhythms through autocrine and /or paracrine actions through its receptors [96].
Gastrin releasing peptide (GRP)
Gastrin releasing peptide (GRP) has also been identified as a neurotransmitter in the SCN. Although GRP and its receptor BB2 are found to be synthesized by rodent SCN neurons [98-100], the role of GRP in circadian rhythm regulation is not well known. Evidence points towards a role for GRP in photic entrainment [101,102], in spite of a number of studies favouring glutamate as the main neurotransmitter [36,37,103]. McArthur and co-workers [104] studied the role of GRP in photic entrainment by using the resetting actions of GRP application on electrical activity rhythms during subjective day, early subjective night, and late subjective night in vitro in rats and hamsters. Their studies have shown phase delay on SCN neuron firing during early subjective night and phase advance during late subjective night with no response on application during subjective day. Phase shifts were blocked by a BB2 receptor antagonist, thereby confirming the role of GRP in the participation of photic entrainment. GRP found within calbindin-containing retinorecipient cells and also causing photic-like phase shifts on application directly to the SCN may be a possible neurotransmitter for intra-SCN communication [105].
Acetylcholine (ACh)
Acetylcholine (ACh) has the distinction of being identified as the first neurotransmitter for the regulation of circadian rhythms. There is evidence in favour and against acetylcholine as a neurotransmitter in the literature. It was suggested that acetylcholine plays a role in the light-input pathway on the basis of some of the studies [16,106]. Electrophysiological studies indicating excitation of some neurons of the SCN by cholinergic agents [107,108] have supported the role of acetylcholine. Use of the acetylcholine receptor agonist carbachol, a non-specific agonist, to mimic the effects of light [109] also added to the supporting evidence for the role of acetylcholine in the SCN. The effect is mediated by muscarinic receptors of the M1 subtype [110]. Intraventricular administration of carbachol, which caused phase shifts in vivo, could be blocked by GluR antagonists [111]. However, acetylcholine does not appear to be directly involved as a neurotransmitter in the light-input pathway. It may act to modulate the photic information reaching the SCN.
Geniculohypothalamic tract
The geniculohypothalamic tract (GHT) is a second afferent photic projection from the intergeniculate leaflet (IGL) to the SCN. The IGL receives input directly from the retina via a separate branch of the RHT. The projection from IGL via GHT terminates in the areas of the SCN that overlap the direct RHT-SCN input. GHT provides a secondary, indirect photic input as well as an alternate input which has an important role in entrainment mediated by non-photic stimuli such as motor activity. While lesions of the IGL block activity induced phase shifts [112], electrical stimulation produces phase shifts similar to those produced by activity [113]. It has been reported that IGL mediates photoperiodic responses as well as non-photic entrainment of circadian rhythms [114,115]. Hence, the IGL may have integration of photic and non-photic information as its function. In addition to neuropeptide Y (NPY), GHT may also have GABA and enkephalin (ENK) as neurotransmitters in rat and hamster [116,117]. ENK was found in cell bodies in the IGL as well as in fibres in the SCN of many mammalian species. High density of delta opioid receptors, which have the highest affinity for ENK, was detected in the hamster SCN [118] and an ENK agonist phase-advanced hamster wheel running activity late in subjective day [119].
Neuropeptide Y (NPY)
RHT and GHT exhibit partial overlapping in the SCN. The SCN exhibits immunoreactivity for NPY [120]. There is evidence that IGL mediates both photoperiodic and non-photic entrainment of circadian rhythms [114]. NPY, the primary transmitter of GHT, acts directly on pacemaker neurons of the SCN in hamsters [121]. IGL neuron projections to the SCN also have GABA/NPY immunoreactivity and those projecting to contralateral IGL have GABA/ENK immunoreactivity [116,122]. There may be co-localization of NPY and GABA in the GHT projections. Since many features of the response to light by the circadian system remain unaffected by IGL lesion in animals, it is suggested that this pathway may not be critical for the photic regulation.
Further evidence is available to emphasize the importance of GHT to cause non-photic phase shifts during the day but not during the night, such as the phase-shifts evoked by activity induced by novel stimuli [123,124]. The phase shifts are abolished by IGL lesions. Both in vitro and in vivo NPY administration produced a similar pattern of phase shifts during the day, which was blocked by bicuculline [125]. NPY has also been found to act presynaptically to inhibit GABA-mediated synaptic transmission through inhibition of calcium currents [126].
Serotonin (5HT)
A dense, robust serotonergic projection from midbrain raphe nuclei terminating predominantly in the retinorecipient region of the SCN has been reported [127]. A reciprocal projection from the SCN to raphe nuclei is also seen [128]. Both in vitro and in vivo, 5HT receptor agonists are found to cause phase shifts of the SCN when administered at times in the circadian cycle during which light does not cause phase shifts [129,130]. Raphe nuclei lesions reduce the amplitudes or "clarity " of rat's circadian activity rhythm [131] with detectable persistent rhythmicity. Serotonergic projection to the SCN terminate to a great extent on vasoactive intestinal peptide(VIP)-containing neurons in the ventrolateral part of the SCN. There is a close relation between retinal afferents and VIP-containing neurons of the SCN in this area [132].
The major function of the serotonergic projection most probably is the modulation of the pacemaker responses to light. Raphe nuclei receive retinal afferents [133] and hence the raphe-retina projection may be viewed as another indirect photic input to the biological clock. In in vitro studies, serotonin advances the phase of the circadian pacemaker during the day and delays it at night, an action similar to that of GABA [134]. Serotonin is also found to regulate SCN neurons by both pre- and post synaptic inhibitory mechanisms [135]. 5HT and 5HT agonists are also found to inhibit optic nerve-induced field potentials in the SCN brain slice preparation, and light-induced Fos expression and phase shifts of the circadian rhythm of wheel-running activity [136]. It is also reported that 5HT antagonists enhance light-induced increases in the firing rates of SCN neurons [137] and light induced phase shifts [138]. Many of the above studies point towards the hypothesis that the serotonergic innervation of the SCN serves to modulate light-induced glutaminergic input. Considering these facts, there exists a possibility of the involvement of 5HT in tonic inhibition of the light-input pathway to the SCN. There is a suggestion that this serotonergic projection from raphe to the SCN may be the anatomical substrate for affective disorders to alter human circadian system/rhythms. This belief is further strengthened by the observation that dysfunction of serotonergic pathways play a role in affective disorders and that these disorders are frequently treated with agents that alter serotonergic neurotransmission. Further studies about this pathway are likely to give more information about the link between disruptions of circadian function and affective disorders.
GABA
It is now widely accepted that gamma amino butyric acid (GABA) is an important neurotransmitter of the SCN for regulating SCN function. Most SCN neurons express the neurotransmitter GABA and are thus GABAergic [12]. GABA receptors and receptor subunits have been described by Castel and Morris [139], Naun and co-workers [140], van den Pol [141], Gao and co-workers [142], and O'Hara and co-workers [143]. In most of the brain regions, GABA primarily acts through interaction with GABAA and GABAB receptors and produces neuronal inhibition through membrane hyperpolarization and increased membrane conductance. Glutamic acid decarboxylase (GAD) is the enzyme required for synthesizing GABA and is found in nearly all neurons of the SCN [116]. Additional support for an inhibitory role of GABA in the rat SCN has come from the studies of Gribkoff and co-workers [144,145]. However, recent investigations have shown that GABA has dual effects on the SCN neurons, excitatory during day and inhibitory at night [146], and this has been attributed to changes in [Cl- ]I during the circadian cycle. This dual inhibitory [147] and excitatory [148] action of GABA has been thought of as the probable reason for the synchronization of spiking in the SCN neurons. Under some circumstances, such as in early development, GABA can also be depolarizing and potentially excitatory [126,149-151]. The excitatory effect of GABA on SCN neurons in the night seems to be complex. While the action of GABA in the day on SCN neurons is uniformly inhibitory, the effects of GABA during the night are heterogeneous due to both depolarizing and hyperpolarizing effects. The GABA-mediated depolarizing effect seen at night is restricted to a subset of SCN neurons. Differential day-night modulation of GABAergic neurotransmission seen in the SCN may provide a time-dependent gating mechanism to counteract propagation of excitatory signals throughout the biological clock during the day and to promote it at night [152]. GABA does not seem to be synthesized in the SCN in a circadian fashion, but in a diurnal pattern as per GAD m RNA basis [153,154]. A circadian rhythm in GABA transmission in the dorsal part of the mouse SCN, with requirement of VIP for the expression of this rhythm, is reported by Itri and co-workers [155]. While considering the action of GABA in the SCN, whether inhibitory or excitatory, extrinsic GABA sources such as from IGL [116] and release of GABA from SCN terminals implicated in transmission of light information should also be looked into to visualize a clearer picture. GABA receptors and receptor subunits are expressed in the SCN [116,139,140]. Although no variation in concentration of GABA in the SCN has been reported, responsiveness of the SCN undergoes daily variation [156].
Neurotransmitters for intra-SCN communication
Integrated output as a result of integrated activity within the SCN in spite of heterogeneity in functional and neurochemical organization explains the efficiency of the SCN, the biological clock. Such a process is likely to involve much coordinated activity, and intra-SCN communication must be strong enough to produce such an action. It is clear that photic information received must be relayed from retinorecipient cells to the oscillator cells in the nucleus. Intra-SCN signals underlying such communication are not known with certainty as yet. The importance of circadian synchrony of the SCN neurons in the normal working of the clock has been highlighted in some animal studies recently [157]. In these studies, loss of coherent daily rhythms has been shown to coincide with loss of circadian synchrony among the constituent neurons [157]. Neurotransmitters have been designated as potential SCN synchronizers and one of them, which is unique since it is expressed by most of the SCN neurons, is GABA. This and other transmitters involved in synchronization, such as VIP, GRP and prokineticin 2, have been studied quite extensively. However, the potential of the latter two, GRP and prokineticin 2 as synchronizing factors require further investigation [23]. Further, it is reported that neurotransmitters released by neurons of the ventral part of the SCN is necessary for maintaining synchrony of the whole SCN [23 ].
Vasoactive intestinal polypeptide (VIP)
VIP, a gut polypeptide, has been identified as one of the main neurotransmitters of SCN neurons and participates in SCN function. These SCN neurons are retinorecipient and are found in the core of the SCN. They are activated by light, and exogenous application of VIP can reset the circadian clock in a manner similar to that of light application, both in vitro and in vivo[6]. It is estimated that 9%–24 % of SCN neurons express VIP [26,158]. It appears that in rats there are two types of VIP neuronal components[159], namely a medial GRP-free group and a lateral group containing GRP. Only the lateral group expresses per1 following a light pulse [159]. However, few VIP-containing cells rhythmically express per1 and per2 [160,161]. VIP is synthesized from prepro VIP and further cleavage of the molecule forms VIP and peptide histidine isoleucine (PHI). PHI is found in abundance in the SCN and is co-localized [162,163]. VIP and PHI are structurally related to PACAP. The receptor for VIP, VPAC2,also known as Vipr2, is expressed in about 60% of the SCN neurons, which respond to VIP with changes in firing rate [164,165]. VIP acting through VPAC2 can participate in both resetting by light and maintenance of ongoing rhythmicity in the SCN [6].
VIP along with GRP and AVP show circadian variation in the level of mRNA in constant environmental conditions [166]. Some earlier studies [167-169] had indicated that VIP and GRP do not show circadian rhythms in DD and only daily rhythms in LD. On the basis of their study, Shinohara et al [168] suggested that changes in the peptide content by light conditions might reflect changes in the synthesis and release of peptides. The release of these peptides also shows circadian variation [170]. It has been reported that treatment of SCN slices with VIP produces phase shifts similar to those induced by light pulses [171]. Nielsen and co-workers [172] showed that VIP induces per1 and per2 gene expression in rat SCN in a phase dependent manner. More recently, VIP has been shown to be necessary for the coordination of the daily rhythms in behaviour and physiology at the level of biological clock in mice [173]. Loss of internal desynchronization and its subsequent restoration were achieved by adding VIP into the mice cells. Thus, VIP signalling through its receptor serves two important functions in the SCN, namely, circadian rhythmicity in a subset of neurons and maintenance of synchrony between intrinsically rhythmic neurons. This may also mean that VIP-expressing neurons themselves are circadian pacemakers in the SCN for establishing and synchronizing rhythmic activity.
Vasopressin (AVP)
AVP neurons occupy a large part of the SCN, mostly in the dorsomedial part of the SCN and are extensively interconnected [174,175], indicating the capacity of the SCN to produce an integrated output. It is estimated that nearly one third of the SCN neurons in rats synthesize AVP. It is one of the major neuropeptides identified in the SCN [16,176,177]. AVP is synthesized and secreted by the SCN in a circadian pattern. AVP has an important excitatory role by activating V1a receptors [177] to increase the amplitude of firing rates in the SCN during subjective day [178,179] and enhance SCN output [176,177]. Although the presence of AVP at the level of the SCN may not be critical for the expression of some of the circadian rhythms, abnormalities can be seen in some of the expressed rhythms in its absence. AVP-deficient Brattleboro rats have served as an excellent model for the demonstration of the absence of AVP and subsequent disturbances in many of the circadian rhythms. Local application of AVP into the SCN does not affect the free running circadian wheel running rhythm in hamsters[180] or entrained circadian food intake [181] and water intake [182]. A convincing supportive role for vasopressin in SCN circadian function has come from transplantation studies of DeCoursey and Buggy [183] as well as that of Lehman and co-workers [184]. Infusion of V1 receptor antagonist has been reported [185] to produce no significant effect on the wheel running activity in rats, thereby indicating no role for VP in the generation of circadian rhythms. Boer and co-workers [186] reported that vasopressin may not be a critical component in the maintenance or in the transfer of circadian activity of the biological clock for drinking activity based on their graft transplant study. In depressed patients, both synthesis and release of AVP in the SCN is reduced, which leads to an impaired functional activity of the circadian clock [187], although there was an increase in the number of AVP-immunoreactive neurons. Arima and co-workers [188] reported AVP transcription in the SCN in long-term organotypic cultures. Transcription exhibits circadian rhythmicity and is dependent on the ongoing electrical and synaptic transmission in the cultures.
It was mentioned earlier that the predominant excitatory actions of AVP within the SCN are mediated by V1 receptors, although it is not yet known with certainty whether V1a or V1b subtypes are involved in the action. Decrease in the AVP neurons and AVP content in the SCN has been reported [189-191], and this has been correlated with decreased amplitude of activity rhythms, increased rhythm fragmentation, and disruption of the normal sleep/wake cycle. However, Hochstetler and co-workers [192] did not find a correlation between differences in activity level and circadian expression and differences in the number of AVP-immunoreactive cells in the SCN. In a study by Kalamatianos and co-workers [193], it was reported that there is a decrease in the amplitude of the daily rhythm in the expression of V1a receptor mRNA along with persistently elevated level for V1b mRNA in aged male rats as compared to young adult ones. A role for AVP in the SCN not only in circadian timing but also in the circadian memory of radical events has been reported by Biemans and co-workers [194]. There have been many attempts in the past to link AVP in the SCN to specific clock function. However, the attempts have not yielded definite results so far. Reduction of AVP neurons of the SCN has been reported to eliminate or reduce the amplitude of many rhythms studied. But at the same time studies in Brattleboro rats have shown that AVP may not be necessary to maintain coherent circadian rhythmicity [195,196]. In house mice, Hochstetler and co-workers [192] reported that there is no relationship between AVP neurons in the SCN and circadian features of wheel running activity. In addition, the SCN also participates in the communication with the rest of the brain. One such output signal, primarily electrical but not exclusively, is AVP [197]. Correlation between SCN-AVP expression and circadian organization of locomotor behaviour has been shown across species including rats [198] and hamsters[199]. However, transplantation studies indicate some other diffusible factor other than AVP in the regulation of circadian rhythmicity [186].
Melatonin
Melatonin, the hormone from the pineal gland, called the "darkness hormone " is of great importance in the functioning of the SCN. The most important target of melatonin in humans appears to be the SCN, as the SCN contains the highest density for melatonin receptors [200]. A double effect of melatonin in the SCN, namely, an immediate effect and long term effect, has encouraged its worldwide use against the ill effects of jet lag. As an immediate effect, melatonin is found to suppress neuronal SCN activity towards night time levels [201]. It also lowers VP secretion from SCN neurons as shown by experiments in rats [202]. Acceleration of sleep initiation in humans at circadian phases when the SCN would normally stimulate waking is another reported action of melatonin [203]. In terms of long term effect, melatonin can phase shift and amplify circadian rhythmicity of the SCN. Melatonin application has been found to be useful in synchronizing the endogenous circadian rhythms not only in people who suffer from jet lag, but also in blind individuals [204,205], patients with dementia [206], and shift workers [207]. Probably recognizing the importance of melatonin as a chronobiotic, many researchers have studied the applications of melatonin on human circadian rhythms. Recently, Revell and co-workers reported that administration of a combination of morning intermittent bright light and afternoon melatonin along with a gradually advancing sleep schedule can advance circadian rhythms almost an hour a day, with very little circadian misalignment [208]. This protocol might be applied before eastward jet travel or for delayed sleep phase syndrome to evoke a phase advance of the circadian clock [208]. In spite of the experimental evidence favouring a very important role for melatonin in the circadian timing system, the exact role of melatonin has not been demonstrated clearly. Melatonin and seasonal rhythms are intimately related in mammals, and this has been well documented [209,210]. Lincoln and co-workers [211] provided evidence for a temporal melatonin-controlled expression of clock genes in specific calendar cells. The retinohypothalamic -pineal (RHP) axis is comparable in animals and humans. In both animals and humans melatonin is secreted exclusively at night. The RHP is capable of detecting changes in night length to make proper adjustments for the duration of nocturnal melatonin secretion so that animals can use this melatonin message to trigger seasonal changes in behaviour [209]. With seasonal changes in night duration, there are parallel changes in the duration of melatonin secretion, and this leads to more secretion as compared to summer.
Neurotransmitters in efferent projections
The output of the SCN by way of efferent projections serves the purpose of conveying the information to the related centres. Outputs are primarily seen to the nearby hypothalamic and thalamic nuclei from the SCN, particularly to the medial preoptic nucleus, the medial part of the paraventricular nucleus of the hypothalamus, the anterior part of the paraventricular nucleus of thalamus, the medial part of the dorsomedial nucleus of hypothalamus, and principally the subparaventricular zone [212,213]. Projections to the ventrolateral preoptic nucleus from the dorsomedial nucleus, the preoptic nucleus and the subparaventricular zone appear to serve as the anatomical basis for the control of sleep and wakefulness, as the ventrolateral preoptic nucleus is implicated in the control of sleep states [214-216]. Efferent projections seem to have mainly AVP and VIP as transmitters. These fibres that originate in the SCN can be seen for long distances within the hypothalamus and have a characteristic morphology. The functional significance of these projections remains to be fully determined, apart from the basic fact that they are necessary for the SCN to exert its overt function. The functional role has been described to some extent earlier [217].
Although the SCN is often designated as the "master" circadian pacemaker that drives most, if not all, rhythmic physiological processes, the importance of oscillators outside the SCN cannot be ignored. Indeed, there is considerable evidence for the existence of circadian pacemakers outside the SCN [218-220] (Figure 2). It is believed that the master circadian pacemaker (the SCN) has peripheral "slave" oscillators that may be individual clocks. It is necessary in such a situation to have a mechanism by which peripheral oscillators are coupled to the master oscillator thereby synchronizing the activity of an organ with the central clock. A humoral substance mediating the circadian signal may be available in the efferent output in such case [221]. One such recently identified diffusible output candidate from the SCN is transforming growth factor α (TGFα) [222]. TGFα is found extensively in the brain and is a member of the epidermal growth factor (EGF) family produced by both neurons and astrocytes [223]. In situ hybridization and immunocytochemistry techniques have demonstrated the presence of TGFα in the SCN of rats [224,225] and Syrian hamsters [222,226,227]. Van der Zee and co-workers [228] reported that the two output systems of the SCN, namely AVP and TGFα, are anatomically separate, having different daily profiles in expression.
Figure 2.
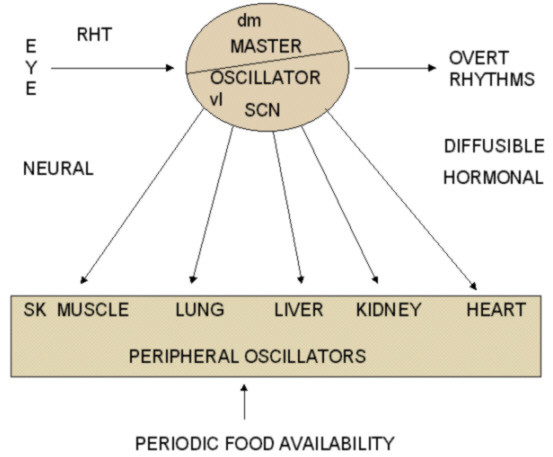
Inter-relation and efferent outputs between central and peripheral oscillators. Notice the neural and diffusible control of the central oscillator. Peripheral oscillators respond to signals from SCN as well as to other inputs like periodic food availability. Diffusible output may have AVP, VIP, prokineticin, and TGF-α. RHT: Retinohypothalamic tract, dm: dorsomedial SCN, vl: ventrolateral SCN.
SCN output pathways in addition to influencing the hypothalamic neighbourhood [229] can be traced to extra hypothalamic sites as far as the liver, thyroid, adrenal, and salivary glands in rats [230,231]. Both neural output pathways and diffusible non-neural pathways become important in elucidating the functional significance of SCN output in terms of the control of the SCN on other oscillators.
Though the various mechanisms used to regulate the activity of other systems of the body is unclear at present, a number of hormones with direct actions on different parts of the body are produced by the SCN. These include AVP, VIP, GRP, and SS. A review by Van Esseveldt and co-workers [9] describes not only the transmitters of RHT but also the output of the SCN. A long neural signalling pathway from the SCN regulates the pineal gland secretion of melatonin. SCN neurons also stimulate gonadotrophin releasing hormone (GnRH) synthesizing neurons of the preoptic area and thereby affect sex hormone cycles [232]. It is generally seen that outputs of the circadian system are rhythmic but not temperature compensated. In spite of the difficulties to understand the mechanisms by which the SCN regulates a wide range of physiological outputs, it has been agreed that there are two types of signals originating from the SCN. These are hormonal and neural outputs. Transplantation experiments [233] in particular have provided highly useful evidences for the suggestion that hormonal /diffusible factors produced by the SCN act as an important output signal for the circadian system [234]. Quick recovery of behavioural rhythms within 4 days of transplantation of the SCN [184], successful placement of transplants at locations distant from the SCN [184], and transplanted dissociated SCN cells capable of restoring rhythmicity [235] are all in favour of hormonal /diffusible factors as the output signal.
It is possible that different physiological systems are controlled by either neural or hormonal output from the SCN. For example, rhythmic secretion of melatonin could be under neural control while locomotor activity might be under hormonal control. Also, a specific physiological function may receive both neural and hormonal signals. As regards to the communication of information from the circadian clock to the centres controlling activity in brain areas, more than one mode is indicated [222,236].
Analysis of the chemoarchitecture of the SCN have shown that, in addition to the above neurotransmitters, the SCN also contain neurons capable of synthesizing a number of other neurochemicals, with the distribution of immunoreactive neurons differing slightly for each neurochemical. A few of these neurochemicals are described here.
Somatostatin (SS)
SS producing neurons of the SCN are located in both the core and shell portions [237] and form a distinct peptidergic neuronal group. These cells are few in the mouse SCN, but are large in size. Part of the SS and Substance P (SP) peptides are co-localized [238]. The shell portion of the SCN, which is likely to be involved in the regulation of overt rhythms, projects within the SCN through SS fibres [239]. In an interesting study, Biemans co-workers [240] reported a significant increase in SS and SP immunoreactivity in aged Wistar rats as compared to young ones, thereby indicating that not all SCN neuropeptidergic systems decline with age. However, another study in Syrian hamsters [241] showed that there is no effect of age on SS mRNA. Aging effects of SCN neuropeptide expression, like the circadian profile of peptide expression, may be species specific as far as the SCN is concerned. Further, it has been reported [242] that SS has a role in phase shifting and is able to reset the phase of the clock [243]. Synapse of SS fibres on VIP and AVP neurons and presence of SS receptors in the SCN is suggestive of a regulatory role for SS on other peptidergic neurons. An inhibitory modulating role of SS on VIP rhythmicity is seen [244]. Increase in SS immunoreactivity could explain the observed VIP decrease with aging, and, if enhanced SS immunoreactivity reflects a release deficit, this may lead to reduction in inhibitory action [240].
Calbindin (CalB)
Calbindin (CalB) neurons are also found throughout the core and shell subdivisions of the SCN. Silver and co-workers [245] reported the presence of CalB-containing cells in the caudal part of the SCN. CalB cells are densely packed and receive direct retinal synaptic input [246] and respond to photic stimuli. Study of Silver and co-workers [245] indicates that CalB cells are present in the input pathway for photic stimuli reaching the SCN. The CalB subregion of the SCN seems essential for the maintenance of circadian locomotor activity rhythm. This comes from the studies of lesion as well as transplantation. Animals with lesions that destroyed CalB neurons but spared other neurons of the SCN lost rhythmicity of locomotor activity, and transplants of SCN tissue containing CalB cells restored rhythmicity. SCN transplants lacking CalB cells failed to restore rhythmicity [247]. CalB cells are found to co-localize with other peptides such as GRP, SP, and VIP. Interconnections of CalB cells with AVP, GRP, CCK, NPY and VIP have been reported [246], and the pattern of interconnection of CalB cells appear specialized in the sense that some are bilateral, while others are not [248]. The strength of linkage between interconnections also varies. Intra-SCN connections are influenced to a great extent by the projections of CalB cells. The connections are direct in some cases and indirect in others. However, indirect communication as is the case with CalB to AVP is equally effective. In addition to intra SCN communication, CalB cells may also project to extra SCN regions [248]. CalB cells appear to be non rhythmic. They, however, receive photic input from the RHT and GHT, and rapid transmission of light information to CalB neurons may facilitate circadian output [226]. Light exposure always increases firing rates of SCN neurons. However, cells in the core of the SCN oscillate in their responsiveness to photic input [5]. Light also induces clock gene expression in the SCN but only during the night [8,249]. This gene expression pattern seems to be mirrored by changes in subcellular localization of CalB, the cellular nucleus being devoid of CalB during the night [249]. Behavioural and molecular responses to nocturnal light pulses also disappeared on decreasing CalB levels [249]. Thus, CalB cells in the SCN function as gates to relay photic signals when open and to block the signals when closed, which suggests a central role for CalB neurons in gating photic input. Further investigation seems essential to elucidate the relation between functional connectivity and a single coherent output from the SCN.
Calretinin (CALR)
Calretinin (CALR) is another variety of neurons found in the SCN (in the core ventral part) that appears to be co-localized with VIP neurons. CALR cells are small to medium sized. Optic tracts also show heavy immunoreactivity for CALR. Like calbindin (CalB), CALR is a calcium binding protein. Apparently, there is a developmental reduction in Calbindin-D28k expression parallel to RHT formation and a developmental increase in calretinin expression which is independent of RHT connections to SCN neurons [250].
Galanin (Gal)
Galanin is yet another peptide associated with the SCN. It is a 29/30 amino acid neuropeptide seen in many parts of the nervous system [251], including the SCN [9,252,253]. In fact, neurons containing both galanin and AVP have been reported in human SCN [253]. In rat SCN, galanin receptor subtype R2 (Gal-R2) expression has been identified [254].
Angiotensin II (ANG II)
Angiotensin II (ANG II) is an octapeptide found in the SCN [15,255] and is likely to be involved in circadian function. The presence of ANG II in the SCN has been demonstrated both at light microscopic [15] and electron microscopic level [256] in normotensive rats. Of late there has been much interest in the physiological, pharmacological, and immunohistological studies of angiotensins of the SCN. At present the available information on the role of angiotensin II at the level of the SCN is much less than that on the role of known transmitters of the SCN. It has been proposed that SCN-derived angiotensin II acts as a neuromodulator as well as neurotransmitter with the effects being mediated through angiotensin1(AT1) receptors located on the endothelial plasma membrane of SCN parenchyma [257].
Met-Enkephalin (mENK)
These neurons are located primarily dorsomedially in the shell of the SCN and overlap with the distribution of AVP neurons. mENK cells are large to medium sized. Enkephalin has been found in the neurons of the IGL [116,117,122,258] projecting to the ventrolateral aspect of the SCN. The role of enkephalin as a putative neurotransmitter has been demonstrated in hamster SCN. Injection of retrograde tracer fluoro-gold into the SCN showed the existence of a population of labelled neurons in the intergeniculate leaflet which are immunoreactive for enkephalin [117]. Among the three main classes of opioid receptors, little δ or μ opioid receptor expression has been identified through autoradiographic techniques in hamster SCN in vitro [259,260]. No direct effect on basal or N-methyl-D-aspartate (NMDA)-evoked firing rates of SCN neurons in hamster has been observed on short -term application of opioid receptor agonists [261]. However, the observation of SCN neurons exhibiting withdrawal responses after the influence of enkephalins had been removed indicates that endogenous opioids may play a role in modulation of SCN function. On the other hand, naloxone injection into the SCN is reported to produce disruption of the circadian pattern of food [262] and water [263] intake.
Prokineticin 2 (PK2)
Prokineticin 2 (PK2) has been identified as an output molecule from the SCN circadian clock [236]. PK2 is a cystein-rich secreted protein. It is reported to be involved in the transmission of behavioural circadian rhythm as well as in local function within the SCN to synchronize the out put [236]. PK2, named for its ability to stimulate intestinal smooth muscle contractility, is proposed to have a major role in inhibiting locomotor activity during the day in nocturnal species [236]. Receptors for PK2, PKR2, are found abundantly in the target nuclei of the SCN output pathway, indicating again that it is an output molecule. A recent study [264] demonstrated that the molecular rhythm of PK2 in the SCN is regulated by both circadian clock and light, with the clock having a predominant role and light having a modulatory role. PK2 expression induced by light independent of the circadian oscillator may also indicate participation of PK2 in the photic entrainment of circadian rhythms. Evidence suggesting diurnality in behavioural patterns based on alterations in PK2 receptors in regions receiving SCN efferents does not seems to provide a complete answer at least in rodents[265]. The action may be downstream of the SCN or may be in parallel to the PK2/PKR2 receptor systems[265].
Relationship between the biological clock of humans and those of other mammals
The majority of studies of SCN function has been conducted on laboratory animals, particularly the rat. Similarities in the anatomical and physiological data available strongly suggest that the SCN of humans and other mammals are functionally similar. Two unique case reports [266,267] suggested that lesions of the SCN lead to disruption of circadian rhythmicity in humans. The location of the SCN, in the anterior hypothalamus, bilaterally next to third ventricle and above the optic chiasm, and the afferent and efferent projections of the SCN are similar in humans and other mammals. In the case of neurotransmitters involved in SCN function, also similar number and well defined subdivisions can be seen. Human SCN additionally contain neurotensin as a neurotransmitter. In general, human SCN has well defined subdivisions with chemically defined neuronal groups comparable to those described in experimental animals [18]. More details can be seen in the review article by Scheer and co-workers [268]. A recent review by Bell-Pedersen and co-workers [269] summarizes the similarities and differences in different organisms and discusses the organization of the circadian system as a composite of multiple oscillators. The endogenous nature of the clock, its entrainment by time cues, its phase response curves for light and melatonin, and its electrophysiological and metabolic activity are other features that are common to humans and other mammals.
Studies on animal models have helped the understanding of many disease conditions seen in humans. The lack of proper functioning of the biological clock seen in the elderly, the ill effects of jet lag, and clinical intolerance in shift workers are all topics of current research in experimental studies. Animal experimentation with new rodent models for circadian study involving transgenic and knockout mice and rats have contributed to our understanding of the above as well as many other conditions. Fu and collaborators [270] showed that the life span of their period2 knockout mice was less than that of wild type sibling mice. They also reported that these mice develop spontaneous lymphomas of high frequency, which suggests that the period2 gene plays an important role in tumor suppression. Studies on liver regeneration after partial hepatectomy in mice showed that regeneration occurs only at certain times of the circadian day [271]. A clear mechanistic link between the clock and tolerance to anti-cancer drugs has been reported [272].
Genes and their linkage with neurotransmitters in the working of the clock
Presence of robust, overt circadian rhythm expression by the SCN depends on a stable pacemaker with period length of approximately 24 hours. This in turn is made possible by the integration of the thousands of individual cellular clocks found in the SCN. Using real-time analysis of gene expression, Yamaguchi and co-workers [30] have shown synchronized rhythms of clock gene transcription across hundreds of neurons within the mammalian SCN. Whether it is the neurotransmitters in the afferent input to the SCN, or of the SCN itself or efferent projection from the SCN, neurotransmitters and their linkage with genes are important, and this has been the basis for the information now available on the molecular basis of the biological clock and its working.
In mammals, the molecular mechanism for generation of circadian rhythms is dependent on the concerted co-expression of specific clock genes [273]. The genes in mammals are the period (per1, per2, and per3), cryptochrome (cry1 and cry2), clock (clk), and brain-muscle-Arnt-like-protein 1 (bmal1) genes. It is now known that light entrainment of the clock involves the induction of c-Fos [274] and clock genes per1 and per2 [275-277]. These genes are rapidly induced in the SCN by light stimulation at those time points at which light phase shifts the clock. Possible genes for glutamate and PACAP thus appear to be the clock genes per1 and per2, which are induced in the SCN by light, glutamate and PACAP at night. Further, Nielsen and co-workers [172] also have shown that VIP induces per1 and per2 expression in a phase-dependent manner, thereby suggesting that VIP is important for the light-induced phase shift at night.
Neurotransmitters and disease conditions
Researchers have started to identify the role of the SCN in certain disease conditions. SCN dysfunction, particularly in terms of neurotransmitter content, has been associated with several chronic diseases such as hypertension, diabetes, and depression [278,279].The anatomical picture with respect to staining in the SCN is changed in spontaneous hypertensive rats (SHR), and transplantation of hypothalamic tissue containing the SCN from SHR to normotensive rats induces hypertension [280]. Decrease in staining for many SCN neurotransmitters along with an enhanced activity of paraventricular nucleus (PVN) CRH neurons have been observed in hypertensive patients [281]. This suggests that, in humans, the SCN may have an inhibitory role in the CRH neurons of the PVN. These observations strongly suggest that a changed SCN may precede the development of hypertension. There is also evidence that circadian disturbances may be detected prior to the development of diabetes or hypertension [282,283]. Further evidence that the functionality of the biological clock may be affected in humans by diseases such as depression and hypertension has been provided by post-mortem analysis of the SCN in human physiological disorders by Zhou and co-workers [187] and Goncharuk and co-workers [280]. One of the possible explanations for the development of hypertension may be that a less active SCN may prepare an individual less effectively for the new period of activity, and that repetition of this strain over the years may result in hypertension. One can find support for this theory in the observation that cardiovascular accidents precipitate during morning hours when the onset of activity occurs.
Conclusion
Neurotransmitters of the circadian clock have been investigated more and more with the unfolding of the understanding of the clock, particularly at the molecular level. From a time when hardly anything was known about neurotransmitter involvement in the working of the clock, we have come to a stage of controlled manipulation on the basis of the properties and nature of neurotransmitters. The ill effects of jet lag, clinical intolerance to shift work, disruptions of the working of the clock with old age and their correction to some extent with the help of chemicals, especially those like melatonin, will hopefully be treated in the near future by interventions developed with knowledge of SCN neurotransmitters. Chronotherapy has become an advantageous therapeutic option for many disease conditions. Chronomodulation methods for chemotherapy, radiotherapy and even immunotherapy have been highlighted by many researchers and physicians in the recent past for treatment of patients with cancer. With the advances in molecular chronobiology and clarification of its mechanisms, chronotherapy is likely to become increasingly accepted as a effective means for treating not only cancer but also many other disease conditions. Thus, knowledge of the workings of the clock and its neurotransmitters appears to be essential not only for disease prevention but for therapeutic practice as well.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
VR and RR contributed equally to this work (literature search, systematization, and writing).
Acknowledgments
Acknowledgements
We thank Mrs Reshmi Menon for her critical analysis of the various aspects of the manuscript.
Contributor Information
Vallath Reghunandanan, Email: vallathr@gmail.com.
Rajalaxmy Reghunandanan, Email: rajalaxmyr@gmail.com.
References
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Morre DJ, Chueh PJ, Pletcher J, Tang X, Wu LY, Morre DM. Biochemical basis for the biological clock. Biochemistry. 2002;41:11941–11945. doi: 10.1021/bi020392h. [DOI] [PubMed] [Google Scholar]
- Meijer JH. Photic entrainment of mammals. In: Takahashi JS, Turek FW, Moore RY, editor. Handbook of Behavioral Neurobiology Circadian clocks. New York, Plenum; 2002. pp. 183–222. [Google Scholar]
- Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biological Reviews. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends in Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Cutler DJ. The roles of vasoactive intestinal polypeptide in the mammalian circadian clock. J Endocrinol. 2003;177:7–15. doi: 10.1677/joe.0.1770007. [DOI] [PubMed] [Google Scholar]
- Van Gelder RN. Recent insights into mammalian circadian rhythms. Sleep. 2004;27:166–171. doi: 10.1093/sleep/27.1.166. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time keeping system revisited. Brain Res Rev. 2000;33:34–77. doi: 10.1016/s0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Card JP, Moore RY. The suprachiasmatic nucleus of the golden hamster: Immunohistochemical analysis of cell and fiber distribution. Neuroscience. 1984;13:415–431. doi: 10.1016/0306-4522(84)90240-9. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Gamma-aminobutyrate, gastrin releasing peptide, serotonin, somatostatin, and vasopressin: ultrastructural immunocytochemical localization in presynaptic axons in the suprachiasmatic nucleus. Neuroscience. 1986;17:643–659. doi: 10.1016/0306-4522(86)90037-0. [DOI] [PubMed] [Google Scholar]
- Rusak B, Bina KG. Neurotransmitters in the mammalian circadian system. Ann Rev Neurosci. 1990;13:387–401. doi: 10.1146/annurev.ne.13.030190.002131. [DOI] [PubMed] [Google Scholar]
- Reghunandanan V, Reghunandanan R, Marya RK. Vasopressin: its possible role in circadian time keeping. Chronobiologia. 1991;18:39–47. [PubMed] [Google Scholar]
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: Immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- Moore RY. Organization and function of a central nervous system oscillator: the suprachiasmatic nucleus. Fed Proc. 1983;42:2783–2789. [PubMed] [Google Scholar]
- Mai JK, Kedziora O, Teckhaus L, Sofroniew MV. Evidence for subdivisions in the human suprachiasmatic nucleus. J Comp Neurol. 1991;305:508–525. doi: 10.1002/cne.903050312. [DOI] [PubMed] [Google Scholar]
- Reghunandanan V, Reghunandanan R, Singh PI. Neurotransmitters of the suprachiasmatic nucleus: role in the regulation of circadian rhythms. Prog Neurobiol. 1993;41:647–655. doi: 10.1016/0301-0082(93)90029-r. [DOI] [PubMed] [Google Scholar]
- Hannibal J. Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res. 2002;309:73–88. doi: 10.1007/s00441-002-0574-3. [DOI] [PubMed] [Google Scholar]
- Harmar AJ. An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei. J Neuroendocrinol. 2003;15:335–338. doi: 10.1046/j.1365-2826.2003.01005.x. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Agostino PV, Plano SA, Ferreyra GA. Signaling in the mammalian circadian clock: the NO/cGMP pathway. Neurochem Int. 2004;45:929–936. doi: 10.1016/j.neuint.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Herzog ED. Come together, right...now: synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY. Entrainment pathways and functional organization of the circadian system. In: Buijs RM, Kasbeek A, Romijn HJ, Pennartz CMA, Mirmiran M, editor. Hypothalamic integration of circadian rhythms Progress in Brain Research. Vol. 111. Amsterdam Elsevier; 1966. pp. 103–119. [Google Scholar]
- Shirakawa T, Honma S, Honma K. Multiple oscillators in the suprachiasmatic nucleus. Chronobiol Int. 2001;18:371–387. doi: 10.1081/cbi-100103962. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Honma S, Nakamura W, Shirakawa T, Honma K. Diversity in the circadian periods of single neurons of the rat suprachiasmatic nucleus depends on nuclear structure and intrinsic period. Neurosci Lett. 2004;358:173–176. doi: 10.1016/j.neulet.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Gonze D, Bernard S, Waltermann C, Kramer A, Herzel H. Spontaneous synchronization of coupled circadian oscillators. Biophys J. 2005;89:120–129. doi: 10.1529/biophysj.104.058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Herzog ED. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J Biol Rhythms. 2004;19:400–413. doi: 10.1177/0748730404268786. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- Mai JK, Tripel J, Metz J. Neurotensin in the human brain. Neuroscience. 1987;22:499–524. doi: 10.1016/0306-4522(87)90349-6. [DOI] [PubMed] [Google Scholar]
- Moore RY. The organization of the human circadian timing system. In: Swaab DF, Holfman MA, Mirmiran M, Ravid R, Van Leeuwen FW, editor. The human hypothalamus in health and disease. Amsterdam, Progress in Brain research Elsevier; 1992. pp. 101–118. [Google Scholar]
- Romijn HJ, van Uum JF, Emmering J, Goncharuk V, Buijs RM. Colocalization of VIP with AVP in neurons of the human paraventricular, supraoptic and suprachiasmatic nucleus. Brain Res. 1999;832:47–53. doi: 10.1016/s0006-8993(99)01468-7. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci. 1996;16:5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pevet P, Buijs RM. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci. 2000;12:3146–3154. doi: 10.1046/j.1460-9568.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nature Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Menaker M. Regulation of circadian rhythms by excitatory amino acids. In: Brann DW, Mahesh VB, editor. Excitatory amino acids: Their role in neuroendocrine function. New York: CRC Press; 1996. pp. 223–252. [Google Scholar]
- Ebling FJP. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Myers MP, Wagner-Smith K, Rothenfluh-Hilfiker A, Young MW. Light induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- Castel M, Belenky M, Cohen S, Ottersen OP, Storm-Mathisen J. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur J Neurosci. 1993;5:368–381. doi: 10.1111/j.1460-9568.1993.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Moller M, Ottersen OP, Fahrenkrug J. PACAP and glutamate are co-stored in the retinohypothalamic tract. J Comp Neurol. 2000;418:147–155. [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Immunoreactive substance P is not part of the retinohypothalamic tract in the rat. Cell Tissue Res. 2002;309:293–299. doi: 10.1007/s00441-002-0564-5. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Samuels RE, Coogan AN, Cutler DJ. Distribution of substance P and neurokinin-1 receptor immunoreactivity in the suprachiasmatic nuclei and intergeniculate leaflet of hamster, mouse, and rat. J Comp Neurol. 2001;438:50–65. doi: 10.1002/cne.1301. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggins HD, Rusak B. Effects of microinjections of substance P into the suprachiasmatic nucleus region on hamster wheel-running rhythms. Brain Res Bull. 1997;42:451–455. doi: 10.1016/s0361-9230(96)00371-1. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Larsen PJ, Mick G, Vrang N, Ebling FJP, Maywood ES, Hastings MH, Moller M. Gating of retinal inputs through the suprachiasmatic nucleus: role of excitatory neurotransmission. Neurochem Int. 1992;27:263–272. doi: 10.1016/0197-0186(95)00039-b. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Vries MJD, Treep JA, Pauw ESDD, Meijer JH. The effects of electrical stimulation of the optic nerves and anterior optic chiasm on the circadian activity rhythm of the Syrian hamster: involvement of excitatory amino acids. Brain Res. 1994;642:206–212. doi: 10.1016/0006-8993(94)90923-7. [DOI] [PubMed] [Google Scholar]
- DeVries MJ, Cardozo BN, van der Want J, de Wolf A, Meijer JH. Glutamate immunoreactivity in terminals of the retinohypothalamic tract of the brown Norwegian rat. Brain Res. 1993;612:231–237. doi: 10.1016/0006-8993(93)91665-f. [DOI] [PubMed] [Google Scholar]
- Gannon RL, Rea MA. In situ hybridization of antisense mRNA oligonucleotides for AMPA, NMDA and metabotropic glutamate receptor subtypes in the rat suprachiasmatic nucleus at different phases of the circadian cycle. Mol Brain Res. 1994;23:338–344. doi: 10.1016/0169-328x(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Hoque S, Hall A, Golombek D, Biello S. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J Neurosci. 1999;19:6637–6642. doi: 10.1523/JNEUROSCI.19-15-06637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Furuno K, Akiyama M, Moriya T, Shibata S. Pituitary adenylate cyclase-activating polypeptide produces a phase shift associated with induction of mPer expression in the mouse suprachiasmatic nucleus. Neuroscience. 2002;113:37–45. doi: 10.1016/s0306-4522(02)00148-3. [DOI] [PubMed] [Google Scholar]
- Bos NP, Mirmiran M. Effects of excitatory and inhibitory amino acids on neuronal discharges in the cultured suprachiasmatic nucleus. Brain Res Bull. 1993;31:67–72. doi: 10.1016/0361-9230(93)90012-z. [DOI] [PubMed] [Google Scholar]
- Scott G, Rusak B. Activation of hamster suprachiasmatic neurons in vitro via metabotropic glutamate receptors. Neuroscience. 1996;71:533–541. doi: 10.1016/0306-4522(95)00438-6. [DOI] [PubMed] [Google Scholar]
- Abe H, Rusak B, Robertson HA. NMDA and non-NMDA antagonists inhibit photic induction of Fos protein in the hamster suprachiasmatic nucleus. Brain Res Bull. 1992;28:831–835. doi: 10.1016/0361-9230(92)90269-4. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Menaker M. NMDA as well as non-NMDA receptor antagonists can prevent the phase shifting effects of light on the circadian system of the golden hamster. J Biol Rhythms. 1992;7:125–136. doi: 10.1177/074873049200700204. [DOI] [PubMed] [Google Scholar]
- Decker K, Reuss S. Nitric oxide -synthesizing neurons in the hamster suprachiasmatic nucleus: a combined NOS -and NADPH-staining and retinohypothalamic tract tracing study. Brain Res. 1994;666:284–288. doi: 10.1016/0006-8993(94)90785-4. [DOI] [PubMed] [Google Scholar]
- Reuss S, Decker K, Rosseler L, Layes E, Scholl-mayer A, Sppessert R. Nitric oxide synthase in the hypothalamic suprachiasmatic nucleus of rat: evidence from histochemistry, immunochemistry and western blot and colocalization with VIP. Brain Res. 1995;695:257–262. doi: 10.1016/0006-8993(95)00829-f. [DOI] [PubMed] [Google Scholar]
- Chen D, Hurst WJ, Ding JM, Faiman le, Mayer B, Gillette MU. Localization and characterization of nitric oxide synthase in the rat suprachiasmatic nucleus: evidence for a nitergic plexus in the biological clock. J Neurochem. 1997;68:855–861. doi: 10.1046/j.1471-4159.1997.68020855.x. [DOI] [PubMed] [Google Scholar]
- Caillol M, Devinoy E, Lacroix MC, Schirar A. Endothelial and neuronal nitric oxide synthases are present in the suprachiasmatic nuclei of Syrian hamsters and rats. Eur J Neurosci. 2000;12:649–661. doi: 10.1046/j.1460-9568.2000.00961.x. [DOI] [PubMed] [Google Scholar]
- Prosser RA, McArther AJ, Gillette MU. cGMP induces phase shifts of mammalian circadian pacemaker at night in antiphase to cAMP effect. Proc Natl Acad Sci USA. 1989;86:6812–6815. doi: 10.1073/pnas.86.17.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S. Blocking NMDA receptors or nitric oxide production disrupts light transmission to the suprachiasmatic nucleus. Brain Res. 1992;586:336–339. doi: 10.1016/0006-8993(92)91644-t. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Hamada T, Shibata S, Watanabe S. Effects of nitric oxide synthase inhibitors on N-methyl-D-aspartate-induced phase delay of circadian rhythm of neuronal activity in the rat suprachiasmatic nucleus in vitro. Brain Res. 1994;646:161–164. doi: 10.1016/0006-8993(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Weber ET, Gannon RL, Michel AM, Gillette MU, Rea MA. Nitric oxide synthase inhibitor blocks light-induced phase shifts of the circadian activity rhythm, but not c-fos expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res. 1995;692:137–142. doi: 10.1016/0006-8993(95)00685-j. [DOI] [PubMed] [Google Scholar]
- Ding JM, Faiman LE, Hurst WJ, Kuriashkina LR, Gillette MU. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate and nitric oxide. J Neurosci. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek DA, Ferreyra GA, Katz ME, Marpegan L, Pinto FD, Alfonso TF, Yannielli PC. Neurochemistry of mammalian entrainment: Signal transduction pathways in the suprachiasmatic nuclei. Biol Rhythm Res. 2000;31:56–70. [Google Scholar]
- Starkey SJ, Grant AL, Hagan RM. A rapid and transient synthesis of nitric oxide (NO) by a constitutively expressed type II NO synthase in the guinea -pig suprachiasmatic nucleus. Br J Pharmacol. 2001;34:1084–1092. doi: 10.1038/sj.bjp.0704330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Demas GE, Lee SE, Dawson TM, Dawson VL, Nelson RJ. Circadian locomotor analysis of male mice lacking the gene for neuronal nitric oxide synthase (nNOS-/-) J Biol Rhythms. 1999;14:20–27. doi: 10.1177/074873099129000407. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Drazen DL, Nelson RJ. Circadian organization in male mice lacking the gene for endothelial nitric oxide synthase (eNOS-/-) J Biol Rhythms. 2001;16:142–148. doi: 10.1177/074873001129001845. [DOI] [PubMed] [Google Scholar]
- Bellingham J, Foster RG. Opsins and mammalian photoentrainment. Cell Tissue Res. 2002;309:57–71. doi: 10.1007/s00441-002-0573-4. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Takatsuji K, Miguel-Hidalgo JJ, Tohyama M. Substance P-immunoreactive innervation from the retina to the suprachiasmatic nucleus in the rat. Brain Res. 1991;568:223–229. doi: 10.1016/0006-8993(91)91401-l. [DOI] [PubMed] [Google Scholar]
- Kim YI, Kim SH, Kim DY, Lee HW, Shin HC, Chung JM, Han HC, Na HS, Hong SK. Electrophysiological evidence for the role of substance P in retinohypothalamic transmission in the rat. Neurosci Lett. 1999;274:99–102. doi: 10.1016/s0304-3940(99)00681-3. [DOI] [PubMed] [Google Scholar]
- Kim DY, Kang HC, Shin HC, Lee KJ, Yoon YW, Han HC, Na HS, Hong SK, Kim YI. Substance p plays a critical role in photic resetting of the circadian pacemaker in the rat hypothalamus. J Neurosci. 2001;21:4026–4031. doi: 10.1523/JNEUROSCI.21-11-04026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomisto L. Involvement of histamine in circadian and other rhythms. In: Watanabe T, Wada H, editor. Histaminergic neurons: Mophology and function. Inc Boca Raton FL CRC Press; 1991. pp. 283–295. [Google Scholar]
- Stephan FK, Berkley KJ, Moss RL. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience. 1981;6:2625–2641. doi: 10.1016/0306-4522(81)90108-1. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Yamatodani A, Timmerman H. Is histamine the final neurotransmitter in the entrainment of circadian rhythms in mammals? Trends Pharmacol Sci. 2000;21:293–298. doi: 10.1016/s0165-6147(00)01504-2. [DOI] [PubMed] [Google Scholar]
- Itowi N, Yamatodani A, Mochizuki T, Wada H. Effects of intracerebroventricular histamine injection on circadian activity phase entrainment during rapid illumination changes. Neurosci Lett. 1991;123:53–56. doi: 10.1016/0304-3940(91)90156-n. [DOI] [PubMed] [Google Scholar]
- Liou SY, Shibata S, Yamakawa K, Ueki S. Inhibitory and excitatory effects of histamine on suprachiasmatic neurons in rat hypothalamic slice preparation. Neurosci Lett. 1983;41:109–113. doi: 10.1016/0304-3940(83)90231-8. [DOI] [PubMed] [Google Scholar]
- Stehle J. Effects of histamine on spontaneous electrical activity of neurons in rat suprachiasmatic nucleus. Neurosci Lett. 1991;130:217–220. doi: 10.1016/0304-3940(91)90400-n. [DOI] [PubMed] [Google Scholar]
- Eaton SJ, Cote NK, Harrington ME. Histamine synthesis inhibition reduces light-induced phase shifts of circadian rhythms. Brain Res. 1995;695:227–230. doi: 10.1016/0006-8993(95)00635-4. [DOI] [PubMed] [Google Scholar]
- Eaton SJ, Eoh S, Meyer J, Hoque S, Harrington ME. Circadian rhythm photic phase shifts are not altered by histamine receptor antagonists. Brain Res Bull. 1996;41:227–229. doi: 10.1016/s0361-9230(96)00179-7. [DOI] [PubMed] [Google Scholar]
- Alexander MJ, Leeman SE. Widespread expression in adult rat forebrain of mRNA encoding high-affinity neurotensin receptor. J Comp Neurol. 1998;402:475–500. [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Francois-Bellan AM, Bosler O, Tonon MC, Wei LT, Beaudet A. Association of neurotensin receptors with VIP-containing neurons and serotonin-containing axons in the suprachiasmatic nucleus of the rat. Synapse. 1992;10:282–290. doi: 10.1002/syn.890100403. [DOI] [PubMed] [Google Scholar]
- Meyer-Spasche A, Reed HE, Piggins HD. Neurotensin phase-shifts the firing rate rhythm of neurons in the rat suprachiasmatic nuclei in vitro. Eur J Neurosci. 2002;16:339–44. doi: 10.1046/j.1460-9568.2002.02067.x. [DOI] [PubMed] [Google Scholar]
- Coogan AN, Rawlings N, Luckman SM, Piggins HD. Effects of neurotensin on discharge rates of rat suprachiasmatic nucleus neurons in vitro. Neuroscience. 2001;103:663–672. doi: 10.1016/s0306-4522(00)00583-2. [DOI] [PubMed] [Google Scholar]
- Schmahl C, Bohmer G. Effects of excitatory amino acids and neuropeptide Y on the discharge activity of suprachiasmatic neurons in rat brain slices. Brain Res. 1997;746:151–163. doi: 10.1016/s0006-8993(96)01220-6. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Piggins HD, Selbie LA, Mason R. Responses to neuropeptide Y in adult hamster suprachiasmatic nucleus neurones in vitro. Eur J Pharmacol. 1998;345:155–162. doi: 10.1016/s0014-2999(98)00012-0. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, van den Pol AN, Yocca FD. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: mediation by different receptor subtypes. J Neurosci. 1998;18:3014–3022. doi: 10.1523/JNEUROSCI.18-08-03014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Hu XY, Wu L, Zhou JN. Neurotensin expressing neurons developed earlier than vasoactive intestinal polypeptide and vasopressin expressing neurons in the human suprachiasmatic nucleus. Neurosci Lett. 2003;335:175–178. doi: 10.1016/s0304-3940(02)01184-9. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hanada R, Murakami N, Teranishi H, Ohgusu H, Fukushima N, Moriyama M, Ida T, Kangawa K, Kojima M. he gut-brain peptide neuromedin U is involved in the mammalian circadian oscillator system. Biochem Biophys Res Commun. 2004;318:156–161. doi: 10.1016/j.bbrc.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Mori K, Miyazato M, Ida T, Murakami N, Serino R, Ueta Y, Kojima M, Kangawa K. Identification of neuromedin S and its possible role in the mammalian circadian oscillator system. EMBO J. 2005;24:325–335. doi: 10.1038/sj.emboj.7600526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ES, Littlewood P, Turnbull Y, Mercer JG, Morgan PJ, Barrett P. Neuromedin -U is regulated by circadian clock in the SCN of the mouse. Eur J Neurosci. 2005;21:814–819. doi: 10.1111/j.1460-9568.2005.03923.x. [DOI] [PubMed] [Google Scholar]
- Battey J, Wada E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 1991;14:524–528. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Jensen RT, Mantey SA, McHugh PR, Moran TH. Receptor heterogeneity for bombesin-like peptides in the rat central nervous system. Brain Res. 1990;537:233–240. doi: 10.1016/0006-8993(90)90363-g. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Jensen RT, Mantey SA, Moran TH. Distinct distributions of two bombesin receptor subtypes in the rat central nervous system. Brain Res. 1992;593:168–178. doi: 10.1016/0006-8993(92)91305-x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Hayashi S, Tamada Y, Ikeda T, Hisa Y, Takamatsu T, Ibata Y. Direct retinal projections to GRP neurons in the suprachiasmatic nucleus of the rat. NeuroReport. 1997;8:2187–2191. doi: 10.1097/00001756-199707070-00020. [DOI] [PubMed] [Google Scholar]
- Aïoun J, Chambille I, Peytevin J, Martinet L. Neurons containing gastrin-releasing peptide and vasoactive intestinal polypeptide are involved in the reception of the photic signal in the suprachiasmatic nucleus of the Syrian hamster: an immunocytochemical ultrastructural study. Cell Tissue Res. 1988;291:239–253. doi: 10.1007/s004410050994. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Rusak B. Intercellular interactions and the physiology of circadian rhythms in mammals. In: Lydic R, Baghdoyan HA, editor. Handbook of behavioral state control: cellular and molecular mechanisms. Boca Raton FL CRC; 1999. pp. 31–44. [Google Scholar]
- McArthur AJ, Coogan AN, Ajpru S, Sugden D, Biello SM, Piggins HD. Gastrin-releasing peptide phase-shifts suprachiasmatic nuclei neuronal rhythms in vitro. J Neurosci. 2000;20:5496–5502. doi: 10.1523/JNEUROSCI.20-14-05496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Kriegsfeld LJ, Silver R. Signaling within the master clock of the brain: localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J Neurosci. 2005;25:2447–2454. doi: 10.1523/JNEUROSCI.4696-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina KG, Rusak B, Semba K. Localization of cholinergic neurons in the forebrain and brainstem that project to the suprachiasmatic nucleus of the hypothalamus in rats. J Comp Neurol. 1993;335:295–307. doi: 10.1002/cne.903350212. [DOI] [PubMed] [Google Scholar]
- Nishino H, Koizumi K. Responses of neurons in the suprachiasmatic nuclei of the hypothalamus to putative transmitters. Brain Res. 1977;120:167–172. doi: 10.1016/0006-8993(77)90509-1. [DOI] [PubMed] [Google Scholar]
- Zatz M, Brownstein MJ. Injection of alpha-bungarotoxin near the suprachiasmatic nucleus blocks the effects of light on nocturnal pineal enzyme activity. Brain Res. 1981;213:438–442. doi: 10.1016/0006-8993(81)90250-x. [DOI] [PubMed] [Google Scholar]
- Earnest DJ, Turek FW. Neurochemical basis for the photic control of circadian rhythms and seasonal reproductive cycles: role for acetylcholine. Proc Natl Acad Sci U S A. 1985;82:4277–4281. doi: 10.1073/pnas.82.12.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Gillette MU. Cholinergic regulation of the SCN circadian rhythm via a muscarinic mechanism at night. J Neurosci. 1996;16:744–752. doi: 10.1523/JNEUROSCI.16-02-00744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Kaufman CM, Menaker M. Phase shifting mechanisms in the mammalian circadian system: new light on the carbachol paradox. J Neurosci. 1993;13:1454–1459. doi: 10.1523/JNEUROSCI.13-04-01454.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik D, Mrosovsky N. Intergeniculate leaflet lesions and behaviorally-induced shifts of circadian rhythms. Brain Res. 1994;651:174–182. doi: 10.1016/0006-8993(94)90695-5. [DOI] [PubMed] [Google Scholar]
- Rusak B, Meijer JH, Harrington ME. Hamster circadian rhythms are phase-shifted by electrical stimulation of the geniculo-hypothalamic tract. Brain Res. 1989;493:283–291. doi: 10.1016/0006-8993(89)91163-3. [DOI] [PubMed] [Google Scholar]
- Menet J, Vuillez P, Jacob N, Pevet P. Intergeniculate leaflets lesion delays but does not prevent the integration of photoperiodic change by the suprachiasmatic nuclei. Brain Res. 2001;906:176–179. doi: 10.1016/s0006-8993(01)02518-5. [DOI] [PubMed] [Google Scholar]
- Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev. 1997;21:705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard J. Organization of the hamster intergeniculate leaflet: NPY and ENK projections to the suprachiasmatic nucleus, intergeniculate leaflet and posterior limitans nucleus. Vis Neurosci. 1995;12:57–67. doi: 10.1017/s0952523800007318. [DOI] [PubMed] [Google Scholar]
- Byku M, Legutko R, Gannon RL. Distribution of delta opioid receptor immunoreactivity in the hamster suprachiasmatic nucleus and intergeniculate leaflet. Brain Res. 2000;857:1–7. doi: 10.1016/s0006-8993(99)02290-8. [DOI] [PubMed] [Google Scholar]
- Byku M, Gannon RL. SNC 80, a delta-opioid agonist, elicits phase advances in hamster circadian activity rhythms. Neuroreport. 2000;11:1449–1452. doi: 10.1097/00001756-200005150-00019. [DOI] [PubMed] [Google Scholar]
- Moore RY, Gustafson EL, Card JP. Identical immunoreactivity of afferents to the rat suprachiasmatic nucleus with antisera against avian pancreatic polypeptide, molluscan cardioexcitatory peptide and neuropeptide Y. Cell Tissue Res. 1984;236:41–46. doi: 10.1007/BF00216511. [DOI] [PubMed] [Google Scholar]
- Biello SM, Golombek DA, Schak KM, Harrington ME. Circadian phase shifts to neuropeptide Y In vitro: cellular communication and signal transduction. J Neurosci. 1997;17:8468–8475. doi: 10.1523/JNEUROSCI.17-21-08468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Card JP. Intergeniculate leaflet: an anatomically and functionally distinct subdivision of the lateral geniculate complex. J Comp Neurol. 1994;344:403–430. doi: 10.1002/cne.903440306. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. A non -photic gateway to the circadian clcok of hamsters. In: Chadwick DJ, Ackrill K, editor. Circadian clocks and their adjustment. New York, John Wiley; 1995. pp. 154–174. [DOI] [PubMed] [Google Scholar]
- Turek FW, Pinto LH, Vitaterna MH, Penev PD, Zee PC, Takahashi JS. Pharmacological and genetic approaches for the study of circadian rhythms in mammals. Front Neuroendocrinol. 1995;16:191–223. doi: 10.1006/frne.1995.1007. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Babagbemi TO, Albers HE. Bicuculline blocks neuropeptide Y-induced phase advances when microinjected in the suprachiasmatic nucleus of Syrian hamsters. Brain Res. 1995;675:333–336. doi: 10.1016/0006-8993(95)00018-l. [DOI] [PubMed] [Google Scholar]
- Chen G, van den Pol AN. Multiple NPY receptors coexist in pre- and postsynaptic sites: inhibition of GABA release in isolated self-innervating SCN neurons. J Neurosci. 1996;16:7711–7724. doi: 10.1523/JNEUROSCI.16-23-07711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- Bons N, Combes A, Szafarczyk A, Assenmacher I. Efferences dextrahypothalamiques de noyau suprachiasmatique chez le rat. C R Acad Sci. 1983;297:347–350. [PubMed] [Google Scholar]
- Medanic M, Gillette MU. Serotonin regulates the phase of the rat suprachiasmatic circadian pacemaker in vitro during the subjective day. J Physiol. 1992;450:629–642. doi: 10.1113/jphysiol.1992.sp019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar DM, Miller JD, Prosser RA, Dean RR, Dement WC. Serotonin and the mammalian circadian system: phase shifting rat behavioral rhythms with serotonergic agonists. J Biol Rhythms. 1993;8:17–31. doi: 10.1177/074873049300800102. [DOI] [PubMed] [Google Scholar]
- Block M, Zucker I. Circadian rhythms of rat locomotor activity after lesions of the midbrain raphe nuclei. J Comp Physiol. 1976;109:235–247. [Google Scholar]
- Okamura H, Takahashi Y, Terubayashi H, Hamada S, Ynaihara N, Ibata Y. VIP-like immunoreactive neurons and retinal projections in the rat suprachiasmatic nucleus. Biomed Res. 1987;8:253–262. [Google Scholar]
- Fite KV, Janusonis S, Foote W, Bengston L. Retinal afferents to the dorsal raphe nucleus in rats and Mongolian gerbils. J Comp Neurol. 1999;414:469–484. doi: 10.1002/(sici)1096-9861(19991129)414:4<469::aid-cne4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Prosser RA. Serotonergic actions and interactions on the SCN circadian pacemaker: In vitro investigations. Biol Rhythm Res. 2000;31:315–339. [Google Scholar]
- Jiang ZG, Teshima K, Yang Y, Yoshioka T, Allen CN. Pre- and postsynaptic actions of serotonin on rat suprachiasmatic nucleus neurons. Brain Res. 2000;866:247–256. doi: 10.1016/s0006-8993(00)02294-0. [DOI] [PubMed] [Google Scholar]
- Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14:3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Rusak B. Effects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleus. Brain Res. 1994;651:37–46. doi: 10.1016/0006-8993(94)90678-5. [DOI] [PubMed] [Google Scholar]
- Rea MA, Barrera J, Glass JD, Gannon RL. Serotonergic potentiation of photic phase shifts of the circadian activity rhythm. Neuroreport. 1995;6:1289–1292. doi: 10.1097/00001756-199507100-00014. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris JF. Morphological heterogeneity of the GABAergic network in the suprachiasmatic nucleus, the brain's circadian pacemaker. J Anat. 2000;196:1–13. doi: 10.1046/j.1469-7580.2000.19610001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naum OG, Fernanda Rubio M, Golombek DA. Rhythmic variation in gamma-aminobutyric acid(A)-receptor subunit composition in the circadian system and median eminence of Syrian hamsters. Neurosci Lett. 2001;310:178–182. doi: 10.1016/s0304-3940(01)02129-2. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Glutamate and GABA presence and action in the suprachiasmatic nucleus. J Biol Rhythms. 1993;8:11–15. [PubMed] [Google Scholar]
- Gao B, Fritschy JM, Moore RY. GABAA-receptor subunit composition in the circadian timing system. Brain Res. 1995;700:142–156. doi: 10.1016/0006-8993(95)00944-l. [DOI] [PubMed] [Google Scholar]
- O'Hara BF, Andretic R, Heller HC, Carter DB, Kilduff TS. GABAA, GABAC, and NMDA receptor subunit expression in the suprachiasmatic nucleus and other brain regions. Brain Res Mol Brain Res. 1995;28:239–250. doi: 10.1016/0169-328x(94)00212-w. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, Park WK, Strecker GJ, deJeu MTG, Pennartz CMA, Dudek FE. A re-examination of the role of GABA in the mammalian suprachiasmatic nucleus. J Biol Rhythms. 1999;14:126–130. doi: 10.1177/074873099129000515. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Dudek FE. GABA receptor -mediated inhibition of neuronal activity in rat SCN in vitro: Pharmacology and influence of circadian phase. J Neurophysiol. 2003;90:1438–1448. doi: 10.1152/jn.01082.2002. [DOI] [PubMed] [Google Scholar]
- Wagner S, Sagiv N, Yarom Y. GABA-induced current and circadian regulation of chloride in neurons of the rat suprachiasmatic nucleus. J Physiol. 2001;537:853–869. doi: 10.1111/j.1469-7793.2001.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker GJ, Wuarin JP, Dudek FE. GABA A-mediated local synaptic pathways connect neurons in the rat suprachiasmatic nucleus. J Neurophysiol. 1997;78:2217–2220. doi: 10.1152/jn.1997.78.4.2217. [DOI] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143:1459–1466. doi: 10.1210/endo.143.4.8724. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- de Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J Neurophysiol. 2002;87:834–844. doi: 10.1152/jn.00241.2001. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Hennessey AC, Albers HE. Rhythms of glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus. J Biol Rhythms. 1996;11:311–316. doi: 10.1177/074873049601100404. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Jasnow AM, Sisitsky AK, Albers HE. Glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus of rats housed in constant darkness. Brain Res. 1999;851:266–269. doi: 10.1016/s0006-8993(99)02167-8. [DOI] [PubMed] [Google Scholar]
- Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J Neurophysiol. 2004;92:311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron. 2000;25:123–128. doi: 10.1016/s0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Nagano M, Kanda F, Chihara K, Shigyoshi Y, Okamura H. Two types of VIP neuronal components in rat suprachiasmatic nucleus. J Neurosci Res. 2003;74:852–857. doi: 10.1002/jnr.10751. [DOI] [PubMed] [Google Scholar]
- Dardente H, Poirel VJ, Klosen P, Pevet P, Masson-Pevet M. Per and neuropeptide expression in the rat suprachiasmatic nuclei: compartmentalization and differential cellular induction by light. Brain Res. 2002;958:261–271. doi: 10.1016/s0006-8993(02)03563-1. [DOI] [PubMed] [Google Scholar]
- Dardente H, Klosen P, Caldelas I, Pevet P, Masson-Pevet M. Phenotype of Per1- and Per2-expressing neurons in the suprachiasmatic nucleus of a diurnal rodent (Arvicanthis ansorgei): comparison with a nocturnal species, the rat. Cell Tissue Res. 2002;310:85–92. doi: 10.1007/s00441-002-0609-9. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh J, Card JP. Vasoactive intestinal peptide (VIP) and peptide histidine isoleucine (PHI) colocalize in neurons of the rat suprachiasmatic nucleus(SCN)[abstract] Soc Neuroscir. 1985;11:s1139. [Google Scholar]
- Stopa EG, Jonassen JA, Minamitani N, King JC, Wolfe H, Mobtaker H, Albers HE. m RNA of vasoactive intestinal peptide (VIP) and peptide histidineisoleucine (PHI) within the suprachiasmatic nucleus(SCN)[abstract] Soc Neurosci. 1986;12:s597. [Google Scholar]
- Reed HE, Cutler DJ, Brown TM, Brown J, Coen CW, Piggins HD. Effects of vasoactive intestinal peptide on neurons of the rat suprachiasmatic nuclei in vitro. J Neuroendocrinol. 2002;14:639–646. doi: 10.1046/j.1365-2826.2002.00826.x. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HMJ, Harmar AJ, Piggins HD. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur J Neurosci. 2003;17:197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- Dardente H, Menet js, chalet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res. 2004;124:143–151. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Takeuchi J, Nagasaki H, Shinohara K, Inouye SIT. A circadian rhythm of somatostatin messenger RNA levels, but not of vasoactive intestinal polypeptide/peptide histidine isoleucine messenger RNA levels in rat suprachiasmatic nucleus. Mol Cell Neurosci. 1992;3:29–35. doi: 10.1016/1044-7431(92)90005-m. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban Y, Shigeyoshi Y, Okamura H. Development of vasoactive intestinal peptide mRNA rhythm in the rat suprachiasmatic nucleus. J Neurosci. 1997;17:3920–3931. doi: 10.1523/JNEUROSCI.17-10-03920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Mitushima D, Kimura F. Effects of gap junction blocker on vasopressin and vasoactive intestinal polypeptide rhythms in the rat suprachiasmatic nucleus in vitro. Neurosci Res. 2000;38:43–47. doi: 10.1016/s0168-0102(00)00141-3. [DOI] [PubMed] [Google Scholar]
- Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Vasoactive intestinal polypeptide (VIP) phase- shifts the rat suprachiasmatic nucleus clock in vitro. Eur J Neurosci. 2001;13:839–843. doi: 10.1046/j.0953-816x.2000.01437.x. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Hannibal J, Fahrenkrug J. Vasoactive intestinal polypeptide induces per1 and per2 gene expression in the rat suprachiasmatic nucleus late at night. Eur J Neurosci. 2002;15:570–574. doi: 10.1046/j.0953-816x.2001.01882.x. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel M, Feinstein N, Cohen S, Harari N. Vasopressinergic innervation of the mouse suprachiasmatic nucleus: An immuno-electron microscopic analysis. J Comp Neurol. 1990;298:172–187. doi: 10.1002/cne.902980204. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN, Gorcs T. Synaptic relationship between neurons containing vasopressin, gastrin releasing peptide, vasoactive intestinal peptide and glutamate decarboxylase immunoreactivity in the suprachiasmatic nucleus: Dual ultrastructural immunocytochemistry with gold-substituted silver peroxidase. J Comp Neurol. 1986;252:507–521. doi: 10.1002/cne.902520407. [DOI] [PubMed] [Google Scholar]
- Ingram CD, Snowball RK, Mihai R. Circadian rhythm of neuronal activity in suprachiasmatic nucleus slices from the vasopressin-deficient Brattleboro rat. Neuroscience. 1996;75:635–641. doi: 10.1016/0306-4522(96)00274-6. [DOI] [PubMed] [Google Scholar]
- Ingram CD, Ciobanu R, Coculescu IL, Tanasescu R, Coculescu M, Mihai R. Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog Brain Res. 1998;119:351–364. doi: 10.1016/s0079-6123(08)61580-0. [DOI] [PubMed] [Google Scholar]
- Mihai R, Juss TS, Ingram CD. Suppression of suprachiasmatic nucleus neuron activity with a vasopressin receptor antagonist: possible role for endogenous vasopressin in circadian activity cycles in vitro. Neurosci Lett. 1994;179:95–99. doi: 10.1016/0304-3940(94)90943-1. [DOI] [PubMed] [Google Scholar]
- Mihai R, Coculescu M, Wakerley JB, Ingram CD. The effects of [Arg8] vasopressin and [Arg8] vasotocin on the firing rate of suprachiasmatic neurons in vitro. Neuroscience. 1994;62:783–792. doi: 10.1016/0306-4522(94)90476-6. [DOI] [PubMed] [Google Scholar]
- Albers HE, Ferris CF, Leeman SE, Goldman BD. Avian pancreatic polypeptide phase shifts hamster circadian rhythms when microinjected into the suprachiasmatic region. Science. 1984;223:833–835. doi: 10.1126/science.6546454. [DOI] [PubMed] [Google Scholar]
- Reghunandanan V, Badgaiyan RD, Marya RK, Maini BK. Suprachiasmatic injection of a vasopressin antagonist modifies the circadian rhythm of food intake. Behav Neural Biol. 1987;48:344–351. doi: 10.1016/s0163-1047(87)90905-8. [DOI] [PubMed] [Google Scholar]
- Reghunandanan V, Reghunandanan R, Marya RK, Singh PI. Vasopressin antagonist disrupts the circadian rhythm of water intake on suprachiasmatic injection. Chronobiol Int. 1992;9:356–361. doi: 10.3109/07420529209064547. [DOI] [PubMed] [Google Scholar]
- Decoursey PJ, Buggy J. Restoration of locomotor activity in SCN-lesioned golden hamsters by transplantation of fetal SCN. [abstract] Neurosci. 1986;12:210. [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibso M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoynev AG, Nagai K. Lack of effect of suprachiasmatic infusion of a vasopressin antagonist on the circadian rhythm of wheel-running activity in rats. Acta Physiol Pharmacol Bulg. 1996;22:39–43. [PubMed] [Google Scholar]
- Boer GJ, van Esseveldt KE, van der Geest BAM, Duindam H, Rietveld WJ. Vasopressin-deficient suprachiasmatic nucleus grafts re-instate circadian rhythmicity in suprachiasmatic nucleus-lesioned arrhythmic rats. Neuroscience. 1999;89:375–385. doi: 10.1016/s0306-4522(98)00300-5. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJ, van Heerikhuize JJ, Hofman MA, Swaab DF. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiatry. 2001;58:655–662. doi: 10.1001/archpsyc.58.7.655. [DOI] [PubMed] [Google Scholar]
- Arima H, House SB, Gainer H, Aguilera G. Neuronal activity is required for the circadian rhythm of vasopressin gene transcription in the suprachiasmatic nucleus in vitro. Endocrinology. 2002;143:4165–4171. doi: 10.1210/en.2002-220393. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994;651:134–142. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. Influence of aging on the seasonal rhythm of the vasopressin-expressing neurons in the human suprachiasmatic nucleus. Neurobiol Aging. 1995;16:965–971. doi: 10.1016/0197-4580(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Hofman MA, Swabb DF. Increased light intensity prevents the age related loss of vasopressin-expressing neurons in the rat suprachiasmatic nucleus. Brain Res. 1995;693:261–266. doi: 10.1016/0006-8993(95)00933-h. [DOI] [PubMed] [Google Scholar]
- Hochstetler KJ, Garland T, Jr, Swallow JG, Carter PA, Bult-Ito A. Number of arginine-vasopressin neurons in the suprachiasmatic nuclei is not related to level or circadian characteristics of wheel-running activity in house mice. Behav Genet. 2004;34:131–136. doi: 10.1023/B:BEGE.0000009482.91124.52. [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Kallo I, Coen CW. Ageing and the diurnal expression of the mRNAs for vasopressin and for the V1a and V1b vasopressin receptors in the suprachiasmatic nucleus of male rats. J Neuroendocrinol. 2004;16:493–501. doi: 10.1111/j.1365-2826.2004.01196.x. [DOI] [PubMed] [Google Scholar]
- Biemans BAM, Van der Zee EA, Gerkema MP, Bult A, Daan S. Chronobiology Gordon Research Conference. Salve Regina University, Newport, Rode Island; A double role for vasopressin in the SCN? 5–10, August 2001. [Google Scholar]
- Groblewski TA, Nunez AA, Gold RM. Circadian rhythms in vasopressin deficient rats. Brain Res Bull. 1981;6:125–130. doi: 10.1016/s0361-9230(81)80036-6. [DOI] [PubMed] [Google Scholar]
- Stoinev AG, Ikonomov OK. Effect of continuous infusion of vasopressin on circadian rhythms of food and water intake, diuresis and electrolyte excretion in Brattleboro rats. Bull Exp Biol Med. 1990;09:122–124. [PubMed] [Google Scholar]
- Castel M, Morris J, Belenky M. Non-synaptic and dendritic exocytosis from dense-cored vesicles in the suprachiasmatic nucleus. NeuroReport. 1996;7:543–547. doi: 10.1097/00001756-199601310-00040. [DOI] [PubMed] [Google Scholar]
- Isobe Y, Nishino H. AVP rhythm in the suprachiasmatic nucleus in relation to locomotor activity under constant light. Peptides. 1998;9:827–832. doi: 10.1016/s0196-9781(98)00021-7. [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Oklejewicz M, Jansen K, Daan S, Gerkema MP. Vasopressin immunoreactivity and release in the suprachiasmatic nucleus of wild-type and tau mutant Syrian hamsters. Brain Res. 2002;936:38–46. doi: 10.1016/s0006-8993(02)02497-6. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Science. 1988;242:78–83. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- Van den Top M, Buijs RM, Ruijter JM, Delagrange P, Spanswick D, Hermes ML. Melatonin generates an outward potassium current in rat suprachiasmatic nucleus neurones in vitro independent of their circadian rhythm. Neuroscience. 2001;107:99–108. doi: 10.1016/s0306-4522(01)00346-3. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yamaoka S, Vanecek J. Melatonin inhibits spontaneous and VIP-induced vasopressin release from suprachiasmatic neurons. Brain Res. 1998;801:216–219. doi: 10.1016/s0006-8993(98)00543-5. [DOI] [PubMed] [Google Scholar]
- Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci USA. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- Mishima K, Okawa M, Hozumi S, Hishikawa Y. Supplementary administration of artificial bright light and melatonin as potent treatment for disorganized circadian rest-activity and dysfunctional autonomic and neuroendocrine systems in institutionalized demented elderly persons. Chronobiol Int. 2000;17:419–432. doi: 10.1081/cbi-100101055. [DOI] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ. Melatonin as a chronobiotic, treatment of circadian desynchrony in night workers and the blind. J Biol Rhythms. 1997;12:595–603. doi: 10.1177/074873049701200615. [DOI] [PubMed] [Google Scholar]
- Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CL. Advancing human circadian rhythms with afternoonmelatonin and morning intermittent bright light. J Clin Endocrinol Metab doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA. Melatonin and seasonal rhythms. J Biol Rhythms. 1997;12:518–527. doi: 10.1177/074873049701200605. [DOI] [PubMed] [Google Scholar]
- Pévet P. Melatonin: From Seasonal to Circadian Signal. J Neuroendocrinol. 2003;15:422–426. doi: 10.1046/j.1365-2826.2003.01017.x. [DOI] [PubMed] [Google Scholar]
- Lincoln GA, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals – a unifying hypothesis. J Endocrinol. 2003;179:1–13. doi: 10.1677/joe.0.1790001. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Teclemariam-Mesbah R, Pevet P. Efferent projections of the suprachiasmatic nucleus in the golden hamster (Mesocricetus auratus) J Comp Neurol. 1993;332:293–314. doi: 10.1002/cne.903320304. [DOI] [PubMed] [Google Scholar]
- Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to the median preoptic nucleus in rat. Brain Res. 2003;987:100–106. doi: 10.1016/s0006-8993(03)03295-5. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Burns J, Semba K. Indirect projections from the suprachiasmatic nucleus to the ventrolateral preoptic nucleus: a dual tract-tracing study in rat. Eur J Neurosci. 2002;16:1195–1213. doi: 10.1046/j.1460-9568.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- Watts AG. The efferent projections of the suprachiasmatic nucleus: anatomical insights into the control of circadian rhythms. In: Klein DC, Moore RY Reppert SM, editor. Suprachiasmatic nucleus: the mind's clock. New York, Oxford Univ Press; 1991. pp. 77–106. [Google Scholar]
- Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, Honma K. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci. 2000;12:4206–4214. [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Allen G, Rappe J, Earnest DJ, Cassone VM. Oscillating on borrowed time: diffusible signals from immortalized suprachiasmatic nucleus cells regulate circadian rhythmicity in cultured fibroblasts. J Neurosci. 2001;21:7937–7943. doi: 10.1523/JNEUROSCI.21-20-07937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Humoral signals mediate the circadian expression of rat period homologue (rPer2) mRNA in peripheral tissues. Neurosci Lett. 1998;256:117–119. doi: 10.1016/s0304-3940(98)00765-4. [DOI] [PubMed] [Google Scholar]
- Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- Junier MP. What role(s) for TGFα in the central nervous system? Prog Neurobiol. 2000;62:443–473. doi: 10.1016/s0301-0082(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Ma YJ, Junier MP, Costa ME, Ojeda SR. Transforming growth factor-alpha gene expression in the hypothalamus is developmentally regulated and linked to sexual maturation. Neuron. 1992;9:657–670. doi: 10.1016/0896-6273(92)90029-d. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Lee DC, Guthrie KM, Gall CM. Cellular localization of transforming growth factor-alpha mRNA in rat forebrain. J Neurochem. 1993;60:1777–1782. doi: 10.1111/j.1471-4159.1993.tb13403.x. [DOI] [PubMed] [Google Scholar]
- Jobst EE, Robinson DW, Allen CN. Potential pathways for intercellular communication within the calbindin subnucleus of the hamster suprachiasmatic nucleus. Neuroscience. 2004;123:87–99. doi: 10.1016/j.neuroscience.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Li X, Sankrithi N, Davis FC. Transforming growth factor-alpha is expressed in astrocytes of the suprachiasmatic nucleus in hamster: role of glial cells in circadian clocks. Neuroreport. 2002;13:2143–2147. doi: 10.1097/00001756-200211150-00031. [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Roman V, Ten Brinke O, Meerlo P. TGFα and AVP in the mouse suprachiasmatic nucleus: Anatomical relationship and daily profiles. Brain Research. 2005;1054:159–166. doi: 10.1016/j.brainres.2005.06.075. [DOI] [PubMed] [Google Scholar]
- Leak RK, Card JP, Moore RY. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Res. 1999;819:23–32. doi: 10.1016/s0006-8993(98)01317-1. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000;141:3832–3841. doi: 10.1210/endo.141.10.7709. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000;871:50–56. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- De la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: Activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci. 2003;23:7412–7414. doi: 10.1523/JNEUROSCI.23-19-07412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Lehman MN. Transplantation: a new tool in the analysis of the mammalian hypothalamic circadian pacemaker. Trends Neurosci. 1991;14:362–366. doi: 10.1016/0166-2236(91)90164-p. [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J. Efferent signals of the suprachiasmatic nucleus. J Biol Rhythms. 1993;8:89–92. [PubMed] [Google Scholar]
- Silver R, Lehman MN, Gibson M, Gladstone WR, Bittman EL. Dispersed cell suspensions of fetal SCN restore circadian rhythmicity in SCN-lesioned adult hamsters. Brain Res. 1990;525:45–58. doi: 10.1016/0006-8993(90)91319-c. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Okamura H, Matsuda T, Shigeyoshi Y, Hisa Y, Chihara K, Ibata Y. Somatostatin neurons form a distinct peptidergic neuronal group in the rat suprachiasmatic nucleus: a double labeling in situ hybridization study. Neurosci Lett. 1996;215:119–122. [PubMed] [Google Scholar]
- Shigeyoshi Y, Maebayashi Y, Okamura H. Co-localization of preprosomatostatin mRNA and preprotachykinin A mRNA in neurons of the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1997;48:159–163. doi: 10.1016/s0169-328x(97)00153-8. [DOI] [PubMed] [Google Scholar]
- Daikoku S, Hisano S, Kagotani Y. Neuronal associations in the rat suprachiasmatic nucleus demonstrated by immunoelectron microscopy. J Comp Neurol. 1992;325:559–571. doi: 10.1002/cne.903250408. [DOI] [PubMed] [Google Scholar]
- Biemans BA, Gerkema MP, Van der Zee EA. Increase in somatostatin immunoreactivity in the suprachiasmatic nucleus of aged Wistar rats. Brain Res. 2002;958:463–467. doi: 10.1016/s0006-8993(02)03703-4. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Herron JM, Hill SA. Aging selectively suppresses vasoactive intestinal peptide messenger RNA expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res Mol Brain Res. 2001;87:196–203. doi: 10.1016/s0169-328x(01)00015-8. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Hamada T, Shibata S, Watanabe S, Aoki K, Inouye SI. Phase advances of circadian rhythms in somatostatin depleted rats: effects of cysteamine on rhythms of locomotor activity and electrical discharge of the suprachiasmatic nucleus. J Comp Physiol [A] 1994;175:677–685. doi: 10.1007/BF00191840. [DOI] [PubMed] [Google Scholar]
- Shibata S, Tsuneyoshi A, Hamada T, Tominaga K, Watanabe S. Effect of substance P on circadian rhythms of firing activity and the 2-deoxyglucose uptake in the rat suprachiasmatic nucleus in vitro. Brain Res. 1992;597:257–263. doi: 10.1016/0006-8993(92)91482-t. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Nishiwaki T, Cagampang FR, Inouye ST. Emergence of VIP rhythmicity following somatostatin depletion in the rat suprachiasmatic nucleus. Brain Res. 1994;645:343–346. doi: 10.1016/0006-8993(94)91671-3. [DOI] [PubMed] [Google Scholar]
- Silver R, Romero MT, Besmer HR, Leak R, Nunez JM. Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport. 1996;7:1224–1228. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- Bryant DN, Le Sauter J, Silver R, Romero MT. Retinal innervation of calbindin-D28K cells in the hamster suprachiasmatic nucleus: ultrastructural characterization. J Biol Rhythms. 2000;15:103–111. doi: 10.1177/074873040001500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Leak RK, Silver R, Moore RY. Hamster suprachiasmatic nucleus: chemoarchitecture and topography of projections. Soc Neurosci Abst. 1999;25:s552. [Google Scholar]
- LeSauter J, Kriegsfels LJ, Hon J, Silver R. Calbindin-D(28 K) cells selectively contact intra-SCN neurons. Neuroscience. 2002;111:575–585. doi: 10.1016/s0306-4522(01)00604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Lokshin M, Romero MT, Yan L, Venuti JM, Silver R. Calbindin influences response to photic input in suprachiasmatic nucleus. J Neurosci. 2003;23:8820–8826. doi: 10.1523/JNEUROSCI.23-26-08820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Allen CN. Developmental changes in calbindin-D28k and calretinin expression in the mouse suprachiasmatic nucleus. Eur J Neurosci. 2003;17:1111–1118. doi: 10.1046/j.1460-9568.2003.02515.x. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. Galanin – a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Gai WP, Geffen LB, Blessing WW. Galanin immunoreactive neurons in the human hypothalamus: colocalization with vasopressin-containing neurons. J Comp Neurol. 1990;298:265–280. doi: 10.1002/cne.902980302. [DOI] [PubMed] [Google Scholar]
- Mitchell V, Bouret S, Howard AD, Beauvillain JC. Expression of the galanin receptor subtype Gal-R2 mRNA in the rat hypothalamus. J Chem Neuroanat. 1999;16:265–277. doi: 10.1016/s0891-0618(99)00011-3. [DOI] [PubMed] [Google Scholar]
- Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology. 1985;40:2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Fleissner G, Stohr M, Hauptfleisch S, Lemmer B. Localization of components of the renin-angiotensin system in the suprachiasmatic nucleus of normotensive Sprague-Dawley rats: part A. angiotensin I/II, a light and electron microscopic study. Brain Res. 2004;1008:212–223. doi: 10.1016/j.brainres.2004.01.086. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Fleissner G, Stohr M, Hauptfleisch S, Lemmer B. Localization of components of the renin-angiotensin system in the suprachiasmatic nucleus of normotensive Sprague-Dawley rats: part B. angiotensin II (AT1)-receptors, a light and electron microscopic study. Brain Res. 2004;1008:224–235. doi: 10.1016/j.brainres.2004.01.085. [DOI] [PubMed] [Google Scholar]
- Takatsuji K, Tohyama M. Geniculo-geniculate projection of enkephalin and neuropeptide Y containing neurons in the intergeniculate leaflet of the thalamus in the rat. J Chem Neuroanat. 1989;2:19–27. [PubMed] [Google Scholar]
- Ostrowski NL, Hill JM, Pert CB, Pert A. Autoradiographic visualization of sex differences in the pattern and density of opiate receptors in hamster hypothalamus. Brain Res. 1987;421:1–13. doi: 10.1016/0006-8993(87)91268-6. [DOI] [PubMed] [Google Scholar]
- Tubbiola ML, Nock B, Bittman EL. Photoperiodic changes in opiate binding and their functional implications in golden hamsters. Brain Res. 1989;503:91–99. doi: 10.1016/0006-8993(89)91708-3. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Mundey MK, Mason R. Electrophysiological effects of opioid receptor activation on Syrian hamster suprachiasmatic nucleus neurones in vitro. Brain Res Bull. 1999;50:119–125. doi: 10.1016/s0361-9230(99)00069-6. [DOI] [PubMed] [Google Scholar]
- Reghunandanan V, Marya RK, Maini BK, Reghunandanan R. Bilateral suprachiasmatic injection of naloxone disrupts circadian rhythm of food intake. Indian J Exp Biol. 1988;26:954–956. [PubMed] [Google Scholar]
- Reghunandanan V, Reghunandanan R, Singh PI. Effect of naloxone injection into the suprachiasmatic nuclei on the circadian rhythm of water intake. J Parasitol Appl Biol. 1992;1:33. [Google Scholar]
- Cheng MY, Bittman EL, Hattar S, Zhou QY. Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neurosci. 2005;6:17. doi: 10.1186/1471-2202-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. Analysis of the prokineticin 2 system in a diurnal rodent, the unstriped Nile grass rat (Arvicanthis niloticus) J Biol Rhythms. 2005;20:206–18. doi: 10.1177/0748730405275135. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Busis NA, Hedley-Whyte ET. A discrete lesion of ventral hypothalamus and optic chiasm that disturbed the daily temperature rhythm. J Neurol. 1986;233:1–4. doi: 10.1007/BF00313981. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Albers HE. Disruption of human circadian and cognitive regulation following a discrete hypothalamic lesion: A case study. Neurology. 1991;41:726–729. doi: 10.1212/wnl.41.5.726. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: neural and neuroendocrine mechanisms in human and rat. Biol Chem. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The Circadian Gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- Green CB. Time for chronotherapy? Clock genes dictate sensitivity to cyclophosphamide. Proc Nat Acad Sci USA. 2005;102:3529–3530. doi: 10.1073/pnas.0500552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Schantz M, Archer SN. Clocks, genes and sleep. J R Soc Med. 2003;96:486–489. doi: 10.1258/jrsm.96.10.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhauser JM, Mayo KE, Takahashi JS. Light, immediate-early genes, and circadian rhythms. Behav Genet. 1996;26:221–240. doi: 10.1007/BF02359382. [DOI] [PubMed] [Google Scholar]
- Field MD, Maywood ES, O'Brien JA, Weaver DR, Reppert SM, Hastings MH. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron. 2000;25:437–447. doi: 10.1016/s0896-6273(00)80906-x. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. 1999;19:1115–1121. doi: 10.1523/JNEUROSCI.19-03-01115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Takekida S, Shigeyoshi Y, Okamura H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience. 1999;94:141–150. doi: 10.1016/s0306-4522(99)00223-7. [DOI] [PubMed] [Google Scholar]
- Pickering TG. The clinical significance of diurnal blood pressure variations. Dippers and nondippers. Circulation. 1990;81:700–702. doi: 10.1161/01.cir.81.2.700. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamasaki Y, Kodama M, Matsuhisa M, Kishimoto M, Ozaki H, Tani A, Ueda N, Iwasaki M, Hori M. Impaired diurnal cardiac autonomic function in subjects with type 2 diabetes. Diabetes Care. 1999;2:2072–2077. doi: 10.2337/diacare.22.12.2072. [DOI] [PubMed] [Google Scholar]
- Goncharuk VD, Van Heerikhuize J, Swaab DF, Buijs RM. Paraventricular nucleus of the human hypothalamus in primary hypertension: activation of corticotropin-releasing hormone neurons. J Comp Neurol. 2002;443:321–331. doi: 10.1002/cne.10124. [DOI] [PubMed] [Google Scholar]
- Goncharuk VD, van Heerikhuize J, Dai JP, Swaab DF, Buijs RM. Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. J Comp Neurol. 2001;31:320–330. doi: 10.1002/1096-9861(20010312)431:3<320::aid-cne1073>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Borghi C, Costa FV, Boschi S, Mussi A, Ambrosioni E. Predictors of stable hypertension in young borderline subjects: a five-year follow-up study. J Cardiovasc Pharmacol. 1986;8:s138–41. doi: 10.1097/00005344-198608005-00030. [DOI] [PubMed] [Google Scholar]
- Eilam R, Malach R, Bergmann F, Segal M. Hypertension induced by hypothalamic transplantation from genetically hypertensive to normotensive rats. J Neurosci. 1991;11:401–411. doi: 10.1523/JNEUROSCI.11-02-00401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


