Abstract
Patients with non-specific limb pain often show signs of nerve mechanosensitivity, i.e. local tenderness over nerve trunks and pain in response to limb movements that cause nerve stretch. In such patients a nerve lesion is not apparent, and it has been suggested that local neural inflammation may be a key factor. The present study examines the extent to which nerve fibres in regions of local inflammation respond to small stretches, and whether functional changes occur throughout the primary afferent neurone. A local neuritis was induced in adult rats by wrapping oxidised cellulose saturated in complete Freund’s adjuvant (CFA) around the peroneal or sciatic nerves. A small cut was made in the perineurium of some of the peroneal lesioned animals. A- and C-fibre recordings were made 2–10 days post-surgery from filaments dissected proximal to the lesion. Local mechanosensitivity was assessed using a glass probe and by small stretches. Responses to stretch and local pressure were recorded in 7% of C- and 8% of A-fibres from the peroneal nerve following CFA treatment with the sheath opened. A smaller proportion of stretch sensitive fibres were seen in sciatic and peroneal nerves after CFA treatment alone (2% of C- and 3% of A-fibres), but such fibres were not seen in control preparations. The most responsive fibres fired to 3% stretch, which is within the range of nerve stretch seen during normal limb movements. Less than 1% of stretch sensitive fibres had peripheral fields, indicating that most had probably degenerated distally.
Keywords: Neuritis, Nerve injury, Nerve stretch, Mechanosensitivity, Repetitive strain injury, Electrophysiology
1. Introduction
Patients with non-specific arm pain (NSAP; or repetitive strain injury) and carpal tunnel syndrome (CTS), frequently show signs of nerve trunk mechanosensitivity. For example, digital pressure over the median nerve can cause an exacerbation of painful symptoms in patients with NSAP and painful responses can also be induced by placing the upper limb in a posture that stretches the median nerve (Lynn et al., 2002).
In animal models, signs of nerve trunk hypersensitivity, such as spontaneous activity and mechanosensitive responses to pressure at the site of injury, can develop in injured afferents following a neuroma or a chronic constriction injury (Chen and Devor, 1998; Tal and Eliav, 1996; Tal et al., 1999). Responses to stretch can also develop in A-fibres in a neuroma (Proske et al., 1995). However, for many patients with widespread painful conditions a nerve lesion is not apparent on clinical examination. It has been suggested that in these patients, pain can occur following relatively minor nerve damage and that inflammation of the nerve trunk alone may play a major role in symptom production (Greening and Lynn, 1998). Studies in the rat have shown that a local neuritis can cause pain-related behavioural changes (Chacur et al., 2001; Eliav et al., 1999) and that intact nerve fibres can become sensitive to pressure at the lesion site (Bove et al., 2003; Eliav et al., 2001). These studies found no obvious axonal damage at the lesion site.
The present study is an electrophysiological examination of nerve trunk mechanosensitivity following induction of a local neuritis along the peroneal or sciatic nerves in the rat. Both pressure and stretch responses are examined, with particular emphasis on the latter. Conduction block at the peroneal lesion site is assessed as well as driving of afferents from the periphery. The up-regulation of activating transcription factor-3 (ATF3), a marker of cell stress, following inflammation is also examined.
2. Methods
All experiments adhered to the guidelines of the Committee for Research and Ethical Issues of the IASP.
2.1. Surgery
A neuritis lesion was induced in the sciatic or peroneal nerve of adult female Sprague–Dawley rats (170–200 g). Animals were anaesthetised and maintained on halothane (2% in oxygen) and nitrous oxide for the duration of the surgery. A blunt dissection was performed through the biceps femoris muscle to expose either the sciatic or peroneal nerve. The length of nerve to be lesioned (5–10 mm) was cleared from its surrounding connective tissue. In one group of rats (n=9), a strip of oxidised cellulose (Surgicel, Ethicon, USA) (approximately, 5×10×1 mm3) saturated in complete Freunds adjuvant (CFA) (approximately, 200 μL) was wrapped loosely around the sciatic nerve proximal to the trifurcation. In a second group of animals (n=4), the same procedure was repeated on the peroneal nerve 3–6 mm distal to where it leaves the sciatic. The reason for using the peroneal nerve for some experiments was to allow a longer conduction distance proximal to the lesion and so to permit electrical stimulation proximal and distal to this (see below). In a third group (n=10), a small longitudinal cut (approximately, 1 mm) was made through the perineurium of the peroneal nerve using a razor blade prior to wrapping the nerve with oxidised cellulose saturated in CFA. In a fourth group of animals (n=4), a small longitudinal cut was made through the perineurium of the peroneal nerve and oxidised cellulose saturated with 0.9% saline was wrapped around the nerve. In a fifth group of control animals (n=9), oxidised cellulose (n=6) or GelFoam (n=3) saturated with 0.9% saline was loosely wrapped around the sciatic nerve. Preliminary electrophysiology experiments produced the same results with either Surgicel or GelFoam (data not shown). In a sixth series of animals for immunohistochemistry (n=5), the sciatic nerve was transected proximal to the trifurcation. A 2 mm segment of sciatic nerve was removed to prevent regeneration between the stumps.
2.2. Electrophysiology
Electrophysiology was performed on 20 CFA-treated rats with various nerves treated as listed in Table 1. In addition, nine rats with saline treatment and five rats with no nerve pre-treatment were examined (again details in Table 1). The experimenter was not blind to the lesion type. Animals were anaesthetised with urethane (1.5 g urethane/kg), the body temperature was monitored by a rectal thermistor probe and maintained at 37 ° C using a heated blanket, and the trachea was cannulated. The sciatic, peroneal and sural nerves were exposed in the thigh by blunt dissection and the lesion site and adjacent nerve segments were freed from surrounding connective tissue and any remaining oxidised cellulose. The surrounding skin was stitched to a metal ring to form a pool filled with liquid paraffin. In rats with sciatic nerve lesions (animals treated with both CFA and saline), paired platinum stimulating electrodes were positioned under the sural nerve distal to the lesion site. In rats with peroneal nerve lesions, and also naïve (untreated) animals, paired stimulating electrodes were positioned under the peroneal nerve both proximal and distal to the lesion site, thereby enabling conduction through the lesion to be determined (Fig. 1).
Table 1.
Summary of lesion types
| Lesion type | n | Average days post-surgery (range) | Fibres examined |
|---|---|---|---|
| Peroneal CFA (sheath cut) | 9 | 4.1 (2–8) | Peroneal |
| Peroneal CFA | 4 | 3.3 (3–4) | Peroneal |
| Sciatic CFA | 7 | 5.7 (2,10) | Sural |
| Peroneal saline (sheath cut) | 4 | 3.3 (2–4) | Peroneal |
| Sciatic saline | 5 | 5.0 (3–8) | Sural |
| Untreated | 5 | N/A | Peroneal |
Fig. 1.
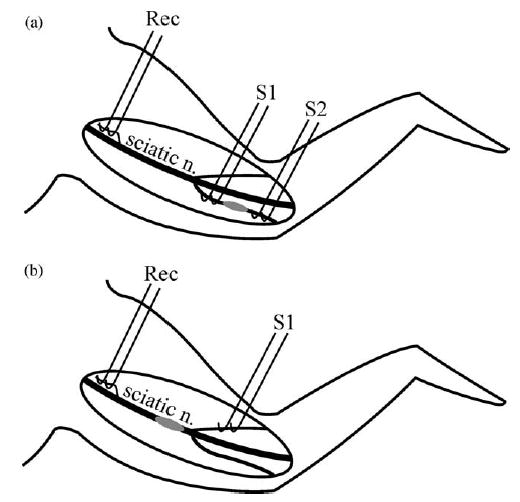
Diagram of electrophysiology setup in animals with (a) a peroneal nerve and (b) a sciatic nerve lesion. In (a) peroneal nerve fibres are stimulated proximal (S1) and distal (S2) to the lesion site (grey ellipse). In (b) sural nerve fibres are stimulated distal (S1) to the lesion site (grey ellipse). In both preparations, recordings are made from fine filaments teased from the sciatic nerve proximal to the lesion site. S1: stimulus 1, S2: stimulus 2; Rec: recording electrodes.
A mirrored platform was positioned under the sciatic nerve in the mid-upper thigh, 6–10 mm proximal to the lesion site. The nerve was desheathed over this segment and fine filaments were teased out on the platform with watchmakers forceps and needles. Filaments were cut centrally but remained in continuity distally. Each filament was suspended onto a platinum recording electrode, referenced to a nearby indifferent electrode. Filaments with sural units (which are all cutaneous) were examined in rats with sciatic nerve lesions, and peroneal units (a mixed nerve, which innervates both cutaneous and deep structures) in animals with peroneal nerve lesions and in untreated rats (Table 1, Fig. 1). Filaments were split until single A- and C-units could be identified using electrical stimulation (usually when filaments contained less than 10 A- and/or less than 15 C-fibres). The total number of units was counted by gradually increasing the stimulus intensity within the A- and C-fibre range (square wave pulses: 0.05–0.5 ms duration, 0–10 V amplitude). The number of units was identified both proximal and distal to the lesion site in peroneal lesioned and untreated rats to determine the percentage of fibres not conducting through the lesion site. Conduction velocities were also determined by dividing the latency of individual units (at approximately twice threshold) by the conduction distance. Conduction velocities across the lesion site (i.e. between the two sets of stimulating electrodes) were also determined. A- and C-units were identified by their conduction velocities. Filaments were watched for approximately 1 min to observe any spontaneous activity. Spontaneous units were identified as A- or C-units by spike shape. C-fibres typically had a biphasic shape and a longer duration compared to A-fibres which were generally monophasic (mean duration at the mid amplitude of the first positive inflection for C-fibres=574 μs (lower quartile= 440 μs) (n=72) and for A-fibres=309 μs (upper quartile= 340 μs) (n=59)). Peripheral fields were identified in peroneal and sural lesioned animals, and in controls, using mechanical and heat/cold stimuli. The dorsum of the foot was searched for mechanical receptive fields (using either a brush, a blunt metal probe or pinching the skin). Squeezing the foot and moving the ankle and digits enabled deep muscle units to be identified. To distinguish cutaneous nociceptive units that respond to strong pressure, from deep mechanical units, the skin was displaced away from the peripheral field. In contrast to deep units, the receptive fields of cutaneous units moved with the skin. A heat contact probe (set to 50–55 ° C) was used to search for polymodal nociceptors when it was not possible to use strong pressure (e.g. close to the ring on which the skin flaps were stitched). To avoid damage to the skin, the heat contact probe was only applied for a brief periods, approximately 1–2 s. Cold units were identified by passing a cold metal probe (approximately 5 ° C) over the surface of the foot. Peroneal nerve muscle spindles were identified by moving the ankle or digits.
The numbers of units with peripheral fields compared to the number of spikes on electrical stimulation were noted. On filaments with a good signal-to-noise ratio and few spikes on electrical stimulation, it was possible to determine the conduction velocity of the drivable units from the periphery. This was achieved by stimulating the receptive field mechanically whilst stimulating the axon electrically. If the electrical stimulus occurred during the relative refractory period of the unit, an action potential would not be initiated or would be delayed (referred to throughout the text as collision). Units with peripheral fields were identified as A- or C-units by a combination of spike shape, receptive field properties and conduction velocity. The spike shape was used to determine whether spontaneous units also had peripheral fields. Changes in the pattern of spontaneous activity during the mechanical stimulation of the peripheral field further indicated that both units were the same.
2.3. Mechanosensitivity testing at the lesion site
Pressure mechanosensitivity was tested using a blunt glass probe with an approximate tip diameter of 1 mm. Light pressure, just sufficient to produce an indentation visible under the dissecting microscope, was applied with the probe successively along the lesion site or along the equivalent stretch of peroneal nerve in untreated rats (the same experimenter performed all experiments to reduce variations in the probe pressure applied). To avoid damaging the axons, strong pressure was not used. A second glass probe was used to support the underside of the nerve during testing.
Stretch mechanosensitivity was assessed at the lesion site and along the equivalent part of the peroneal nerve in untreated rats using fine watch makers forceps. The nerve was held by its sheath either side of the lesion (5–10 mm apart) and small local stretches were applied. Under the dissecting microscope, the nerve contents could be seen to slide with the sheath. The forceps were positioned adjacent to locally fixed points (such as the electrodes) to allow the amount of stretching to be easily measured. One pair of the forceps was kept stationary, whilst the other pair were used to apply the stretch. Distance between the forceps was 7–12 mm and stretches 0.2–1.5 mm were used. Percentage strain (stretch) was calculated. For example, a stretch of 0.3 mm with a distance of 10 mm between the forceps would represent a 3% stretch. The nerve was usually stretched close to a stimulating electrode so that the amount of stretch could be estimated relative to the diameter of the electrode (0.32 mm). Stretches could be reproduced to within ± 0.1 mm. To avoid damage to the nerve fibres, the maximum stretch did not exceed 15%.
The numbers of units driven by mechanical stimulation at the lesion site were compared to the number of spikes on electrical stimulation proximal to the lesion site in peroneal lesioned animals. In sciatic nerve lesioned animals, where there was no proximal stimulation, the proportion of units drivable by mechanical stimulation was calculated from the number of spikes on the distal count. Results from the peroneal CFA group showed that with the sheath intact, very few fibres failed to conduct through the lesion site (< 12% conduction failure, see Section 3). The proportion of mechanosensitive pressure units in these animals was similar when calculated from distal or proximal stimulation for both A-fibres (8.5% (11/130) on distal and 7.9% (11/139) on proximal count; P=0.95) and C-fibres (6.6% (8/121) on distal and 6.3% (8/128) on proximal count; P=0.89). Therefore, it was considered reasonable to compare the proportion of mechanosensitive units based on distal counts in sciatic animals where the sheath is also intact, with the proportion on proximal counts in peroneal animals.
Units that were driven by mechanical stimulation at the lesion site could often be identified when electrically stimulated. Spike identification was based on spike shape or by collision (refer to section above). Repeating these methods with electrical stimulation either side of the lesion site, it was sometimes possible to determine whether a particular unit was conducting through the lesion site. Therefore, in rats with a peroneal neuritis, the total number of units on distal stimulation (i.e. conducting through the lesion) was compared to the total number of units on proximal stimulation. Mechanosensitive units on filaments with the same number of units either side of the lesion site must be conducting through the lesion site. (Note only filaments with good signal-to-noise and reliable unit counts were used.) Conversely, mechanosensitive units on filaments totally blocked at the lesion site were clearly not conducting through the lesion site. Finally, spike shape and collision were used to determine whether mechanosensitive units possessed a peripheral receptive field.
In sciatic lesioned animals, filaments dissected at the recording site tended to be from the same fascicle, i.e. the sural nerve. Therefore, mechanosensitive units, and also spontaneous units, that could not be identified from electrical stimulation (perhaps due to conduction failure at the lesion site) were still considered to be sural fibres.
The sequence of tests on all animals was as follows: (1) The numbers of A- and C-units were determined from electrical stimulation (from both stimulus locations in peroneal experiments) and latencies were noted. (2) The number of spontaneous units was counted. (3) The periphery was examined for cutaneous and deep receptive fields. (4) The lesion site was checked for pressure and then stretch mechanosensitivity. (5) Any mechanosensitive units were identified by collision and the periphery was searched for their receptive fields.
2.4. Histology
In a number of the peroneal-treated (n=12) and untreated animals (n=3), a 2 mm length of nerve centred on the lesion site (or the equivalent segment of nerve in untreated animals) was removed for histology 2–9 days post-surgery. The tissue was fixed by submersion in 4% paraformaldehyde in 0.1 M phosphate-buffered saline. Following osmium treatment and dehydration, the tissue was embedded in epoxy resin, and transverse semi-thin sections (1 μm) were cut and stained with toluidine blue. Sections were viewed under a light microscope (Olympus, Japan) and photographed using a digital camera (Nikon, Japan). The extent of nerve damage was determined blind from the area within each cross-section that appeared either (1) normal with myelinated fibres regularly spaced, or (2) with sparse myelinated fibres (poorly packed with clear oedematous spaces between profiles or normal looking profiles with interspersed degenerated profiles) or (3) completely degenerated with no myelinated fibres visible. The results were ranked in order of the extent of damage (with 1 as the least affected).
2.5. Immunohistochemical analysis of activating transcription factor-3 (ATF3)
Dorsal root ganglia (DRG) were harvested from rats with CFA (n=6) and saline (n=7) sciatic nerve treatments or post-transection (n=5) on days 2–10 post-surgery. A number of these rats also underwent electrophysiological examination (four CFA and three saline treated). All animals were perfused intracardially with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline. L4 and L5 DRG from the lesioned side were removed for immunochemical analysis and stored in 4% paraformaldehyde or 4% paraformaldehyde/2% gluteraldehyde prior to use.
Activating transcription factor-3 (ATF3) expression was determined using immunohistochemistry. DRG were placed in 30% sucrose overnight and then embedded in Tissue Tek™ cooled by crushed dry ice. Frozen sections were cut at 5 μm directly onto Superfrost gelatin coated slides. Slides were rinsed in distilled water. Tissue previously fixed in gluteraldehyde was treated for 10 min in 1% sodium borohydride to remove any autofluorescence. This was followed by washes in PBS and PBS with 0.2% Triton X-100. After a 30-min incubation in 2% donkey serum (in PBS with 0.2% Triton X-100) at room temperature, the sections were incubated in ATF3 primary antibody (1:500) (Santa Cruz Biotechnologies, USA) overnight at 4 ° C. Following three washes in PBS, the sections were incubated in secondary donkey anti-rabbit antibody (1:500) (Alexa Flour 488, Molecular Probes, USA) in the dark at room temperature for 1 h. After three further washes in PBS, the sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI) nuclear counter-stain (1:10,000) in the dark at room temperature for 45 min. Slides were subsequently washed three times with PBS, and then mounted (using 90% glycerol and 10% PBS as a mounting reagent). Sections were viewed under a fluorescence microscope (Olympus) at 488 and 350 nm excitation and photographed at each wavelength using a digital camera (Nikon). To avoid repeated counting of nuclei, the sections examined were at >15 μm intervals. The number of ATF3 and DAPI positive neuronal profiles were counted in each section, and the percentage of ATF3 positive nuclei calculated.
2.6. Statistical analysis
All comparisons between frequency data were made using chi-square tests. Comparisons between mean conduction velocities were made using the non-parametric Mann–Whitney and Kruskal–Wallis tests because the distributions of C-fibre conduction velocities were markedly positively skewed. Correlation coefficients between histological appearance of nerves and degree of conduction block were calculated using the Spearman rank correlation coefficient. Where multiple comparisons were made, the Bonferroni correction was applied in assessing significance.
3. Results
3.1. Pressure mechanosensitivity
Both C- and A-fibre responses to pressure were seen in CFA treated animals, but not following saline treatment of intact nerves or in untreated nerves (Fig. 2). Amongst sural fibres isolated following sciatic nerve CFA treatment, 4.0% of C-fibres and 2.0% of A-fibres were pressure sensitive. Amongst peroneal fibres following peroneal treatment, 6.3% of C-fibres and 7.9% of A-fibres were pressure sensitive. When a small cut was made in the perineurium at the time of CFA treatment a larger proportion of fibres became pressure sensitive, 12.6% of C-fibres and 19.0% of A-fibres. This difference was statistically significant for the A fibres (P<0.01) but not quite significant for the C-fibres (P=0.07). Interestingly, if the nerve sheath was opened in this way, but only saline was applied, considerable numbers of pressure sensitive fibres were still observed (10.4% of C-fibres and 16.3% of A-fibres). Units tended to respond from small defined fields (<1 mm diameter) located within the lesion site, although two pressure sensitive A-units responded to light pressure along a narrow band extending the length of the exposed peroneal nerve. In early (2–3 days) and late lesions (6–10 days), there were similar numbers of pressure sensitive units.
Fig. 2.
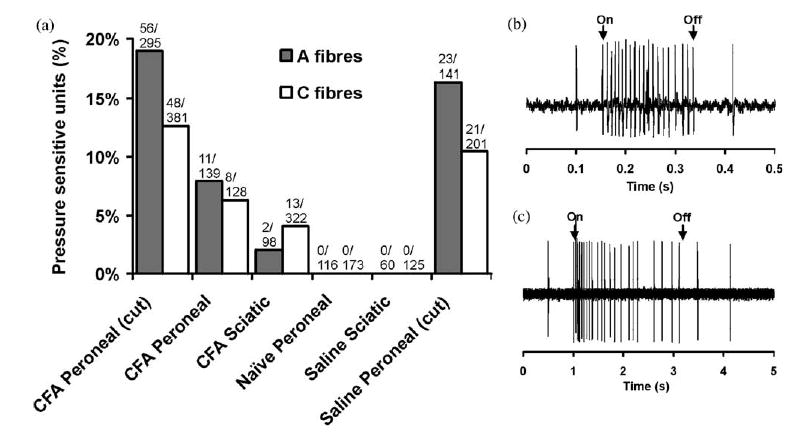
Summary of local pressure responses following neuritis. (a) Proportion of A- and C-fibres responding to local pressure at the lesion in the CFA-treated peroneal lesion with the sheath opened (CFA peroneal (cut)) and intact (CFA peroneal), CFA-treated sciatic lesion, untreated animals, saline-treated sciatic lesion and saline-treated peroneal lesion with the sheath opened (saline peroneal (cut)). (b and c) Typical responses to local pressure at the treatment site for an A-fibre (b) and a C-fibre (c). The total number of units sampled and the number responding to pressure are shown for each group.
3.2. Stretch mechanosensitivity
In all the preparations investigated, approximately half of the pressure sensitive units were also sensitive to small stretches of the peroneal or sciatic nerves (compare Figs. 2 and 3). Following CFA treatment, 1.8% of C-fibres and 1.3% of A-fibres were stretch sensitive in sciatic nerve lesioned animals and 1.6% of C- and 3.3% of A-fibres in the peroneal nerve lesioned group with the sheath intact. As with pressure responses, more stretch sensitive units were seen in the preparations where the nerve sheath had been opened. Thus in the peroneal CFA lesion group with the nerve sheath opened 6.5% of C-fibres and 8.1% of A-fibres responded to stretch while after saline treatment with the sheath opened 3.8% of C- and 6.2% of A-fibres became stretch sensitive (Fig. 3). All the stretch sensitive units also responded to pressure at the lesion site. The mean stretch resulting in A- and C-fibre responses was 6% (2% SD; range=3–10%; measured for 14 A- and 12 C-fibres). Fifty-four percent of units responded to less than 5% stretch across the lesion site. In early (2–3 days) and late lesions (6–10 days), there were similar numbers of stretch sensitive units.
Fig. 3.
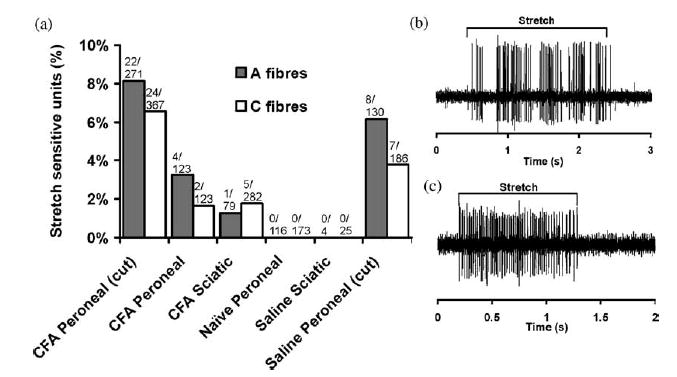
Summary of stretch responses following neuritis. (a) Proportion of A- and C-fibres responding to stretch across the lesion in the CFA-treated peroneal lesion with the sheath opened (CFA peroneal (cut)) and intact (CFA peroneal), CFA-treated sciatic lesion, untreated animals and saline-treated sciatic lesion and saline-treated peroneal lesion with the sheath opened (saline peroneal (cut)). A typical (b) A-fibre and (c) C-fibre response to stretch at the lesion site. The erratic firing in (b) is caused by small fluctuations in the amount of stretch applied to the nerve. The total number of units sampled and the number responding to stretch are shown for each group.
3.3. Conduction across the lesion site
The proportion of fibres failing to conduct beyond the lesion site was examined in the peroneal nerve experiments (Fig. 4). In CFA treated nerves with the sheath intact conduction through the lesion site was good with only 12% of A-fibres and 3% of C-fibres not being excited at a stimulus location immediately distal to the treatment zone. In untreated preparations the proportion of C-fibres that could not be excited distally was similar (3%) although the proportion of A-fibres was significantly smaller (2%, P< 0.01). In preparations where the sheath had been opened there were many fibres that did not conduct through the treatment site. In these animals, 80% of A-fibres and 33% of C-fibres stimulated immediately proximal to the CFA treatment site could not be excited by maximal stimuli applied immediately distal to it. This is a significantly higher percentage than when the sheath was intact (P<0.01 for A- and C-fibres). In the saline treated group with the sheath opened there was also a large increase in the proportions of A- and C-fibres failing to conduct through he lesion site (51% of A-fibres and 17% of C-fibres). In the CFA group with the sheath opened, there was a further increase in the number of A- and C-fibres failing to conduct through the lesion site in late lesions (7–8 days) (96% (111/116) of A-fibres) compared to early lesions (2–3 days) (73% (195/266) of A-fibres) (P<0.01). There was a similar trend for C-fibres, although not significant (40% (31/78) of C-fibres failed to conduct in late lesions compared to 31% (103/328) in early lesions (P=0.2)).
Fig. 4.
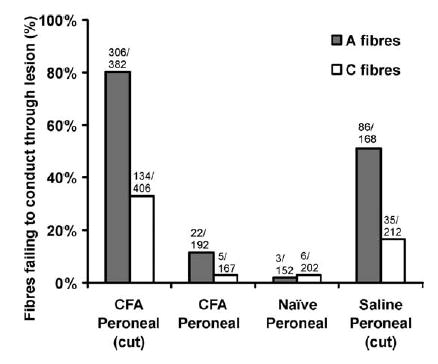
Proportion of A- and C-fibres failing to conduct through the lesion site in the CFA-treated peroneal lesioned animals with the sheath opened (CFA peroneal (cut)), intact (CFA peroneal), in untreated animals and in the saline-treated peroneal lesion with the sheath opened (saline peroneal (cut)). The total number of units sampled and the number not conducting through the lesion site are shown for each group.
With the sheath opened, approximately 54% (19/35) of C- and 77% (24/31) of A-mechanosensitive fibres could not be electrically stimulated at the distal electrodes, even though all could be excited by pressure within the treatment zone. Thus only a minority of fibres remained conducting through the lesion site when the sheath was opened. Following peroneal CFA treatment with the sheath intact, all of the A- or C-mechanosensitive fibres (0/10) could be excited from the distal electrodes, indicating that they conducted through the lesion site
Following CFA treatment (combined results with the sheath intact and opened) and in saline treated animals with the sheath opened there was no significant difference in A-fibre conduction velocity across the lesion site compared to untreated animals (summarised in Table 2). There was however a 14% reduction in C-fibre conduction velocity across the lesion site after CFA-treatment (P<0.01, Mann–Whitney test) and an 18% reduction in saline treated animals with the sheath opened (P<0.01). These changes in C-fibre conduction velocity were statistically significant (P<0.01, Mann–Whitney test). There was no significant difference in the conduction velocities of C-fibres following CFA treatment with sheath intact or cut.
Table 2.
Mean conduction velocities across the lesion site for each lesion type
| Mean conduction velocity
|
||
|---|---|---|
| Lesion type | A-fibre (SEM) (m/s) | C-fibre (SEM) (m/s) |
| CFAa | 23.2 (2.1) (n=36) | 0.69 (0.04)** (n=64) |
| Saline (sheath cut) | 24.6 (3.4) (n=10) | 0.65 (0.05)** (n=39) |
| Untreated | 23.5 (2.3) (n=26) | 0.80 (0.04) (n=35) |
Non-parametric ANOVA (Kruskal-Wallis test) showed significant differences between groups (P=0.001).
P<0.01 compared to untreated animals (Mann–Whitney test).
Pooled results for CFA animals with both intact and opened sheaths.
3.4. Driving from the periphery
Following CFA treatment there was a marked reduction in the proportion of fibres with peripheral receptive fields (Fig. 5). In CFA treated nerves with the sheath intact, 81.0% of C-fibres conducting through the lesion site and 65.9% of conducting A-fibres were without peripheral fields compared to 54.5% of C-fibres and 41.3% of A-fibres in untreated nerves (P<0.01 for C- and A-fibres). It was not possible to find fields for all units sampled in untreated animals, since a proportion of peroneal fibres are either motor or sympathetic.
Fig. 5.
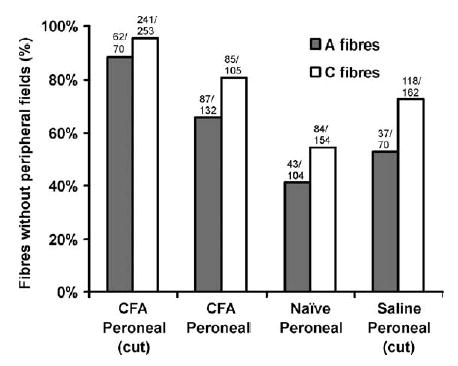
Proportion of conducting A- and C-fibres without peripheral fields in the CFA-treated peroneal lesioned animals with the sheath opened (CFA peroneal (cut)) and intact (CFA peroneal), in untreated animals and in the saline-treated peroneal lesion with the sheath opened (saline peroneal (cut)). The total number of units sampled and the number without peripheral fields are shown for each group.
Cutting the nerve sheath prior to CFA application caused a further reduction in driving from the periphery, and an increase in the proportion of fibres without peripheral fields (95.3% of C- and 88.6% of A-fibres). In the saline treated group with the sheath cut, there was a much smaller increase in the number of fibres without peripheral fields (72.8% of C- and 52.9% of A-fibres) and for this measure there was significantly less change with saline treatment than with CFA (P<0.01 for C- and A-fibres). In early (2–3 days) and late lesions (6–10 days), the proportion of units with peripheral fields was similar.
Peripheral fields for units that showed local mechanosensitive properties at the lesion site were extremely uncommon. No fields have been found for C- mechanosensitive fibres (0/77) and only one field has been found for an A-mechanosensitive fibre (1/90), a muscle spindle responding to digit flexion. Note this count is based on the total number of mechanosensitive units, including those that are blocked at the lesion site (approximately 49% of C- and 61% of A-fibres).
3.5. Spontaneous activity
Spontaneous activity was recorded in a number of C-and A-fibres following neuritis and in control animals (naïve and saline treated) (Fig. 6). In peroneal nerves, the proportion of C-fibre spontaneous activity ranged from 8.8 to 10.4%, with no significant difference between control and CFA treatment groups (P=0.9). In the Sciatic nerve lesioned animals, there was also no significant difference in the proportion of spontaneously active C-fibres from the sural nerve following CFA-treatment (10.1%; 42/414) compared to the saline-treated animals (7.1%; 10/130) (P=0.4). In peroneal nerves, the proportion of A-fibre spontaneous units ranged from 4.0 to 8.2%, with no significant difference between control and CFA treatment groups (P=0.5). In sciatic nerve lesioned animals, there was no spontaneous A-fibre activity from the sural nerve following CFA (0/136) or saline (0/107) treatment. Following CFA treatment, there was more C-fibre spontaneous activity in early lesions (2–3 days) compared to late lesions (6–10 days). In the CFA peroneal group with the sheath opened, 12.3% (39/318) of C-fibres were spontaneous in early lesions compared to 4.8% (5/104) of in late lesions (P<0.05). In the CFA sciatic group, 17.4% (21/121) of C-fibres were spontaneous in early lesions compared to 7.2% (21/293) in late lesions (P<0.01). Interestingly, following pressure testing at the lesion site, many of the mechanosensitive units developed spontaneous activity.
Fig. 6.
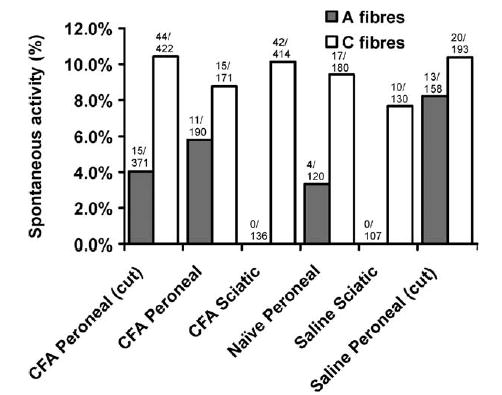
Proportion of spontaneously active A- and C-fibres following different nerve treatments or in untreated (naïve) nerves.
3.6. Light microscopic appearance of the treatment sites in the peroneal nerve
The lesion severity was ranked according to the extent of A- fibre damage (lowest rank=least damage) (for examples, see Fig. 7). There was a positive correlation between the lesion severity and the proportion of recorded fibres that could not be excited distally for both A-fibres (Spearman rank correlation coefficient (r)=0.91) and C-fibres (r=0.87) (Fig. 8). In untreated nerves where most fibres conducted distally to the treatment site, the lesion rank was low, with most sections appearing normal. In CFA treated animals with the sheath intact, the lesion rank increased with increasing proportion of fibres that could not be electrically stimulated distally. When the sheath was opened, the lesion rank was high with massive amounts of degeneration, consistent with the few fibres that could be excited distally. There was one exception in this group of animals where there was no A- or C-fibre conduction block and reassuringly, in this preparation, the lesion site also appeared normal.
Fig. 7.
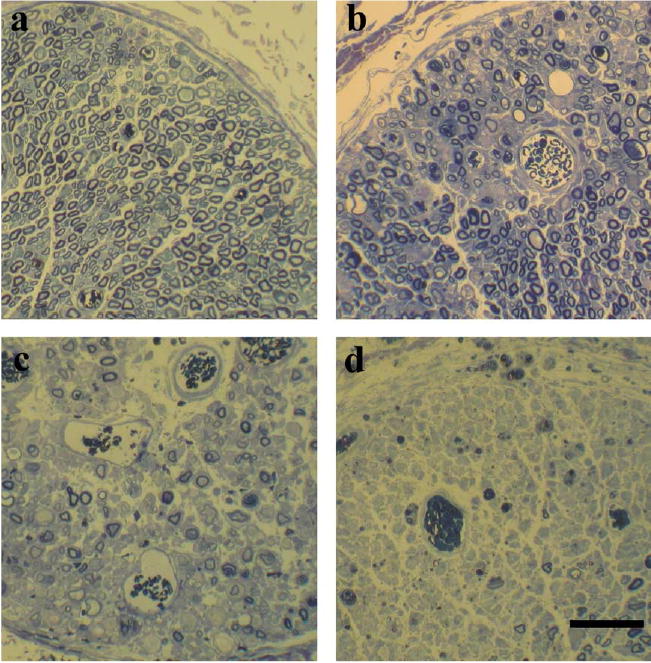
Light microscopic appearance of the peroneal nerve at the treatment site (b–d) or the equivalent part of an untreated nerve (a). Treatments were CFA with the sheath intact (b and c) and CFA with the sheath opened (d). In the untreated nerve (a) myelin profiles appear normal. In (b) the myelinated profiles appear relatively normal with only a few absent profiles, whereas in (c) myelinated profiles appear sparse. Thus (b) and (c) give an indication of the variability of changes seen from animal to animal. In most CFA-treated animals with the sheath opened, there were very few surviving myelinated profiles at the lesion site, as can be seen in (d). Bar=50 μm.
Fig. 8.
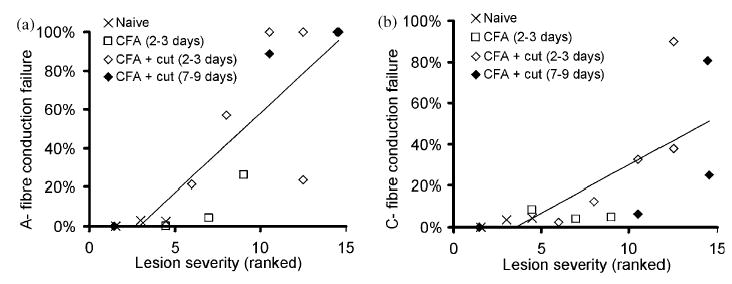
Relationship between lesion severity and A- and C-fibre conduction failure across the lesion site (Lowest rank=least damaged). The number of days post surgery is shown for each group. Trend lines have been fitted to all data points (rank correlation coefficient (r) for A-fibres=0.91, correlation coefficient (r) for C-fibres=0.87). Note that in a single CFA-treated animal with the sheath cut there was no A- or C-fibre conduction block and correspondingly this animal had one of the lowest rank values.
3.7. ATF3 expression
ATF3 expression was similar in animals that had undergone electrophysiological examination compared to those animals that did not, and therefore these groups have been combined. ATF3 was up-regulated in L4 and L5 DRG following local inflammation of intact sciatic nerves (Fig. 9). There was a significant increase in ATF3 expression in CFA treated animals (n=6; mean: 13.5% (SEM 3.1%)) compared to saline treated controls (n=7; mean: 3.6% (SEM 0.8%); P<0.05, unpaired t test). ATF3 expression following sciatic nerve transection (n=5) was significantly raised compared to the neuritis (mean ATF3 expression following sciatic nerve transection: 43.1% (SEM 5.2%); P<0.05, unpaired t test). In a single untreated animal, there was no ATF3 expression, as described previously (Tsujino et al., 2000).
Fig. 9.
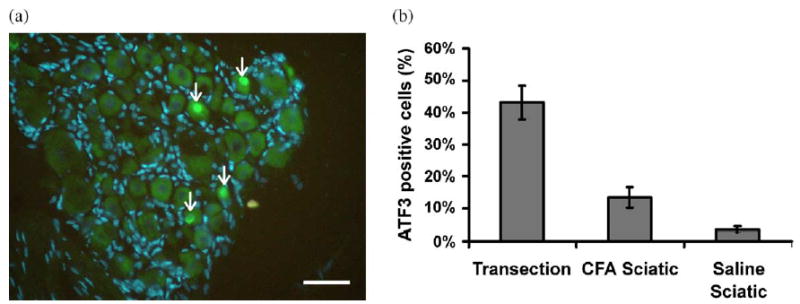
Immunofluorescence staining of DRG following CFA treatment of the sciatic nerve (a). The nuclei of DAPI positive neuronal cells appear as large circular pale blue structures compared to the smaller elongated brighter blue nuclei of the satellite cells; nuclei of ATF3 positive cells appear bright green (arrows). ATF3 and DAPI images were overlaid using Photoshop (Adobe, USA). ATF3 expression following CFA treatment was 15.3%. Scale bars=100 μm. (b) Summary of ATF3 expression following nerve transection (n=5), CFA treatment (n=6) and saline treatment (n=7). Error bars=SEM.
4. Discussion
4.1. Inflammation can cause fibres to become mechanosensitive
In the present study, inflammation of the nerve trunk caused small numbers of myelinated and unmyelinated afferent fibres to develop local mechanosensitivity to pressure and stretch. Pressure mechanosensitivity at lesion sites has been demonstrated in rats following nerve transection, chronic constrictive injury and nerve crush (Chen and Devor, 1998; Michaelis et al., 1995; Tal and Eliav, 1996; Tal et al., 1999). These lesions however, cause extensive axonal damage. More recent studies have shown that local inflammation of the nerve trunk without apparent axonal damage can cause intact A- (Bove et al., 2003; Eliav et al., 2001) and C-fibres (Bove et al., 2003) to develop pressure mechanosensitive properties at the lesion site. The present results confirm that mechanosensitivity does occur in functionally intact A- and C-fibres in locally inflamed nerves, since many of the mechanosensitive fibres found here conducted with near normal conduction velocity through the inflamed region. Mechanical damage to the nerve fibres during pressure and stretch testing was unlikely to be a cause of increased local mechanosensitivity, since none of the fibres in untreated animals developed similar properties after repeated testing.
Nerve fibres responding to stretch across the lesion site were a sub population (approximately half) of pressure mechanosensitive units. The majority of these fibres responded to less than 5% stretch, with the most sensitive fibres firing to 3% stretch. These values are within the range of nerve stretch that occurs during normal limb movements (Dilley et al., 2003).
With the nerve sheath intact only small numbers of fibres developed mechanosensitive properties, probably because most of the inflammatory response occurred away from the endoneurium at the sheath itself (Bove et al., 2003). When a small longitudinal cut was made through the perineurium prior to CFA treatment a significantly higher proportion of fibres developed mechanosensitive properties (13–19%). With the sheath cut a substantial loss of conduction across the lesion site developed (80% of A- and 33% of C-fibres) probably indicating that a lot of fibres had degenerated locally. Interestingly, opening the nerve sheath in the absence of CFA was also sufficient to cause a high proportion of fibres to develop mechanosensitive properties (10–16%) and for a substantial loss of conduction to develop (51% of A- and 17% of C-fibres). Previous studies have shown that cutting a window in the perineurium of untreated rats can cause fibres in close proximity to the window to undergo demyelination (Nukada et al., 1992; Spencer et al., 1975). The present study is the first to show changes in C-fibre function following opening of the perineurium. The causes of these changes following opening of the perineurium are unclear, although it has been suggested that changes to the nerve microenvironment (e.g. altered pressure within the nerve, changes to the endoneurial fluid or reduced endoneurial blood flow) may play a role (Nukada et al., 1992; Spencer et al., 1975). Alternatively, damage to the perineurium when it is opened might be sufficient to initiate an inflammatory response similar to the effects of CFA.
4.2. Intact and degenerated fibres can become mechanosensitive
Two populations of mechanosensitive fibres can be differentiated, those which conduct through the lesion site, and those which fail to conduct through the lesion. Mechanosensitive A- and C-fibres that do not conduct through the lesion site are likely to be axons which have degenerated distally, as also shown by histology, responding to pressure and stretch from their proximal, regenerating end. These fibres are essentially like regenerating terminals in neuromas that have been shown to be pressure and stretch sensitive (e.g. Chen and Devor, 1998; Proske et al., 1995; Tal et al., 1999). In addition, it is possible that some of these fibres are nervi nervorum fibres innervating the nerve sheath perhaps sensitized by the inflammation.
The fibres conducting through the lesion site are like those described previously as developing pressure mechanosensitivity without axonal damage (Bove et al., 2003; Eliav et al., 2001). All of the pressure units examined in the study by Bove et al. (2003) conducted through the lesion and had peripheral fields. In the present study, it was not possible to find peripheral fields for the majority of mechanosensitive units.
The growth of regenerating axonal sprouts across the lesion site might explain why a high proportion of fibres in the CFA treated animals, including the mechanosensitive units, conducted through the lesion site but lacked peripheral fields. However, conduction along regenerating axonal sprouts is slower then that along the uninjured segment of nerve fibres (e.g. following nerve transection there is a 30–40% reduction in C-fibre velocity (Lisney, 1987)). In the present study, there were no obvious signs of A-fibre conduction slowing and following CFA treatment, C-fibres were only slowed by 14%. Interestingly, mechanosensitive responses to pressure and stretch from regenerating axonal sprouts have been reported (Gorodetskaya et al., 2003; Johnson and Munson, 1991; Proske et al., 1995). This is unlikely to be a major cause of local mechanosensitivity in the fibres that conduct through the treatment site, since the majority of mechanosensitive hotspots were at the lesion site rather than distal to it. Hotspots distal to the lesion would be expected if the nerve fibres were sprouts, which can regenerate up to 3.8 mm/day (Lozeron et al., 2004).
The lack of functioning peripheral terminals in conducting fibres might have resulted from a disruption of axonal transport at the lesion site, preventing factors required for axonal survival from reaching the terminals. In these fibres, conduction through the lesion site would be preserved despite non-functioning terminals. In previous studies, local inflammation of nerves in vivo and treatment of neurites in vitro with histamine has been found to disrupt axonal transport (Amano et al., 2001; Armstrong et al., 2004).
4.3. Nerve trunk inflammation can cause widespread changes to the nerve fibres
The lack of peripheral fields in conducting fibres and the expression of ATF3 in the cell bodies suggests that local inflammation along a short segment of the nerve trunk can cause widespread changes. Disruption of the peripheral terminals of both A- and C-fibres is consistent with studies in patients with peripheral sensory neuropathies. As many as a quarter of patients with sensory neuropathies show signs of epidermal denervation but have normal nerve fibre density and sensory conduction amplitudes (Herrmann et al., 1999).
4.4. Mechanisms of nerve trunk mechanosensitivity
The accumulation of sodium channels at nerve fibre endings in neuromas is considered to play a major role in the development of spontaneous activity (Devor et al., 1993; Novakovic et al., 1998). In the present study, the accumulation of channels at the endings of degenerated fibres is probably a major cause of the local mechanosensitivity. Disruption of axonal transport in intact fibres might also result in the accumulation of channels at the lesion site leading in the development of mechanosensitive properties.
The up regulation of ATF3 in the nuclei of a small proportion of DRG cells following neuritis, suggests a change in gene expression in response to local nerve inflammation. In degenerated fibres, these changes might include the synthesis of proteins required for regeneration. It may also be related to the development of nerve trunk mechanosensitivity, for example, the synthesis of mechanosensitive channels. A number of mechanosensitive channels have been identified which are expressed in DRG neurones (e.g. acid sensing ion channel-2, ASIC2, and dorsal root acid sensing ion channel, DRASIC). The up-regulation of such channels and their subsequent accumulation at the lesion site may be a cause of local mechanosensitivity.
4.5. Clinical implications
Inflammation of nerve trunks could play a major role in many widespread painful conditions such as NSAP, CTS and also chronic back pain from nerve root irritation, and other neuropathic conditions such as complex regional pain syndrome type 1 where there is often no obvious physical nerve injury (Janig and Baron, 2003). In these conditions, inflammation could cause widespread changes to nerve fibres resulting in increased mechanosensitivity as well as dysfunction at the peripheral terminals. Both intact and damaged fibres can become mechanosensitive, and it is likely to be these fibres that contribute to mechanosensitive symptoms, including nerve trunk tenderness and painful responses to nerve stretch during joint movements. Although the percentage of fibres that develop mechanosensitive properties appears small, 4.0% of all C-fibres is in fact a large number of units, especially when most of these are likely to be nociceptive.
Acknowledgments
We would like to thank Dr Jonathan Gale for his help with the immunohistochemistry and Professor Martin Koltzenburg for his helpful advice. This work was supported by the Wellcome Trust (068691).
References
- Amano R, Hiruma H, Nishida S, Kawakami T, Shimizu K. Inhibitory effect of histamine on axonal transport in cultured mouse dorsal root ganglion neurons. Neurosci Res. 2001;41:201–6. doi: 10.1016/s0168-0102(01)00275-9. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Lee M, Chhith S, Gomariz RP, Waschek JA. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience. 2004;29:93–9. doi: 10.1016/j.neuroscience.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Bove GM, Ransil BJ, Lin HC, Leem JG. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol. 2003;90:1949–55. doi: 10.1152/jn.00175.2003. [DOI] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–44. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Devor M. Ectopic mechanosensitivity in injured sensory axons arises from the site of spontaneous electrogenesis. Eur J Pain. 1998;2:165–78. doi: 10.1016/s1090-3801(98)90009-x. [DOI] [PubMed] [Google Scholar]
- Devor M, Govrin-Lippmann R, Angelides K. Na+ channel immunolocalization in peripheral mammalian axons and changes following nerve injury and neuroma formation. J Neurosci. 1993;13:1976–92. doi: 10.1523/JNEUROSCI.13-05-01976.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley A, Lynn B, Greening J, DeLeon N. Quantitative in vivo studies of median nerve sliding in response to wrist, elbow, shoulder and neck movements. Clin Biomech (Bristol Avon) 2003;18:899–907. doi: 10.1016/s0268-0033(03)00176-1. [DOI] [PubMed] [Google Scholar]
- Eliav E, Herzberg U, Ruda MA, Bennett GJ. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. Pain. 1999;83:169–82. doi: 10.1016/s0304-3959(99)00102-5. [DOI] [PubMed] [Google Scholar]
- Eliav E, Benoliel R, Tal M. Inflammation with no axonal damage of the rat saphenous nerve trunk induces ectopic discharge and mechanosensitivity in myelinated axons. Neurosci Lett. 2001;311:49–52. doi: 10.1016/s0304-3940(01)02143-7. [DOI] [PubMed] [Google Scholar]
- Gorodetskaya N, Constantin C, Janig W. Ectopic activity in cutaneous regenerating afferent nerve fibers following nerve lesion in the rat. Eur J Neurosci. 2003;18:2487–97. doi: 10.1046/j.1460-9568.2003.02974.x. [DOI] [PubMed] [Google Scholar]
- Greening J, Lynn B. Minor peripheral nerve injuries: an understanding source of pain? Man Ther. 1998;3:187–94. [Google Scholar]
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–40. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- Janig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol. 2003;2:687–97. doi: 10.1016/s1474-4422(03)00557-x. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Munson JB. Regenerating sprouts of axotomized cat muscle afferents express characteristic firing patterns to mechanical stimulation. J Neurophysiol. 1991;66:2155–8. doi: 10.1152/jn.1991.66.6.2155. [DOI] [PubMed] [Google Scholar]
- Lisney SJ. Functional aspects of the regeneration of unmyelinated axons in the rat saphenous nerve. J Neurol Sci. 1987;80:289–98. doi: 10.1016/0022-510x(87)90163-8. [DOI] [PubMed] [Google Scholar]
- Lozeron P, Krarup C, Schmalbruch H. Regeneration of unmyelinated and myelinated sensory nerve fibres studied by a retrograde tracer method. J Neurosci Methods. 2004;138:225–32. doi: 10.1016/j.jneumeth.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Lynn B, Greening J, Leary R. Sensory and autonomic function and ultrasound nerve imaging in RSI patients and keyboard workers. CRR 417/2002. 2002. Health and Safety Executive.
- Michaelis M, Blenk KH, Janig W, Vogel C. Development of spontaneous activity and mechanosensitivity in axotomized afferent nerve fibers during the first hours after nerve transection in rats. J Neurophysiol. 1995;74:1020–7. doi: 10.1152/jn.1995.74.3.1020. [DOI] [PubMed] [Google Scholar]
- Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18:2174–87. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukada H, Powell HC, Myers RR. Perineurial window: demyelination in nonherniated endoneurium with reduced nerve blood flow. J Neuropathol Exp Neurol. 1992;51:523–30. doi: 10.1097/00005072-199209000-00007. [DOI] [PubMed] [Google Scholar]
- Proske U, Iggo A, Luff AR. Mechanical sensitivity of regenerating myelinated skin and muscle afferents in the cat. Exp Brain Res. 1995;104:89–98. doi: 10.1007/BF00229858. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Weinberg HJ, Raine CS, Prineas JW. The perineurial window—a new model of focal demyelination and remyelination. Brain Res. 1975;96:323–9. doi: 10.1016/0006-8993(75)90742-8. [DOI] [PubMed] [Google Scholar]
- Tal M, Eliav E. Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain. 1996;64:511–8. doi: 10.1016/0304-3959(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Tal M, Wall PD, Devor M. Myelinated afferent fiber types that become spontaneously active and mechanosensitive following nerve transection in the rat. Brain Res. 1999;824:218–23. doi: 10.1016/s0006-8993(99)01190-7. [DOI] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–82. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]


