Abstract
Purpose
This study contrasts language lateralization between pediatric epilepsy patients and healthy children.
Methods
Eighteen epilepsy patients (8–18 years of age) completed an fMRI study with a silent verb generation task. The imaging data was compared to 18 age-gender-handedness matched healthy children and also to a group of 336 healthy children age 5–18 years.
Results
A significant difference in hemispheric lateralization index was found (t=6.278, p<0.0001) between children with epilepsy (mean LI =−0.017) and the age-gender-handedness matched healthy control subjects (mean LI = 0.267). A dramatic difference was also observed in the percentage of children with epilepsy (67.7%) that had atypical lateralization index (right-hemispheric or bilateral, LI<0.1) when compared with the age-gender-handedness matched group (0%, χ2=22.9, p<0.001) or to the entire group of healthy children (18.5%, χ2=35.7, p<0.001). A linear regression analysis showed that the hemispheric lateralization of language function increased with age (R2=0.09, p<0.0001) in the entire healthy control group, while this correlation with age was not significant in pediatric epilepsy subjects (R2=0.005, p>0.7). The residual analysis for the linear regression showed a greater variability for LI in epilepsy patients (residual SD =0.15) than that observed in healthy cohorts (0.11 and 0.13 for the age-gender-handedness matched and the entire healthy children group, respectively).
Conclusions
This study shows that epilepsy during childhood is associated with neuroplasticity and reorganization of language function.
Keywords: fMRI, language development, pediatric, epilepsy, lateralization index
Introduction
Modern studies of language localization and lateralization began with the first reports by Broca and Wernicke, who introduced the concept of unilateral left hemispheric control of language functions (1,2). Since then, the localization of language processing in healthy and diseased brain has become the subject of intense research. The “classical model” of language organization based on data from aphasic patients with brain lesions, popularized in the 19th century, remains in common use (3–5). The general principle of this “classical model” is supported by studies of individuals who have lost language secondary to focal brain lesions. In addition to lesion studies, other studies such as neuropsychological studies, direct electrocortical stimulation experiments, and imaging studies have all found that language functions are primarily distributed in the inferior frontal gyrus (Broca’s area) and in the superior and middle temporal gyri, supramarginal and angular gyri (Wernicke’s area) with strong left hemispheric dominance (3, 6–17). More recently, this left dominant pattern has been documented even during infancy (18).
It is known that neurological disorders, such as stroke, brain tumors, and, as related to the present study, epilepsy, can have a profound and chronic impact on language function (e.g., 3, 19–21). Adult epilepsy patients often demonstrate significant changes in terms of the location, strength, and variability of language activation (3, 22). “Atypical” language distribution patterns have also been observed in many fMRI studies of adults (23–34). One comprehensive fMRI study (22) that compared language dominance in a large number of adult subjects (100 healthy control subjects and 50 epilepsy subjects) found that a significantly larger percentage of epilepsy patients had bilateral or right-hemispheric language dominance than that observed in healthy control subjects. In that study and for our purpose here, bilateral language lateralization is defined by an fMRI lateralization index (LI) that is close to zero (|LI| < 0.1 in our study), representing nearly equal amount of activation in the left and right hemispheres. A more recent fMRI study also showed similar findings: in comparison to healthy subjects, those adults with left temporal lobe epilepsy (TLE) tended to have greater language activation in right hemisphere and their overall language representation was more bilateral (35).
Although the current understanding of the impact of epilepsy in adults is far from conclusive, the above studies provide a degree of quantification of the differences in language localization and lateralization between healthy adult controls and adult epilepsy subjects and have offered plausible explanations for the etiology of these differences. At this point, however, we do not know when atypical language circuitry begins to emerge during the course of epilepsy. In fact, it is not yet known whether reorganization of language in the epileptic brain is stimulated by the repeated seizure activity or whether pre-existing neuropathology provides a common cause for the epilepsy and the redistribution of language.
The differences in the neural substrates of language between healthy children and children with epilepsy are even less well documented. Aside from the technical difficulties involved in the MR scanning of children (36), the fact that language develops rapidly during childhood adds an additional level of complexity to the effort of distinguishing neuropathological changes from normal language developments. Language activation patterns in healthy children, even as early as infancy, are believed to be distributed in a similar pattern to their adult counterparts (e.g., 18, 37–39). In addition to variables such as gender, handedness, and other factors that need to be considered in adults, studies have shown that age plays an important role in determining language activation patterns and lesion-related neuroplasticity (e.g., 38, 40). Controversy still exists regarding a specific cut-off age after which language development complete (22,41–43). Some studies suggest that language development may not be complete until the third decade of life (44, 45).
The convolution of epilepsy and development, combined with other factors, makes it extremely difficult to distinguish the actual impact of epilepsy on the neural substrates of language based only on language function distribution patterns observed in pediatric epilepsy patients. Due to the limitation in the control of experimental condition in clinical research, the question of cause and effect remains. Does repeated seizure activity in epilepsy cause the reorganization of language in the developing brain, or, does epilepsy result from underlying neuropathology, which, although invisible by neuroimaging, causes early redistribution of language? Studies in animals suggest this first question is closer to the answer. There is evidence showing that seizures in developing mouse brain can cause immediate neural cell loss, synaptic circuitry reorganization, as well as long-term neurological consequences such as lowering of seizure threshold, impaired learning capacity, and cognitive deficit in later life (46–51).
A logical first step to investigate this question in humans would be to make group comparisons to quantitatively contrast fMRI imaging results between pediatric epilepsy patients and healthy control subjects. Based on the extent to which these patterns differ, it might be possible to extrapolate further information about the relationship of the abnormal patterns and epilepsy-related neuroplasticity in the pediatric patients. Surprisingly, no such study has been reported to date. The published fMRI literature about the impact of epilepsy on language lateralization in children has either been clinical research that focused on evaluating the accuracy of pre-surgical and post-surgical fMRI assessment, or, studies comparing the language organization patterns among epilepsy patients (31, 52–55). No fMRI study thus far has reported about the differences between pediatric epilepsy patients and healthy children.
The lack of normative MRI/fMRI data for children has been a major factor for the absence of direct comparison between patients and healthy controls. While some previous fMRI studies have collected normative data in healthy children, they either included a small number of subjects (38, 39, 56–58), or they combined pediatric and adult subjects in the study and data analysis (25, 53).
Recently we have completed a large-scale fMRI study of language development (NIH-R01-HD38578-05) that provides a reference frame for better understanding neuroimaging findings in pediatric patients. This study investigated language localization and lateralization and other trends in language function in over 300 healthy children, age 5 to 18 years. This study provides strong evidence that the cerebral distribution of language function changes with age, exhibiting patterns that are influenced by development as well as other factors including gender, handedness, IQ, SES, etc. (38, 40). These discoveries shed light on previous ambiguity in the literature which was due in part to the lack of consideration of brain development as a confounding factor influencing hemispheric dominance of language. Most importantly, our normative data can now serve as a reference for the interpretation of fMRI language studies in pediatric patients with various neurological disorders, e. g., brain injury, lesion, trauma, and here, epilepsy.
In this study, we present fMRI data obtained from children with epilepsy and examine the patterns of language lateralization (Lateralization Index: LI) among these patients by comparing with patterns obtained in healthy control subjects. We were also interested in studying the influence of epilepsy on the correlation between LI and age in children. We expected to find significant differences in LI between patients and healthy controls and a weaker correlation with age, if any, in patients than in the healthy counterparts. Our approach is unique in that we contrasted fMRI activation in pediatric epilepsy patients with a large group of healthy control subjects (n=336, age=5–18 years). As a subgroup of this large healthy cohort, an equal number (n=18) of age-gender-handedness matched healthy subjects were also selected for comparison with the patients. These comparisons provide new insight into the growth trend of language lateralization and the extent to which the brain reorganizes language function to compensate for damage associated with epilepsy during childhood.
Methods
This is a retrospective study in which the fMRI data for the patients and healthy subjects were obtained on two different MR scanners at different time periods between 1997 and 2004. Some of the data has been reported previously (36, 38, 40, 59, 60). Since fMRI data acquisition techniques have evolved and been refined over these years, differences in methodology will be clarified whenever is necessary. The fMRI data from epilepsy patients and healthy control subjects were analyzed using identical methodology in order to minimize cross-platform variability. For example, a region-of-interest (ROI)-based objective-thresholding method (described below) was used to calculate a lateralization index (LI), providing a data analysis scheme that was robust and portable across scanners and field strength.
Subjects
Twenty three pediatric patients (age 8–18 years) diagnosed with epilepsy by a pediatric neurologist (RHS) were recruited for an fMRI language study. FMRI data from a silent verb generation task (described below) was successfully obtained from 18 of these patients (age 8–18 years). Patient information is summarized in Table 1. These patients were recruited irrespective of their race, gender, epilepsy syndrome, lesion site, or handedness. After a complete description of the study was given, written consent was obtained from the parents, and the subjects gave either verbal or written consent. This study was approved by the Institutional Review Board at the Cincinnati Children’s Hospital Medical Center (CCHMC).
Table 1.
The summary about the information in the pediatric epilepsy patients and the age-gender-handedness matched healthy controls. LI: Lateralization Index; AVM: Arteriovenous Malformation; BRE: Benign Rolandic Epilepsy; ENCEPH: Encephalomalici
| Sex | Handedness | Age (in Months) (Patient/Matching) | LI (Patient) | MRI Structure (Patient) | Epilepsy Diagnosis (Patient) | LI (Matching ) | |
|---|---|---|---|---|---|---|---|
| 1 | M | L | 101/98 | −0.165 | L HIPPOCOMPAL ATROPHY | PARTIAL L | 0.135 |
| 2 | F | R | 111/111 | 0.010 | R FRONTAL ATROPHY | PARTIAL R | 0.188 |
| 3 | M | R | 133/134 | 0.013 | NORMAL | IDIOPATHIC GENERALIZED | 0.325 |
| 4 | F | R | 136/137 | 0.064 | R TEMPORO-PARIETAL DYSPLASIA | PARTIAL R | 0.256 |
| 5 | F | R | 141/141 | 0.172 | NORMAL | PARTIAL R | 0.203 |
| 6 | M | L | 141/147 | −0.342 | L OCCIPITAL ENCEPH | PARTIAL L | 0.367 |
| 7 | M | R | 149/149 | −0.108 | NORMAL | BRE | 0.154 |
| 8 | M | R | 150/150 | −0.101 | NORMAL | PARTIAL, 2ND GEN | 0.138 |
| 9 | F | R | 168/167 | 0.290 | NORMAL | PARTIAL | 0.149 |
| 10 | M | R | 176/174 | 0.026 | N/A | N/A | 0.396 |
| 11 | M | R | 176/179 | −0.025 | L TEMPORAL GLIOMA | PARTIAL L | 0.211 |
| 12 | F | R | 177/177 | −0.051 | R TEMPORO-PARIETAL MIGR | PARTIAL R | 0.210 |
| 13 | M | R | 179/179 | 0.144 | NORMAL | PARTIAL R, 2ND GEN | 0.565 |
| 14 | F | R | 183/182 | −0.172 | NORMAL | IDIOPATHIC GENERALIZED | 0.315 |
| 15 | M | R | 194/198 | 0.194 | NORMAL | PARTIAL | 0.326 |
| 16 | M | R | 208/208 | −0.090 | NORMAL | PARTIAL R | 0.361 |
| 17 | M | L | 219/218 | −0.025 | L TEMPORAL AVM | PARTIAL L | 0.336 |
| 18 | F | R | 228/227 | −0.155 | NORMAL | PARTIAL R | 0.171 |
|
Pediatric Patients
No. of Subjects: 18 No. of Male: 11, No. of Female: 7 No. of Left Handedness: 3 No. of Right Handedness: 15 Age in months: Mean = 165, SD = 35.15 LI: Mean=−0.018, SD=0.154 |
Matching Controls
No. of Subjects: 18 No. of Male: 11, No. of Female: 7 No. of Left Handedness: 3 No. of Right Handedness: 15 Age in months: Mean = 164.83, SD = 35.03 LI: Mean=0.267, SD=0.115 |
||||||
The control group consisted of 336 healthy children (age = 5–18 years, 166 males, 170 females) who were part of a large-scale fMRI language development study (NIH-R01-HD38578-05). The description of this cohort has been presented in previous publications (36, 38, 40, 59, 60). As part of our data comparison, a subgroup was selected from this large healthy control group. This smaller healthy control group consisted of 18 subjects and was determined as follows: for each pediatric epilepsy patient, one healthy child with the same gender, handedness, and with the closest age in months was selected from the entire healthy control subjects group (N=336). This age-gender-handedness matched healthy control group was used to demonstrate the contrast between patients and healthy controls with the presumption that matching the subjects would eliminate the confounding influences of age, gender, and handedness on language development and allow us to focus the analysis on other factors including language lateralization and localization.
Verb Generation Task and Language Paradigm in fMRI Scanning
The language task used in the study was a child-friendly word-fluency paradigm based on the verb generation task introduced by Peterson (61) and Benson et al. (62). This task involves the presentation of a series of concrete nouns to the subject via a MRI-compatible audio system. The subjects were asked to covertly generate verbs corresponding to the presented noun. For example, when the subjects heard the noun “ball”, they would think of the verbs like “kick”, “play”, “hit”, etc. The subjects were instructed to generate the verbs silently to avoid head motion artifacts that could result from overt speech. This language task has been used consistently by our group for the past ten years. Additional details about the task are described in our earlier publications (38, 40).
The language paradigm was administered in a periodic block design where 30 seconds of verb generation was inter-leaved with 30 seconds of control task. The control task for healthy children was bilateral finger tapping cued by a tone at 5-second interval (FM tones centered on 400 Hz with 25% modulation), while patients were instructed to remain in silence during this period of the experiment. Functional MRI scanning proceeded during the alternating periods of activation and control at a rate of one EPI (Echo-planar Imaging) acquisition every 3 seconds. In healthy children, a total of 110 time points were acquired during five activation periods and six control periods, resulting in a total fMRI acquisition time of 330 seconds. The first ten image volumes of control periods were discarded in post-processing to eliminate non-equilibrium effect. In epilepsy patients, the initial control period was not included and only 100 EPI images (five activation periods and five control periods were acquired with scanning time being 300 seconds in total.
Imaging Data Acquisition
MRI/fMRI scans for epilepsy patient group were performed on a 1.5 Tesla, GE Signa Horizon MRI scanner with Echospeed gradients. Each fMRI scan consisted of 100 single-shot EPI gradient echo images acquired with TR/TE = 3000/40 msec, FOV=220 × 220 mm, matrix = 64 × 64. Six sagittal slices were acquired in each hemisphere for each time point, leading to a total of 1200 slices of fMRI data. Slice thickness was 5 mm with a 1 mm gap. The slices were positioned such that the outermost slice on each hemisphere extended to the most lateral aspect of the temporal lobe, as illustrated in Figure 1. A T2-weighted image was obtained in the same location for anatomical superposition. T2-weighted 2D axial images and T1-weighted whole brain sagittal images were also acquired to define the parameters necessary for the transformation into Talairach reference frame.
Figure 1.
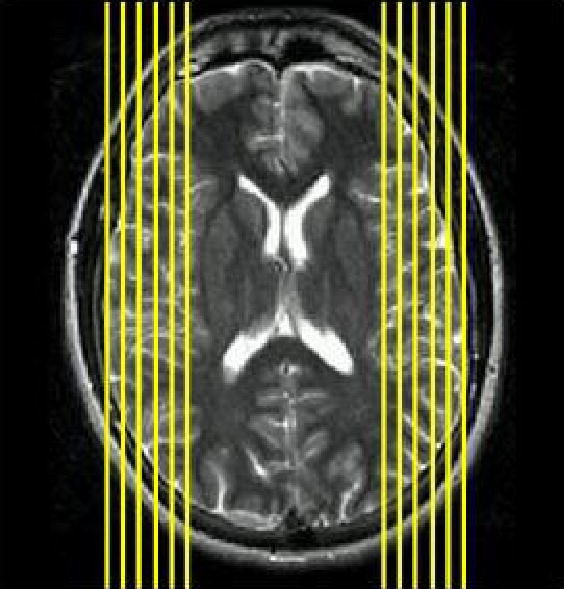
The axial view of slice selection in the MR imaging on 1.5 T GE scanner for pediatric patients with epilepsy. Only the pixels on slices with |x|>35 on Talaraich coordinate were included for the calculation of lateraization index.
MRI/fMRI scans for healthy control subjects were performed on a 3T Bruker Biospec 30/60 MRI scanner (Bruker Medizintechnik, Karsruhe, Germany). The whole brain anatomical images were obtained using a 3D MDEFT (Modified Driven Equilibrium Fourier Transform) in axial plane (63, 64) with the parameters as follows: TR/TE/tau = 15.7/4.3/550 msec, FOV = 192x256x160 mm, matrix = 256 × 192 × 128. Functional MRI scans were acquired in the transverse plane using a T2* weighted, gradient-echo, EPI sequence (TR/TE = 3000/38msec, FOV = 25.6cm × 25.6cm, matrix = 64x64, slice thickness = 5 mm). Each scan consisted of 24 slices.
The Differences in fMRI Data Acquisition
As noted earlier, one of the limitations in this retrospective study comes from the fact that the fMRI data for epilepsy patients and healthy controls were collected using different MR scanners with different magnetic field strength. Understanding that this is not an ideal situation, we believe the comparison is still valid since the calculation of lateralization index (as outlined in the following sections) is based on a ratio of t-statistics in the two hemispheres for the same individual and in the same scan. By definition, the lateralization index (LI) represents a relative hemispheric difference for an individual that is self-normalizing in terms of relative BOLD activity corresponding to the neuronal recruitment necessary to perform the language task. As noted later, the brain areas included for LI calculation were determined so that they were consistent in the two populations while still conforming to the traditional standard of ROI selection for language lateralization. Every effort has been made to insure that the methodologies applied in the LI calculation of two subject groups are compatible.
fMRI Post-processing and Data Analysis
FMRI image post-processing was conducted using Cincinnati Children’s Hospital Image Processing Software (CCHIPS) developed in the Imaging Research Center at CCHMC in the IDL environment (Research Systems Inc., Boulder, CO). A Hamming filter was applied in k-space data prior to Fourier transformation to reduce truncation artifacts and high frequency noise (65). Then, the images were co-registered to reduce the influence of motion artifact based on a pyramid co-registration algorithm developed by Thevenaz and Unser (66). Thereafter, a quadratic baseline correction algorithm was used to correct baseline drift (67, 68). Finally, Talairach transformation was performed on each subject’s anatomical and functional data to put them in a common reference frame for further data analysis.
On a pixel-by-pixel basis, t-statistics were calculated after grouping the time series data into active and control states. A hemispheric lateralization index was calculated for each subject by first counting the activated pixels within the pre-defined region of interest (ROI). For epilepsy patients and healthy control subjects, the ROIs used for the calculation included all pixels with Talairach coordinate |x|>35 in either hemisphere. Identical ROIs were used for data processing and analysis in both epilepsy patients and healthy control subjects for consistency. A threshold was determined by calculating the mean value of the t-statistics for all pixels within the ROIs. The number of pixels exceeding this threshold was counted for both the left and right side ROI. The lateralization index was calculated as follows: LI = (∑NL−∑NR)/(∑NL+∑NR), where ∑NL and ∑NR represented the sum of the fMRI pixels that exceeded the threshold for the left and right hemispheric ROI, respectively. Calculating the LI in this manner avoided the biases introduced by arbitrary thresholding and clustering schemes, as well as possible differences in BOLD contrast-to-noise ratio between the two scanners operating at different field strength. Both features were crucial for the present study to insure the consistency in our between-group data analysis.
This approach yields LIs that range between −1 (right-sided activation only/maximum right hemispheric dominant) and 1 (left-sided activation only/maximum left dominant). Values close to “0” (i.e., −0.1 ≤ LI ≤ 0.1) define bilateral language distribution (38, 64). A subject with LI>0.1 is categorized as left dominant, while a subject with LI<−0.1 is categorized as right-side dominant.
Results
For both pediatric epilepsy patients and healthy children, cortical activation during the verb generation task was observed in all subjects in the classical language areas including the inferior frontal gyrus, superior and middle temporal gyri, angular gyrus, and supramarginal gyrus. In general, brain activation in Broca’s area and Wernicke’s areas are stronger and more concentrated in healthy children than that in children with epilepsy.
Several notable differences are evident in the quantitative subject group comparison of lateralization index (LI). As shown in Table 1, among 18 children with epilepsy, eight patients (44.4%) had bilateral language distributions (−0.1< LI < 0.1); six patients (33.3%) had right-side language dominance (LI < −0.1); only four patients (22.2%) demonstrated left-side language dominance (LI > 0.1). In contrast, all 18 age-gender-handedness matched healthy children (100%) were categorized as left dominant for language (LI>+0.1). In the entire healthy subject group (N=336), the majority (274 children, 81.6%) were left dominant with only a small portion being right-side dominant (7 subjects, 2.1%) or bilateral (55 subjects, 16.4%). The frequency of atypical language lateralization was significantly between the patient group and the age-gender-handedness matched healthy control group (χ2=22.9, p<0.001) and between the patient group and the entire healthy group (χ2=35.7, p<0.001). The Welch Modified Two-sample t-test showed that the mean LI for the epilepsy patients was −0.018 (SD=0.154, n=18), a value significantly different (t=6.278, P<0.0001) when compared with the age-gender-handedness controlled healthy children (Mean LI=0.267, SD=0.115, n=18). The average LI value for the epilepsy patients was also significantly different (t=6.294, p<0.0001) from the entire healthy subject group (Mean LI=0.216, SD=0.135, n=336). This is our most striking finding and is also reflected in the graph of Figure 2.
Figure 2.
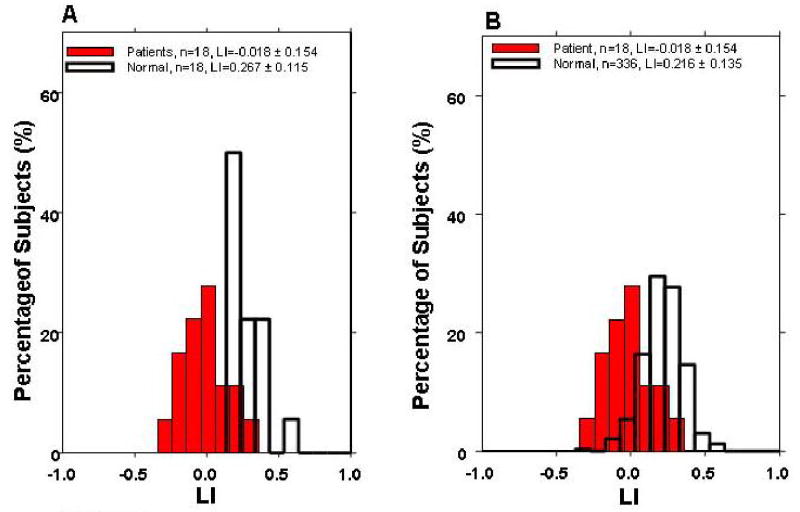
Comparison of LI for (A) pediatric epilepsy patient (n-18) vs. age-gender-handedness matched healthy control subjects (n=18) and (B) pediatric epilepsy patients (n=18) vs. the entire healthy control subject group (n=336).
Figure 2 shows percentage histograms comparing the distribution of LI values from 18 epilepsy patients with A) the age-gender-handedness matched healthy subjects (N=18), and B) with the whole healthy children group (N=336). In both Figure 2A) and 2B), the lateralization indices from the two subject groups form two distinct distributions with leftward shift on the histogram for the epilepsy patients by approximately 0.2 – 0.3. This shift suggests relatively more language activation in the right hemisphere for the pediatric epilepsy patients than healthy controls.
A linear regression model was used to examine the relationship between lateralization index (LI) and age. As shown in Figure 3, the LI in the healthy control subjects (N=336) was found to increase with age (R2=0.09, p=1.01x10−8), which was consistent with our previous reports (38, 40). A correlation with similar strength was also found in the age-gender-handedness matched healthy children group (R2 =0.12) but the correlation in this smaller group of health children did not reach statistical significance (p=0.16). No correlation between LI and age was found for the epilepsy patients (R2=0.005, p>0.7). Comparison of residuals from the linear regression in the three cohorts found all three groups were unbiased with a mean value of zero. No increasing or decreasing spread about the regression line was observed as the age increased. The standard deviation of the residual for the children with epilepsy (SD=0.15) was larger than both the age-gender-handedness matched healthy children group (SD=0.11) and the entire healthy children group (SD=0.13).
Figure 3.
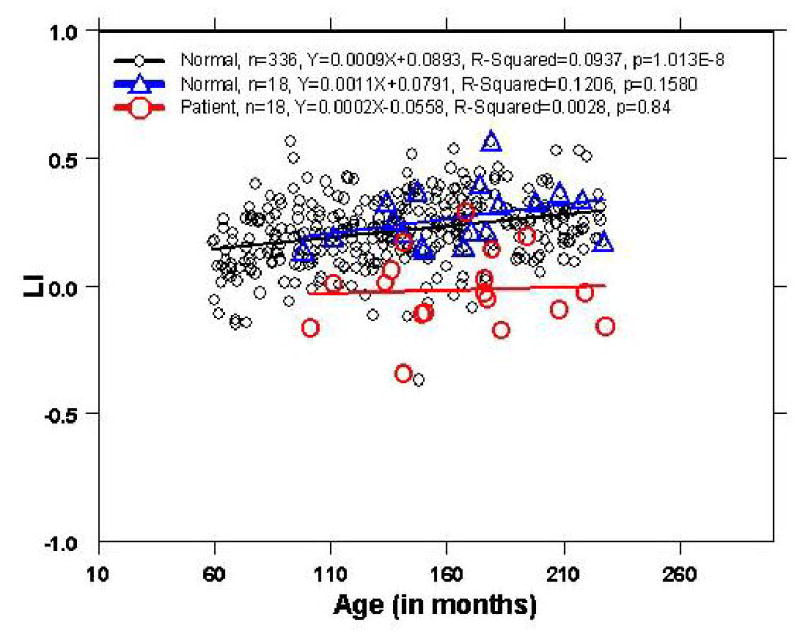
The linear regression for the pediatric patients with epilepsy (n=18), the age-gender-handedness matched healthy group (n=18), and the entire healthy group (n=336).
Discussion
The neuroplasticity of language function associated with childhood epilepsy has been a long-term interest in our research since it can serve as a model that allows for examination of the effect of chronic injury on brain development. This study focuses on using the lateralization index computed from fMRI images to quantify the influence of childhood epilepsy on the re-distribution of language functions.
Our results demonstrate that the language distribution patterns in epilepsy patients differ significantly from those found in healthy control subjects in terms of the mean value and the variability of lateralization index. Similar to the findings in adults (22, 35), we observed that pediatric epilepsy patients have higher percentage of atypical (bilateral or right hemispheric) language dominance than healthy controls. These findings suggest that the recurrent ictal and/or interictal activity in epilepsy subjects causes redistribution of language function in the developing brain to compensate for injury to traditionally left dominant language areas or connections to them (i.e., cortical plasticity). Alternatively, our results could be interpreted as suggesting that preexisting brain abnormalities leading to the development of epilepsy cause aberrant language localization/lateralization.
Consistent with our previous findings (38, 40), the increasing of left language lateralization in healthy children was found to be associated with the increase of age. Even though the relationship between LI and age may not be as simple as being purely linear, the linear regression result (Figure 3) shows the general trend in LI along with age throughout childhood and adolescence. Younger healthy children are more likely to have atypical (relative to adult findings) language distribution than older healthy children, indicating an increasing specialization of language functions to the left hemisphere as age increases. Yet, a similar relationship was found in our epilepsy patients. This is likely due to the limited number of subjects within the group, as in the age-gender-handedness matched healthy children group. Nevertheless, a closer examination revealed that, even though the correlation between LI and age in age-gender-handedness matched healthy group was not significant, this group actually had a slightly higher correlation coefficient between LI and age than the entire healthy children group (Figure 3). The distribution of LI along the regression line in the matching group (N=18) followed very closely with the LI distribution of entire healthy group (N=336). It should also be noted that this group had a tighter LI distribution than the patient group as evidenced in Figure 3, as well as by the standard deviations of the residuals of the linear regression. From the perspective of neural development, the lack of correlation of LI with age in epilepsy patients suggests a disruption caused by epilepsy to the normal specialization and consolidation of language functional areas in the normal developmental process in which the convergence toward certain preferred areas (such as Brocas’area and Wernicke’s area in left hemisphere) coincides with age in healthy controls. Another possible explanation is that the damage caused by epilepsy stimulates the brain to either activate pre-existing connections to the dormant language functional areas or to develop new areas in the right hemisphere to compensate for the loss in the left hemisphere language centers.
Similar to the conclusion from adult studies, our research shows that, LI can serve as a simple yet robust method for demonstrating differences in brain activation patterns between epilepsy patients and healthy control subjects during development. The question remains as to whether atypical language lateralization in epilepsy patients is due to the chronic effect of seizures on the brain, or whether preceding brain pathologies cause both the atypical lateralization and the epilepsy. Due to the limitations of this study in terms of the variability in seizure onset age, inhomogeneous epilepsy pathologies, and lesion site, as well as other confounding factors, we were not able to establish a conclusive cause-and-effect relationship between the atypical lateralization and epilepsy. Further longitudinal study, such as a design that involves pediatric subjects with new onset seizures, will be necessary to help clarify the role and consequence of epilepsy on neuroplasticity in language development.
Acknowledgments
This study is supported in part by NIH grant RO1-HD38578-05 (SKH).
References
- 1.Broca P. Remarques sur le siege de la faculte du langage articule; suivies d'une observation d'aphemie. Bull Soc Anat Paris. 1861;6:398–407. [Google Scholar]
- 2.Wernicke C. The symptom of complex aphasia. Diseases of the nervous system. A. E. Church. New York, 1911: Appleton: 265–324.
- 3.Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;30(299):355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- 4.Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985;42(5):428–59. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- 5.Loring DW, Meador KJ. Cerebral language lateralization: evidence from intracarotid amobarbital testing. Neuropsychologia. 1990;28(8):831–8. doi: 10.1016/0028-3932(90)90007-b. [DOI] [PubMed] [Google Scholar]
- 6.Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–77. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Roux FE, Boulanouar K, Lotterie JA, Mejdoubi M, LeSage JP, Berry I. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52(6):1335–45. doi: 10.1227/01.neu.0000064803.05077.40. discussion 1345–7. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13(2):170–7. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- 9.Luke KK, Liu HL, Wai YY, Wan YL, Tan LH. Functional anatomy of syntactic and semantic processing in language comprehension. Hum Brain Mapp. 2002;16(3):133–45. doi: 10.1002/hbm.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuest J, Karbe H. Cortical activation studies in aphasia. Curr Neurol Neurosci Rep. 2002;2(6):511–5. doi: 10.1007/s11910-002-0038-x. [DOI] [PubMed] [Google Scholar]
- 11.Nakai T, Matsuo K, Kato C, Matsuzawa M, Okada T, Glover GH, Moriya T, Inui T. A functional magnetic resonance imaging study of listening comprehension of languages in human at 3 tesla-comprehension level and activation of the language areas. Neurosci Lett. 1999;19(2631):33–6. doi: 10.1016/s0304-3940(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 12.Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. Neurol Neurosurg Psychiatry. 1998;64(4):492–8. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13(2):170–7. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- 14.Binder JR. Neuroanatomy of language processing studied with functional MRI. Clin Neurosci. 1997;4(2):87–94. [PubMed] [Google Scholar]
- 15.Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17(1):353–62. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojemann GA. Cortical organization of language. J Neurosci. 1991;11(8):2281–7. doi: 10.1523/JNEUROSCI.11-08-02281.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strauss E, Wada J. Lateral preferences and cerebral speech dominance. Cortex. 1983;19(2):165–77. doi: 10.1016/s0010-9452(83)80012-4. [DOI] [PubMed] [Google Scholar]
- 18.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298(5600):2013–5. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 19.Pulvermuller F, Mohr B, Lutzenberger W. Neurophysiological correlates of word and pseudo-word processing in well-recovered aphasics and patients with right-hemispheric stroke. Psychophysiology. 2004;41(4):584–91. doi: 10.1111/j.1469-8986.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 20.Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, Basso A, Fazio F. A fMRI study of word retrieval in aphasia. Brain Lang. 2003;85(3):357–68. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- 21.Schirmer A, Alter K, Kotz SA, Friederici AD. Lateralization of prosody during language production: a lesion study. Brain Lang. 2001;76(1):1–17. doi: 10.1006/brln.2000.2381. [DOI] [PubMed] [Google Scholar]
- 22.Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122 (Pt 11):2033–46. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 23.Vingerhoets G, Deblaere K, Backes WH, Achten E, Boon P, Boon PJ, Hofman P, Vermeulen J, Vonck K, Wilmink J, Aldenkamp AP. Lessons for neuropsychology from functional MRI in patients with epilepsy. Epilepsy Behav. 2004;(Suppl 1):S81–9. doi: 10.1016/j.yebeh.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Vezina LG, Frattali C, Theodore WH. fMRI language task panel improves determination of language dominance. Neurology. 2004;26(638):1403–8. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- 25.Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Jabbari B, Vezina LG, Frattali C, Theodore WH. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;23(592):256–65. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- 26.Rutten GJ, Ramsey NF, van Rijen PC, Alpherts WC, van Veelen CW. FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. Neuroimage. 2002;17(1):447–60. doi: 10.1006/nimg.2002.1196. [DOI] [PubMed] [Google Scholar]
- 27.Theodore WH, Gaillard WD. Hippocampal volume and glucose metabolism in temporal lobe epileptic foci. Epilepsia. 2001;42(1):130–2. doi: 10.1046/j.1528-1157.2001.080874.x. [DOI] [PubMed] [Google Scholar]
- 28.Billingsley RL, McAndrews MP, Crawley AP, Mikulis DJ. Functional MRI of phonological and semantic processing in temporal lobe epilepsy. Brain. 2001;124(Pt 6):1218–27. doi: 10.1093/brain/124.6.1218. [DOI] [PubMed] [Google Scholar]
- 29.Gaillard WD, Hertz-Pannier L. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54(1):180–5. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- 30.Bookheimer SY, Zeffiro TA. Activation of language cortex with automatic speech tasks. Neurology. 2000;55(8):1151–7. doi: 10.1212/wnl.55.8.1151. [DOI] [PubMed] [Google Scholar]
- 31.Hertz-Pannier L, Gaillard WD, Mott SH, Cuenod CA, Bookheimer SY, Weinstein S, Conry J, Papero PH, Schiff SJ, Le Bihan D, Theodore WH. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48(4):1003–12. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- 32.Bookheimer SY, Zeffiro TA. A direct comparison of PET activation and electrocortical stimulation mapping for language localization. Neurology. 1997;48(4):1056–65. doi: 10.1212/wnl.48.4.1056. [DOI] [PubMed] [Google Scholar]
- 33.Cuenod CA, Bookheimer SY. using conventional equipment: a potential tool for language localization in the clinical environment. Neurology. 1995;45(10):1821–7. doi: 10.1212/wnl.45.10.1821. [DOI] [PubMed] [Google Scholar]
- 34.Theodore WH, Fishbein D. Patterns of cerebral glucose metabolism in patients with partial seizures. Neurology. 1988;38(8):1201–6. doi: 10.1212/wnl.38.8.1201. [DOI] [PubMed] [Google Scholar]
- 35.Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18(2):423–38. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 36.Byars AW, Holland SK, et al. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17(12):885–90. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balsamo LM, Xu B, Grandin CB, Petrella JR, Braniecki SH, Elliott TK, Gaillard WD. Arch Neurol. 2002;59(7):1168–74. doi: 10.1001/archneur.59.7.1168. [DOI] [PubMed] [Google Scholar]
- 38.Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–43. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- 39.Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, Xu B, Petrella JR, Balsamo L, Basso G. Cortical localization of reading in normal children: an fMRI language study. Neurology. 2001;10(571):47–54. doi: 10.1212/wnl.57.1.47. [DOI] [PubMed] [Google Scholar]
- 40.Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, Holland SK. BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15(17):2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janszky J, Jokeit H, Heinemann D, Schulz R, Woermann FG, Ebner A. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003;126(Pt 9):2043–51. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- 42.Saltzman-Benaiah J, Scott K, Smith ML. Factors associated with atypical speech representation in children with intractable epilepsy. Neuropsychologia. 2003;41(14):1967–74. doi: 10.1016/s0028-3932(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 43.Saltzman J, Smith ML, Scott K. The impact of age at seizure onset on the likelihood of atypical language representation in children with intractable epilepsy. Brain Cogn. 2002;48(2–3):517–20. [PubMed] [Google Scholar]
- 44.Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002 May 24;296(5572):1476–9. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- 45.Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005 Mar;15(3):275–90. doi: 10.1093/cercor/bhh129. Epub 2004 Aug 5. [DOI] [PubMed] [Google Scholar]
- 46.Kubova H, Druga R, Lukasiuk K, Suchomelova L, Haugvicova R, Jirmanova I, Pitkanen A. Status epilepticus causes necrotic damage in the mediodorsal nucleus of the thalamus in immature rats. J Neurosci. 2001;21(10):3593–9. doi: 10.1523/JNEUROSCI.21-10-03593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh S, Storey TW, Santos TC, Mian AY, Cole AJ. Early-life seizures in rats increase susceptibility to seizure-induced brain injury in adulthood. Neurology. 1999;53(5):915–21. doi: 10.1212/wnl.53.5.915. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z, Yang Y, Silveira DC, Sarkisian MR, Tandon P, Huang LT, Stafstrom CE, Holmes GL. Consequences of recurrent seizures during early brain development. Neuroscience. 1999;92(4):1443–54. doi: 10.1016/s0306-4522(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 49.Huang L, Cilio MR, Silveira DC, McCabe BK, Sogawa Y, Stafstrom CE, Holmes GL. Long-term effects of neonatal seizures: a behavioral, electrophysiological, and histological study. Brain Res Dev Brain Res. 1999;118(1–2):99–107. doi: 10.1016/s0165-3806(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 50.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44(6):845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 51.Stafstrom CE, Chronopoulos A, Thurber S, Thompson JL, Holmes GL. behavioral deficits after kainic acid seizures. Epilepsia. 1993;34(3):420–32. doi: 10.1111/j.1528-1157.1993.tb02582.x. [DOI] [PubMed] [Google Scholar]
- 52.Liegeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha-Khadem F, Baldeweg T. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127(Pt 6):1229–36. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- 53.Sabbah P, Chassoux F, Leveque C, Landre E, Baudoin-Chial S, Devaux B, Mann M, Godon-Hardy S, Nioche C, Ait-Ameur A, Sarrazin JL, Chodkiewicz JP, Cordoliani YS. Functional MR imaging in assessment of language dominance in epileptic patients. Neuroimage. 2003;18(2):460–7. doi: 10.1016/s1053-8119(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 54.Liegeois F, Connelly A, Salmond CH, Gadian DG, Vargha-Khadem F, Baldeweg T. A direct test for lateralization of language activation using fMRI: comparison with invasive assessments in children with epilepsy. Neuroimage. 2002;17(4):1861–7. doi: 10.1006/nimg.2002.1327. [DOI] [PubMed] [Google Scholar]
- 55.Hertz-Pannier L, Chiron C, Jambaque I, Renaux-Kieffer V, Van de Moortele PF, Delalande O, Fohlen M, Brunelle F, Le Bihan D. Late plasticity for language in a child's non-dominant hemisphere: a pre- and post-surgery fMRI study. Brain. 2002;125(Pt 2):361–72. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- 56.Wood AG, Harvey AS, Wellard RM, Abbott DF, Anderson V, Kean M, Saling MM, Jackson GD. Language cortex activation in normal children. Neurology. 2004;63 (6):1035–44. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- 57.Blanton RE, Levitt JG, Peterson JR, Fadale D, Sporty ML, Lee M, To D, Mormino EC, Thompson PM, McCracken JT, Toga AW. Gender differences in the left inferior frontal gyrus in normal children. Neuroimage. 2004;22(2):626–36. doi: 10.1016/j.neuroimage.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Lee BC, Kuppusamy K, Grueneich R, El-Ghazzawy O, Gordon RE, Lin W, Haacke EM. Hemispheric language dominance in children demonstrated by functional magnetic resonance imaging. J Child Neurol. 1999 Feb;14(2):78–82. doi: 10.1177/088307389901400203. [DOI] [PubMed] [Google Scholar]
- 59.Yuan W, Holland SK, Szaflarski J, Schmisthorst VJ, Byars AW, Strawsburg RH. fMRI Shows Atypical Language Lateralization in Children with Epilepsy, ISMRM 13th Scientific Meeting & Exhibition 2005, Accepted.
- 60.Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage. 2003;20(1):202–15. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]
- 61.Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;18(3316157):585–9. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 62.Benson RR, FitzGerald DB, LeSueur LL, Kennedy DN, Kwong KK, Buchbinder BR, Davis TL, Weisskoff RM, Talavage TM, Logan WJ, Cosgrove GR, Belliveau JW, Rosen BR. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;10(524):798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- 63.Duewll S, Wolff SD, Wen H, Balaban RS, Jezzard P. MR imaging contrast in human brain tissue: Assessment and optimization at 4T. Radiology. 1996;199:780–786. doi: 10.1148/radiology.199.3.8638005. [DOI] [PubMed] [Google Scholar]
- 64.Ugurbil K, Garwood M, Ellermann J, Hendrich K, Hinke R, Hu X, Kim SG, Menon R, Merkle H, Ogawa S, Salmi R. Imaging at High Magnetic Fields: Inital Experience at 4T. Magn Reson Quart. 1993;9:259–277. [PubMed] [Google Scholar]
- 65.Lowe MJ, Sorenson JA. Spatially filtering functional magnetic resonance imaging data. MRM. 1997;37:723–729. doi: 10.1002/mrm.1910370514. [DOI] [PubMed] [Google Scholar]
- 66.Thevenaz P, Unser M. A pyramid approach to subpixel Registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- 67.Le TH, Hu X. Retrospective estimation and correction of physiological artifacts in fMRI by direct extraction of physiological activity from MR data. Magn Reson Med. 1996;35(3):290–8. doi: 10.1002/mrm.1910350305. [DOI] [PubMed] [Google Scholar]
- 68.Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med. 1995;34(2):201–12. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]


