Abstract
Objective:
The objective of this study was to evaluate the effect of systematic utilization of extended donor criteria liver allografts (EDC), including living donor allografts (LDLT), on patient access to liver transplantation (LTX).
Summary Background Data:
Utilization of liver allografts that do not meet traditional donor criteria (EDC) offer immediate expansion of the donor pool. EDC are typically allocated by transplant center rather than regional wait-list priority (RA). This single-institution series compares outcomes of EDC and RA allocation to determine the impact of EDC utilization on donor use and patient access to LTX.
Methods:
The authors conducted a retrospective analysis of 99 EDC recipients (49 deceased donor, 50 LDLT) and 116 RA recipients from April 2001 through April 2004. Deceased-donor EDC included: age >65 years, donation after cardiac death, positive viral serology (hepatitis C, hepatitis B core antibody, human T-cell lymphotrophic), split-liver, hypernatremia, prior carcinoma, steatosis, and behavioral high-risk donors. Outcome variables included patient and graft survival, hospitalization, initial graft function, and complication categorized as: biliary, vascular, wound, and other.
Results:
EDC recipients were more frequently diagnosed with hepatitis C virus or hepatocellular carcinoma and had a lower model for end-stage liver disease (MELD) score at LTX (P < 0.01). Wait-time, technical complications, and hospitalization were comparable. Log-rank analysis of Kaplan-Meier survival estimates demonstrated no difference in patient or graft survival; however, deaths among deceased-donor EDC recipients were frequently the result of patient comorbidities, whereas LDLT and RA deaths resulted from graft failure (P < 0.01). EDC increased patient access to LTX by 77% and reduced pre-LTX mortality by over 50% compared with regional data (P < 0.01).
Conclusion:
Systematic EDC utilization maximizes donor use, increases access to LTX, and significantly reduces wait-list mortality by providing satisfactory outcomes to select recipients.
Extended donor criteria liver allografts do not meet traditional donor criteria for liver transplantation. This single-institution series demonstrated systematic utilization of extended donor criteria liver allografts maximized donor use, increased access to liver transplantation, reduced wait-list mortality, and provided satisfactory outcomes in select recipients.
Donor availability limits the application of liver transplantation (LTX). Logarithmically increasing demand within the last decade has resulted in a critical donor scarcity.1 In response, select transplant centers have relaxed customary restrictions to donation, thereby expanding the potential donor pool through increased recipient risk.2,3 These criteria have been loosely termed “extended donor” criteria (EDC) or “marginal” donors; however, guidelines defining this category of donor or level of acceptable risk have not been defined.
Liver allograft allocation within the United States is dependent on 2 variables: a calculated disease severity score, termed the Model for End Stage Liver Disease score (MELD),4 and geographic location. The state of New York has a particularly large disparity between recipient demand and donor supply.5 EDC allografts become available for export out of the region of donor origin after being declined by all transplant centers within geographic proximity to the donor and are typically offered to a transplant center in an area of greater donor scarcity for allocation at the discretion of the accepting physician. This is distinctly different from the allocation of standard criteria allografts (RA) that remain within the region of donor origin and are allocated by MELD score according to the United Network for Organ Sharing (UNOS) match list. We hypothesized the benefit of earlier access to LTX afforded by an EDC allograft outweighs the risk of waiting for an RA allograft. This investigation assesses the impact of systematic EDC utilization on donor use and patient access to liver transplantation.
METHODS
Recipient Data Collection
Retrospective review of 225 initial LTX recipients from April 2001 through April 2004 was performed using an internal registry approved by the New York Presbyterian Hospital (NYPH) Institutional Review Board (Approval #: AAAA9189). Recipients were categorized as RA or EDC. EDC allografts were procured from a deceased donor (EDC-dd) or living donor (LDLT). Living donor liver transplantation (LDLT) fulfills EDC criteria by allocation outside of the UNOS match list and outcomes that do not meet expectations of standard criteria deceased donors. Specifically, the incidence of technical complications is higher among LDLT recipients and outcomes, when applied to patients in urgent medical need of LTX, are inferior.20–22 Limited application and a higher frequency of technical complications increase risk for the LDLT recipient when compared with standard criteria deceased donor allografts; however, allocation of standard criteria allografts to potential LDLT recipients is unlikely. Thus, patients assume an earlier increased risk to avoid the morbidity and mortality associated with waiting, a rationale identical to the utilization of EDC-dd.
EDC-dd allografts originated outside UNOS region 9 as an “open offer” with allocation at the discretion of the accepting transplant surgeon. These organs were declined by all transplant centers within a region before NYPH allocation because of increased potential donor-associated risk. Potential risk was broadly categorized as impaired allograft function (potential parenchyma injury) or donor-transmitted disease. All LTX recipients were fully informed of the risks and benefits of the procedure, including donor information, before signing an appropriate consent.
Donor characteristics associated with a higher risk of delayed graft function or primary nonfunction include: age >65 years,2,6–8 macrovesicular steatosis >40%,9–11 donation after cardiac death,12–14 donor serum sodium >155 meq/dL,3,15,16 split-liver transplantation,17,18 and cold ischemia time exceeding 12 hours.19 Donors with an increased risk of disease transmission include positive serologic data (hepatitis C [HCV], hepatitis B [HBV] core antibody, human T-cell lymphotrophic virus [HTLV] I/II), carcinoma outside of the liver, and Centers for Disease Control and Prevention (CDC) high-risk behavior.
Demographic, operative, clinical, and pathologic variables included: gender, age, indication, waiting time, physiological MELD with exception points negated, and disposition at LTX. Allograft variables included allograft type, cold ischemia and warm ischemia times, organ procurement organization, blood type, body mass index, serum sodium, alanine aminotransferase, aspartate aminotransferase, total bilirubin, serologic data, and biopsy estimation of macrovesicular steatosis.
All candidates participate in routine surveillance screening for hepatocellular carcinoma (HCC) while listed for LTX that includes cross-sectional imaging (ultrasound, computed tomography, magnetic resonance imaging) and serum alpha-fetoprotein at 6-month intervals. Recipients with known or suspected HCC are staged by AJCC criteria23 as well as the currently recognized criteria for LTX as outlined by Mazzaferro.24
Outcomes analysis included: initial graft function, graft loss, retransplantation, intensive care unit length of stay, total hospitalization, occurrence of a complication, HCV recurrence, and death. Complications were classified as biliary, vascular, wound, and other with further subclassification of biliary complications as leak or stricture. Vascular complications were subclassified as hepatic artery thrombosis, portal vein thrombosis, and hepatic vein thrombosis. Biliary complications occurring subsequent to the diagnosis of hepatic artery thrombosis were not treated as independent events. Primary nonfunction was defined as medical indication for retransplantation within 24 hours of allograft reperfusion. Delayed graft function was defined by the necessity for prolonged metabolic support, transaminases >25× upper limit of normal, or significantly delayed recipient recovery. Pathologic data of HCV recurrence was derived from protocol biopsies performed on all HCV-positive recipients at 6 and 12 months after LTX. Follow up was terminated on the date of last known visit for patients lost to follow up, death, or April 1, 2005, whichever is earlier.
Extended Donor Criteria Participation
EDC participation is voluntary. On completion of a standard evaluation to determine candidacy and listing for LTX, recipient candidates receive a separate surgical consultation to discuss the option of EDC, including living donation. This is followed by a specific consent that documents patient understanding of the risks and benefits of participation and permits the individual to be eligible for EDC listing at NYPH. The EDC list is reviewed weekly at a multidisciplinary conference where candidates are advocated and prioritized by their clinical condition. When an EDC-dd allograft becomes available, the candidate is given specific donor information as well as pertinent literature detailing the risk assumed before surgical consent. A copy of the information provided is retained in the medical record and the category of EDC detailed in the operative note.
Posttransplant Screening
Select recipients of an allograft with an increased risk of donor-transmitted disease receive additional screening after LTX. Serology positive for HTLV I/II warrants confirmatory Western blot analysis. CDC high-risk allograft recipients are tested at 2 months for HBV, HCV, and human immunodeficiency virus (HIV). HBV core antibody-positive allograft recipients are treated indefinitely with lamivudine (Epivir; GlaxoSmithKline, Middlesex, U.K.); however, no specific screening for recipients of HCV or HBV core antibody-positive allografts is performed unless clinically indicated. Recipients of an allograft from a donor with a history of extrahepatic carcinoma do not receive specific screening unless clinically indicated.
Living Donor Evaluation and Selection
Preoperative living donor evaluation and selection at our center have been previously described.25,26 All donor candidates participate in a standardized 3-phase evaluation process that is in compliance with the New York State Committee on Quality Improvement in Living Liver Donation.27
Surgical Techniques
Technical aspects for the transplantation of deceased donor whole organs, split-liver allografts, and the performance of adult-to-adult living donor liver transplantation have been described.28–33 Venovenous bypass was not used. Whether the inferior vena cava was included in the native hepatectomy was dictated by the clinical scenario, except for recipients with known or suspected HCC in whom the retrohepatic inferior vena cava was always included to achieve an adequate surgical margin. In this study, all adult recipients of split-liver grafts received a Couinaud segment IV–VIII allograft.34,35 LDLT allografts comprised 49 right lobe (Couinaud segment IV–VIII) and 1 left lobe (Couinaud segment II–IV) allograft.
Immunosuppression
Immunosuppressive therapy was administered equally to all NYPH recipients and has been previously described in detail.36 Briefly, the standard regimen for adults consists of microemulsion cyclosporine (Neoral; Novartis Pharmaceuticals, Basel, Switzerland), corticosteroids, and mycophenolate mofetil (Cellcept; Roche, Nutley, NJ). Induction therapy is not routinely applied.
Statistical Analysis
Means, medians, standard deviations, and ranges are provided to summarize data distribution. Unless indicated, all mean data are expressed as mean ± standard deviation. Comparisons of continuous measures were assessed by one-way analysis of variance followed by the t test for parametric data or the nonparametric Wilcoxon rank sum test for data not approximated by a Gaussian distribution.37 Categorical variables were analyzed by the chi-squared test. Survival was estimated by the Kaplan-Meier method38 for 1) time to recipient death (recipient survival), and 2) time to either recipient death or graft loss, whichever occurs earlier (graft survival). Kaplan-Meier survival estimations were compared by the log-rank test for nonparametric data. The statistical package used was StatView version 5.01 (SAS Institute Inc., Cary, NC). Statistical significance is assumed for P < 0.05.
RESULTS
Recipient Populations
Between April 2001 and April 2004, a total of 225 primary liver transplant procedures were performed on adults aged 18 years or older, including 116 RA, 49 EDC-dd, and 50 LDLT. Indications for classification as EDC-dd are detailed in Table 1. Predictors of potential parenchyma injury were present in 31 donors (63%), 30% of which donors had multiple indications for classification as EDC. Among donors in the latter group, all had hypernatremia in addition to advanced age or HBV core-positive serology. Elderly donors ranged from 68 to 83 years with a mean of 72 ± 5 years and a median of 71 years. Serum sodium in hypernatremic donors’ sodium ranged from 156 to 173 meq/dL with a mean of 163 ± 5 meq/dL and a median of 160 meq/dL. Hypernatremia was analyzed as a separate variable only when present as an isolated indicator of potential parenchyma injury. Carcinoma outside the liver included a donor with a history of prostate cancer (Gleason score 6)39 who had undergone successful prostatectomy and a donor with a 4-cm well-differentiated renal cell carcinoma of the left kidney discovered at procurement.
TABLE 1. Deceased Donor Extended Donor Criteria Indication (n = 49)
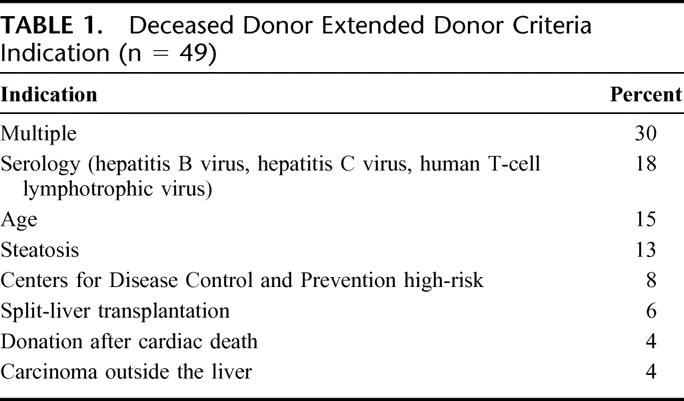
Cold ischemia time was not significantly different between EDC-dd and RA (P = 0.65), despite the increased travel time required to recover EDC-dd allografts. EDC-dd cold ischemia time ranged from 5 to 15 hours with a mean of 9.6 ± 2.6 hours and a median of 10 hours; 14% had a cold ischemia time >12 hours. RA cold ischemia time ranged from 2 to 15.5 hours with a mean of 8.0 ± 2.8 hours and a median of 8 hours; <3% of these allografts had a cold ischemic time >12 hours.
LDLT allografts included 49 right lobe and 1 left lobe allograft. The most frequent LDLT donor was an adult child (28%), followed by sibling (26%), spouse (23%), extended family (13%), parent (8%), and friend (2%).
Recipient demographic data are summarized in Table 2. EDC recipients were more frequently diagnosed with HCV or HCC than RA recipients. Significantly more males were EDC-dd recipients, reflecting greater difficulty in identifying appropriate LDLT donors for males as well as an increased incidence of HCV and HCC among male candidates at NYPH.40 Follow up was significantly shorter among RA recipients (P < 0.01) for the study period, reflecting the increased growth of deceased donor liver transplantation with a concomitant decline in LDLT.
TABLE 2. Recipient Demographics
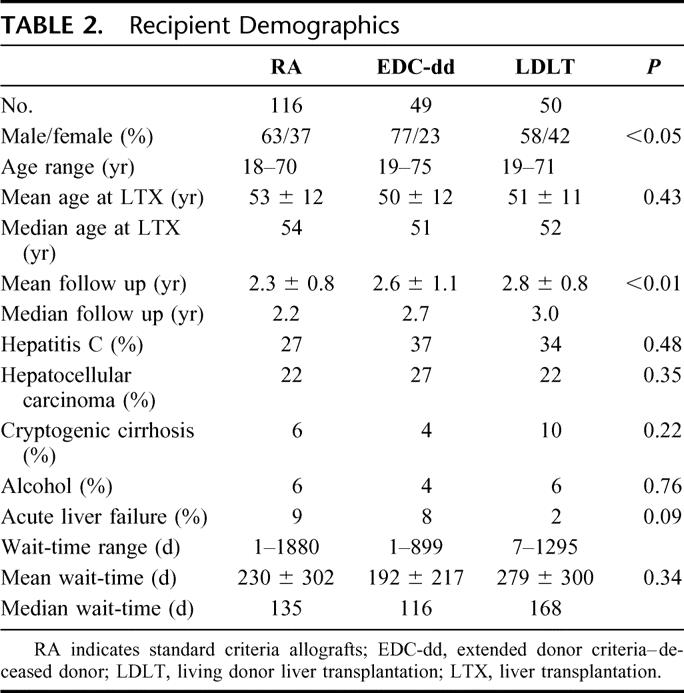
Highly significant differences were observed in allocation characteristics of RA and EDC recipients (Table 3). RA recipients were more frequently hospitalized and required intensive care at LTX; however, of EDC-dd recipients arriving from home at LTX, over one-third had required hospitalization at least once within the preceding 90 days for manifestations of end-stage liver disease, half of whom had multiple admissions within that period. Analysis of physiological MELD at LTX demonstrates RA recipients with a significantly higher MELD at LTX versus EDC recipients. This results from LDLT recipients’ MELD at LTX that were significantly lower than RA (P < 0.001) or EDC-dd (P < 0.01) recipients.
TABLE 3. Allocation Characteristics
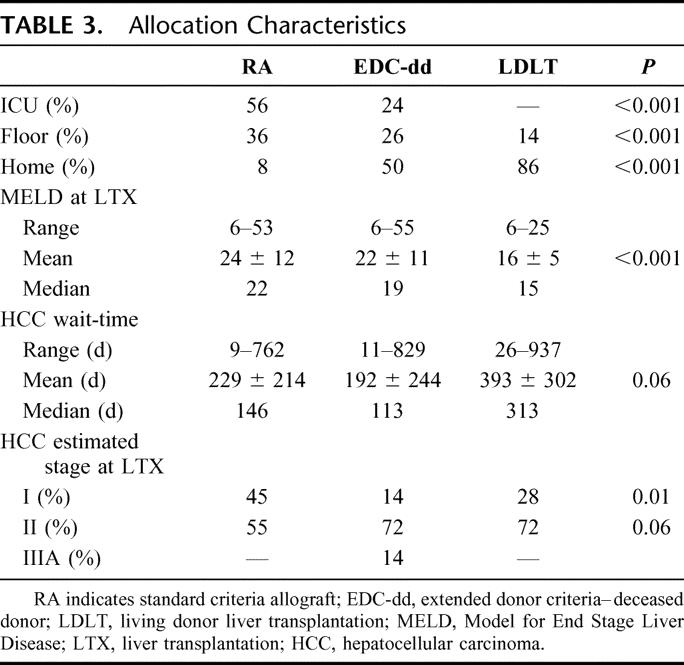
LDLT recipients demonstrated longer wait-times than RA or EDC-dd (Table 3). For LDLT recipients diagnosed with HCC, the wait-time was more than double that of either group. LDLT recipients represent 2 distinct groups: candidates with stable disease who developed HCC while awaiting LTX and those diagnosed with suspected advanced HCC at the time of LTX evaluation. LDLT recipients have longer wait-time and more advanced HCC stage at LTX. Indeed, 72% of LDLT recipients were an estimated AJCC stage II at LDLT versus 55% of RA recipients, an observation that approached statistical significance (P = 0.06).
Recipient and Graft Survival
Kaplan-Meier estimations of recipient and graft survival were compared by log-rank analysis.38 Comparison groups included: RA, EDC, EDC subdivided by potential parenchyma injury, and all subgroups of potential parenchyma injury. Six-month, 1- and 3-year RA recipient survival was 94%, 84%, and 80%, respectively, whereas EDC recipient survival was 89%, 83%, and 78%, respectively (P = NS). EDC recipients demonstrated a higher frequency of graft failure resulting in retransplantation or death, but the difference did not achieve statistical significance. Six-month, 1- and 3-year graft survival for RA recipients (Fig. 1A) were 94%, 84%, and 80%, respectively, whereas EDC graft survival at the same time points was 85%, 79%, and 78%, respectively.
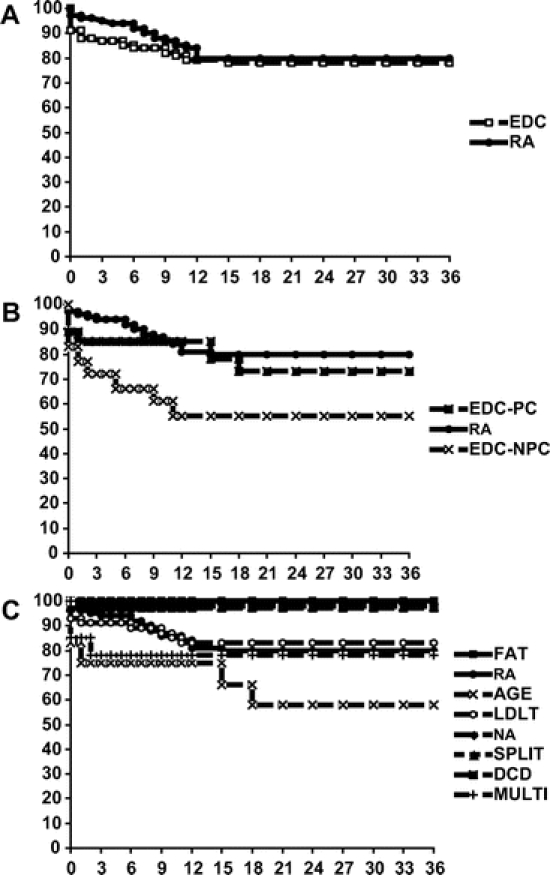
FIGURE 1. A, Kaplan-Meier graft survival RA versus EDC. B, Kaplan-Meier graft survival RA versus EDC-PC versus EDC-NPC. C, Kaplan-Meier graft survival.
Among RA recipients, 24 died: 5 from graft-related complications within the first 6 months after LTX and 19 from comorbidities, including recurrent HCV, recurrent HCC, and cardiopulmonary disease. No mortality after 6 months was attributed to graft function. One RA recipient required retransplantation for graft dysfunction and 5 underwent later retransplantation for recurrent HCV. Twenty-two EDC recipients died: 3 within the first week after LTX, 9 between 1 week and 6 months, and 10 after 6 months after LTX. Six of the 12 deaths occurring within 6 months were related to LTX complications with no late EDC deaths attributed to graft dysfunction. Six EDC-dd recipients underwent retransplantation: 3 within the first month after LTX for graft dysfunction and 3 over 1 year after LTX as a result of recurrent HCV. LDLT recipients required retransplantation for primary nonfunction, small-for-size syndrome, and hepatic artery thrombosis at post-LTX days 12, 18, and 26, respectively.
Classification of EDC by potential parenchyma injury (PC) versus increased risk of donor disease transmission (NPC) demonstrated lower patient and graft survival (Fig. 1B) among the latter group of recipients that achieved statistical significance (P = 0.02). This was the result of recipient comorbidities because mortality resulting from graft failure was only observed in 1 NPC recipient. However, overall short-term function of all EDC allografts remained lower than RA.
Analysis of predictors of potential parenchyma injury (steatosis, hypernatremia, split-liver transplantation, LDLT, donation after cardiac death, and age) demonstrates lower patient and graft survival among recipients with multiple indications for EDC classification or advanced donor age compared with RA recipients (Fig. 1C). EDC-dd donors with multiple predictors all exhibited hypernatremia in addition to age or HBV core-positive serology (n = 15). Of this group, 3 recipients died within 6 months of transplantation: 2 from complications of graft dysfunction at 30 days and 62 days after LTX, and 1 from a cerebrovascular accident.
Decreased patient and graft survival among recipients of allografts from donor aged >65 years recipients principally reflect allocation of these organs to an older recipient group. Over 40% of these recipients were ≥60 years of age at LTX. Two recipients died from graft dysfunction at 30 days and 60 days after LTX; however, 1 of the recipients had experienced a significant perioperative myocardial infarction that contributed to graft failure. The other recipient mortality was the result of comorbidities that included pneumonia, myocardial infarction, stroke, and recurrent HCV. Donation after cardiac death (n = 2) and split-liver (n = 3) transplantation have yielded outcomes that exceed RA.
EDC allografts with an increased risk of donor-transmitted disease included positive serology for HCV, HBV core antibody, or HTLV I/II, CDC high-risk behavior, and extrahepatic carcinoma. Within this group, 1 primary nonfunction occurred in an HBV core antibody-positive allograft that necessitated retransplantation and 3 early deaths were attributed to graft dysfunction. The deaths occurred at 25, 36, and 44 days after LTX among a hospitalized recipient of an HBV core antibody-positive allograft, an HCV/HCC recipient of an HCV-positive allograft who developed inferior vena cava thrombosis complicated by renal failure, and a CDC high-risk allograft recipient with a physiological MELD score of 34. Two additional late graft failures were the result of recurrent HCV (1 HCV-positive and 1 HCV-negative donor) and 3 late deaths resulted from recurrent HCV or HCC.
Hospitalization
RA recipients demonstrated greater mean and median intensive care unit length of stay as well as total post-LTX hospitalization. Statistical significance is not observed between groups as a result of widespread variation within the groups that correlates with immediate graft function. However, given that 92% of RA recipients required intensive care or hospitalization at LTX, overall outcomes are satisfactory in this group with a median intensive care unit stay of 4 days and a median post-LTX hospital stay of 15 days.
Complications
Postoperative complications were broadly categorized as vascular, biliary, wound, and other. In addition, the incidence of primary nonfunction and delayed graft function were compared. Wound complications included hernia and dehiscence. Wound complications occurred in 11% of EDC compared with 10% of RA recipients (P = 0.72). The incidence of vascular complications in both RA and EDC recipients was 5% and included 1 hepatic arterial thrombosis and 2 portal vein thromboses among LDLT recipients; and 1 portal vein and 1 inferior vena cava thrombosis among EDC-dd recipients, 1 hepatic vein stenosis, inferior vena cava thrombosis, portal vein thrombosis, and portal vein stenosis among RA recipients.
Biliary complications occurred more frequently among EDC recipients (34%) than RA recipients (10%). The incidence of biliary stricture was significantly higher in EDC-dd (22%) and LDLT (26%) recipients than RA (7%) recipients (P < 0.01), whereas biliary leaks occurred predominantly in LDLT recipients.
Two EDC-dd recipients of multiple predictor allografts died from complications of poor initial graft function; both were recipients who required intensive care hospitalization at LTX with a physiological MELD score of 39 and 54. All other recipient deaths occurred greater than 1 year after transplantation and were not related to initial graft function.
Graft dysfunction was the principal cause of LDLT recipient mortality and contributed to all LDLT recipient deaths within 1 year of LTX. Three grafts failed within the first 60 days of LDLT, resulting in 1 retransplantation and 2 LDLT recipient deaths. Three additional graft failures led to LDLT mortality at 7, 8, and 12 months after LTX. Three late LDLT recipient deaths resulted from recurrent HCC.
Disease Recurrence
Posttransplant disease recurrence was assessed by evaluating histologic HCV recurrence among HCV recipients of an HCV-positive donor versus recipients of an HCV-negative donor, HCC recurrence, and documentation of a donor-transmitted disease. Of the 53 HCV-positive EDC recipients, all 25 LDLT recipients and 19 of the 28 EDC-dd recipients received an HCV-negative allograft. HCV recurrence was diagnosed by histology of 6- and 12-month protocol biopsies. All EDC-dd recipients of an HCV-positive allograft had histologic recurrence by 6 months versus 95% of EDC-dd recipients of an HCV-negative allograft and 90% of LDLT recipients. HCV recurrence was not significantly different among RA recipients of an HCV-negative allograft. Histologic recurrence did not correlate with survival within any group.
Although preoperative data suggested EDC recipients overall had more advanced HCC than RA, HCC recurrence was not significantly different between groups. By explant pathology, each recipient was restaged by AJCC and Mazzaferro criteria. Stage I HCC was present in 37% of LDLT, 50% of EDC-dd, and 33% of RA recipients. Recurrence in these patients averaged 12% (P = 0.87). Stage II HCC was present in 63% of LDLT, 50% of EDC-dd, and 41% of RA recipients. HCC recurrence occurred in 25% of LDLT, 20% of RA, and none of 5 EDC-dd recipients with stage II disease (P = 0.48). Recurrence among the 6 RA recipients with stage IIIA HCC was 80%. Mazzaferro criteria24 was exceeded in 57% LDLT, 43% EDC, and 41% of RA recipients. Recurrence in all groups significantly correlated to explant pathology that exceeded Mazzaferro criteria (P = 0.03).
Potential donor-transmitted disease had no effect on post-LTX outcomes. Recipients of HBV core antibody-positive donors receive prophylaxis with lamivudine and have done well with no clinical sequelae. There were 4 recipients of HTLV I/II donors: 2 represented false-positives, 1 true-positive was allocated to an HIV/HCV recipient who died at 12 months from recurrent HCV, and 1 HIV/HBV-coinfected recipient is healthy 8 months after LTX. No donors with extrahepatic carcinoma or CDC high-risk behavior have transmitted disease to a recipient.
Access to Liver Transplantation
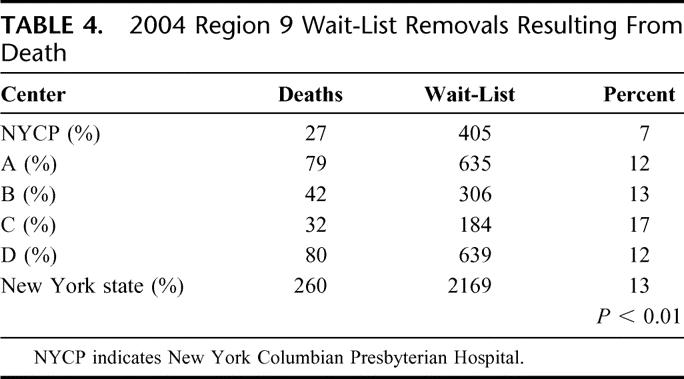
Routine implementation of an EDC program increased transplant volume by 99 allografts over a 3-year period and patient access to LTX by 77%. The observed 50% reduction in wait-list removal as a result of death was significantly lower than regional data (Table 4) and overall 1-year patient survival significantly higher than SRTR-predicted 1-year survival within the region (P < 0.001).5
TABLE 4. 2004 Region 9 Wait-List Removals Resulting From Death
DISCUSSION
Utilization of extended criteria donors has been advocated for over a decade.2 During this period, numerous single-center reports have identified predictors of potentially poor graft function.14,41,42 The increasing discrepancy between organ supply and demand resulting from the successful application of LTX has motivated select transplant centers in regions of particular scarcity to adopt strategies for the utilization of these organs with overall satisfactory results.3,13 As organ scarcity intensifies, further emphasis on expanding donor criteria will occur.
Incumbent on expansion of the donor pool is the establishment of guidelines that define standard and extended donor criteria. To date, this has not been achieved. This study attempts to define extended donor criteria based on risk assessment incurred by the recipient. Using such an approach, increased risk incurred by a recipient could include a recognized higher risk of early graft failure or delayed graft function; alternatively, the recipient may be assuming an added risk of known or potential donor-transmitted disease. Although all deceased donor organ recipients assume a theoretical risk associated with the process of organ transplantation, the transplantation of an allograft with positive serologic analysis or an extrahepatic carcinoma significantly elevates the level of risk.
Accepting potentially increased risk must be accompanied by a process of informed consent that specifically addresses the concerns of the patient with respect to the particular donor as well as the transplant procedure. The procedure described here achieves informed consent through a 2-staged process that is initiated at the time of routine listing for LTX. Informed consent is based on estimates from experience and aggregate data provided to the candidate.
Inclusion of adult-to-adult LDLT as an EDC allograft is supported by data on allocation, probability of a complication, and risk of poor immediate graft function. LDLT allocation occurs at the transplant center and is optimal among candidates who do not experience acute decompensation of chronic liver disease or fulminant hepatic failure. LDLT outcomes do not equal outcomes from deceased donor whole organ transplantation.20,21 Therefore, LDLT recipients assume increased risk when compared with the expectations of LTX using a standard criteria allograft; however, allocation of such an allograft is unlikely given the current donor scarcity. The clinical paradox that motivates patients to participate in EDC is identical for LDLT.
Review of recipient demographic data highlights our allocation patterns with respect to EDC. EDC recipients were typically male with HCV and concomitant HCC. When present, HCC was often at an advanced stage compared with RA recipients. Furthermore, the mean and median wait-time of LDLT recipients transplanted for HCC was particularly long, suggesting disease progression while awaiting LTX. This reflects a frequent observation with respect to LDLT in which an individual is listed for transplantation and matures to an LDLT candidate while waiting, usually after a significant clinical event such as the diagnosis of HCC or clinical deterioration from end-stage liver disease. The onset of an additional clinical indication may affect willingness of the recipient or recipient's social support system to ultimately participate in LDLT. Our data on wait-times, MELD scores, and HCC stage at LTX suggest MELD was the principal predictor for LTX in RA and EDC-dd groups, whereas wait-time and the diagnosis of HCC were the principal influence for LTX among LDLT recipients.
EDC-dd donors were principally used to meet the demand of candidates who were hospitalized at the time of organ offer or recently hospitalized for manifestations of end-stage liver disease. The identification and transplantation of candidates as they begin to clinically deteriorate is supported by EDC-dd physiological MELD scores that were not statistically different from RA recipients.
EDC donors were broadly categorized according to risk of potential parenchyma injury and delayed graft function or donor-transmitted disease. This study population included 50 LDLT recipients and 31 EDC-dd recipients of allografts with 1 or more indicators of potential parenchyma injury. Our strategy to reduce the likelihood of delayed graft function involved minimizing cold ischemia time through the deployment of procurement surgeons experienced in EDC allograft assessment. Experience in medical resuscitation, assessment, and efficient donor procurement using advanced techniques is central to successful application of EDC.
Patient and graft survival among recipients of EDC allografts with potential parenchyma injury have equaled RA outcomes. Although 2 EDC-dd recipients died from graft dysfunction, all other late EDC-dd recipient deaths were the result of cardiovascular complications or recurrent HCV. This was not true for LDLT recipients in whom the principal cause of mortality was graft dysfunction.
Several authors have documented similar outcomes between HCV recipients transplanted with HCV-positive and -negative allografts.14,42 Our data mirrored these data demonstrating histologic evidence of HCV recurrence in the majority of recipients of HCV-positive and -negative allografts. Routine liver biopsies are not obtained in donors with normal or mildly elevated (<4 × upper limit of normal) transaminase levels; rather, visualization by an experienced recovery surgeon determines utilization. Situations in which visualization of the liver warranted biopsy rarely resulted in utilization. Empiric evidence indicates most HCV-positive donors under 50 years of age are usable, approximately one-half of HCV-positive donors between 50 and 60 years of age are usable, and rare HCV-positive donors over age 60 years are usable.
Excellent results were also observed among recipients of HBV core antibody-positive donors14,42,43 in agreement with substantial data demonstrating effective long-term prophylaxis against HBV activation with lamivudine. In the current climate of organ scarcity, HCV-positive allografts to HCV recipients and HBV core antibody donors to all adult recipients do not warrant categorization as EDC.
A total of 4 HTLV I/II-positive donors have been used with confirmatory Western blot obtained in situations in which the history appeared compatible (n = 2) such as travel or intravenous drug use and hepatitis serologies were positive, Western blot confirmed the diagnosis. However, the 2 donors with an isolated HLTV-positive serology in the setting of negative hepatitis serologies and a history that does not support exposure proved to be false-positives.
All CDC high-risk donors were engaged in intravenous drug use and/or prostitution until their death. We have routinely screened CDC high-risk allograft recipients for potential donor-transmitted disease. All recipients were already HCV-positive and 2 had previously been exposures to HBV; HIV testing in each of these recipients was negative. The other 2 CDC high-risk allograft recipients were negative for HBV and HIV. Each recipient of an allograft from a donor with extrahepatic malignancy has had no evidence of donor-derived malignancy.
The incidence of biliary complications was significantly higher among EDC recipients. Increased biliary complications, both leak and stricture, within LDLT recipients would be expected. What was not expected was the observation of a significantly higher incidence of biliary stricture among EDC-dd recipients. This observation suggests underlying ischemia and reaffirms our dedication to minimizing periods of cold and warm ischemia.
The systematic utilization of EDC allografts increased patient access to LTX by 77% and reduced wait-list mortality by greater than 50%. Satisfactory outcomes, as demonstrated by patient and graft survival, length of hospitalization, incidence of complications, and occurrence of donor-transmitted disease, have been achieved. Although not statistically significant, the incidence of impaired immediate graft function was higher among EDC recipients, and our finding likely reflects a type II error secondary to insufficient power. This is expected because our goal is not to equal standard criteria allograft performance, but rather to surpass survival expectations from continued waiting for organ allocation by MELD score.
These outcomes data must be compared with available national outcomes. To put our outcomes in perspective, UNOS data for 2002 through 2004 demonstrate a patient survival exceeding the risk-adjusted survival by approximately 20%. Overall actuarial survival was 86% compared with a predicted survival for our patient population of 68% (P < 0.01).
In conclusion, EDC allografts provide an immediate and significant expansion of the existing donor pool. Because organ scarcity persists, additional pressure will build to use a greater proportion of the existing donor pool. Fundamental to further progress in the utilization of EDC allografts is prospective, multicenter data collection to clearly define risk and delineate guidelines for standard and extended criteria donors. Such data would likely broaden the existing deceased donor pool by encouraging the utilization of donors that are currently deemed unsuitable for transplantation. This should be prioritized just as the significance of LDLT has been recognized by the National Institutes of Health.
Lastly, EDC utilization has impacted the dynamics of transplantation at our center. By closely following the clinical progress of EDC candidates, we can frequently identify patients who are beginning to clinically deteriorate and bring them to transplantation earlier. This has actually translated into decreased reliance on and utilization of region-allocated organs such that we are transplanting our waiting-list as much from the center as from the sickest end.
ACKNOWLEDGMENTS
This manuscript is dedicated to the memory of Thomas Fernandez.
Discussions
Dr. Christoph Broelsch (Essen, Germany): Patient access to liver transplants in view of a registered waiting list mortality of more than 20% presents a major trouble in today's transplants activities. Dr. Renz and his group have systematically used donor organs rejected by other centres for quality reasons carrying a high risk of complications, the most serious one being the necessity of a retransplantation.
Guided by the fact that the worse complication in liver transplantation, however, is not being transplanted at all they picked donor grafts on the basis of an informed consent process which exceeded allograft criteria set by UNOS. For this task I want to congratulate them!
High volume transplant centres cannot do without extended graft criteria providing the opportunity of choices for individual candidates. The authors actually describe 3 categories of extended grafts; live donor grafts, extended UNOS criteria grafts, and marginal grafts. In their series 31 patients received marginal grafts.
First, Dr. Renz, could you elaborate on the selection criteria of those patients who received marginal grafts with the exception of HCC patients? Interestingly, they had a lower MELD score than the ones who received UNOS allocated grafts. Are they being picked earlier with a lower MELD score to potentially sustain complications?
Second, with your excellent results obtained by the application of extended donor criteria, aren't you challenging the UNOS MELD score and in particular the UNOS graft criteria?
It has been shown by our group and others, that organs retrieved from donors aged more than 65 years with a sodium over 155 mE/dl and cold ischemia time exceeding 12 hours could function appropriately. For example, in the EURO TRANSPLANT area 1109 liver transplants were performed in 2004. Of them, 78.6% were allocated through ET standard allocation equivalent to UNOS standard allocation process. In total, 283 organs (21.4%) were allocated as organ rescue offers equivalent to UNOS extended donor criteria. In our center, 24 livers were transplanted as marginal grafts. In this subgroup, patient survival and graft survival were 89% and 82%, respectively. The retransplantation rate was low, however, the rate of primary disfunction was 20%, requiring a higher rate of dialysis of 18% and reexploration of 16%. These facts increased the costs of the procedure significantly. Does our experience correspond with your experience?
Third, should we plot the live donors procedures into the category of the extended donor criteria? Basically, this is a group of patients being transplanted electively with appropriately calculated and primarily functioning grafts. Interestingly, in your series they had the longest waiting time and you mentioned that they matured into the living procedure. Shouldn't they have the benefit of immediate transplant avoiding the morbidity and mortality of the waiting list?
I would like to thank the Association for the privilege of having the floor and I thank Dr. Renz for giving me the opportunity of reviewing his manuscript prior to the meeting.
Dr. John Renz (New York, New York): I would begin with the first question. And that is how do we talk about allocation and how do we individualize it? It is really a function of the patient's blood type and what their expected weight in that region will be, as well as their etiology and the ability of us to potentially get an expanded donor liver for them.
If you exclude help hepatoma and hepatitis C, the 3 principal indications for transplanting in this group where (1) Older individuals who may have been presenting perhaps over the age of 65 with several comorbidities and we didn't want them to wait too long and essentially eliminate the ability to transplant them; (2) Patients who were hospitalized recently for complications of end-stage liver disease; and (3) Special indications; for example, the gentleman who was working full-time, had his own business and was up against disability, and extreme changes in his family and his ability to provide. So it is tailored to the recipient as to what exactly the risk we will ask them to accept.
As far as the cost, we are actually beginning to look at costs. And we don't have the complete data. But there is no question that the allocation of the organ acquisition cost is a lot higher. Because what I didn't talk about in the presentation was the importance of the donor team and the sophistication you need in your donor team, because basically you are asking them to operate very far from home and evaluate a lot of different organs and perhaps do some medical resuscitation down there to optimize their quality. So I think our allocation costs are higher. But that may be offset by the overall lower incidence of their hospital stay and by trimming their morbidity versus a wait.
Lastly, for the living donors, I think, given the current data, it is appropriate to consider the living donors within the ECD realm because of their application and because of their complications to date, although the results are getting better. Our EDC list and why they waited so long is principally the result of three reasons. One is there is some historical overlap as we evolve a living donor program for patients who are on the list. But also some of those patients didn't mature on the list in the sense that they were listed for liver transplantation and then developed either a medical complication that prioritized their need for transplantation, either within their mind or their social support system's mind, or they developed hepatocellular carcinoma. So I think there are 2 different populations within the population data that I presented.
Dr. Nancy L. Ascher (San Francisco, California): It is clear that cold ischemic time has a negative impact on the outcome of both ECD donors and regularly distributed donors. Do you have data or an approach to minimize the cold ischemic time in this group of donors? Do you think your data will support use of these donors by using other transplant centers throughout the U.S.?
Dr. John Renz (New York, New York): There is no question. Thank you for bringing it up. And I deleted that slide in the interest of brevity. But when you look at cold ischemia time there actually was no difference in our group. The region allocateds were about 8.5 hours and the ECDs were about 9 hours even though the average length to procurement was hundreds of miles more, over 400 miles, versus local.
The bottom line is the technique to make any of these livers work has to function on cold ischemia time. And that means sending your own team, someone you trust, to look at these older livers or these hep-C livers or these funny analysis things, then as soon as they say it is okay, to actually begin the operation regardless of what time it is, to actually keep that cold time down. And we have used a lot of livers almost transcontinental with under 10 hours pretty routinely just by trying to put a lot of emphasis on coordination and utilization. We try not to do any biopsying, because that will contribute to the prolonged time.
Dr. Nancy L. Ascher (San Francisco, California): The other issue, though, in all 3 of your groups you have had patients with MELDs as low as 6. As you know, the SRTR data clearly shows that when patients undergo transplantation patients with MELDs below 10, their chance of dying after transplant exceeds their chance chance of dying on the list. I am sure you thought about that; I wonder how you rationalize the use of these extended donors in those patients?
Dr. John Renz (New York, New York): There are 2 answers to that question. The first is that they actually aren't the MELDs that were transplanted because I deleted all the exceptions to try to get to the physiology. That is the easy part.
The harder part is that some of that data in the lower MELDs frankly came when we didn't have good MELD data. We now have a very powerful tool to tell us who needs to be done. And I think when you take away the hepatomas and you have a patient with hepatitis C and no other indication to go, my bias in using EDC has gone up into the 20s where I would say a year and a half ago it may have been 17. Unless they give me a very good reason to move below 20, unless they have some other indication, I will let them mature a little bit. Because I think that you are absolutely right.
Dr. Byers W. Shaw, Jr. (Omaha, Nebraska): One of the things that confuses me a little bit is that historically our rate of primary nonfunction, if you go back 10 years ago, at its peak was maybe 10%. That is about the worst that anybody presented, I think. We started having the luxury with UW solution of doing preoperative biopsies and we detected preexisting disease, some of which was steatosis, which at a level of 40–50% increased the risk of primary nonfunction. But those livers were already in that 10%. So when we eliminated them, we lowered the risk even further.
So one of the things that bothers me about the way the data is being analyzed, and it may be a disadvantage to you, is that you are not looking at the end result of using a liver and associating it with the most likely result from 1 of the criteria.
For instance, a fatty liver generally does not cause biliary strictures, and it generally does not cause arterial thrombosis, but it can be associated with a higher incidence of primary nonfunction. Likewise, cold ischemia time, at least within the limits of what you are presenting, generally doesn't result in an increased risk of primary non-function. It is primarily associated with a higher risk of biliary stricture. So have you broken this down to look at the indications for the ECD and determined whether or not these variables correlate with the outcome?
Dr. John Renz (New York, New York): I actually did. But the real problem I have is one of power. Because I don't have a lot of the individual indications, enough in my series, to actually make a statement that I am confident in presenting.
Footnotes
MELD exception points are afforded patients with indications that require prioritization (ie, hepatocellular carcinoma) within a region. Unadjusted or physiological MELD is a more accurate indicator of potential liver failure.
This work was supported by the Rice Research Fund.
Reprints: John F. Renz, MD, PhD, Center for Liver Disease and Transplantation, New York Presbyterian Hospital, 622 West 168th St., Room PH-14C, New York, NY 10032. E-mail: jfr2103@columbia.edu.
REFERENCES
- 1.Brown R, et al. Liver and intestine transplantation. American Journal of Transplantation. 2004;4(suppl 9):81–92. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, Vaughn W. The use of ‘marginal’ donors for organ transplantation. Transplantation. 1991;51:135–141. [DOI] [PubMed] [Google Scholar]
- 3.Markmann J, et al. Preoperative factors associated with outcome and their impact on resource use in 1148 consecutive liver transplants. Transplantation. 2001;72:1113–1122. [DOI] [PubMed] [Google Scholar]
- 4.Wiesner RH, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. [DOI] [PubMed] [Google Scholar]
- 5.UNOS, United Network for Organ Sharing. Available at: http://www.UNOS.org. 2004.
- 6.Alexander J, Vaughn W, Carey M. The use of marginal donors for organ transplantation: the older and younger donors. Transplant Proc. 1991;23:905–909. [PubMed] [Google Scholar]
- 7.De Carlis L, et al. Marginal donors in liver transplantation: the role of donor age. Transplant Proc. 1999;31:397–400. [DOI] [PubMed] [Google Scholar]
- 8.Baccarani U, et al. Liver transplantation from old donors into HCV and non-HCV recipients. Transplant Proc. 2004;36:527–528. [DOI] [PubMed] [Google Scholar]
- 9.Todo S, et al. Primary nonfunction of hepatic allografts with preexisting fatty infiltration. Transplantation. 1989;47:903–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Alessandro A, et al. The predictive value of donor liver biopsies on the development of primary nonfunction after orthotopic liver transplantation. Transplant Proc. 1991;23:1536–1537. [PubMed] [Google Scholar]
- 11.Adam R, et al. The outcome of steatotic grafts in liver transplantation. Transplant Proc. 1991;23:1538–1540. [PubMed] [Google Scholar]
- 12.Casavilla A, et al. Experience with liver and kidney allografts from non-heart-beating donors. Transplant Proc. 1995;27:2898. [PubMed] [Google Scholar]
- 13.Reich D, et al. Controlled non-heart-beating donor liver transplantation. Transplantation. 2000;70:1159–1166. [DOI] [PubMed] [Google Scholar]
- 14.Busuttil R, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez F, et al. Predictive factors of early postoperative graft function in human liver transplantation. Hepatology. 1994;20:565–573. [DOI] [PubMed] [Google Scholar]
- 16.Figueras J, et al. The deleterious effect of donor high plasma sodium and extended preservation in liver transplantation. A multivariate analysis. Transplantation. 1996;61:410–413. [DOI] [PubMed] [Google Scholar]
- 17.Yersiz H, et al. One-hundred in situ split-liver transplantations: a single center experience. Ann Surg. 2003;238:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts J, et al. he influence of graft type on outcomes after pediatric liver transplantation. American Journal of Transplantation. 2004;4:373–377. [DOI] [PubMed] [Google Scholar]
- 19.Adam R, et al. Deleterious effect of extended cold ischemia time on the posttransplant outcome of aged livers. Transplant Proc. 1995;27:1181–1183. [PubMed] [Google Scholar]
- 20.Renz JF, Busuttil RW. Adult-to-adult living-donor liver transplantation: a critical analysis. Semin Liver Dis. 2000;20:411–424. [DOI] [PubMed] [Google Scholar]
- 21.Trotter J, et al. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. [DOI] [PubMed] [Google Scholar]
- 22.Pomfret E. Early and late complications in the right-lobe adult living donor. Liver Transpl. 2003;9:S45–49. [DOI] [PubMed] [Google Scholar]
- 23.Yabro J, et al. American Joint Committee on Cancer prognostic factors consensus conference. Cancer. 1999;86:2436–2446. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferro V, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 25.Renz JF, et al. Donor selection limits use of living-related liver transplantation. Hepatology. 1995;22:1122–1126. [DOI] [PubMed] [Google Scholar]
- 26.Renz J, Roberts J. Long-term complications of living donor liver transplantation. Liver Transpl. 2000;6:73–76. [DOI] [PubMed] [Google Scholar]
- 27.A report to the New York State Transplant Council and New York State Department of Health. 2002.
- 28.Klintmalm G, Busuttil R. The recipient hepatectomy. In: RAK, Busuttil RK, eds. Transplantation of the Liver. Philadelphia: WB Saunders Co; 1996:405–418. [Google Scholar]
- 29.Busuttil RW, Goss J. Split liver transplantation. Ann Surg. 1999;229:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renz J, et al. Changing faces of liver transplantation: partial-liver grafts for adults. J Hepatobiliary Pancreat Surg. 2003;10:31–44. [DOI] [PubMed] [Google Scholar]
- 31.Wachs ME, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313–1316. [DOI] [PubMed] [Google Scholar]
- 32.Marcos A, et al. Surgical management of anatomical variations of the right lobe in living donor liver transplantation. Ann Surg. 2000;231:824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcos A, et al. Functional venous anatomy for right-lobe grafting and techniques to optimize outflow. Liver Transpl. 2001;7:845–852. [DOI] [PubMed] [Google Scholar]
- 34.Couinaud C. Les enveloppes vasculobiliares de foie ou capsule de Glisson. Leur interet dans la chirurgie vesiculaire, les resections hepatiques et l'abord du hile du foie. Lyon Chir. 1954;49:589–615. [Google Scholar]
- 35.Couinaud C. Le Foie: Etudes anatomiques et chirurgicales. Paris: Masson; 1957. [Google Scholar]
- 36.Gaglio P, et al. Increased risk of cholestatic hepatitis C in recipients of grafts from living versus cadaver liver donors. Liver Transpl. 2003;9:1028–1035. [DOI] [PubMed] [Google Scholar]
- 37.Freedman D, Pisani R, Purves R. Statistics, 3rd ed. Philadelphia: Harcourt; 2004. [Google Scholar]
- 38.Kaplan GL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 39.Pepe P, et al. Prediction by quantitative histology of pathologic stage in prostate cancer. Eur J Surg Oncol. 2005;31:309–313. [DOI] [PubMed] [Google Scholar]
- 40.Russo M, et al. Impact of adult living donor liver transplantation on waiting time survival in candidates listed for liver transplantation. American Journal of Transplantation. 2004;4:427–431. [DOI] [PubMed] [Google Scholar]
- 41.Karatzas T, et al. Expanded liver donor age over 60 years for hepatic transplantation. Transplant Proc. 1997;29:2830–2831. [DOI] [PubMed] [Google Scholar]
- 42.Feng S, et al. Organ donors with positive viral serology or malignancy: risk of disease transmission by transplantation. Transplantation. 2002;74:1657–1663. [DOI] [PubMed] [Google Scholar]
- 43.Burton JJ, Shaw-Stiffel T. Use of hepatitis B core antibody-positive donors in recipients without evidence of hepatitis B infection: a survey of current practice in the United States. Liver Transpl. 2003;9:837–842. [DOI] [PubMed] [Google Scholar]


