Abstract
Background:
Recent studies suggest local excision may be acceptable treatment of T1 adenocarcinoma of the rectum, but there is little comparative data with radical surgery to assess outcomes and quantify risk. We performed a retrospective evaluation of patients with T1 rectal cancers treated by either transanal excision or radical resection at our institution to assess patient selection, cancer recurrence, and survival.
Methods:
All patients who underwent surgery for T1 adenocarcinomas of the rectum (0–15 cm from anal verge) by either transanal excision (TAE) or radical resection (RAD) between January 1987 and January 2004 were identified from a prospective database. Data were analyzed using Fisher exact test, Kaplan-Meier method, and log-rank test.
Results:
Three hundred nineteen consecutive patients with T1 lesions were treated by transanal excision (n = 151) or radical surgery (n = 168) over the 17-year period. RAD surgery was associated with higher tumor location in the rectum, slightly larger tumor size, a similar rate of adverse histology, and a lymph node metastasis rate of 18%. Despite these features, patients who underwent RAD surgery had fewer local recurrences, fewer distant recurrences, and significantly better recurrence-free survival (P = 0.0001). Overall and disease-specific survival was similar for RAD and TAE groups.
Conclusion:
Despite a similar risk profile in the 2 surgical groups, patients with T1 rectal cancer treated by local excision were observed to have a 3- to 5-fold higher risk of tumor recurrence compared with patients treated by radical surgery. Local excision should be reserved for low-risk cancers in patients who will accept an increased risk of tumor recurrence, prolonged surveillance, and possible need for aggressive salvage surgery. Radical resection is the more definitive surgical treatment of T1 rectal cancers.
T1 adenocarcinoma of the rectum can be treated by local excision or radical surgery, but there is little comparative data to assess outcomes and quantify risk. In this study of 319 patients, the patients undergoing radical surgery had fewer local recurrences, fewer distant recurrences, and significantly better recurrence-free survival.
Local excision is an important treatment option for T1 adenocarcinomas of the rectum.1–3 When accomplished by transanal excision, local excision has low morbidity, avoids permanent colostomy, and preserves anorectal function.4,5 In contrast, radical surgery, even in expert hands, carries significant risk of perioperative morbidity and may compromise long-term bowel and sexual function.6–13
The concern about local excision is whether it can be offered to patients with confidence that treatment results are equivalent to radical resection. Recent publications have documented a variable risk of tumor recurrence after transanal excision of T1 rectal cancers.1–3 Local recurrence rates have been reported as low as 0% to 4%1,2,14,15 and as high as 17% to 31%,16–19 raising the question of what factors account for this variation in outcome results. Tumor size, depth of penetration, regional lymph node detection, grade, vessel invasion, tumor fragmentation, and margin status have all been used to define patient populations at varying risk of tumor recurrence after local excision.20,21 Despite such selection criteria, local excision does not remove regional lymph nodes and thus inevitably carries the risk of unresected regional disease and incomplete pathologic staging. At present, preoperative imaging cannot completely exclude the risk of occult regional lymph node metastasis.22,23 Furthermore, salvage resection for recurrence frequently requires multiorgan resection and appears to have only a modest cure rate.19,24,25
No randomized trials of local excision and radical resection have been performed, and there is little comparative data in the literature to assess cancer outcomes and quantify risk. We performed a retrospective evaluation of patients with T1 rectal cancers treated by either transanal excision or radical resection at our institution to assess patient selection, cancer recurrence, and survival.
METHODS
All patients who underwent surgery for T1 adenocarcinomas of the rectum (0–15 cm from anal verge) by either transanal excision (TAE) or radical resection (RAD) as definitive surgical treatment between January 1987 and January 2004 were identified from a prospective database. Review of clinicopathologic features and follow up of all patients for this study was approved by the Institutional Review Board. Pathology reports and operative notes were reviewed to confirm patient age, gender, operative procedure, tumor size and location, and absence of preoperative chemotherapy or radiotherapy.
In all cases, the preoperative workup included endoscopic examination of the rectum, biopsy of the tumor, computed tomography scan of the abdomen and pelvis, and chest x-ray. Tumor location was recorded as the distance from the anal verge to the lower edge of the tumor. Height above the anal verge was recorded whenever possible from the surgeon's initial clinic notes. For 33 cases, the clinic note was missing or did not document tumor location, and for these cases, location was assigned based on the operative report or data entered in a prospective clinical database. In cases of local excision, endorectal ultrasound was performed routinely.
Local excision was performed under general anesthesia using either dorsal lithotomy or the prone jackknife position. Tumors were excised from the rectal wall using electrocautery. Full-thickness excision was performed in all cases. Radical surgery was performed as total mesorectal excision. The rectum and mesorectum were dissected sharply in the areolar spaced between the visceral and parietal fascial planes of the pelvis. The length of the distal resection margin below the lower edge of the cancer was variable based on the surgeon's judgment of adequate tumor clearance. Pathologic features were recorded from the original pathology report. Tumor grade was assigned as poor, moderate, or well based on the extent of glandular architecture within the tumor.26 Vessel invasion was scored as present when dysplastic cancer cells were identified within the lumen of blood vessels or lymphatic vessels. Tumor size was assigned as the largest diameter recorded on the original pathology report. If a particular morphologic feature such as lymphovascular invasion was not mentioned, then that feature was presumed to be absent. In 25 cases, tumor size was not recorded and is therefore unknown.
After completion of therapy, patients were followed according to the preference of their physicians. Most patients were examined at 3- to 6-month intervals for the first 3 years. The excision site was routinely monitored by digital and endoscopic examination, and a minority of patients had surveillance with endorectal ultrasound. Tumor recurrences were documented from clinic notes, radiology reports, and pathology reports. Local recurrence (LR) was defined as any tumor recurrence within the true pelvis. Distant recurrence (DR) was defined as any tumor metastasis identified outside the true pelvis. The pattern of recurrence was assigned as local, local plus distant, or distant and was based on all sites of disease documented within 6 months from the first evidence of disease recurrence. Salvage resection for LR was recorded.
The Kaplan-Meier estimates of overall and disease-specific survival time in various prognostic factor categories were calculated and compared by means of the log-rank test. Differences of P < 0.05 were considered significant. Time to recurrence or death was calculated from the date of surgery to date of first recurrence, death, or last follow up. Multivariable analysis of recurrence was performed using Cox regression analysis. The association of variables with type of operation was tested using Fisher exact test for dichotomous covariates and the t test for continuous variables. Statistical analysis was performed using SPSS software (Chicago, IL).
RESULTS
Three hundred nineteen consecutive patients with T1 lesions were treated by transanal excision (n = 151) or radical surgery (n = 168) over the 17-year period. The T1 rectal cancer cases were distributed evenly over the 17-year accrual period. There was a trend toward increased use of local excision in the early and mid-1990s. Of the 319 patients, 34 had a local or distant recurrence of cancer. Among the patients with recurrence, 11 remain with no evidence of disease (NED), 7 are alive with disease (AWD), and 16 are dead of disease. Thirteen patients died of other causes. The median follow up was 51 months.
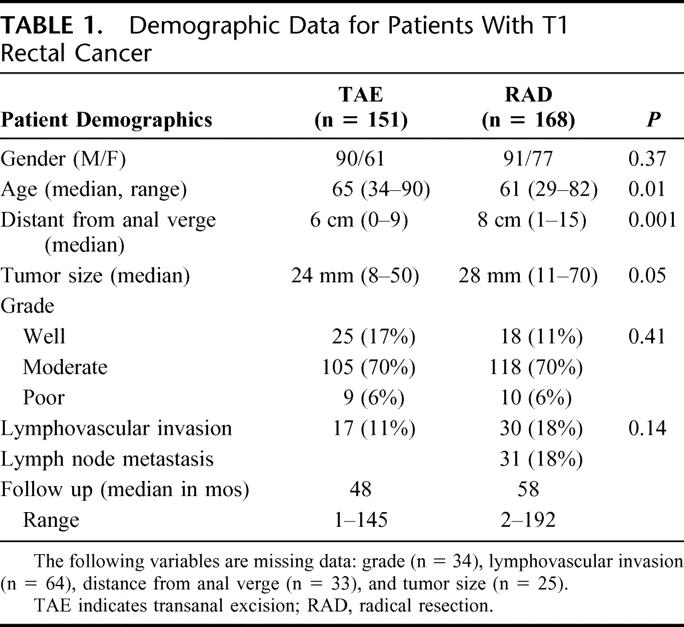
The clinical features of the study population are described in Table 1. The median tumor size was 2.4 cm for patients who underwent TAE and 2.8 cm for patients in the radical surgery group (P = 0.05). The median tumor location recorded as the distance from the anal verge for the TAE and radical surgery groups was 6 cm and 8 cm (P = 0.001), respectively. High-risk pathology was found with equal frequency, with lymphovascular invasion or poorly differentiated (high-grade) tumor identified in 11% and 6% of patients undergoing local excision and in 18% and 6% of patients undergoing radical surgery (P = 0.14 and P = 0.41, respectively).
TABLE 1. Demographic Data for Patients With T1 Rectal Cancer
The use of multimodality adjuvant therapy for these patients is summarized in Table 2. In general, patients receive 46.8 Gy to the whole pelvis followed by a boost to 50.4 Gy according to previously published techniques.27 For those who received combined modality therapy, most received concurrent 5-FU/leucovorin using either bolus or continuous infusion. In the local excision cohort, the indication for adjuvant radiotherapy was the presence of high-risk pathology. In the radical surgery cohort, the indication was positive mesenteric lymph nodes. Full-dose, adjuvant systemic chemotherapy was used exclusively in the radical surgery group for patients with positive lymph nodes. Twenty-nine patients with positive lymph nodes received adjuvant chemotherapy, and 16 received radiation therapy. No patients treated by local excision received adjuvant systemic chemotherapy as part of their initial treatment.
TABLE 2. Multimodality Therapy of T1 Rectal Cancer

The estimated 5-year recurrence rate for all patients was 12% (95% confidence interval [CI], 8–16%). At 5 years, the estimated overall recurrence rate was 23% (95% CI, 13–29%) for the TAE group and 6% (95% CI, 2–9%) for the radical surgery group (P < 0.001) (Fig. 1). The median time to any recurrence for all patients who recurred was 19.5 months (range, 4–78 months). Time to any recurrence was typically longer in the TAE group (median, 22 months; range, 4–78 months) than in the RAD group (median, 16 months; range, 8–48 months). At 5 years, the estimated local recurrence rate was 15% for patients treated with local excision and 3% for patients treated with radical surgery (P = 0.0001). The actuarial rates of distant recurrence at 5 years for the TAE and radical surgery groups were 12% and 3%, respectively (P = 0.01).
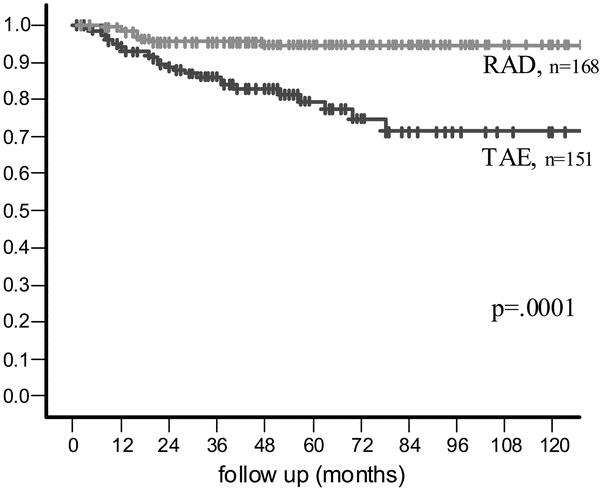
FIGURE 1. Recurrence-free survival.
Estimated disease-specific and overall survival rates were similar for RAD and TAE groups. At 5 years, the estimated disease-specific survival rate was 93% for patients undergoing TAE and 97% for the radical surgery group (P = 0.05) (Fig. 2A). At 5 years, the estimated overall survival rate was 89% for patients undergoing TAE and 93% for the radical surgery group (P = 0.17) (Fig. 2B).
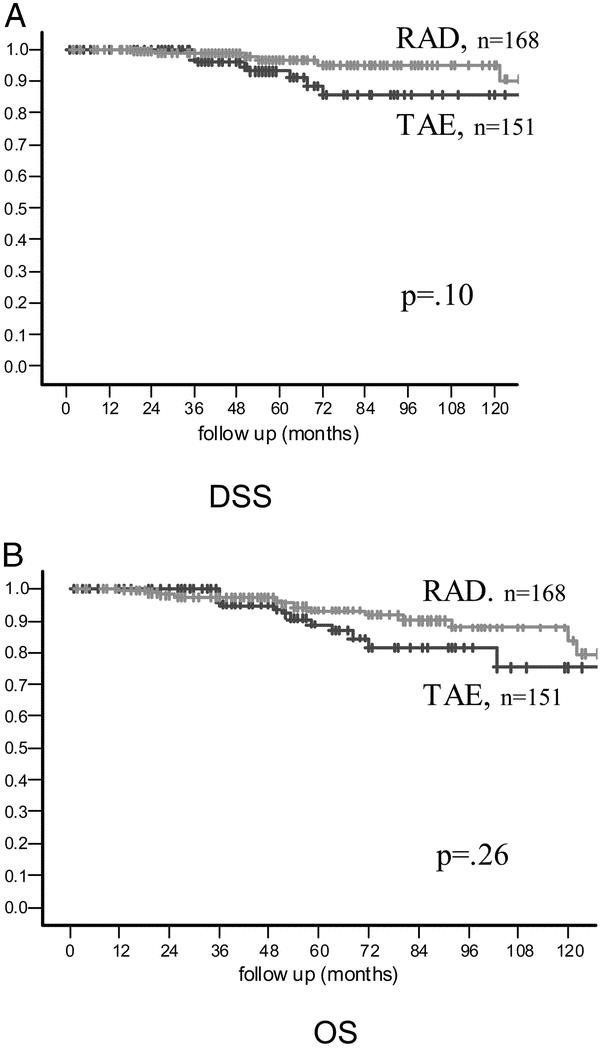
FIGURE 2. (A) Disease-specific survival. (B) Overall survival.
In the local excision group, 26 patients had a recurrence: 14 local recurrences, 5 combined local and distant recurrences, and 7 distant recurrences (Table 3). In the radical surgery group, 8 patients had a recurrence: 4 local recurrences and 4 distant recurrences. Of the patients who recurred after local excision, 16 of 19 patients with local recurrence underwent complete resection, including 3 of 5 patients who also had a distant site of recurrent disease (Table 3). Salvage surgery was used aggressively for both local and distant recurrence (Table 4). Complete resection (CR) was accomplished in 77% of recurrences in the TAE group and 50% of recurrences in the RAD group. In the TAE group, 5-year disease-specific survival (actuarial) after CR for isolated local recurrence (N = 12) was 58% (95% CI, 27–90%). However, among the 16 patients still alive after tumor recurrence, only 10 remain NED at last follow up, whereas 6 are AWD. Therefore, the disease-specific survival rates quoted here are not yet mature and will be considerably lower when the AWD patients ultimately succumb to their recurrent cancer. Six of 14 salvage operations (43%) for local recurrence after TAE resulted in permanent colostomy.
TABLE 3. Pattern of First Recurrence
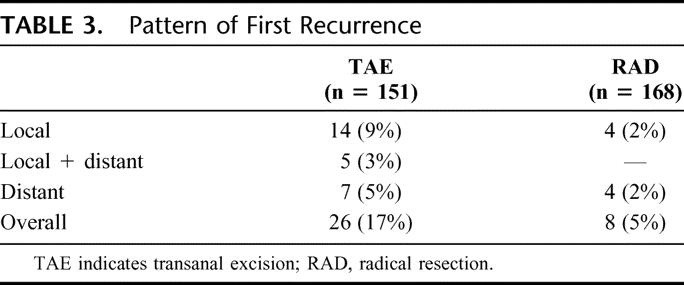
TABLE 4. Surgical Salvage of Patients With Recurrence
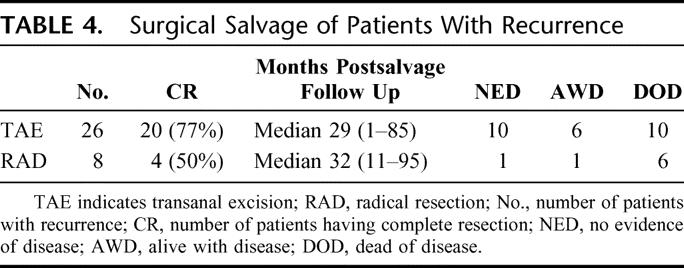
Five parameters, including type of operation, were evaluated for statistical correlation with cancer recurrence (Table 5). On univariate analysis for the entire cohort of 319 patients, advanced age, high-grade cancer, and the presence of lymphovascular invasion were not associated with recurrence of disease. Distance from the anal verge and type of operation were each significant predictors of recurrence (P = 0.002 and 0.0001). On multivariate analysis, only type of operation reached statistical significance as a predictor of recurrence. Statistical analysis of the local excision group alone revealed no statistically significant predictors of recurrence (data not shown).
TABLE 5. Univariate/Multivariate Analysis of Recurrence
DISCUSSION
When considering local excision for fit patients with T1 rectal cancers, surgeons face a dilemma. On the one hand, a large majority (70–85%) of patients are cured by this safe and relatively simple procedure. On the other hand, the risk of cancer recurrence is substantially higher compared with radical surgery. Predictors of who will and who will not recur after local excision are not entirely reliable, and the risk period for recurrence extends beyond 5 years. Therefore, if local excision is performed as definitive therapy, one must rely on prolonged postoperative surveillance and salvage surgery to minimize the impact of increased tumor recurrence on quality of life and survival. Whether aggressive surgical salvage cures enough people to achieve an overall cure rate equivalent to upfront radical surgery is uncertain. This study was undertaken to provide some objective data to address these issues.
In assessing our data, one should recognize it comes from a retrospective study. We sought to compare the risk of tumor recurrence observed in a local excision cohort and a radical surgery cohort treated over the same timeframe at one institution. Secondary objectives were to evaluate patient selection and cancer survival. Because our study is a retrospective analysis and not a randomized trial, it is possible that factors other than the difference in efficacy of the 2 operations influenced the outcome results. Differences in patient selection could play a role. Other potential pitfalls are whether the size of each patient cohort is adequate, use of adjuvant therapy, whether all cancer recurrences and cancer deaths have been detected, and whether the length of follow up is sufficient. Our study is the largest of its kind yet reported in the literature. Our follow up is sufficient to analyze recurrence but may not be sufficient for a confident assessment of ultimate cancer cure rates.
Our data show that although there are clear differences in the selection of patients for local excision versus radical surgery, these differences are of relatively small magnitude. The local excision patients are only slightly older (median, 65 vs 61 years), and their tumors are only slightly smaller (median, 24 mm vs 28 mm) and lie only somewhat lower in the rectum (median, 6 cm vs 8 cm from anal verge). From these data, we conclude that there was a preference for local excision for older patients and for low-lying tumors, whereas radical surgery was favored for larger cancers and for cancers lying higher in the rectum. These observations are similar to previously published reports.16,18 However, it is not evident that these biases in patient selection created either 1) a significant difference in the risk profile in the 2 groups of rectal cancers, or 2) a major impact on the treatment results for each group. No difference was found in the frequency of adverse tumor pathology (grade or lymphovascular invasion) in the 2 surgical cohorts. Median patient follow up was over 4 years and was actually longer in the radical surgery group. On multivariate analysis, the dominant predictor of tumor recurrence was the type of surgery performed. These data present a compelling argument that the differences we found in cancer recurrence rates are the result of the superior efficacy of radical resection as a surgical treatment of T1 rectal cancer.
As observed in prior studies, the major problem with local excision is the high rate of local recurrence. We observed a 15% actuarial rate of local failure at 5 years (19 of 151 patients) compared with 3% (4 of 168 patients) for radical surgery.
This difference is not surprising given that local excision neglects the substantial risk of spread to regional lymph nodes by T1 rectal cancers. In the current study, 18% of patients undergoing radical surgery were observed to have at least one lymph node metastasis in the resected specimen, and we speculate there was a similar rate of occult lymph node spread in the local excision cohort. It should be recognized that the rectum is an unfavorable site for local excision. T1 adenocarcinomas of the rectum have a substantially higher risk of lymph node spread (15–25%) than T1 adenocarcinomas located more proximally in the colon (3–8%).28,29 This is most likely a reflection of differences in cancer biology between cancers that arise in the proximal versus the distal colon.30,31 Of considerable interest, our study also showed a higher 5-year actuarial rate of distant recurrence for the local excision group (12% actuarial, 7 of 151 patients) compared with the radical resection group (3% actuarial, 4 of 168 patients).
What reasons can we point to for the superior treatment results observed for radical surgery? The first and most obvious is the therapeutic benefit of wide lymphadenectomy. As demonstrated by Takahashi, the regional spread of superficial rectal cancer is nearly always confined to the mesorectum.32 Thus, a properly performed rectal resection can provide local control for most of early rectal cancers, even when regional lymph nodes are involved.10,12 A second reason is that radical surgery provides far superior staging because it identifies the patients with positive lymph nodes. These patients can then be selected for treatment with adjuvant radiotherapy and adjuvant chemotherapy with the expectation of significant benefit.33 On the other hand, because the lymph node status of individual patients is unknown in the local excision group, there is no proven method for directing adjuvant therapy to patients who truly need it. We speculate that the combination of unresected occult nodal disease and inadequate adjuvant therapy are the 2 factors that explain the higher rate of local and distant recurrence we have documented in the local excision group.
We observed similar rates of disease-specific survival and overall survival for the local excision and radical surgery cohorts. Thus, one can argue that when the impact of aggressive surgical salvage is considered, local excision does not represent a significant compromise in treatment of T1 rectal cancer. Indeed, our data show that among the 26 patients who recurred after local excision, 77% were able to have a complete resection of disease and 50% were still alive at last follow up with a median follow up of 34 months from recurrence. However, we believe our follow up is not adequate to assure the durability of salvage surgery. At last follow up for the 26 patients who recurred after local excision, 10 were dead of disease, 6 were AWD, and only a minority (10 patients) remained NED. We suspect that with longer follow up, our data will reveal inferior disease-specific survival in the group of patients treated by TAE.
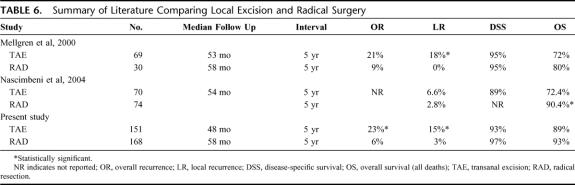
There are 2 previously published comparisons of local excision and radical resection for T1 rectal cancers (Table 6). Investigators at the University of Minnesota compared 69 local excision cases (all T1) with 30 radical resection cases (all T1N0).16 Their 5-year actuarial rates for local recurrence for local excision (18%) and radical surgery (0%) are nearly identical to our data. Despite a large difference in recurrence rates, they could not document a significant difference in disease-specific or overall survival. However, the median follow up after salvage resection was only 2.9 years, and the investigators questioned the durability of surgical salvage. Recent data from the Mayo Clinic did not show a significant difference in recurrence rate for local excision (70 patients) versus radical resection (74 patients) but did demonstrate better disease-free and overall survival (median follow up of 8.1 years) for radical surgery.18 The benefit of radical surgery was greatest for deep T1 lesions that invaded to the lower third of the submucosa. Unlike our study, these 2 studies excluded all or some of the radical surgery cases with positive lymph nodes.
TABLE 6. Summary of Literature Comparing Local Excision and Radical Surgery
In summary, we have observed that the overall rate of postoperative tumor recurrence for T1 rectal cancers treated by local excision is approximately 3 to 5 times higher than for T1 rectal cancers treated by radical surgery. This difference appears to be primarily the result of the therapeutic benefit of regional lymphadenectomy, but may also reflect superior staging and better use of adjuvant therapy for patients treated by radical resection. Tumor recurrence after local excision can frequently be resected, but less than half of the patients with recurrence are ultimately cured. Cancer-specific survival at 5 years is over 90% for both local excision and radical surgery groups. However, the survival rates diverge somewhat after 5 years, raising suspicion that the cancer cure rate of the local excision group is less durable over time. A larger study with longer follow up is required to address the question of cure beyond 5 years.
Based on the available data, what can we conclude about the appropriate use of local excision for T1 rectal cancer in fit patients? We believe the data favor radical surgery as the more definitive cancer treatment but do not eliminate local excision as a reasonable choice for many patients. Careful case selection remains paramount. Local excision should be reserved for low-risk cancers in patients who will accept an increased risk of tumor recurrence, a prolonged period of postoperative cancer surveillance, and the important role played by salvage surgery. Through the informed consent process, the surgeon and patient must select the operation best suited to the patient's goals and expectations for rectal cancer treatment.
Discussions
Dr. David A. Rothenberger (Minneapolis, Minnesota): It has been said that misery loves company, so when Dr. Paty called me to ask if I would discuss his paper I readily accepted.
As you noted, in 2000 our group at the University of Minnesota published the results of a similar although smaller experience with T1 rectal cancers treated only by local excision showing what we thought was a somewhat surprising and shocking 18% local recurrence rate. I guess this is an example of the ultimate transparency discussed earlier today by our president.
For a time we stood out as the only group reporting such dismal results. And we wondered whether we would retain that devious distinction forever. But subsequent reports from other centers, including the Cleveland Clinic and Memorial Sloan-Kettering Cancer Center, have shown similar equally worrisome outcomes following local excision of rectal cancer.
We certainly would agree with the conclusions offered in this retrospective review of their 319 patients with T1 rectal cancers. If local excision is used in isolation, the risk of recurrence is significantly higher rate than that observed after radical surgery alone. If there is a silver lining, it is that both your study and our study showed no differences in overall and disease-specific survivals. However, as you point out and as we pointed out, the numbers are small, the follow-up is short, and caution is certainly in order.
I have a few questions for the authors.
First, only 15% of your cases experienced either death or recurrence and I am concerned that there is not adequate power to detect a difference between these 2 groups unless that difference is very large. What did your statistician tell about the power to detect differences in these 2 groups?
Secondly, what is the distribution of cases over the 17 years that you did this study? The median follow-up was 51 months, and yet your patients were accrued over a larger period of time than the median would suggest.
Thirdly, I am certain that the indications and enthusiasm for local excision varied considerably over the 17-year period of your study and perhaps the techniques of local excision and radical surgery may also have changed. Did you attempt to assess the impact of surgery on your outcomes?
Fourthly, the retrospective use of prospectively collected databases has limitations. Did you have a single pathologist review the pathology slides? Did you encounter missing data? If so, what assumptions were made regarding the missing data? That is, did you consider it missing at random, ignorable or nonignorable?
Finally, could you comment on the type of local recurrences observed in your series and do you believe that neoadjuvant chemoradiation could decrease the local recurrences and make local excision a more attractive alternative for therapy of these distal favorable cancers?
I enjoyed the paper. It confirms much of our earlier work. I again send a word of caution to all of our members about using local excision in isolation.
Dr. Philip B. Paty (New York, New York): I would like to thank Dr. Rothenberger for his very excellent questions.
The first question was about the number of cases and the power of the study. You have to distinguish between recurrence and survival. In our study with 34 recurrences and a hazard ratio of over 5, the study was adequately powered for recurrence. For survival, we observed 29 deaths, only 16 of them cancer deaths. The hazard ratio for overall survival was 1.6. So the power of the study was not adequate to evaluate survival, hence my reservations about concluding too much from the survival data.
Based on our data, one would need 120 cancer deaths to show a survival difference at a P value less than 0.05 and 80% power. One hundred twenty deaths represents a study size about 4-fold larger. So an adequately powered study would require close to a thousand patients with 5- to 10-year follow-up. So it would take a big study with substantial follow-up.
Your second question was about the distribution of cases over the 17-year period. The cases were fairly evenly distributed, about 18 to 20 per year. What did change somewhat was the ratio between radical surgery and local excision. There was more enthusiasm for local excision in the early to mid 1990s. Over the past 5 years when we recognized the higher recurrence rates, and also the fact that adjuvant postoperative radiation doesn't reliably prevent local failure, the enthusiasm for local excision has diminished.
Regarding the year of surgery as predictor of occurrence, we did not look at that. I can say that crudely the recurrences and survivals are distributed all through the timeperiod.
Regarding the use of a retrospective database and missing data, the biggest limitation was looking at the pathological parameters: grade and vessel invasion. We did have missing data. We did review the clinical files and pathology reports in all cases. But the slides were not reviewed, so we didn't have standard pathological assessment.
The one other important variable is that sometimes we noted variable information on the height of the tumor above the anal verge. What was said in the clinic note might be different than the operative note. In those cases we accepted the number at initial assessment in the clinic.
Finally, you mentioned the type of local recurrence seen in our series. We did not record whether these were intramural recurrences or extramural. In my own experience the majority are true pelvic recurrences, primarily in the mesentery. However, the great majority are still resectable. So the recurrences tend to be in the central pelvis, not in the pelvic sidewall.
As far as the role of neoadjuvant therapy, I am enthusiastic about it to some extent. In the radical surgery literature we see that adjuvant radiation has a greater impact when it is given preoperatively than postoperatively. It is more efficient in reducing recurrences, and may even have some impact on survival. On the other hand, the cost of therapy if we are going to treat everybody with preoperative chemoradiation is going to be very high. This strategy also ignores the fact 70% of the patients are cured by local excision, we just don't know who they are. So if we had better ways to stratify patients I think that would be very important.
Dr. Harold J. Wanebo (Providence, Rhode Island): Dr. Paty, I compliment you and your group for pointing out the persistent recurrence rate which others have demonstrated with use of local excision. And obviously the interest in this has waxed and waned over the last years.
My question to you is: Should we develop a better algorithm for local excision in these patients? Is there a role for neoadjuvant therapy even for T1, and especially the early T2 patients? This raises the question of the use of ultrasound and other imaging techniques to demonstrate the slight differences clinically between T1 and T2, especially in view of the fact that 19% of the patients in the radical resection group had lymph node metastases that suggested these patients are basically under treated by local excision. In view of the data from Hama-Gaber of Brazil that suggests a certain number of rectal cancer patients can have a complete response with neoadjuvant therapy, such patients may not require resection. Could this approach be used similar to the algorithm of the Nigro protocol for early ano-rectal squamous cancer? So I raise this question: Whether it is worth exploring neoadjuvant therapy with selective excision in early rectal cancer? Your group would appear to have the numbers to initiate such a trial.
Dr. Philip B. Paty (New York, New York): I agree with you that we need a better algorithm. However, algorithms require data to make decisions and I think we don't have great data to stratify small cancers into high risk and low risk groups.
Your point about the nodal metastases and whether they can be imaged preoperatively is very relevant. To my review of the literature, neither endorectal ultrasound nor endorectal MRI has sufficient reliability to stratify patients. However, there is insufficient published data on large numbers of T1 cancers with imaging and pathologic correlations on a node-by-node basis.
So I agree, a better algorithm is needed. But exactly what that algorithm is has yet to be determined. I think molecular markers could be valuable but would require substantial work to validate them.
Dr. Merril T. Dayton (Buffalo, New York): Dr. Paty, the fairly high recurrence rates that you quote in your paper could relate to any one of a couple of factors. One is obviously the 15% of these early tumors that had positive lymph nodes from the metastases. But the other possible factor could be a technically inadequate operation in which the margins were too small or one didn't resect deep enough. I didn't see anything in your paper regarding margins. Would you address what you did to rule out inadequate margins as a possible reason for the recurrences here and what role margins may play in the recurrences that you observed?
Dr. Philip B. Paty (New York, New York): That is an excellent question. We had a positive margin based on the pathology reports in 4 cases of local excision, which is about a 3% rate. However, all of these cases had immediate salvage resection and are in the radical surgery group. I think margins are a concern, although when you have excised what looks like a relatively stable piece of tissue from the rectal wall, it can fall apart when it comes out. You have to pin it out, there can be a little bit of pulling and tearing of the tissues. So margins are somewhat difficult to assess.
The reason I think that margins are not the number 1 issue is that, in my experience, the failures tend to be mesenteric, not intramural failures. These T1 cancers are not penetrating through the muscular wall, they are contained within the rectum. We do a full thickness excision. So these recurrences are either due to the implantation of shed cancer cells, or there is lymphatic dissemination beyond the rectal wall. I don't think extending local excision an additional centimeter will assure a better outcome.
Dr. William W. Turner, Jr. (Jackson, Mississippi): This is a thought-provoking analysis of radical excision versus transanal excision with salvage surgery. The piece that is missing for me to translate this to care is the incidence of complications following the 2 operations and some analysis of patient satisfaction. That would clearly tip the scale in 1 direction or another. If you have preliminary information in that regard, it would be helpful.
Dr. Philip B. Paty (New York, New York): I agree this is a 1-sided presentation looking only at cancer endpoints, and the benefit of local excision is clearly in the other direction. I can't present to you specific complication data, the excellent functional recovery from TAE but I think is well documented in the literature. There is a huge difference compared to radical surgery. I also don't think that our data or any other published data necessarily contraindicates local excision. We hope our data will be helpful in coming to an agreement with patients during the informed-consent process about the risks and in making informed judgments.
Footnotes
Reprints: Philip B. Paty, MD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021. E-mail: patyp@mskcc.org.
REFERENCES
- 1.Steele GD Jr, Herndon JE, Bleday R, et al. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol. 1999;6:433–441. [DOI] [PubMed] [Google Scholar]
- 2.Bleday R, Breen E, Jessup JM, et al. Prospective evaluation of local excision for small rectal cancers. Dis Colon Rectum. 1997;40:388–392. [DOI] [PubMed] [Google Scholar]
- 3.Kim HK, Jessup JM, Beard CJ, et al. Locally advanced rectal carcinoma: pelvic control and morbidity following preoperative radiation therapy, resection, and intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1997;38:777–783. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberger DA, Garcia-Aguilar J. Role of local excision in the treatment of rectal cancer. Semin Surg Oncol. 2000;19:367–375. [DOI] [PubMed] [Google Scholar]
- 5.Kim DG, Madoff RD. Transanal treatment of rectal cancer: ablative methods and open resection. Semin Surg Oncol. 1998;15:101–113. [DOI] [PubMed] [Google Scholar]
- 6.Rothenberger DA, Wong WD. Abdominoperineal resection for adenocarcinoma of the low rectum. World J Surg. 1992;16:478–485. [DOI] [PubMed] [Google Scholar]
- 7.Petrelli NJ, Nagel S, Rodriguez-Bigas M, et al. Morbidity and mortality following abdominoperineal resection for rectal adenocarcinoma. Am Surg. 1993;59:400–404. [PubMed] [Google Scholar]
- 8.Williamson ME, Lewis WG, Finan PJ, et al. Recovery of physiologic and clinical function after low anterior resection of the rectum for carcinoma: myth or reality? Dis Colon Rectum. 1995;38:411–418. [DOI] [PubMed] [Google Scholar]
- 9.Averbach AM, Chang D, Koslowe P, et al. Anastomotic leak after double-stapled low colorectal resection. Dis Colon Rectum. 1996;39:780–787. [DOI] [PubMed] [Google Scholar]
- 10.Enker WE, Havenga K, Polyak T, et al. Abdominoperineal resection via total mesorectal excision and autonomic nerve preservation for low rectal cancer. World J Surg. 1997;21:715–720. [DOI] [PubMed] [Google Scholar]
- 11.Havenga K, Enker WE, McDermott K, et al. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg. 1996;182:495–502. [PubMed] [Google Scholar]
- 12.Enker WE, Merchant N, Cohen AM, et al. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg. 1999;230:544–552; discussion 552–554. [DOI] [PMC free article] [PubMed]
- 13.Schmidt CE, Bestmann B, Kuchler T, et al. Ten-year historic cohort of quality of life and sexuality in patients with rectal cancer. Dis Colon Rectum. 2005. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez QH, Heslin MJ, Shore G, et al. Results of long-term follow-up for transanal excision for rectal cancer. Am Surg. 2003;69:675–678; discussion 678. [PubMed]
- 15.Varma MG, Rogers SJ, Schrock TR, et al. Local excision of rectal carcinoma. Arch Surg. 1999;134:863–867; discussion 867–868. [DOI] [PubMed]
- 16.Mellgren A, Sirivongs P, Rothenberger DA, et al. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064–1071; discussion 1071–1074. [DOI] [PubMed]
- 17.Garcia-Aguilar J, Mellgren A, Sirivongs P, et al. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg. 2000;231:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nascimbeni R, Nivatvongs S, Larson DR, et al. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47:1773–1779. [DOI] [PubMed] [Google Scholar]
- 19.Paty PB, Nash GM, Baron P, et al. Long-term results of local excision for rectal cancer. Ann Surg. 2002;236:522–529; discussion 529–530. [DOI] [PMC free article] [PubMed]
- 20.Morson BC, Bussey HJ, Samoorian S. Policy of local excision for early cancer of the colorectum. Gut. 1977;18:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleday R. Local excision of rectal cancer. World J Surg. 1997;21:706–714. [DOI] [PubMed] [Google Scholar]
- 22.Brodsky JT, Richard GK, Cohen AM, et al. Variables correlated with the risk of lymph node metastasis in early rectal cancer. Cancer. 1992;69:322–326. [DOI] [PubMed] [Google Scholar]
- 23.Blumberg D, Paty PB, Guillem JG, et al. All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum. 1999;42:881–885. [DOI] [PubMed] [Google Scholar]
- 24.Friel CM, Cromwell JW, Marra C, et al. Salvage radical surgery after failed local excision for early rectal cancer. Dis Colon Rectum. 2002;45:875–879. [DOI] [PubMed] [Google Scholar]
- 25.Weiser MR, Landmann RG, Wong WD, et al. Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum. 2005. [DOI] [PubMed] [Google Scholar]
- 26.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. [DOI] [PubMed] [Google Scholar]
- 27.Wagman R, Minsky BD, Cohen AM, et al. Conservative management of rectal cancer with local excision and post-op radiation ± chemotherapy. Int J Radiat Oncol Biol Phys. 1999;44:841–846. [PubMed] [Google Scholar]
- 28.Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–206. [DOI] [PubMed] [Google Scholar]
- 29.Okabe S, Shia J, Nash G, et al. Lymph node metastasis in T1 adenocarcinoma of the colon and rectum. J Gastrointest Surg. 2004;8:1032–1039; discussion 1039–1040. [DOI] [PubMed]
- 30.Delattre O, Olschwang S, Law DJ, et al. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet. 1989;2:353–356. [DOI] [PubMed] [Google Scholar]
- 31.Breivik J, Lothe RA, Meling GI, et al. Different genetic pathways to proximal and distal colorectal cancer influenced by sex-related factors. Int J Cancer. 1997;74:664–669. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Ueno M, Azekura K, et al. Lateral node dissection and total mesorectal excision for rectal cancer. Dis Colon Rectum. 2000;43(suppl):S59–68. [DOI] [PubMed] [Google Scholar]
- 33.Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388–396. [DOI] [PubMed] [Google Scholar]
- 34.Tepper JE, O'Connell M, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control—final report of intergroup 0114. J Clin Oncol. 2002;20:1744–1750. [DOI] [PubMed] [Google Scholar]