Abstract
Objective:
A previous study of patients with stage I to III breast cancer showed that those patients whose tumors were in the highest tertile of eIF4E overexpression experienced a higher risk for recurrence. This study was designed to determine whether high eIF4E overexpression predicts cancer recurrence independent of nodal status by specifically targeting patients with node-positive disease.
Methods:
The prospective trial was designed to accrue 168 patients with node-positive breast cancer to detect a 2.5-fold increase in risk for recurrence. eIF4E level was quantified by Western blots as x-fold elevated compared with breast tissues from noncancer patients. End points measured were disease recurrence and cancer-related death. Statistical analyses performed include survival analysis by the Kaplan-Meier method, log-rank test, and Cox proportional hazard model.
Results:
One hundred seventy-four patients with node-positive breast cancer were accrued. All patients fulfilled study inclusion and exclusion criteria, treatment protocol, and surveillance requirements, with a compliance rate >95%. The mean eIF4E elevation was 11.0 ± 7.0-fold (range, 1.4–34.3-fold). Based on previously published data, tertile distribution was as follow: 1) lowest tertile (<7.5-fold) = 67 patients, 2) intermediate tertile (7.5–14-fold) = 54 patients, and 3) highest tertile (>14-fold) = 53 patients. At a median follow up of 32 months, patients with the highest tertile had a statistically significant higher cancer recurrence rate (log-rank test, P = 0.002) and cancer-related death rate (P = 0.036) than the lowest group. Relative risk calculations demonstrated that high eIF4E patients had a 2.4-fold increase in relative risk increase for cancer recurrence (95% confidence interval, 1.2–4.1; P = 0.01).
Conclusions:
In this prospective study designed to specifically address risk for recurrence in patients with node-positive breast cancer, the patients whose tumors were in the highest tertile of eIF4E overexpression had a 2.4-fold increase in relative risk for cancer recurrence. Therefore, eIF4E overexpression appears to be an independent predictor of a worse outcome in patients with breast cancer independent of nodal status.
eIF4E is a protein initiation factor that is overexpressed in breast cancer. The degree of overexpression appears to be of prognostic importance. In this prospective study designed to address the risk for recurrence in patients with node-positive breast cancer, the group with the highest eIF4E overexpression had a 2.4-fold increase in relative risk for recurrence. eIF4E overexpression appears to be a prognostic marker independent of nodal status.
In women, breast cancer is the most common malignancy and is the second leading cause of cancer death.1 Current American Joint Committee on Cancer (AJCC, 6th edition) staging of breast cancer is based on the TNM status (Tumor size, presence of Nodal disease, presence of Metastasis) at diagnosis.2 At present, the single most important prognosticator for breast cancer outcome is the status of the axillary lymph nodes.3,4 Unfortunately, even in patients with node-negative breast cancer, 20% will develop systemic disease.3,5 Conversely, long follow up of node-positive patients demonstrated that up to 35% of patients do not develop systemic disease.3 Thus, clinicians deciding on adjuvant therapy based on tumor size and nodal disease continue to long for more refined prognostic markers that can better stratify patients with breast cancer and their risk for cancer recurrence.
The evidence for the importance of translation control in malignant transformation and tumor progression is accumulating. Translation control refers to the regulatory control of mRNA translation into polypeptides. Important in this regulatory role are the initiation factors, one of which is eukaryotic initiation factor 4E, or eIF4E.6
eIF4E is a 25 Kilodalton (Kd) cap-binding protein. It recognizes the 7-methylguanosine cap in the 5′ untranslated regions (5′ UTRs) of mRNAs. By binding to the cap, eIF4E facilitates the attachment of the “RNA helicase complex,” eIF4F.7,8 Figure 1 illustrates the cap-binding protein eIF4E and its attachment to the mRNA cap at the start of the 5′ UTR. This facilitates eIF4F binding and the subsequent recruitment of ribosomes to initiate protein synthesis. The binding of eIF4F leads to the unwinding of the 5′ UTR and hence the reduction of steric hindrance associated with long 5′ UTRs. eIF4E is limited in quantity; thus, eIF4E binding is a rate-limiting step in translation initiation in mRNAs with long and/or complex 5′ UTRs.9,10

FIGURE 1. This figure illustrates eIF4E binding with the 7-methylguanosine cap at the start of the 5′ untranslated region (UTR) (represented by the wavy line before the AUG codon). Binding by eIF4E facilitates the binding by the “RNA helicase complex,” eIF4F. This leads to the unwinding of the 5′ UTR and hence the reduction of steric hindrance, allowing the recruitment of the ribosomes and other machinery of protein synthesis.
eIF4E elevation does not result in global increase in protein synthesis; rather, only mRNAs with long 5′ UTRs appear to be upregulated.11 Some of these upregulated gene products are associated with malignant transformation and include cyclin D1,12 angiogenic factors such as VEGF and FGF2,13 and TLK1B, a Tousled-like kinase that appears to confer radioresistance in malignant cell lines.14 Additionally, transformed but nonmalignant cell lines such as CREF and NIH3T3 cells acquire malignant phenotypic changes when eIF4E overexpression is induced through a BK viral eIF4E transfection.10,15,16
In human carcinoma, Kerekatte et al were the first to report that eIF4E is elevated in breast carcinoma specimens, but not in benign breast tissue from noncancer patients.17 Subsequently, others have reported the presence of eIF4E elevation in head and neck squamous cell carcinoma,18 mesenchymal tumors and sarcomas,19 as well as in colorectal cancer specimens.20 Additionally, the degree of eIF4E overexpression may have prognostic significance.21
In our initial retrospective study, 59 breast cancer specimens were examined for eIF4E overexpression. High eIF4E overexpression was defined as elevation of eIF4E that is greater than 7-fold higher compared with benign breast tissue from noncancer patients. In the 38 patients whose tumors had high eIF4E overexpression, 14 patients experienced cancer recurrence. In contrast, only one of 21 patients recurred in the group with low eIF4E (defined as less than 7-fold elevated).21
Small retrospective studies are limited by their inherent potential biases. Thus, a follow-up prospective trial was designed to study whether eIF4E overexpression predicts cancer recurrence in patients with stage I to III breast cancer. One hundred ninety-one patients were prospectively accrued with treatment and surveillance protocols standardized to ensure compliance and study homogeneity. The degree of eIF4E overexpression was grouped into tertile distribution. Patients whose tumors were in the highest tertile of eIF4E overexpression (defined as >15-fold elevated) were 7.2-fold more likely to have cancer recurrence than those patients with the lowest eIF4E tertile (defined as <7-fold elevated). On multivariate analysis for cancer recurrence risk, nodal status and high eIF4E elevation were independent predictors.
One critical limitation of that study was although high eIF4E overexpression appears to be an independent predictor of cancer recurrence, the patients studied had stage I to III disease. As such, it included node-positive as well as node-negative patients. Thus, there remains the possibility that eIF4E cosorted with nodal disease and thus predicted a worse clinical outcome. By studying only patients with node-positive breast cancer in a prospective trial, this current study will test the hypothesis that high eIF4E predicts cancer recurrence independent of nodal status.
METHODS
Based on prior data and pretrial power analysis, it was determined that 168 patients with node-positive breast cancer were needed to detect a statistically significant 2.5-fold increase in relative risk for cancer recurrence in the group of patients whose tumors were in the highest tertile of eIF4E overexpression versus the lowest. The research protocol was approved by our Institutional Review Board (IRB) before patient accrual. Patient treatment and surveillance protocols were standardized to ensure study homogeneity and compliance. Definitive surgical therapy consisted of either a modified radical mastectomy or breast conservation therapy (lumpectomy with tumor-free margin, axillary lymph node dissection and breast irradiation; a subset of patients with T1 lesion were subjected to sentinel node biopsy, with complete axillary lymph node dissection reserved only for those patients with positive sentinel node[s]). Adjuvant axillary irradiation, systemic chemotherapy, and antiestrogen therapy were offered and administered as indicated per current standards.
The surveillance protocol consisted of a history and physical examination every 3 months for 3 years, every 6 months in years 4 and 5, and annually thereafter. Patients had annual mammograms, chest x-rays, complete blood count, and liver function tests. Additional laboratory and radiographic evaluation was performed as indicated by abnormal findings. End points for this study were cancer recurrence (locoregional, distant, or both) and death. Clinical data were accrued and recorded prospectively, including demographic data, stage of disease, treatment rendered, surveillance protocol compliance, and study end points.
Tissue Procurement
Patients were approached before definitive treatment by our staff to solicit consent for study enrollment. Only patients with pathologically proven node-positive disease after definitive surgery were accrued for the study. From each patient, a cancer specimen of at least 100 mg was obtained from the tumor core at the time of surgical intervention. This was identified and verified by the study pathologist (FA) and immediately frozen in liquid nitrogen and stored at −70°C. Using a unique code, each specimen was tracked. A set of note cards, accessible only to the principal investigator, linked the specimen code with patient data. The codes were revealed only at final data analysis to link the eIF4E data with patient outcome to avoid inadvertent biases.
Assay for eIF4E
The Western blot assay for eIF4E protein level has been previously described in detail.17,21 Briefly, protein lysate from each breast specimen was prepared using 10 mg of tissue cut into tiny pieces, suspended in 0.5 mL RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 50 mM Tris [pH 8.0], 0.1 mM PMSF), and mechanically macerated using a Savant Bio 101 Fastprep FP120 system (Savant Instruments, Inc., Holbrook, NY). Centrifugation was then performed at 10,000 g for 10 minutes (at 4°C). Protein concentration was determined using the BCA protein assay kit (Pierce, Rockford, IL) against a standard graph of known BSA protein concentrations and standardized.
Equivalent quantity of protein lysate from each breast specimen (20 μg diluted in 1:10 RIPA) were loaded onto and separated by using 4% to 20% denaturing gel Tris HCl polyacrylamide gel electrophoresis. Electroblotting onto a nylon membrane (Immobilon PVDF; Millipore, Bedford, MA) was then performed and the membranes blocked with 5% nonfat milk for 1 hour. Primary incubation of the membrane was carried out using a 1:500 dilution monoclonal mouse antieIF4E antibody (Transduction Laboratories, San Diego, CA). Secondary incubation of the membrane was carried out using a 1:1000 dilution of goat antimouse horseradish peroxidase conjugate. Blot development was then accomplished using Opti 4CN (4-chloro-1-naphthol; Bio-Rad Laboratories, Hercules, CA). Using the Biophotonics system (Biophotonics Corp., Ann Arbor, MI), the blots were scanned and the band intensity evaluated using Intelligent Quantifier software (Bio Image, Ann Arbor, MI). Quantification of eIF4E level in each cancer specimen was expressed relative to: 1) a standard curve generated from known concentrations of eIF4E, and 2) as x-fold elevated over a control from a breast tissue specimen of a noncancer patient (Fig. 2). Triplicates of each specimen were run and the results were averaged.

FIGURE 2. This is a Western blot for eIF4E quantification. Lanes 2, 3, and 4 are the 3 internal controls with known eIF4E concentrations. Lanes 5 through 9 are varying degrees of eIF4E overexpression in cancer specimens diluted at a ratio of 1:10. Lane 10 is a benign control from a noncancer patient that was used as baseline eIF4E expression.
Hormone Receptor Assay
The receptor status determination for estrogen (ER) and progesterone (PR) was accomplished using immunohistochemical staining. This was performed on a Dako autostainer using standard protocols. Slides were evaluated using the Automated Cellular Imaging System. ER and PR results were reported based on the degree and intensity of nuclear staining with a positive result defined as greater than or equal to 10%.
Statistical Analysis
Data was collected and stored in secured computers using Microsoft Excel for eIF4E data and Microsoft Access for clinical data. Statistical analyses were performed using StatView software (SAS Institute, Inc.). Survival analysis was performed using the Kaplan Meier method. The log-rank test was used to compare survival data. Determination of relative risk for cancer recurrence was performed using Cox proportional hazard model. Chi-squared test was used to correlate T stage and N stage eIF4E overexpression as grouped by tertile distribution.
RESULTS
One hundred seventy-four patients were prospectively accrued for the study. The mean overexpression of eIF4E was 11.0 ± 7.0-fold (mean ± standard deviation). Compliance with the treatment protocol was 94%. Compliance with the surveillance protocol was achieved in 99% of participating patients. The median age of the study population was 51 years of age at the time of diagnosis. The median follow up was 31 months (Table 1).
TABLE 1. Demographics
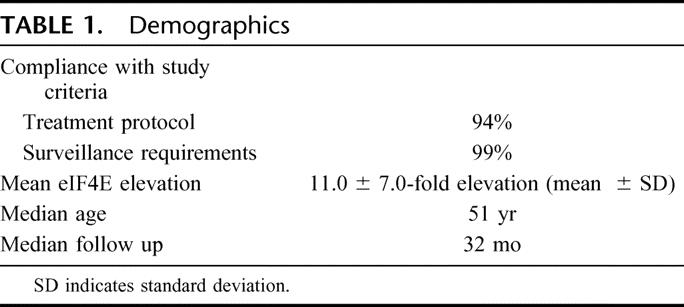
Figure 2 is a typical Western blot for eIF4E quantification. Lanes 2, 3, and 4 are the 3 internal controls with known eIF4E concentrations used to generate a standard curve for eIF4E quantification. Lane 10 is from benign tissues of noncancer patients used as a baseline for eIF4E expression to determine the relative elevation of eIF4E in cancer specimens (expressed as x-fold overexpression). Cancer specimen protein lysates, diluted at a ratio of 1:10, are shown in lanes 5 through 9. Varying degrees of eIF4E overexpression can beseen.
Figure 3 is a pie chart grouping of patients by tertiles based on the degree of eIF4E overexpression. We have previously established a 7.5-fold and a 14-fold elevation as cutoffs for low, intermediate, and high eIF4E overexpression.22 We maintained these cutoffs for our present study. There were 39% of patients with low eIF4E (less than 7.5-fold) elevation. Thirty-one percent (31%) of patients had intermediate eIF4E overexpression (7.5–14-fold). Thirty percent (30%) of patients had high eIF4E overexpression (greater than 14-fold). Thus, the cutoffs as previously determined conformed well to the tertile distribution (39%, 31%, and 30%, respectively).
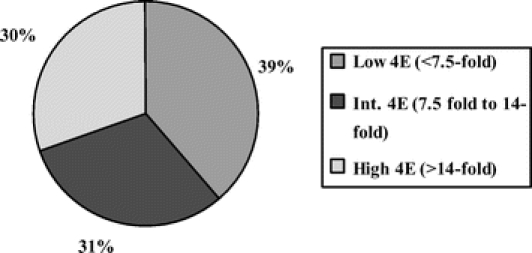
FIGURE 3. This is a pie chart representation of eIF4E overexpression grouped by tertile distribution. There were 39% of patients whose tumors had low eIF4E overexpression (less than 7.5-fold), 31% of patients whose tumors had intermediate eIF4E overexpression (7.5–14-fold), and 30% of patients whose tumors had high eIF4E overexpression (greater than 14-fold).
The breakdown of patients as grouped by tumor size (T stage) is shown in Table 2: 1) T1 lesions—35 patients, 2) T2 lesions—95 patients, 3) T3 lesions—31 patients, and 4) T4 lesions—13 patients. The majority (54.6%) of patients had T2 lesions. There is no correlation between T stage and the degree of eIF4E overexpression grouped by tertile distribution (chi-squared test).
TABLE 2. T stage, N stage, and eIF4E
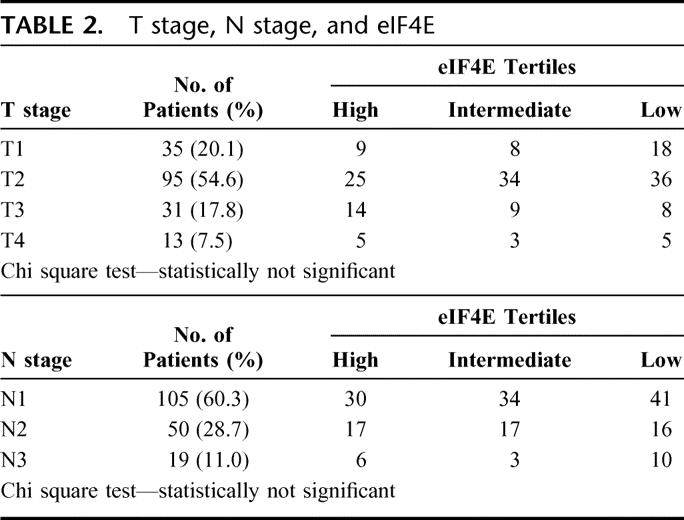
The breakdown of patients by nodal stage is also shown in Table 1: 1) N1—105 patients, 2) N2—50 patients, and 3) N3—19 patients. The majority (60.3%) of the patients had N1 disease. There is no correlation between N stage and the degree of eIF4E overexpression grouped by tertile distribution (chi-squared test).
At a median follow up of 31 months, there have been 65 cancer recurrences and 51 deaths (Table 3). Of the recurrences, 14 were locoregional, 46 were systemic, and 5 were locoregional and systemic. As would be expected for patients with node-positive disease, most recurrences were systemic. In patients whose tumors had low eIF4E overexpression, only 15 of 67 patients (22%) developed systemic disease; in contrast, 29 of 53 patients (55%) whose tumors had high eIF4E overexpression developed systemic disease.
TABLE 3. Cancer Outcomes

Multivariate analysis was performed comparing eIF4E, T stage, tumor grade, positive ER status, and positive PR status for risk of cancer recurrence (Table 4). Except for high eIF4E overexpression in the tumor specimen, none of the other variables predicted cancer recurrence.
TABLE 4. Cox Multivariate Analysis for Cancer Recurrence
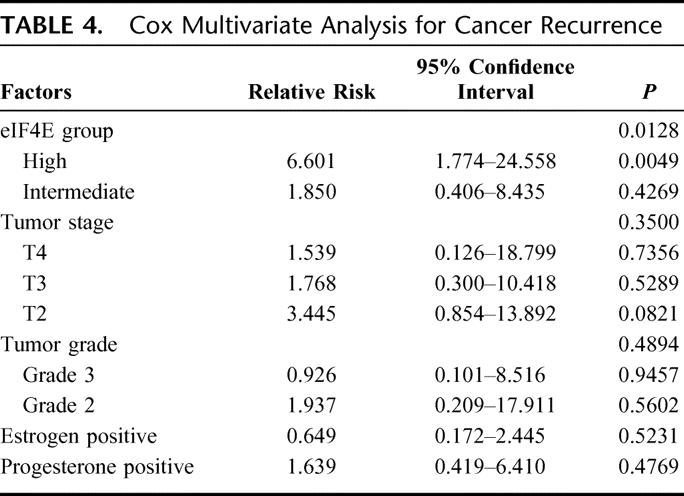
Figure 4 shows the Kaplan-Meier disease-free survival (DFS) curve for the different tertiles of eIF4E overexpression. As shown, patients whose tumors were in the low eIF4E tertile had a statistically significant lower rate of cancer recurrence when compared with patients whose tumors were in the high eIF4E tertile (P = 0.023, log-rank test). The actuarial 5-year DFS for patients whose tumors were in the low eIF4E tertile was 64.3% versus 47.8% for patients whose tumors were in the high eIF4E tertile. The relative risk calculation for cancer recurrence, using the Cox proportional hazard model, is shown in Table 5. Compared with patients whose tumors were in the low eIF4E tertile, patients whose tumors were in the high eIF4E tertile had a 2.4-fold (1.21–4.08, P = 0.01) increase in relative risk for cancer recurrence.
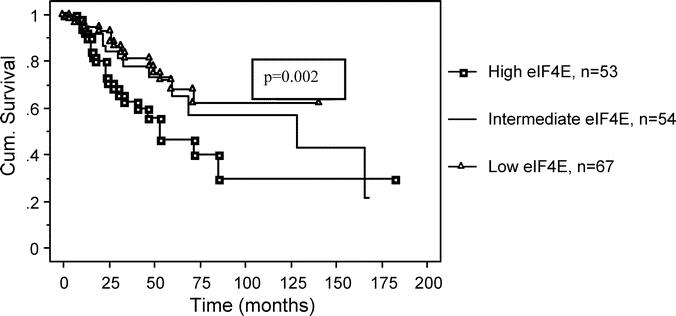
FIGURE 4. This is a Kaplan-Meier graph comparing disease-free survival (DFS) for all tertiles of eIF4E overexpression. Patients whose tumors were in the lowest eIF4E tertile had a lower rate of cancer recurrence when compared with patients whose tumors were in the highest eIF4E tertile (P = 0.023, log-rank test). The actuarial 5-year DFS for patients whose tumors were in the low eIF4E tertile was 64.3% versus 47.8% for patients whose tumors were in the high eIF4E tertile.
TABLE 5. Relative Risk: eIF4E Overexpression and Cancer Recurrence
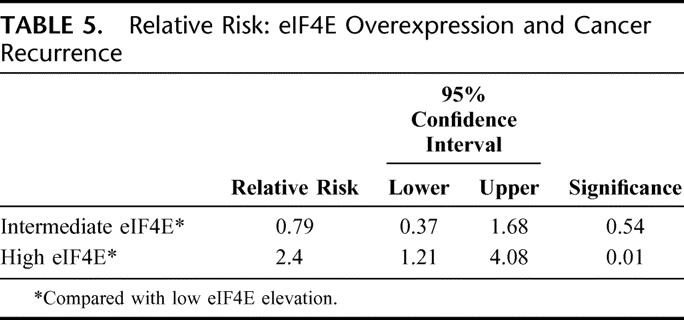
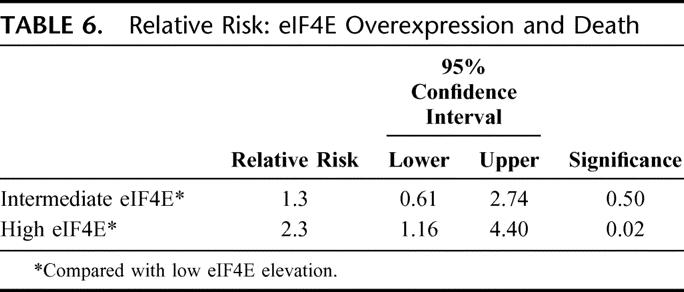
Figure 5 shows the Kaplan-Meier overall survival (OS) curve. Similar to the DFS curve, patients whose tumors were in the high eIF4E overexpression tertile had a higher rate of death than patients whose tumors were in the low and intermediate tertiles (P = 0.036, log-rank test). The actuarial 5-year OS for patients whose tumors were in the low eIF4E tertile was 68% versus 46.5% for patients whose tumors were in the high eIF4E tertile. As shown in Table 6, patients whose tumors were in the high eIF4E tertile had a 2.3-fold higher relative risk of death (1.16–4.4, P = 0.02) than patients whose tumors were in the low eIF4E tertile.
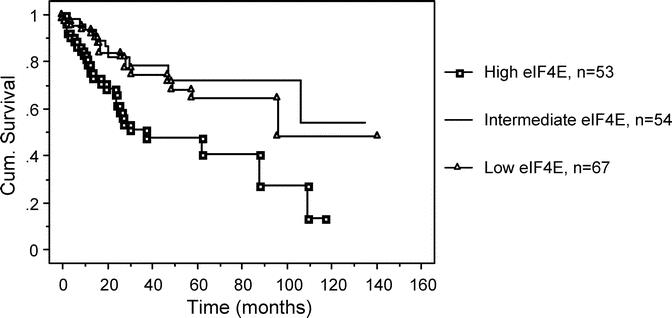
FIGURE 5. This is a Kaplan-Meier graph comparing overall survival (OS) for all tertiles of eIF4E overexpression. Patients whose tumors with the highest eIF4E tertile had a higher rate of death than patients whose tumors had low and intermediate tertiles (P = 0.036, log-rank test). The actuarial 5-year OS for patients whose tumors were in the low eIF4E tertile was 68% versus 46.5% for patients whose tumors were in the high eIF4E tertile.
TABLE 6. Relative Risk: eIF4E Overexpression and Death
DISCUSSION
The search for a “molecular signature,” in addition to AJCC TNM staging, that will allow clinicians to better stratify breast cancer patients’ risk for recurrence continues. Although adjuvant chemotherapy has demonstrable efficacy, with annual odds reduction for recurrence of 25% to 35%, there are well-documented associated morbidities as well as mortalities.23 Furthermore, even in patients with node-positive breast cancer, as high as 35% of patients will not develop systemic disease.3 Therefore, identifying a subset of patients at lower risk for developing recurrence may be helpful in treatment decisions.
eIF4E is critical for translation initiation of mRNAs with long 5′ UTRs. The overexpression of eIF4E can induce malignant phenotypic changes in cell lines. Additionally, cDNA subtraction library of mRNAs in cell lines with inducible eIF4E overexpression have identified a number of gene products that are known oncogenes or are commonly associated with malignant transformation. These include VEGF, cyclin D1, TLK1B, and c-myc.12–14,16 Thus, it was not surprising that eIF4E has been found to be overexpressed in a variety of human malignancies.7
More importantly, the presence of eIF4E overexpression in the cancer specimen,22 or in the surrounding histologically normal tissue and stroma,25 may hold prognostic significance. Nathan et al reported that in patients with head and neck squamous cell carcinoma resected with curative intent, if eIF4E overexpression was present in the histologically tumor-free surgical margins, the patients were 8 times more likely to recur than those whose surgical margins did not have eIF4E overexpression.25
In a prospective trial of patients with stage I to III breast cancer, we found that patients whose cancer specimens were in the highest tertile of eIF4E overexpression were at a 7.2-fold higher risk for cancer recurrence than those with low eIF4E overexpression. The patients in that study were treated and kept on surveillance for cancer recurrence with a high compliance rate. Treatment variability was kept to a minimum. Multivariate analysis suggested that high eIF4E overexpression and the presence of nodal disease were independent predictors for cancer recurrence. That study, however, included node-positive as well as node-negative patients. Therefore, there was still the possibility that high eIF4E overexpression was a surrogate marker for nodal disease and thus associated with a worse clinical outcome. The current study was designed prospectively to examine exclusively patients with node-positive breast cancer to test our hypothesis, that high eIF4E overexpression predicts a worse cancer outcome, independent of nodal status.
In this study, DFS analysis using the Kaplan-Meier method demonstrated that patients whose tumors were in the lowest eIF4E tertile had a statistically significant lower rate of cancer recurrence when compared with those patients whose tumors were in the highest eIF4E tertile (P = 0.023, log-rank test). Similarly, patients whose tumors had the highest eIF4E overexpression had more deaths than those patients whose tumors had the lowest eIF4E overexpression (P = 0.036, log-rank test). As such, patients whose tumors with high eIF4E overexpression had a 2.4-fold (1.21–4.08, P = 0.01) increase in relative risk for cancer recurrence and a 2.3-fold relative risk for death (1.16–4.4, P = 0.02) when compared with patients whose tumors had low eIF4E overexpression.
An important finding in this study is that patients with node-positive breast cancer are not a homogeneous group in their tumor behavior. At present, 123 of 174 (71%) have not developed systemic disease. In patients with low eIF4E, only 15 of 67 (22%) have developed systemic disease. This figure is quite similar to that of patients with stage I breast cancer. Further examination of this data set demonstrated that eIF4E overexpression was not correlated with T stage or N stage. Indeed, multivariate analysis demonstrated that tumor size, grade, and receptor status did not predict cancer recurrence.
In contrast, 29 of 53 (55%) patients with high eIF4E overexpression have developed systemic disease. This subset of patients behaved more like patients with advanced stage breast cancer. Because tumor size and N stage (ie, N1 vs N2 vs N3) did not correlate with the degree of eIF4E overexpression, the fact that these patients with high eIF4E overexpression did poorly cannot be fully attributed to bulkier tumors (T3 or T4 lesions) or higher degree of nodal disease (N2 and N3). Thus, the degree of eIF4E overexpression played an independent role in further identifying risk for cancer recurrence.
Some recent investigators have evaluated the use of multigene assays to predict cancer recurrence. Paik et al reported on patients with node-negative breast cancer treated with tamoxifen.26 Using RNA extracts from paraffin-embedded tumor specimens of the National Surgical Adjuvant Breast and Bowel Project B-14 trial (NSABP B14) tissue archive, the levels of expression of 16 cancer-related genes were used to calculate a risk for recurrence score. These were grouped as low, intermediate, and high risk. They reported that based on the expression profile of this 16 genes, a high-risk score portends a higher likelihood of distant recurrence.
The use of multiple genes to assess risk for recurrence is an attractive idea, because cancer is a “multigene” disease. Although eIF4E overexpression may be a dysregulation of a single gene, it plays a critical role in the initiation of mRNA translation. Thus, its dysfunction alters multiple gene products downstream. Therefore, whether a single dysfunctional gene with multiple downstream regulatory effects is better at predicting cancer outcome, or relative risk calculations derived from multiple gene profiles, remains to be determined.
A critical limitation of the report by Paik et al was that it remains unclear if the worse clinical outcome associated with a specific recurrent risk profile is associated with the actual natural biologic behavior as expressed by that particular array of genes, or if that unique profile actually predicts response to tamoxifen therapy.15 Similarly, all of our patients received adjuvant therapy. We do not have the data to address whether eIF4E is a prognostic versus a “therapeutic” marker. A parallel trial of patients with node-negative breast cancer currently underway, which contains patients who did not receive adjuvant therapy, may help answer the poignant question: Does eIF4E overexpression serve as a therapeutic marker to determine potential response to adjuvant therapy?
The findings in this study need to be validated in a large-scale clinical trial such as afforded by the NCI Cooperative Group Programs. If confirmed, the clinical implications are not inconsequential. Low eIF4E patients may be spared aggressive adjuvant therapy and hence avoid the associated risks. Patients with early disease but high eIF4E may be targeted for intensive adjuvant therapy to lower their risk for recurrence.
Additionally, that eIF4E is overexpressed in breast cancer, but not in benign tissue, may be exploited therapeutically. Tumor-specific molecular target-directed therapy holds great clinical promise, like for example, in the development of imatinib mesylate (Geevec) and kit-associated gastrointestinal stromal tumors (GIST).27,28 DeBenedetti et al has reported on the effectiveness of eIF4E as a tumor-specific target. By splicing the 5′ UTR of FGF-2 upstream of the herpes simplex thymidine kinase (HSV-UTK) gene and subsequently cloning this into the BK-shuttle episomal vector, they were able to limit the expression of HSV-TK to cells that overexpress eIF4E. Using a murine adenocarcinoma cell line, they were able to show reduction in subcutaneous implanted tumors and lung metastases after transfection with BK-UTK and subsequent treatment with ganciclovir (GCV).29
More recently, Mathis et al demonstrated that HSV-TK expression could successfully be limited to cells that overexpress eIF4E. Cell lines, which were found to exhibit a normal level of eIF4E expression failed to express HSV-TK after transfection with the Ad-HSV-UTK vector and displayed minimal toxicity to therapeutic levels of GCV.30 To that end, a rat model for metastatic adenocarcinoma has been developed. We are currently examining the use of an adenovirus with the HSV-UTK (Ad-HSV-UTK) suicide gene construct as a target-specific therapeutic modality for rats after cytoreductive surgery. The introduction of Ad-HSV-UTK, thus allowing only malignant cells with eIF4E overexpression to produce thymidine kinase, and the administration of sequential GCV into a minimal residual disease animal may improve DFS.
In conclusion, patients whose tumors had the highest tertile of eIF4E overexpression had a 2.4-fold increase in relative risk for cancer recurrence. In this study, which specifically was designed to evaluate only pathologically node-positive patients and thereby eliminate nodal status as a potential confounder, we found that high eIF4E continues to predict cancer recurrence. Therefore, eIF4E appears to be an independent predictor of a worse outcome in patients with breast cancer, independent of nodal status.
ACKNOWLEDGMENTS
This study was supported in part by a grant from the National Cancer Institute (CA79082). The authors thank Monica Gauthier, BS, MS, RD, and Mousadak Abbas, MD, for their maintenance of the database, and Dr. Mary Lowery-Nordberg for her clinical laboratory assistance.
Discussions
Dr. Nicholas J. Petrelli (Newark, Delaware): Clinicians have always been dependent on tumor size and lymph node status for the decision of adjuvant therapy in breast cancer. However, no one would argue that more accurate prognostic markers that can better stratify breast cancer patients and their risk for cancer recurrence are needed. Translation control, which is the regulatory control of messenger RNA’s translation into polypeptides is important in malignant transformation. Important in this regulatory role are the initiation factors, one of which is 4E.
Dr. Li and his colleagues have involved this study in an orderly fashion from the 59 breast cancer specimens examined for 4E overexpression, then in a series of 191 patients prospectively with Stage I, II, and III breast carcinoma. As you heard, it was demonstrated that 4E overexpression appears to be an independent predictor of breast cancer recurrence. However, because of his previous studies containing both lymph node positive and lymph node negative patients in order to test his hypothesis that 4E predicts cancer independent of nodal status, the present report studies only node positive breast cancer in a prospective trial. As you have heard, he has demonstrated that 4E appeared to be an independent predictor of worse outcome in breast cancer patients independent of nodal status.
I have 4 questions.
One, as would be expected for patients with node positive disease, most recurrences in this prospective series were systemic. However, a small number of patients had local regional recurrence. Do you know if 4E overexpression present in normal breast tissue at the surgical margin that was histologically negative can hold prognostic significance?
Two, as you are aware, Soon Paik and associates of the NSABP have reported the cancer-related gene microarray panel in node negative postmenopausal breast cancer patients treated with Tamoxifen from NSABP B14 with the ability to calculate a risk score of recurrence into low, intermediate, and high risk groups. Since breast cancer is a multi-gene disease, is evaluating the dysfunction of a single gene felt to be as predictive as a panel described by Paik and associates?
Thirdly, because of the inherent biases in single institution trials, it is important that successful results be reproduced in the multi-institutional setting, which many times those results are not reproduced. Do the authors have plans to evaluate Initiation Factor 4E in 1 of the NCI Cooperative Group Programs?
Lastly, number 4, what, if any, is the potential therapeutic application of Initiation Factor 4E?
Dr. Benjamin D. Li (Shreveport, Louisiana): Your first question really boils down to, if we looked at the tumor margin, does 4E overexpression predict a higher rate of local recurrence? And the answer is we did, but it doesn’t.
In head and neck cancer, Nathan et al reported that if the head and neck tumors were resected for cure, and that the margin was histologically negative, if you find 4E overexpression at the margin, the local recurrence was something like 8-fold higher than those who did not have 4E overexpression. We did not find a similar finding in breast cancer. Perhaps in breast we have much wider margins, as in greater than 1 centimeter, or perhaps it is because of different cancer biology. But we did not find local recurrence or 4E overexpression at the tumor margin.
The second question is a very good question, regarding whether gene profile is a superior method of determining recurrence. I think the jury is certainly out on that. I think that there are some significant issues with the gene array technology at present. I think it is an exciting and very promising field. But in the study that you mentioned, RTPCR was used to amplify extracted RNA in the tumor tissue from archival blocks. We all know that there are concerns about the stability of RNA and the quantification of archival RNA using amplification by RTPCR.
I want to stress that overexpression of 4E is not just a single oncogene dysfunction; the effect is multiple gene products are downstream. About 10% of mRNAs have long and complex S’UTRs and some of these very gene products are known oncogenes and tumor suppressor genes, as well as factors such as VEGF and FGF-2 et cetera. So dysfunction in 4E overexpression leads to a whole bunch of different gene products downstream that may have significant impact on clinical outcome.
You are absolutely right, sir. We need validation of this trial via the intergroup cancer trial mechanism. And I hope that some of the leadership in this organization would help us get our work introduced into those groups.
Finally, on therapeutics. If the validation tests on 4E suggest that 4E overexpression is an important marker of cancer recurrence independent nodal status, then obviously this would become part of the stratification of risk for potential recurrence and will likely impact therapeutica decisions, ie, who should be receiving adjuvant therapy, etcetera.
Additionally, only malignant cells in breast cancer overexpress 4E. We can certainly exploit that mechanism for therapy. We have linked a long S’UTR to the suicide gene, thymidine kinase. We have cloned that into an adenovirus vector. Using that vector, we have introduced the virus into a rat minimal residual disease model for peritoneal carcinomatosis after cytoreductive surgery. What we hope to do with that model is to study whether cytoreductive surgery and gene therapy plus gangcilovir can improve disease outcome by exploiting malignant cells’ ability to overexpress thymidine kinase.
Dr. Tien C. Ko (Galveston, Texas): I have 2 questions.
First, since all the breast cancer patients in this study are node-positive, they should have received either systemic or hormonal therapy. Have you analyzed each of these 2 subsets of patients to determine whether high levels of eIF4E can predict therapeutic outcome?
The second question is regarding the methodology for detecting eIF4E expression in the clinical setting. In your study, Western blotting analyses were performed on fresh specimen to detect eIF4E expression. This methodology can be difficult to standardize for clinical pathology laboratories. Can you tell us whether there are other methods that are being developed that can effectively detect high eIF4E expression in breast cancer and can be easily applied in a community setting?
Dr. Benjamin D. Li (Shreveport, Louisiana): This is a study that was undertaken where patients are treated with adjuvant therapy per standard protocol or for clinical indications. As such we cannot tell whether 4E overexpression is a predictive factor for response to adjuvant therapy or if H is a true prognostic marker. We have a parallel trial looking at node-negative breast cancer patients. In that study, a large number of patients did not receive chemotherapy. Hopefully we can tease out whether 4E overexpression this is a predictor of chemotherapy response or not.
In terms of Western blot, you are absolutely right. Western blot is a rather time-consuming and complex procedure and we would love to see this quantification test be performed in a relatively short and straightforward manner. To that end we have worked in the past with IHC, or immunohistochemical staining as well as developing an ELIZA assay. Those efforts are still continuing.
Dr. Leigh A. Neumayer (Salt Lake City, Utah): I would like to congratulate you on trying very hard to solve this dilemma that a lot of us face every day. Would there be any utility to looking at the 4E levels in the positive nodes?
Dr. Benjamin D. Li (Shreveport, Louisiana): We did. Unfortunately, lymphocytes inherently have a high variability in 4E overexpression. So that makes quantification in lymph nodes quite difficult as we are not sure of what to do with the results. So we have not used nodal 4E expression in clinical outcome conditions.
Footnotes
This study was supported by NCI Grant CA79082.
Reprints: Benjamin D. Li, MD, FACS, Professor of Surgery, Chief, Surgical Oncology, Louisiana State University Health Sciences Center, and the Feist-Weiller Cancer Center, 1501 Kings Highway, Shreveport, LA 71130.
REFERENCES
- 1.Jemal A, Murray T, Ward E, et al. Cancer Statistics 2005. CA Cancer J Clin. 2005;55:10–30. [DOI] [PubMed] [Google Scholar]
- 2.AJCC Staging Manual, 6th ed. Springer-Verlag; 2002. [Google Scholar]
- 3.Weidner N, Cady B, Goodson WH III. Pathologic prognostic factors for patients with breast carcinoma: which factors are important. Surg Oncol Clin North Am. 1997;6:415–453. [PubMed] [Google Scholar]
- 4.Silverstein MJ, Skinner KA, Lomis TJ. Predicting axillary nodal positivity in 2282 patients with breast carcinoma. World J Surg. 2001;25:767–772. [DOI] [PubMed] [Google Scholar]
- 5.Jatoi I, Hilsenbeck SG, Clark GM, et al. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. 1999;17:2334–2340. [DOI] [PubMed] [Google Scholar]
- 6.Sonenberg N. mRNA 5′ cap-binding protein eIF4E and control of cell growth. In: Hershey JWB, Masters MB, Sonenberg N, eds. Translational Control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996:245–269. [Google Scholar]
- 7.DeBenedetti A, Harris AL. EIF4E expression in tumors: its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999;31:59–72. [DOI] [PubMed] [Google Scholar]
- 8.Rhoads RE, Joshi-Barve S, Rinker-Schaeffer C. Mechanism of action and regulation of protein synthesis initiation factor 4E: effects of mRNA discrimination, cellular growth rate, and oncogenesis. Prog Nucleic Acid Res Mol Biol. 1993;46:183–219. [DOI] [PubMed] [Google Scholar]
- 9.Duncan R, Milburn SC, Hershey JB. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF4E suggest a role in translational control. J Biol Chem. 1987;262:380. [PubMed] [Google Scholar]
- 10.DeBenedetti A, Joshi-Barve S, Rinker-Schaeffer C, et al. Expression of antisense RNA against initiation factor eIF4E mRNA in HeLa cells results in lengthened cell division times, diminished translation rates, and reduced levels of both eIF4E and p220 component of eIF4F. Mol Cell Biol. 1991;11:5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koromilas AE, Lazaris-Karatzas A, Sonenberg N. mRNAs containing extensive secondary structure in their 5′ non-coding region translate efficiently in cells overexpressing initiation factor 4E. EMBO J. 1992;11:4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenwald IB, Lazaris-Karatzas A, Sonenberg N, et al. Elevated levels of cyclin D1 in response to increased expression of eukaryotic initiation factor 4E. Mol Cell Biol. 1993;13:7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kevil C, DeBenedetti A, Payne DK, et al. Translational regulation of vascular permeability factor by eukaryotic initiation factor 4E: implications for tumor angiogenesis. Int J Cancer. 1996;65:786. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, DeFatta R, Anthony C, et al. A translationally regulated tousled kinase phosphorylates histone H3 and confers radioresistance when overexpressed. Oncogene. 2001;20:726. [DOI] [PubMed] [Google Scholar]
- 15.DeBenedetti A, Rhoads RE. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc Natl Acad Sci U S A. 1990;87:8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBennedetti A, Joshi B, Graff JR, et al. CHO cells transformed by the translation factor eIF-4E display increased c-myc expression, but requires overexpression of max for tumorigenicity. Mol Cell Diff. 1994;2:347. [Google Scholar]
- 17.Kerekatte V, Smiley K, Hu B, et al. The proto-oncogene/translation factor eIF4E: a survey of its expression in breast carcinomas. Int J Cancer. 1995;64:27. [DOI] [PubMed] [Google Scholar]
- 18.Nathan CO, Liu L, Li BDL, et al. Detection of the proto-oncogene eIF4E in surgical margins may predict recurrence in head and neck cancer. Oncogene. 1997;15:579. [DOI] [PubMed] [Google Scholar]
- 19.Chu QD, Turnage R, McClusky D, et al. Overexpression of eukaryotic initiation factor 4E (eIF4E) in soft tissue neoplasms. Proceedings of the AACR. 2004;45:4285. [Google Scholar]
- 20.Vazquez SH, Byrnes K, Chu Q, et al. Eukaryotic factor 4E (eIF4E) expression in malignant versus inflammatory colon tissue. Ann Surg Oncol. 2005;12:S90–291. [Google Scholar]
- 21.Li BDL, McDonald JC, Nassar R, et al. Clinical outcome in stage I to III breast carcinoma and eIF4E overexpression. Ann Surg. 1998;227:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li BDL, Gruner JS, Abreo F, et al. Prospective study of eukaryotic initiation factor 4E protein elevation and breast cancer outcome. Ann Surg. 2002;235:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Early Breast Cancer Trialist’s Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 24.Deleted in proof.
- 25.Nathan CO, Franklin S, Abreo F, et al. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J Clin Oncol. 1999;17:2909–2914. [DOI] [PubMed] [Google Scholar]
- 26.Paik S. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. [DOI] [PubMed] [Google Scholar]
- 27.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. [DOI] [PubMed] [Google Scholar]
- 28.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. [DOI] [PubMed] [Google Scholar]
- 29.DeFatta RJ, Chervenak RP, De Benedetti A. A cancer gene therapy approach through translational control of a suicide gene. Cancer Gene Ther. 2002;9:505–512. [DOI] [PubMed] [Google Scholar]
- 30.Mathis JM, Sibley DA, Li J, et al. Cancer-specific targeting of an adenovirus-delivered herpes simplex virus thymidine kinase suicide gene using protein translational control. Cancer Gene Therapy. In press. [DOI] [PubMed]


