Abstract
Objective:
The objective of this study was to summarize the evolution of multivisceral transplantation over a decade of experience and evaluate its current status.
Summary Background Data:
Multivisceral transplantation can be valuable for the treatment of patients with massive abdominal catastrophes. Its major limitations have been technical and rejection of the intestinal graft.
Methods:
This study consisted of an outcome analysis of 98 consecutive patients who received multivisceral transplantation at our institution. This represents the largest single center experience to date.
Results:
The most common diseases in our population before transplant were intestinal gastroschisis and intestinal dysmotility syndromes in children, and mesenteric thrombosis and trauma in adults. Kaplan Meier estimated patient and graft survivals for all cases were 65% and 63% at 1 year, 49% and 47% at 3 years, and 49% and 47% at 5 years. Factors that adversely influenced patient survival included transplant before 1998 (P = 0.01), being hospitalized at the time of transplant (P = 0.05), and being a child who received Campath-1H induction (P = 0.03). Among 37 patients who had none of these 3 factors (15 adults and 22 children), estimated 1- and 3-year survivals were 89% and 71%, respectively. Patients transplanted since 2001 had significantly less moderate and severe rejections (31.6% vs 67.6%, P = 0.0005) with almost half of these patients never developing rejection.
Conclusions:
Multivisceral transplantation is now an effective treatment of patients with complex abdominal pathology. The incidences of serious acute rejection and patient survival have improved in the most recent experience. Our results show that the multivisceral graft seems to facilitate engraftment of transplanted organs and raises the possibility that there is a degree of immunologic protection afforded by this procedure.
Multivisceral transplantation (stomach, pancreaticoduodenal complex, intestine ± liver, kidneys, spleen, and abdominal wall) is a formidable procedure for the treatment of complex abdominal pathologies. Evolution of immunosuppression, graft monitoring, and techniques have decreased the incidence and severity of rejection and improved survival, which is approaching that of other complex organ transplants.
Multivisceral transplantation is the concurrent transplantation of the stomach, pancreaticoduodenal complex, and intestine, with (MVTx) or without (modified multivisceral [MMVTx]) the liver. “Mass homotransplantation of abdominal organs” was introduced in 19601 as a model to study the behavior of a large denervated homograft in which the lymphatic drainage was interrupted. The boldness of the concept was evident in the cataclysmic postoperative course. The longest survival reported in 19 dogs was 9 days.
The first patient to undergo the procedure in 1983 was a previously healthy 6-year-old girl who developed short gut syndrome after a swimming pool accident and was terminally ill from liver failure.2 The child died of hemorrhage immediately after the procedure. No observations beyond the grave technical difficulties were possible.
The first 2 patients to survive beyond the immediate postoperative period were reported in 1989.2,3 It was possible to demonstrate normal function of all the organs under cyclosporine immunosuppression. Unfortunately, both patients developed posttransplant lymphoproliferative disorder (PTLD) and died 192 and 109 days after transplantation. The first patient to achieve hospital discharge was transplanted in December 1989.4 He sustained life at home without any parenteral support and died of metastatic pancreatic cancer 10 months after transplantation.
The Achilles heel of multivisceral transplantation has been its intestinal component. Indeed, it was not until intestinal acute rejection was more effectively prevented and controlled with tacrolimus that it became clinically feasible.5–7 Notwithstanding this, multivisceral transplantation remains a rare procedure. One hundred seventy cases were reported worldwide through May 2003 (Grant D, Smith R, personal communication, April 5, 2005) and have been blended with the growing experience in intestinal transplantation. This study presents a decade experience with 100 such transplants at the University of Miami/Jackson Memorial Hospital.
METHODS
Eras
Our experience is divided into 3 eras based on the evolution of the surgical techniques, immunosuppression and monitoring of the graft: August 1994–December 1997 (first era), January 1998–December 2000 (second era), and January 2001–present (third era).
Patients
We studied patients who received a multivisceral transplant as the primary treatment, whereas patients who received a multivisceral graft for rescue after failure of a prior isolated intestinal or combined liver intestinal transplant were not included. All patients were transplanted and followed by the same group of clinicians throughout the study. Donors were of the same blood type with the exception of one recipient who was blood type AB and received organs from a type B donor. Cytotoxic crossmatch and tissue typing were performed but not considered in the choice of the donors.
Surgical Techniques
The abdominal organs are transplanted in tandem. The full spectrum of the procedure is based on the “cluster” principle,8 which has been the foundation of modern intestinal transplantation. According to the “cluster” principle, all abdominal organs are suspended from a central stem, which is composed of the celiac axis, superior mesenteric artery, and the corresponding portal drainage. The “cluster” is transplanted as a single unit in toto or in part. Detailed descriptions have been reported elsewhere.5,8,9 Briefly, the multivisceral transplant procedure includes 2 stages: resection of the native organs (abdominal exenteration) and implantation of a composite graft. Resection includes all affected abdominal organs, intra- and retroperitoneally. The selection of the organs to be replaced is based on careful pre- and intraoperative evaluation. Particular attention is required in the evaluation of the native liver and kidneys even if they are spared from the primary disease because they may sustain injury from total parenteral nutrition (TPN), intraabdominal sepsis, coagulopathies, medications, or prior surgeries.
In the case of a multivisceral transplant, abdominal exenteration is greatly facilitated by early dearterialization. This is accomplished by mass clamping and division of the celiac axis and superior mesenteric artery just above their takeoff from the aorta. Access to their pedicle is obtained after transection of the esophagus or after exposure of the left renal vein. The dearterialized viscera can then be mobilized. The liver is stripped from the inferior vena cava (IVC) (piggyback technique) or resected en bloc with the IVC (conventional total hepatectomy). Extracorporeal venovenous bypass was not used in this series.
If the native liver is preserved, the hepatic artery, including aberrant branches, is dissected and carefully preserved. The common bile duct and arterial branches, including the gastroduodenal and splenic arteries, are ligated and divided as are the feeding arteries of the organs to be resected. The latter are mobilized and removed after the portal vein is transected at its confluence. The liver can be sustained on arterial flow alone without demonstrable ischemic injury until portal flow is reestablished.
Arterialization of the composite graft is usually performed by anastomosis of the infrarenal aorta of the donor to the infrarenal aorta of the recipient, directly or with an interposition graft. The suprarenal aorta is an alternative site. The venous outflow of an MMVTx is constructed by anastomosis of the graft and native portal veins. By comparison, the venous outflow of an MVTx is the donor inferior vena cava (supra-, infra-, or retrohepatic), which is drained by either a piggyback10,11 or conventional technique.
The gastrointestinal reconstructions are performed using standard surgical techniques. A pyloroplasty is performed routinely because the graft is denervated. During the third era, we have made increasing use of gastrogastrostomy rather than esophagogastrostomy as the preferred proximal gastrointestinal anastomosis to preserve the native esophagogastric junction.
The abdominal wall is often damaged by the underlying pathology (desmoid tumor, trauma, fistulae) and/or multiple surgeries. Abdominal closure is facilitated by selection of donors who are smaller than the recipients, reduction of the size of the graft, and by using plastic surgery techniques.12 However, at times, there are simply not enough tissues available for closure. In these cases, we have used a composite graft of a cadaveric abdominal wall with intact inferior epigastric vessels, which are recovered from the donor en bloc with the iliac vessels.13 This graft is vascularized by anastomosis of the donor and recipient pelvic vessels and can be used as a free flap to cover abdominal wall defects (Fig. 1). It can be performed either contemporaneously or several days after the visceral grafting. In the latter case, the donor is from a separate cadaveric origin.
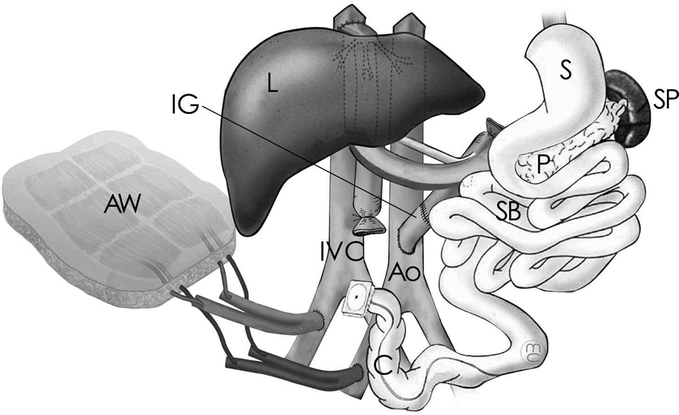
FIGURE 1. Multivisceral transplant, including the small bowel (SB), stomach (S), pancreas (P), liver (L), colon (C), and spleen (SP). Arterial supply is provided by the recipient's aorta (Ao) through an interposition graft (IG). The venous drainage is at the recipient's inferior vena cava (IVC) through the hepatic veins’ confluence (Piggyback technique). An abdominal wall allograft (AW) is used to cover defects of the recipient's abdominal wall.
In the third era, we have used multivisceral transplantation as the procedure of choice for very small babies (<2 years of age) who had extensive abdominal pathologies. Finally, the spleen has been included as part of the multivisceral graft in the third era.
Immunosuppression
Maintenance immunosuppression has been based on tacrolimus (Prograf; Fujisawa Pharmaceuticals, Deerfield, IL) in all patients. In the first era, we were aiming at 12-hour trough tacrolimus levels of 15 to 20 ng/mL. When Daclizumab (Zenapax; Roche Pharmaceuticals, Nutley, NJ) was used for induction,14 target levels were decreased to 10 to 15 ng/mL (second and third eras), and when alemtuzumab (Campath-1H; Berlex Laboratories, Montville, NJ) was used they were decreased to 5 to 10 ng/mL (third era). Tacrolimus was gradually reduced after the third month posttransplantation if there was no rejection.
Steroids (methylprednisolone; Solu-Medrol; Pharmacia and Upjohn Co., Kalamazoo, MI; 1000 mg intravenously at the time of transplantation, followed by 200 mg/d and tapered to 20 mg/d within 5 days for adults and half this dose for children) were used in all cases except with Campath-1H induction. Steroids were gradually withdrawn after the third month posttransplantation if there was no rejection.
Tacrolimus-related toxicity or persistent rejection was treated with dose reduction and addition of sirolimus (Rapamycin; Wyeth-Ayerst, Philadelphia, PA) or mycophenolate mofetil (MMF; CellCept; Roche Pharmaceuticals). Alternatively, tacrolimus was converted to cyclosporine (Neoral; Novartis Pharmaceuticals, East Hanover, NJ) aiming at 12-hour trough levels of 150 to 200 ng/mL.
Induction of immunosuppression was not routinely used during the first era except for sporadic use of muromonab-CD3 (OKT3; Ortho-Biotech, Raritan, NJ) (5 mg/d × 14 days).16 Some of the patients received donor-derived bone marrow infusion (5 × 108 cells/kg).16
During the second era, Zenapax15 was used as induction of immunosuppression at 2 mg/kg at days 0, 7, and 14 and every 2 weeks thereafter during the first 3 months. It was continued at 1 mg/kg every 2 weeks for the next 3 months and then discontinued.
During the third era, we introduced Campath-1H for induction of the immunosuppression. It was administered at 0.3 mg/kg just before and at the end of the transplant procedure and then on posttransplant days 3 and 7. Corticosteroids were given only as premedication for the administration of C1H (Solu-Medrol; methylprednisolone; Pharmacia and Upjohn Co., Peapack, NJ): 500 mg intravenously before the first dose, 250 mg intravenously before the day 3-dose, and 125 mg intravenously before the day-7 dose.17
After April 2004, Campath-1H was administered in just 2 doses of 30 mg on posttransplant days 1 and 4 based on observations of increased intraoperative bleeding in liver transplantation when a preoperative dose was used (unpublished data).
The immunosuppression regimen selected varied by era and by whether the patient was a child or an adult. The composition of graft, including whether an abdominal wall graft was used, did not influence the preferred regimen.
Monitoring of the Graft
In the first era, monitoring of the graft was performed with endoscopies and biopsies only if there was a clinical suspicion of rejection (fever, abdominal distention, increased or decreased or bloody stomal output).
In the second and third eras, we added frequent protocol endoscopies. They were performed twice weekly during the patient's initial posttransplant hospitalization, then weekly over the next 3 months, and then monthly until stoma closure. During the course of any rejection, biopsies were performed at least twice a week. At the same time, we also introduced the routine use of the magnifying endoscope for all but the smallest recipients (<2 years of age).18
The abdominal wall graft was introduced in the third era. Monitoring of the graft was accomplished by visual inspection of the graft skin and by hand-help Doppler assessment of arterial blood flow. Graft skin biopsies were obtained when rejection was suspected on clinical grounds.
We are currently in the process of evaluating citrulline as a serum marker of intestinal injury, including graft rejection.19 Low citrulline levels have been associated with acute rejection. Serum samples and dried blood spots are being collected at the same time as endoscopic biopsies as well as at the planned times of blood testing after the patient's hospital discharge.
Rejection, Diagnosis, Grade of Severity, Duration, and Treatment
Diagnosis
Patients were considered to have a rejection episode if they had a biopsy-proven rejection, which necessitated treatment.
Grades
Acute rejections were classified according to the criteria established in 2003 at the 8th International Small Bowel Transplant Symposium.20 Grades of acute rejection ranged from: 1) no evidence of rejection (grade 0); 2) indeterminate for acute rejection (grade IND) (which in this study was considered as no rejection); 3) acute cellular rejection, mild, grade 1; 4) acute cellular rejection, moderate, grade 2; and 5) acute rejection, severe, grade 3. The severity of each rejection episode was defined by the highest histopathologic grade detected in biopsies during that episode.
Duration
The duration of each rejection episode was defined as the time between the first positive biopsy until the first of a series of 2 or more negative biopsies. In case the patient was treated for rejection and was discharged from the hospital without another biopsy, the time of discharge was considered as the end of the rejection episode.
Treatment
Mild rejections were treated with a bolus (1000 mg Solu-Medrol) and/or a short course of steroids (20–40 mg/day Solu-Medrol for 3–5 days). Tacrolimus levels were readjusted to within the therapeutic range if they were found to be lower than planned. During the first era, moderate rejections were treated with a steroid bolus (1000 mg Solu-Medrol) followed by 200 mg/d Solu-Medrol intravenously, which was tapered to 20 mg/d over 5 days and then slowly decreased as tolerated. Persistent moderate rejections and severe rejections were treated with OKT3 monoclonal antibody (muromonab-CD3; Ortho-Biotec). During the second and third era, moderate and severe rejections were treated with OKT3 monoclonal antibody (muromonab-CD3; Ortho-Biotec) or a repeat dose of Campath-1H.
When rejection of the abdominal wall graft was suspected by visual inspection, the diagnosis was confirmed histologically. Isolated rejection of the abdominal wall was treated in a fashion similar to that for mild rejection of the intestine with a bolus and a short course of steroids.
Infections
Definition
Patients were considered to have an episode of infection when they presented with a positive culture. Blood/catheter cultures were considered positive if there was any microorganism growth/>100 colony-forming units in the culture. Urine culture was considered positive if there were more than 105 microorganisms present. Wound, bronchoalveolar lavage (BAL), and intestinal cultures were not considered positive if there was growth of an unquestionable contaminant (respiratory, normal intestinal flora, coagulase-negative staphylococcus).
A patient was considered to have a viral infection if the virus was detected in a viral culture sample and/or histopathology. Cases of PTLD were analyzed separately and were not included with the viral infections.
Classification
Infections were classified according to the causal agent as bacterial, viral, and fungal. According to the source, they were classified as respiratory, catheter-induced, blood, wound, intraabdominal (abscess, ascites), urinary, and intestinal.
Duration of Infection
The duration of an infection episode was defined as the time from the first positive culture until either the resolution of clinical symptoms or a negative culture. If a patient had 2 or more positive cultures of the same agent that occurred within the same 2-week period, then these cultures were considered to be part of just one infection episode.
Infection Prophylaxis
Cytomegalovirus (CMV) prophylaxis consisted of a combination of ganciclovir (Cytovene; Roche Pharmaceuticals) and cytomegalovirus immune globulin (CytoGam; MedImmune, Gaithersburg, MD) for 1 to 4 months depending on the CMV risk (CMV-negative recipients with a CMV-positive donor received the longest treatment). All patients received Mycostatin (Nystatin; Bristol-Myers Squibb, Princeton, NJ) orally for approximately 1 month posttransplant for prevention of fungal infections. Liposomal amphotericin (Abelcet; Enzon, Piscataway, NJ) was administered after reoperations and until the patient was discharged from the hospital. Cotrimoxazole (Bactrim; Roche Pharmaceuticals), or in case of allergy, dapsone (Avlosulfon; Jacobus Pharmaceuticals, Princeton, NJ), was given routinely for prophylaxis against Pneumocystis carinii.
Graft Versus Host Disease
The diagnosis was based on clinical suspicion and confirmed by biopsy of the involved organs. Treatment of graft versus host disease (GVHD) consisted of an increase in baseline immunosuppression and steroids.
Posttransplant Lymphoproliferative Disorder
PTLD is not considered a single disease, but is rather a syndrome that includes a wide range of atypical hyperplastic and neoplastic lymphocyte growths ranging from a benign indolent form of lymphoproliferation to an aggressive, broadly disseminated disease.21 A predominance of these growths is of B-lymphocyte origin, and a majority contains the Epstein-Barr virus (EBV). Treatment of PTLD included a decrease or withdrawal of immunosuppression, antiviral therapy for EBV-positive tumors, and Rituximab (Rituxan; Genentech, San Francisco, CA, 375 mg/m2 × 4 doses biweekly), which was introduced in 1999.
Primary Graft Dysfunction
Graft loss within the first 2 weeks posttransplantation was for nontechnical and nonimmunologic reasons.
Cause of Death
Deaths were assigned according to the triggering event, which led to the death. For example, a patient who developed severe rejection requiring graft removal and who subsequently died of its consequences was coded as a death as a result of rejection, regardless of the immediate cause of death. On the other hand, patients who died of infectious complications with no ongoing rejection were classified as a death resulting from infection.
An intense effort was made to obtain an autopsy after mortality.
Patient Nutrition and Quality of Life
Data on nutrition of the patients were collected monthly, and data on work and schooling were collected quarterly.
Statistical Methods
The date of last follow up was March 1, 2005. The hazard rates of death (patient survival), intestinal graft failure or death (graft survival), death resulting from rejection, death resulting from nonrejection, development of a severe rejection, and development of any rejection (freedom from rejection) were analyzed. Because there were a sufficient number of patients who died of nonrejection, a multivariable analysis was performed using Cox stepwise regression. Subgroup differences in these hazard rates were graphically displayed by Kaplan-Meier curves. Tests of association were performed using t tests and Pearson (uncorrected) chi-squared tests. Means and estimated survival percentages were reported along with their standard errors. Glomerular filtration rate (GFR) was calculated for each patient over time using distinct formulae for adults22 and children.23
RESULTS
Patients, Immunosuppression, and Techniques
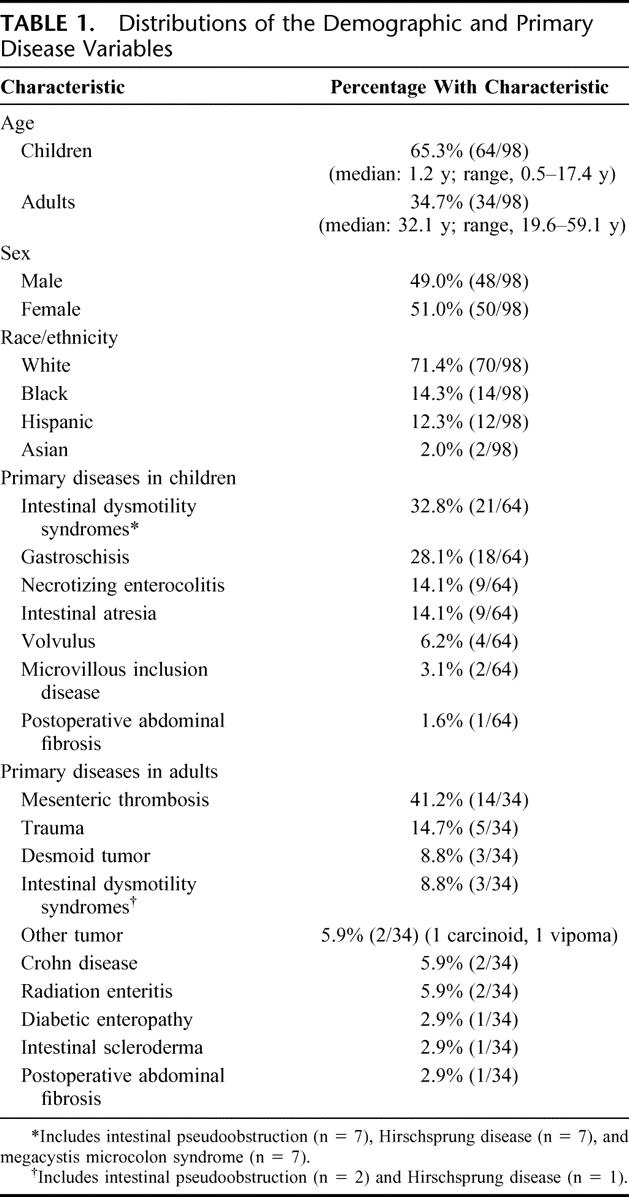
One hundred patients received a primary multivisceral transplant (MTX) at the University of Miami Medical Center between December 1994 and April 2005. We studied 98 patients who underwent transplantation before February 2005 to provide a minimum follow up of 1 month. More than half of the patients, 65% (64 of 98), were children. The most common primary diseases were intestinal gastroschisis and intestinal dysmotility syndromes in children, and mesenteric thrombosis and trauma in adults (Table 1). Organs transplanted were the small intestine and pancreas in all cases and the stomach in 96 of 98 cases. Other organs included in the transplant procedure were the liver (n = 83), large intestine (n = 29), one (n = 6) or 2 (n = 6) kidneys, and the spleen (n = 32). A composite graft of abdominal wall was used for abdominal closure in 5 patients.
TABLE 1. Distributions of the Demographic and Primary Disease Variables
Sixteen patients were transplanted during the first era. Two of these patients received OKT3 as induction. The remaining 14 patients received no induction agent. Eighteen patients were transplanted during the second era. All of these patients received induction with Zenapax. Sixty-four patients were transplanted during the third era. Among the 50 children who were transplanted during this latter era, Zenapax was used as induction in 36 patients and Campath-1H in 14 patients. All of the 14 adults who were transplanted during the third era received induction with Campath-1H. In total, 54 patients in the second and third eras received induction with Zenapax and 28 patients received induction with Campath-1H.
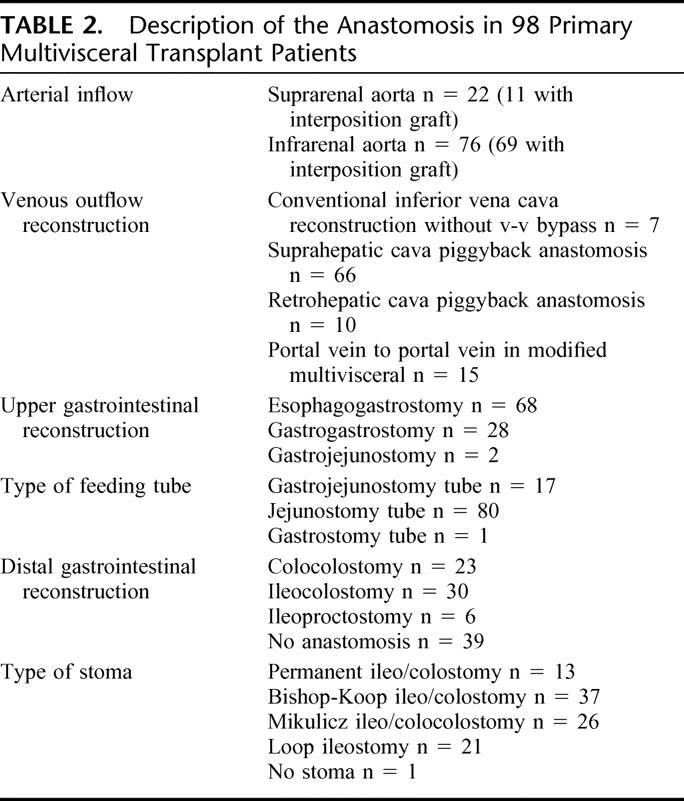
The various types of anastomoses used in the 98 primary multivisceral transplants are shown in Table 2.
TABLE 2. Description of the Anastomosis in 98 Primary Multivisceral Transplant Patients
Patient Survival, Graft Survival, and Cause of Death
Fifty-three patients are alive with a median follow up of 37.5 months (range, 1–116 months). Estimated patient survival at 1, 3, and 5 years was 65% ± 5%, 49% ± 5%, and 49% ± 5%, respectively. Estimated graft survival at the same time points was almost identical (63% ± 5%, 47% ± 5%, and 47% ± 5%).
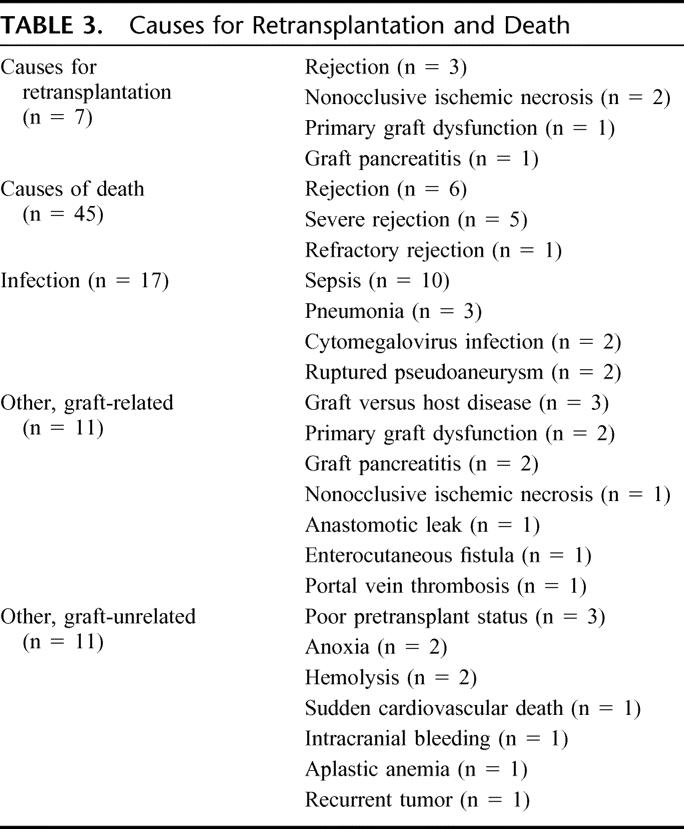
Infection was the leading cause of mortality, followed by rejection (Table 3). Seven patients required retransplantation (Table 3), with 5 of these patients having subsequently died.
TABLE 3. Causes for Retransplantation and Death
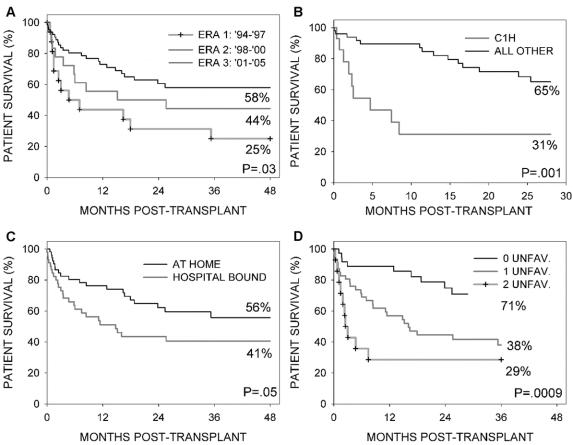
A comparison of patient survival by era (Fig. 2a) indicates a more favorable outcome with the more recent era (P = 0.03). Estimated survival at 1 and 3 years was 44% ± 12% and 25% ± 11% for era 1 (n = 16), 56% ± 12% and 44% ± 12% for era 2 (n = 18), and 73% ± 6% and 58% ± 7% for era 3 (n = 64). Figure 2B shows that among the 64 patients who were transplanted during the third era, 9 of the 14 children who received induction with Campath-1H died within the first year posttransplant and had a significantly poorer survival (P = 0.001). They received their transplants under particularly perilous conditions (8 of them were hospital-bound before the transplant, 6 were less than 1 year of age, and 3 received organs from a neonatal donor (donor <3 months of age). Mortalities in this particular pediatric group were the result of fatal infection (n = 3), rejection, graft pancreatitis, primary graft dysfunction, aplastic anemia, enterocutaneous fistula, and anoxia (one each). The exact role of Campath-1H in the occurrence of this alarmingly high mortality rate could not be determined, and consequently, its use in pediatric intestinal patients was discontinued. Excluding the 14 children who received Campath-1H induction, the estimated survival at 1 and 3 years for the remaining 50 patients in era 3 was 85% ± 5% and 65% ± 8%, respectively.
FIGURE 2. Comparison of patient survival: By era (Fig. A); in the third era between children who received Campath-1H versus all other (B); by pretransplant hospital status (C). According to the number of unfavorable patient characteristics, ie, transplanted before 1998 or being a child who received Campath-1H and being in the hospital pretransplant (D).
There were no significant differences in patient survival according to other organs received, liver versus no liver (P = 0.68), spleen versus no spleen (P = 0.31), between adults and children (P = 0.40), or by induction agent used in the adults (P = 0.56). Patients who were in the hospital fared worse than patients who were at home before transplantation (Fig. 2C, P = 0.05). The proportion of patients who were at home pretransplant did not significantly increase or decrease with era. There was no association of any of these variables with the hazard rate of death resulting from rejection.
A multivariable analysis of death resulting from nonrejection found 3 variables with a significantly unfavorable prognostic value: 1) if the patient was transplanted during the first era (P = 0.006), 2) if the patient was in the hospital immediately before the date of transplant (P = 0.01), and 3) if the patient was a child who received induction with Campath-1H (P = 0.02). The 3 Cox model coefficients were similar in magnitude. Kaplan-Meier survival curves for all deaths according to the number of unfavorable patient characteristics shows a clear separation in prognosis (Fig. 2D, P = 0.0009). Among the 37 patients having no unfavorable characteristics, only 9 deaths were observed. The estimated 1- and 3-year survivals for this subgroup were 89% ± 5% and 71% ± 8%, respectively.
Rejection Episodes and Their Impact
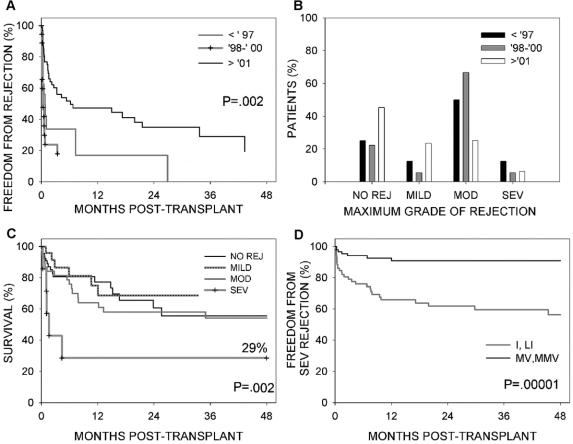
There was a greater freedom from rejection in the third era (Fig. 3A, P = 0.002). In fact, a higher percentage of patients in the third era never developed rejection than in the first 2 eras combined, 45.3% (29 of 64) versus 23.5% (8 of 34). The percentage of patients who developed a moderate or severe rejection was significantly less in the third era in comparison with the first 2 eras combined, 31.6% (20 of 64) versus 67.6% (23 of 34) (P = 0.0005) (Fig 3B). Survival for patients who had no rejection, mild or moderate rejection was similar. Survival after severe rejection was significantly poorer than survival with the other grades of rejection (P = 0.002, Fig. 3C).
FIGURE 3. (A) Comparison of freedom from any rejection by era. (B) Comparison of the maximum grade of rejection observed in each patient by era. (C) Comparison of patient survival according to the maximum observed grade of rejection. (D) Comparison of the hazard rate of developing severe rejection between patients who received a primary multivisceral transplant versus nonmultivisceral transplant.
Figure 3D shows a comparison of the hazard rate of developing severe rejection between patients who received a multivisceral graft (with or without liver) versus the other types of intestinal transplant (ie, isolated intestine or liver–intestine) performed at our center during the same eras. There was a protective effect of the multivisceral graft (P = 0.0001); less than one tenth (7 of 98) of multivisceral transplant patients developed severe rejection in comparison with approximately one third (29 of 91) of nonmultivisceral intestinal transplant patients. Among 115 patients who received a liver graft, the hazard rate of developing severe rejection was significantly higher in patients who received a combined liver–intestinal transplant in comparison with those who received a multivisceral transplant (9 of 32 vs 4 of 83, P = 0.0001). As expected, the hazard rate of death resulting from rejection was significantly different between the multivisceral and nonmultivisceral groups (P = 0.002), with 6 of 98 patients having undergone multivisceral transplant dying of rejection in comparison with 21 of 91 patients having undergone nonmultivisceral intestinal transplant. There were no significant differences in the nonrejection mortalities.
Rejection of Other Organs
Stomach
Twenty-four of the patients who received a stomach (n = 96) developed an acute rejection of that organ. The maximum grade was mild in 18 patients and moderate in 5 patients. No severe rejections were observed. Most of these rejections occurred simultaneously with an intestinal rejection.
Liver
A total of 6 of 83 patients who received a liver as part of a multivisceral transplant developed an acute rejection; the maximum grade was mild in 5 patients and moderate in one patient.
Pancreas
Mild rejection was found in one pancreas allograft explant. No rejection of the pancreas was found in any of the 30 autopsies performed. Another patient presented with transient hyperglycemia that responded to steroid treatment.
Abdominal Wall
Four MVTx recipients and one MMVTx recipient received an abdominal wall graft. Of the 3 who survived with the abdominal wall graft for at least 6 months, one patient never experienced rejection, one experienced rejection that did not coincide to rejection of the intestine, and one experienced simultaneous rejection of the abdominal wall and the intestine. In all cases, rejection responded to treatment.
Infections
Nearly all of the patients (97%, 95 of 98) developed an infection during their posttransplant follow up. The median number of infections per patient was 5 (range, 0–15). Half of the infections observed (251 of 495, 50.7%) occurred within the first 3 months posttransplant, one fourth of them (124 of 495, 25.1%) between 3 to 12 months, and the remaining one fourth (120 of 495, 24.2%) after 12 months posttransplant. There were no significant differences in the mean number of infections per patient by era (4.1 ± 0.7 for era 1, 6.1 ± 0.7 for era 2, and 4.8 ± 0.4 for era 3, P = 0.16).
The great majority of the infections (90.7%, 449 of 495) had a bacterial component: 429 were strictly bacterial, 17 were also fungal, and 3 were also viral. Gram-positive and Gram-negative organisms were equally frequent (47.9% vs 43.4%, 8.7% mixed). The most common pathogens were Klebsiella pneumoniae (13.4%) and Pseudomonas aeruginosa (12.5%). Forty-five of the infections (9.1%) included a fungal component, 28 being solely fungal. Twenty-one (4.2%) of the infections included a viral component, 18 being solely viral.
Analysis by location of infection shows that 201 (40.6%) were found in blood, 88 (17.8%) respiratory, 51 (10.6%) wound, 49 (10.1%) intraabdominal, 40 (8.3%) in the urine, 36 (7.5%) were at the catheter site, 29 (5.9%) were intestinal, and one was in the cerebrospinal fluid. Among the 201 infections that were found in the blood, 47 had the catheter site as an additional location and another 52 contained Staphylococcus-only bacteria. Thus, approximately half of the bacteremias (49.3%, 99 of 201) were determined to have originated at the catheter site.
There was no association of the infections with the era, induction agent, other organs transplanted (liver, spleen), age, or pretransplant status. There was no specific infection associated with an increased risk of death.
Other Complications
Graft Versus Host Disease
Six patients, the majority of them children (n = 5), developed GVHD. Of these patients, 4 were recipients of MVTx and 2 of modified MVTx, including the sole adult. Organs involved were: skin (6 of 6 cases), liver, large intestine, and lungs (one each). Median time of development of GVHD was 50 days (range, 14–160 days). GVHD was responsible for the death of 3 children. Of the remaining 3 patients, 2 died of infection (pneumonia, sepsis n = 1 each). One patient survived. The peripheral blood chimeric study in this patient showed only 0.05% cells from the donor. A greater proportion of patients who received a spleen developed GVHD (3 of 32 vs 3 of 66). The difference was not significant (P = 0.32).
Posttransplant Lymphoproliferative Disorder
Seven patients, the majority of them children (n = 5), developed PTLD. Six of these patients were recipients of a multivisceral graft, and one of a modified multivisceral graft. Organs involved were the transplanted gastrointestinal tract in all but 2 cases: one pediatric patient had a nasopharyngeal mass and an adult patient had a mass in one of her native kidneys. The median time to the development of PTLD was 25.3 months (range, 2.9–57.7 months). None of these patients had a spleen included in the graft, and all survived.
Nephrotoxicity
Figure 4A shows that in children, as the mean tacrolimus 12-hour trough levels decreased by more than 40% over the first 2 years posttransplant, the mean calculated GFR (n = 36) recovered from an initial drop to remain over 100 mL/min beyond the first year posttransplant. The results in adults were distinctly different (Fig. 4B); here, although the mean tacrolimus 12-hour trough levels decreased in a similar fashion over time, the mean calculated GFR (n = 21) dropped significantly from the pretransplant level (>50% reduction, P < 0.05) with no subsequent recovery over time.
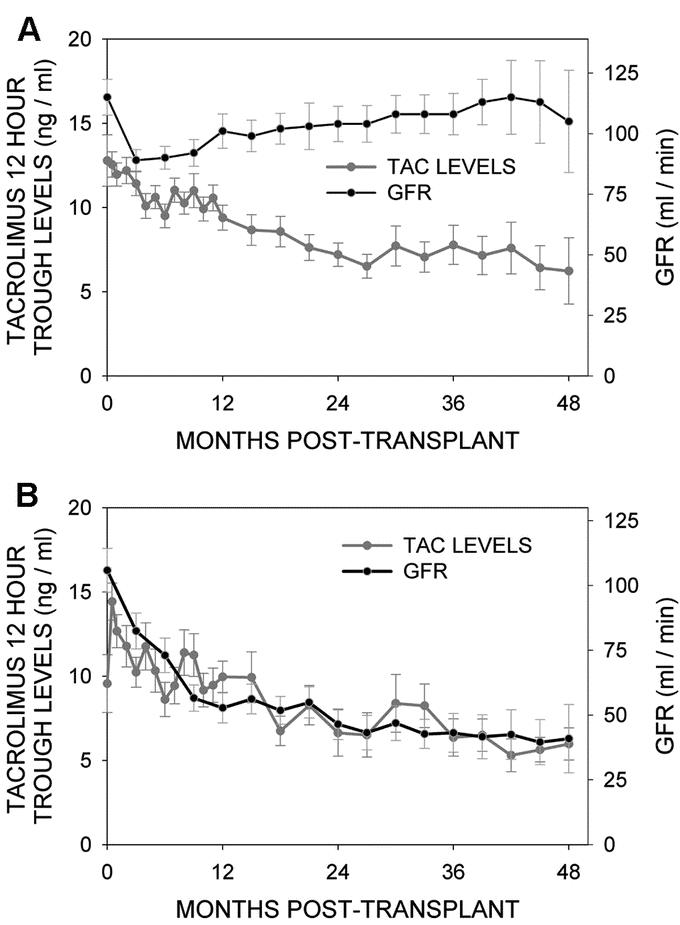
FIGURE 4. Mean tacrolimus 12-hour trough levels and mean calculated glomerular filtration rate in children (A) and adults (B) plotted over time after transplantation.
Six patients ultimately developed renal failure with 5 receiving a kidney graft at a median time of 27 months (range, 5–52 months) posttransplant; one patient is currently on dialysis, awaiting a kidney graft.
Disease Recurrence
Two patients had recurrence of desmoid tumors (n = 2) at the abdominal and thoracic walls, which were surgically removed. Thus far, there has been no intraperitoneal recurrence of the desmoid tumors. One patient died of recurrence of a carcinoid tumor at 24 months posttransplantation.
Vascular Complications
Two patients developed disruption of the aortic anastomosis and died 1 month after transplantation. A third patient, recipient of a multivisceral transplant that included the liver, developed portal vein thrombosis after discharge (at 6 months) and then died.
Graft Function
As of the last day of follow up, all survivors were fed exclusively through their transplanted intestine except for 5 children. The latter require total parenteral nutrition (TPN) supplementation: 4 of them on a temporary basis as a result of rejection (n = 2) or recent transplant (n = 2) and one on a long-term basis as a result of high intestinal output.
Quality of Life
Of the surviving children, 12 are of school age. Ten of them attend school normally, one at a delayed grade level, and one is still recovering from the transplant. Of the 16 adults currently alive, 12 maintain their expected normal daily activities, and 4 are partially disabled.
Preliminary Analysis of the Citrulline Data
There were 16 occasions when citrulline levels were available within 30 days before moderate or severe rejections and also during rejection-free time in the same patients. The average citrulline level immediately before (within 30 days of) rejection was 11.7 ± 1.3 μmol/L versus 16.5 ± 2.1 μmol/L in the rejection-free time. A paired t test of this difference was statistically significant (P = 0.03).
DISCUSSION
Our study shows that, with increasing experience, survival after multivisceral transplantation improved at the same time as the incidence and severity of rejections declined. Survival for better risk candidates is now analogous to the overall results of other complex solid organ transplants. Although rejection per se is not the most common cause of death, patient death can frequently be the final event in a cascade that starts with an under- or overestimation of the level of acute rejection that is present in any of the transplanted viscera. In this regard, rejection of the intestinal graft appears to be the most susceptible and vulnerable target of acute rejection in the patient undergoing multivisceral transplant. Clearly, there has been an improvement in the handling of acute rejection in MMVT patients since the use of tacrolimus became instituted, thus underscoring its importance as a maintenance immunosuppression in this complex transplant system (Grant D, Smith R, personal communication, April 5, 2005).5,6,8,9,15,17,24
The use of antilymphocyte globulins has also been shown to contribute to improvement in survival.6,25 Campath-1H seems to be particularly effective in this group of patients.17 Results with this powerful immunosuppressant are likely to keep improving with growing experience, although serious caution has to be raised by our failed attempt to introduce Campath in the pediatric transplant recipient group. Another contribution of the antilymphocyte globulins is their use for timely control of significant acute rejection when it occurs. In the 2 most recent eras, we used an antilymphocyte globulin for the treatment of all but the mild rejections. Campath-1H may offer an additional advantage because of its capacity for prolonged immunosuppression in the host, an action that is curiously not associated with an increase in the number of infections.
Almost half of our patients in our third era of multivisceral transplantation never developed significant acute rejection. This would have been an incredible result just a few years ago; however, it is still far from being perfect. Severe rejection of the intestine is the final stage of acute rejection and poses as a fatal complication for the recipient or one at least requiring retransplantation. This emphasizes the need for prompt diagnosis and control of earlier stages of acute rejection so that a preempting of the evolution to severe rejection does not take place. Frequent protocol biopsies, particularly with the use of the magnifying endoscope, evaluated by a closely interwoven and experienced multidisciplinary team, have been instrumental in helping achieve the goal of rejection intervention.
The magnifying endoscope allows visualization of the intestinal villi in situ. It supplements the pathologic examination because it can visualize a large intestinal surface in detail. Interpretation has been very accurate and allows treatment to be initiated even before the pathologic diagnosis is available. The opposite is also true and perhaps just as important: the reaction to a pathologic diagnosis of rejection can be tempered by the endoscopic findings. We have found that in as many as 25% of endoscopic biopsies,26 the grade of rejection could be downgraded from mild to indeterminate or normal, a correction confirmed by subsequent endoscopy and biopsy. This adjustment prevented unnecessary treatment. Our findings indicate that citrulline may be a useful marker of impending rejection as has been noted before.19 These results are still not conclusive and require further study.
Our study indicates that the multivisceral graft confers protection to its intestinal component from severe rejection. The low incidence of rejection in the other allograft organs of the multivisceral graft is also suggestive of this protection. This fact was predicted by previous studies.27,28 Previous studies have inferred that the liver allograft can supply a protective effect to other simultaneously transplanted organs.6,29,30 However, our experience has demonstrated no difference in the incidence of severe rejection (in the gastrointestinal viscera) between liver containing and liver-free multivisceral grafts. Moreover, combined liver–intestinal grafts transplanted at our center have not revealed any protective effect on the bowel as a result of having the hepatic transplant in comparison to isolated intestinal grafts. Thus, it is a yet undetermined aspect of multivisceral transplantation that confers the advantage of having lower rejections as compared with isolated intestinal grafts.
Infection, which has been identified as the major cause of mortality, remained the most serious complication in our series. Approximately half of the postoperative bacteremias observed originated from the central venous catheters. The latter have been traditionally a significant cause of morbidity and mortality,31 particularly because these patients are frequently depleted of venous access sites. These complications are unlikely to be diminished unless patients are referred earlier for transplantation and also until the postoperative course is simplified.
A majority of the infections are the result of the surgical procedure itself and the powerful immunosuppression. The spleen was included as part of the multivisceral graft in part to avoid the higher prevalence of sepsis associated with the asplenic state. An additional reason for supplying allograft splenic tissue would be to possibly confer an immunologic advantage (possible immunomodulation),32 although the theoretical risk of GVHD could also potentially increase.33 To date, we have not seen increased GVHD in association with the transplanted splenic tissue and this is supported in previous studies,34 although our experience remains premature to provide conclusive evidence regarding this issue. It remains apparent that the risks of GVHD and PTLD, although significant, are not nearly as great as once feared. Clearly, our understanding of the complex interplay of the donor and host immune systems, the allograft organs involved and modern immunosuppressive agents remains at a superficial level.
Among the complications that arise in multivisceral transplantation, nephrotoxicity is one of the most troubling. This complication is being increasingly identified as a clear hazard for all solid organ recipients.35 Our observation that children seem to be relatively spared still does not address the long-term changes in renal function in this patient population because the follow up in this study is only a small portion of their expected survival.
Although some of the kidney damage is the result of the patient's medical condition before transplantation, the use of tacrolimus, a calcineurin inhibitor known to have potential nephrotoxic effects, has been identified as the principal risk factor in developing renal dysfunction. The need to reduce tacrolimus levels has been the impetus to implement newer immunosuppressive treatments for patients having undergone multivisceral transplant even before they are tested in more conventional solid organ transplants. In this regard, an induction with antilymphocyte globulin, particularly Campath-1H, may help reduce tacrolimus levels for graft maintenance and therefore also decrease the likelihood of potential nephrotoxicity.
The need for further perfection of the surgical technique as well as surgical innovations cannot be overemphasized. Transplantation of the abdominal wall may be such an example. It has facilitated closure specifically when the abdominal compartment is small and the native abdominal wall is irreparably damaged. It is hoped that its addition will reduce the incidence of intractable wound problems that can prolong the patient's convalescence and result in fatal complications.
In the third era, we have used multivisceral transplantation as the procedure of choice for small children with extensive abdominal pathologies. Some of these children could have been ostensibly considered for combined liver–intestinal transplantation with preservation of the native stomach, pancreas, and duodenum. However, these latter native organs are often far from normal. They are frequently affected by the underlying pathology, prior surgical manipulations, dense adhesions, chronic intestinal obstruction, and portal hypertension. When preserved and not removed, they can necessitate a portocaval shunt, which can be precarious as a result of pathologic changes of the inferior vena cava from intravenous catheters. Their retention forces the new viscera to a paratopic position. By comparison, the multivisceral transplant contains healthy organs, is orthotopic, and carries a smaller risk of technical imperfections. It does not require backbench alterations, which could endanger its minute vascular network. If the findings reported here stand the test of time, the added risk from the addition of the stomach and pancreaticoduodenal complex is small.
It is apparent that the multivisceral transplant method has come of age. This multifaceted surgical procedure can be of great use for patients who have complex abdominal pathologies. In our experience, patients who are hospital-bound in the pretransplant period fared worse than those who were at home, an observation also made by review of the world intestinal transplant experience.24 This implies that referral of patients for multivisceral transplantation at an earlier stage as well as continuous improvements of our methods should result in improved outcomes and greater frequency of its use.
ACKNOWLEDGMENTS
The authors thank Mary Campos for her drawings and Jennie Benson for her assistance in the preparation of the manuscript.
Discussions
Dr. Goran B. Klintmalm (Dallas, Texas): This landmark paper by Dr. Tzakis firmly establishes multivisceral transplantation as a realistic concept for the treatment of catastrophic abdominal disease. The paper is filled with painfully gained experience and wisdom that benefits the whole transplant community. Dr. Tzakis’ writings evoke memories of my own sleepless nights and nightmarish efforts in trying to help bring a conceptual treatment to fruition.
Readers of this manuscript would benefit from Dr. Tzakis’ team's observations and careful deductions of “do's” and “don'ts.” The current success brings multivisceral transplantation into the realm of a therapeutic modality. Having read the manuscript, I have many, many questions. Among those, I will pick 3.
Campath 1-H is currently regarded as the most promising induction agent in organ transplantation. It is even regarded as having the potential of setting the stage for operational or prope tolerance. In this series, Dr. Tzakis reports a significantly reduced survival in children receiving Campath 1-H, but not so in adults. And this is in spite of the more immature immune system in children. I would like to hear Dr. Tzakis’ speculations on the cause of this observation.
My second question is in regards to abdominal wall transplants. This is a most visionary and bold solution to an incredibly serious problem, the inability to obtain primary abdominal closure of a contracted abdomen. Did you have any rejections of this allograft? What were the signs of rejection? Did the graft rejections respond to treatment? Did you experience any chronic rejections?
My final question is in regards to multiple organs and the risk of rejection. For 30 years there has been the assumption that with multiple organs the incidence of rejection decreased, especially the concept that the liver allograft exerts a protective effect against rejection. This was not what you did find. What are your thoughts and explanations for this observation? Were the previous reports erroneous, or is the situation in the modified multivisceral transplant biologically unique?
Dr. Andreas G. Tzakis (Miami, Florida): Thank you, Dr. Klintmalm. There are 2 possible reasons why the attempt to improve the outcome with Campath failed in children. One is that we indeed chose the children that were most likely to fail. Because the results already were getting quite good in pediatric recipients, we thought we would try the most difficult cases first. In retrospect, this was probably not a good idea. The second reason was the inability to ascertain the exact dose of Campath that was required for these children. In making the decision about the dose of Compath we consulted with the most experienced of our colleagues. Nevertheless, I believe that it might have been an important reason for this failure.
The second question, rejection of the abdominal walls. Yes, we have seen it. It presents as a rash confined to the abdominal wall graft. It can be diagnosed and graded with a punch biopsy. When the abdoinal wall is from the same donor as the visceral graft, it can serve as a marker of a contemporaneous intestinal rejection. It has not been a serious or a difficult problem to treat. We have not as yet seen chronic rejection of the abdominal graft.
As to the protective effect of the liver versus the whole multivisceral allograft. Our experience is too small to allow a precise response to that, but it is certainly an area of very intense inquiry in our institution.
Dr. Thomas E. Starzl (Pittsburgh, Pennsylvania): I am not going to discuss this remarkable report, but I did want to make a few notations about the colorful history of this unusual operation which I described in dogs and reported at the 1960 Surgical Forum of the American College of Surgeons. Any illusions I might have had about the contribution were deflated by the discussion by Bill Longmire that followed my presentation. He asked, “Wouldn't it have been cheaper and simpler to simply anesthetize the dog and have a laboratory assistant carry the animal from 1 table to the other?”
Actually, multivisceral transplantation that we have heard about today, and its modifications, were applied in humans almost 30 years later and now are part of the conventional armamentarium of advanced organ transplant centers. This was made possible in the late 1980s and early 1990s by 3 friends and surgeons from Pittsburgh: Andy Tzakis (now in Miami) who gave today's paper, Satoru Todo (who is back in Japan), and Kareem Abu-Elmayd (still in Pittsburgh). Seminal contributions also were made in London, Ontario, by David Grant and Bill Wall. All 5 men engaged in these efforts between 1987–1990 have since become members of the American Surgical Association. I am looking forward to some more discussions of Tzakis's presentation by the other 4.
Dr. Kareem M. Abu-Elmagd (Pittsburgh, Pennsylvania): The take home message, regardless of the specific eras, is the continuous improvement in survival after multivisceral transplantation due to evolution of surgical techniques, immunosuppressive protocols, and postoperative management. Four years ago, we reported a similar observation with better survival outcome during the annual meeting of this prestigious society. Since then, the results continue to improve in Pittsburgh by adopting a new tolerogenic protocol with a current 1-year survival rate of more than 90%. Equally impressive, is the successful use of minimal posttransplant immunosuppression with spaced doses of tacrolimus as a single agent. Nearly half of these recipients are currently receiving 2 to 3 single doses of prograf per week with no maintenance steroid therapy.
The many faces of the multivisceral operation by including different abdominal organs en-bloc have been clearly and comprehensively described by Dr. Starzl in 1991. However, the indications for intestinal transplantation only versus the intestine in combination with a variety of other visceral organs have yet to be standardized. Such decisions should be dictated by the extent of the candidate abdominal pathology. In Pittsburgh, in the largest series of intestinal transplants worldwide, the multivisceral procedure was required for only 23% of a total of 337 intestinal recipients. Also, none of these patients required abdominal wall transplant. In Miami, the multivisceral operation was performed in more than 50% of the cases with the need for abdominal wall transplant in some recipients. Why is it necessary to transplant all these organs in children with primary benign disorders such as gastroschisis? Could you please define for us your adopted selection criteria for the multivisceral procedure and the need for transplanting the abdominal wall?
A second question concerns preservation of the donor spleen. We never transplant the spleen. Rather, we tailor the recipient operation and preserve the native spleen, when possible, due to its protection against posttransplant lymphoma or infections. The high fatality associated with graft versus host disease, reported in your manuscript, does not justify inclusion of the spleen. Has your team had the chance to conduct chimeric studies or observe a higher incidence of graft versus host reaction in these unique recipients?
A third question concerns the survival risk factor of immunosuppression. In 1995 and 1998, we identified and published significant immunologic and nonimmunologic risk factors for patient and graft survival at our institution. Today, Dr. Tzakis reported 3 significant unfavorable variables, including the use of campath in children. Is such poor outcomes with campath related to excessive doses or due to other undetermined factors?
Finally, the role of the liver as part of the visceral graft is an important question. Everyone is aware of the immunoprotective effect of the liver when transplanted with other organs. However, the data in the Miami experience challenges this concept, and also brings into question whether it is a liver-specific effect. First, the observation was made that rejection is no different in recipients of intestine-alone versus intestine-liver recipients. Second, the results highlight an immunologic benefit of the multivisceral graft independent of the contained liver. In the manuscript, the statistical analysis showed a significant reduction in rejection with the full multivisceral graft compared to that with the combined liver/intestinal graft. Because the stomach represents the only difference in the components of these 2 kinds of multivisceral grafts, I would like to ask Dr. Tzakis if he truly believes that the stomach adds to the graft's tolerogenicity, or (by implication) even has an immunoprotective effect that is superior to that of the liver.
Dr. Andreas G. Tzakis (Miami, Florida): Thank you, Dr. Abu-Elmagd. In regards to the frequency of the multivisceral transplant performed at our institution, I think it reflects 2 facts. One is a pattern of referral of patients who require multivisceral transplantation to our center; and second, the expanded use of these multivisceral grafts for very small babies.
I have to emphasize that at this moment we propose the expanded use of these grafts for very small babies, and for the reasons I explained in my presentation. Briefly, the native stomach, pancreas and duodenum are not always healthy, the back-table procedure in these very small grafts is quite dangerous, particularly if there are abnormalities of the vasculature of the graft and finally the native organs displace the graft to the right side of the abdomen which could cause anatomical problems.
We did not perform abdominal wall transplants until the third era. The need for an abdominal wall graft came from our observation that significant number of these patients had an open abdomen and a granulating intestine, which prolonged the patient's recovery and caused potentially fatal complications like intestinal fistulas.
Concerning the GVHD and chimeric studies we have been stunned to see that patients with obvious GVHD (with or without the spleen) had zero chimera that we could detect. We were expecting very high chimeric levels. I don't think that the chimeric studies are able to fully explain the appearance of GVHD.
I believe I explained our theories about the experience of the Campath in children.
As far as the immunologic effect of the multivisceral graft, it is well known to exist. Dr. Noriko Murase from the University of Pittsburgh has done very elegant studies demonstrating this effect. I don't think I can go further based on the clinical observations.
Dr. David Grant (Toronto, Ontario, Canada): About 45 years ago, Owen Wagenstein commented on a paper Richard Lillihi about multivisceral transplantation in dogs predicting the procedure would be an adventure in search of adversity. Dr. Tzakis, I would like to congratulate you on your pioneer exploring work in this area and contributions to improving outcomes.
I have 2 questions. One, have changes in techniques reduced the cost of this procedure at your institution? And 2, how does the stomach function over the long term? Do you have problems with reflux, diarrhea, or dumping?
Dr. Andreas G. Tzakis (Miami, Florida): The cost is very high, probably in the range of half a million dollars or more per case. Some of these patients have already run an equally high cost by the time they come in for the multivisceral transplant.
In regards to the stomach, contrary to our fears the stomach has been a very good organ to transplant. The rejection rate, and we have only seen mild to moderate rejections at worst, was 25% in our series. And although the motility is not normal, the emptying of the stomach is quite satisfactory. People can eat normal meals.
Reflux has been a problem, particularly in patients who had a dilated the esophagus from the underlying disease. We tend to perform a gastrogastrostomy rather than an esophagogastrostomy and maintain the native gastroesophageal junction.
As far as dumping, I suspect that this might be a problem. It is hard to tell, because some of these patients might have a high output of the gastric graft.
Dr. John S. Najarian (Minneapolis, Minnesota): I would like to congratulate Dr. Tzakis on an outstanding technical tour de force and for the information that he has provided us. I have 2 questions.
Did you find in your series that there is a hierarchy of rejection of organs? In other words, as we see in pancreas transplants. In the 2000 pancreas transplants we have done at Minnesota, the kidney is the most likely to reject even though the pancreas and the duodenum have not, and then next rejection would occur in the pancreas. And finally, the one that is least likely of the 3 organs to reject is the intestine. I wonder if you saw something similar in the multivisceral transplants?
The next question is: Do you think that one of the reasons why the multivisceral organ transplant does so well is because you are transplanting so many organs and you are overwhelming the immune response of the recipient? We know from the work of Martinez and Good back in 1960 (published in Proc. Soc. Exp. Biol. and Med.) where they reported using total body skin transplants in mice. The authors showed definitely that you can overwhelm the recipient immune response with excessive donor tissue. Or, do you think that your success in this type of transplantation is primarily due to the fact you are replacing the recipient's lymphoid tissue with donor lymphoid tissue?
Dr. Andreas G. Tzakis (Miami, Florida): Thank you, Dr. Najarian. Yes, I do believe there is a hierarchy of rejection. Our data suggests that the intestine is number 1 in rejection. Actually, within the intestine, the distal intestine is probably most prone to it and the other organs follow in frequency and severity.
In regards to the explanation of why the multivisceral graft is accepted perhaps better than isolated organs, I am not sure of the answer. The massive graft could be overwhelming the native immune system, in addition a significant part of the native lymphatics are removed as part of the evisceration.
Dr. Donald D. Trunkey (Portland, Oregon): In a time where the potential recipients are going up every year, and in order to have transparency and accountability, these people are very sick and they receive multiple organs. Has anybody asked the question: If you gave these organs individually to people who weren't so sick, would you benefit more people?
Dr. Andreas G. Tzakis (Miami, Florida): Thank you, Dr. Trunkey, for this question. From the organs that we are using, the only one in extremely short supply is the liver and for that we follow the National allocation rules set by UNOS.
Footnotes
This study was partially supported by NIH grant 1 R03 DK061445-01 A2.
Reprints: Andreas G. Tzakis, MD, PhD, Department of Surgery, University of Miami Miller School of Medicine, Miami, FL 33136.
REFERENCES
- 1.Starzl TE, Kaupp HA Jr. Mass homotransplantation of abdominal organs in dogs. Surg Forum. 1960;11:28–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Rowe MI, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261. [PMC free article] [PubMed] [Google Scholar]
- 3.Williams JW, Sankary HN, Foster PF, et al. Splanchnic transplantation. JAMA. 1989;261. [DOI] [PubMed] [Google Scholar]
- 4.Margreiter R, Konigsrainer A, Schmid T, et al. Successful multivisceral transplantation. Transplant Proc. 1992;24:1226–1227. [PubMed] [Google Scholar]
- 5.Todo S, Tzakis A, Abu-Elmagd K, et al. Abdominal multivisceral transplantation. Transplantation. 1995;59:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: a decade of experience at a single center. Ann Surg. 2001;234:404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzakis T, Webb M, Nery J, et al. Experience with intestinal transplantation at the University of Miami. Transplant Proc. 1996;28:2748–2749. [PubMed] [Google Scholar]
- 8.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335–344. [PMC free article] [PubMed] [Google Scholar]
- 9.Kato T, Ruiz P, Thompson JF, et al. Intestinal and multivisceral transplantation. World J Surg. 2002;26:226–237. [DOI] [PubMed] [Google Scholar]
- 10.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishida S, Pinna A, Verzaro R, et al. Domino liver transplantation with end-to-side infrahepatic vena cavocavostomy. J Am Coll Surg. 2001;192:237–240. [DOI] [PubMed] [Google Scholar]
- 12.Alexandrides IJ, Liu P, Marshall DM, et al. Abdominal wall closure after intestinal transplantation. Plast Reconstr Surg. 2000;106:805–812. [DOI] [PubMed] [Google Scholar]
- 13.Levi DM, Tzakis AG, Kato T, et al. Transplantation of the abdominal wall. Lancet. 2003;361:2173–2176. [DOI] [PubMed] [Google Scholar]
- 14.Tzakis AG, Nery JR, Thompson J, et al. New immunosuppressive regimens in clinical intestinal transplantation. Transplant Proc. 1997;29:683–685. [DOI] [PubMed] [Google Scholar]
- 15.Pinna AD, Weppler D, Nery J, et al. Intestinal transplantation at the University of Miami—five years of experience. Transplant Proc. 2000;32:1226–1227. [DOI] [PubMed] [Google Scholar]
- 16.Ricordi C, Karatzas T, Selvaggi G, et al. Multiple bone marrow infusions to enhance acceptance of allografts from the same donor. Ann N Y Acad Sci. 1995;770:345–351. [DOI] [PubMed] [Google Scholar]
- 17.Tzakis AG, Kato T, Nishida S, et al. Alemtuzumab (Campath-1H) combined with tacrolimus in intestinal and multivisceral transplantation. Transplantation. 2003;75:1512–1517. [DOI] [PubMed] [Google Scholar]
- 18.Kato T, O'Brien CB, Nishida S, et al. The first report of the use of a zoom video-endoscopy for the evaluation of small bowel graft mucosa in a human following intestinal transplantation. Gastrointest Endosc. 1999;50:257–262. [DOI] [PubMed] [Google Scholar]
- 19.Pappas PA, Saudubray JM, Tzakis AG, et al. Serum citrulline and rejection in small bowel transplantation: a preliminary report. Transplantation. 2001;72:1212–1216. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz P, Bagni A, Brown R, et al. Histological criteria for the identification of acute cellular rejection in human small bowel allografts: results of the pathology workshop at the VIII International Small Bowel Transplant Symposium. Transplant Proc. 2004;36:335–337. [DOI] [PubMed] [Google Scholar]
- 21.Nalesnik MA, Jaffe R, Starzl TE, et al. The pathology of post-transplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173–192. [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Haycock GB, Edelmann CM, et al. Simple estimate of glomerular-filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 24.Intestinal Transplant Registry Data. Available at: http://www.intestinaltransplant.org/.
- 25.Grant D, Abu-Elmagd K, Reyes J, et al., on behalf of the Intestine Transplant Registry. 2003 Report of the Intestine Transplant Registry: a new era has dawned. Ann Surg. 2005;241:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato T, Nishida S, Tzakis A. Small bowel and multivisceral transplantation. Transplant Rev. In press.
- 27.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small-bowel allotransplantation under FK 506. Surgery. 1991;110:87–98. [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Gaynor JJ, Selvaggi G, et al. Intestinal transplantation in children: a summary of clinical outcomes and prognostic factors in 108 patients from a single center. J Gastrointest Surg. 2005;9:75–89; discussion 89. [DOI] [PubMed]
- 29.Calne R, Davies H. Organ graft tolerance: the liver effect. Lancet. 1994;343:67–68. [DOI] [PubMed] [Google Scholar]
- 30.Edwards-Smith C, Goto S, Lord R, et al. Allograft acceptance and rejection, mediated by a liver suppressor factor, LSF-1, purified from serum of liver transplanted rats. Transplant Immunol. 1996;4:287–292. [DOI] [PubMed] [Google Scholar]
- 31.Tzakis AG, Todo S, Abu-Elmagd K, et al. Clinical intestinal transplantation: focus on complications. Transplant Proc. 1992;24:1238–1240. [PMC free article] [PubMed] [Google Scholar]
- 32.Dor FJ, Tseng YL, Kuwaki K, et al. Pig spleen transplantation induces transient hematopoietic cell chimerism in baboons. Xenotransplantation. 2004;11:298–300. [DOI] [PubMed] [Google Scholar]
- 33.Deierhoi MH, Sollinger HW, Bozdech MJ, et al. Lethal graft versus host disease in a recipient of a pancreas–spleen transplant. Transplantation. 1986;41:544–545. [PubMed] [Google Scholar]
- 34.Kimball P, Ham J, Eisenberg M, et al. Lethal graft versus host disease after simultaneous kidney–pancreas transplantation. Transplantation. 1997;63:1685–1688. [DOI] [PubMed] [Google Scholar]
- 35.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. [DOI] [PubMed] [Google Scholar]