Abstract
Objective:
Marked variations in sentinel lymph node dissection (SLND) technique have been identified, and definitive qualifications for SLND performance remain controversial. Based on previous reports and expert opinion, we predicted that 20 to 30 cases of SLND with axillary lymph node dissection (ALND) would enable surgeons to identify sentinel lymph nodes (SLN).
Summary Background Data:
In 1999, the American College of Surgeons Oncology Group initiated a prospective trial, Z0010, to evaluate micrometastatic disease in the SLN and bone marrow of women with early-stage breast cancer. Eligible patients included women with biopsy-proven T1/T2 breast cancer and clinically negative lymph nodes who were candidates for lumpectomy and SLND.
Methods:
Participating surgeons were required to document 20 to 30 SLNDs followed by immediate ALND with failure rates less than 15%. Prior fellowship or residency training in SLND provided exemption from skill requirements. Data for 5237 subjects and 198 surgeons were available for analysis.
Results:
Surgeons from academic (48.4%), community (28.6%), or teaching-affiliated (19.8%) institutions qualified with 30 SLND + ALND cases (64.6%), 20 cases (22.2%), or exemption (13.1%). Participants used blue dye + radiocolloid in 79.4%, blue dye alone in 14.8%, and radiocolloid alone in 5.7% of cases, achieving a 98.7% SLN identification rate. Patient factors associated with increased SLND failure included increased body mass index and age, whereas tumor location, stage, and histology, presence of nodal metastases, and number of positive nodes were not. Surgeon accrual of fewer than 50 patients was associated with increased SLND failure; however, SLND technique, specific skill qualification, and institution type were not.
Conclusions:
Using a standard skill requirement, surgeons from a variety of institutions achieved an acceptably low SLND failure rate in the setting of a large multicenter trial, validating the incorporation of SLND into clinical practice.
Marked variations in sentinel lymph node dissection (SLND) practice and performance have been reported. Qualifications for SLND credentialing remain controversial. Using a standard skill requirement, ACOSOG Z0010 surgeons achieved an acceptably low SLND failure rate in the context of a large multicenter trial, validating incorporation of this technique into clinical practice.
For the last century, axillary dissection and histopathologic evaluation of the axilla has represented the gold standard for determining the status of the regional lymph nodes, the prognosis, and the appropriate treatment of patients with early-stage breast cancer.1,2 More recently, the role of axillary lymph node dissection (ALND) has come into question, particularly in the clinical T1 N0 M0 patient, in whom the axillary nodes fail to contain metastases in over 75% of cases.3 In this setting, sentinel lymph node dissection (SLND) has emerged as an accurate and feasible alternative to ALND and is rapidly becoming the standard of care for the patient with breast cancer with a clinically negative axilla.4
The goals of SLND include avoiding the unnecessary removal of uninvolved lymph nodes with a standard ALND, preventing the morbidity of ALND and improving the pathologic examination by focusing on fewer lymph nodes. The success of the procedure depends on several factors, including surgeon experience, patient and tumor characteristics, specific SLND techniques used, and coordinated collaboration with nuclear medicine and pathology. In 1993, using a radiocolloid alone, Krag and colleagues first reported SLN identification in 18 of 22 patients with breast cancer.5 From 1991 to 1994, Giuliano performed SLND on 174 patients with breast cancer using blue dye alone followed by completion ALND.6,7 While the SLND technique continued to evolve, this group observed a technical learning phase such that SLN identification improved greatly with surgeon experience to an overall success rate of 97%.8 Krag et al conducted the first multicenter validation study of SLND with ALND using radiotracer alone. Eleven surgeons achieved an overall success rate of 93%, sensitivity of 89%, and specificity of 100%; however, there were marked variations between institutions and surgeons.9 Cox et al evaluated the performance and technical failure data of 5 individual surgeons in over 700 cases, concluding that an average of 23 cases would be required for a surgeon to achieve a SLND success rate of 90% and 53 cases to reach 95%.10 After examining a multiinstitutional registry of 2148 SLND cases, McMasters et al suggested that surgeons perform at least 20 cases of SLND followed by completion ALND with “acceptable results” before abandoning routine ALND.11,12
The incorporation of new technologies into routine practice has not always followed the traditional route of randomized clinical trials. In the case of laparoscopic cholecystectomy (LC), this technique became the gold standard for the surgical management of symptomatic cholelithiasis without a published multicenter, randomized comparison to open cholecystectomy. After the adoption of this technology by the surgical community, several reports have emerged on the observed learning curve and proposed criteria for institutional credentialing. Persistent decreases in both operative time and complication rates have been observed for individual surgeons in up to 200 consecutive cases.13
For SLND skill acquisition, there is an ongoing debate regarding the number of cases required to ensure safe and appropriate implementation with reasonably low failure and accuracy rates. Stricter hospital credentialing standards may not necessarily correlate with improved patients outcomes; however, surgeons across subspecialties agree that the application of new skills and technologies must not occur at the expense of patient safety or compromise patient outcomes.14–16 Critics of the SLND technique point to the wide range of false-negative events reported in the literature and the lack of standardized techniques and performance criteria. Meanwhile surgical training requirements and standardization of evidence-based techniques are driving the procedural accuracy of SLND toward that of ALND, with current reported diagnostic accuracy rates over 97% and false-negative rates less than 5%.4
In 1999, the American College of Surgeons Oncology Group (ACOSOG) initiated the Z0010 trial to determine the clinical significance of sentinel node and bone marrow micrometastases for patients with early-stage carcinoma of the breast. No specific surgical SLND technique was mandated by this trial. This study allowed use of radioisotope, isosulfan blue dye, or a combination of both agents according to surgeon discretion. Z0010 required documented SLND skill verification initially of 30 cases and subsequently 20 cases of SLND with completion ALND before surgeon participation in the trial. We postulated that this experience would likely result in a high rate of SLN identification. This report discusses the skill requirements for participating surgeons and the technical results of the Z0010 trial.
METHODS
Study Design
The schema for the ACOSOG Z0010 clinical trial is shown in Figure 1. This prospective study was approved by the National Cancer Institute (NCI) as well as by the Institutional Review Board (IRB) for each participating institution. Each subject signed an approved informed consent document before participation. Eligible patients included women with clinical stage T1 or T2, N0, M0 breast carcinoma with biopsy-proven invasive disease. Initially, surgeons were requested to submit a case list detailing the results of 30 sequential SLND followed by immediate completion ALND, demonstrating at least an 85% SLN identification rate and at least a 95% accuracy rate. Subsequently, as the use of SLND increased in clinical practice, the number of required completion ALND cases was decreased to 20. Alternatively, surgeons were allowed to submit documentation of SLND training through a surgical residency or fellowship program or through an institution-wide validation study of SLND.

FIGURE 1. ACOSOG Z0010 schema.
Surgical Intervention
The operative procedure for the ACOSOG Z0010 trial has been described previously.17 Protocol guidelines for the SLND procedure were provided; however, each surgeon was permitted to perform the procedure according to individual preference and training. SLND was performed with isosulfan blue dye, a radiopharmaceutical, or a combination of the 2 agents. The volume of injection of each agent, the timing of the injections, and the location of the injections were left to surgeon preference. Recommendations for the blue dye technique involved 4 to 5 mL of 1% isosulfan blue dye injected into the breast parenchyma around the tumor or biopsy cavity followed by gentle breast compression. The radiopharmaceutical technique used 0.25 to 1.0 mCi of radiolabeled technetium sulfur colloid injected into the breast parenchyma around the tumor or biopsy cavity either the day of or the day before the planned SLND. Segmental mastectomy or lumpectomy was performed after SLND.
If the primary tumor was located in the medial hemisphere of the breast, lymphoscintigraphy or documented intraoperative gamma counting was required to confirm axillary drainage. At the completion of the identification of all sentinel nodes, any remaining suspicious axillary nodes identified by palpation were removed and labeled as sentinel lymph nodes. If an axillary sentinel lymph node could not be identified, a full ALND was required. On pathologic evaluation, a sentinel node was considered to be positive if at least one focus of tumor was identified either by frozen section or on permanent hematoxylin and eosin (H&E) staining. Micrometastatic disease measuring less than 0.2 mm, or tumor foci identified by immunohistochemistry alone, was not considered sufficient to establish sentinel node positivity in this study.
Statistical Analysis
Frequency distributions were used to summarize the characteristics of patients, surgeons, and the surgical procedure. Contingency tables, chi-squared tests, and logistic regression were used to model the effect of individual patient characteristics (patient age, body mass index [BMI], nodal status, tumor stage, and number of positive nodes) and surgeon characteristics (Z0010 accrual rate, SLND technique used, institution type, and skill qualification) on the risk of a failed SLND procedure. Multivariate logistic regression was also used to examine the joint effect of clinical and surgeon characteristics on the risk of SLND procedure failure. The management of subjects with positive SLNs, and their clinical outcome, will be discussed in a separate report.
RESULTS
A total of 198 surgeons, 96 from academic medical centers (48.4%), 57 from community practices (28.6%), 39 from teaching-affiliated institutions (19.8%), and 6 from other types (ie, military) (3%), accrued patients to the Z0010 trial. Participants included surgeons from a total of 126 institutions, including surgical practices from 37 states in the United States as well as 2 centers in Ireland and one in Australia. Of the 198 surgeons, the majority (N = 128) qualified with 30 cases of SLND with completion ALND (64.6%), 44 qualified with 20 reported cases (22.2%), and 26 were awarded exemption for prior SLND training (13.1%).
Demographic data for eligible and evaluable patients (N = 5327) have been reported previously and are summarized in Table 1.17 Median subject age was 56 years (range, 23–95 years), and median subject BMI was 26.4 kg/m2 (range, 14.6–72.6 kg/m2). Preoperatively, 4406 patients were clinically staged as T1 (82.7%) and 881 patients were clinically staged as T2 (16.5%). After pathologic assessment, 3956 patients were staged as T1 (77.7%), 1070 patients were staged as T2 (21.0%), and 68 patients proved to have T3 lesions (1.3%).
TABLE 1. ACOSOG Z0010 Patient Characteristics (n = 5327)
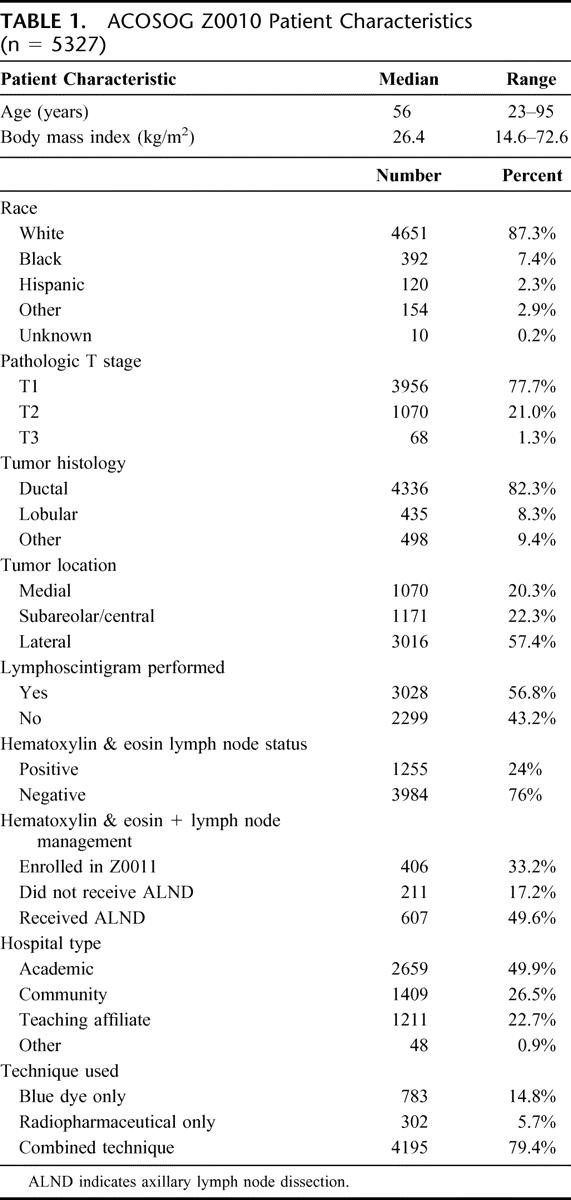
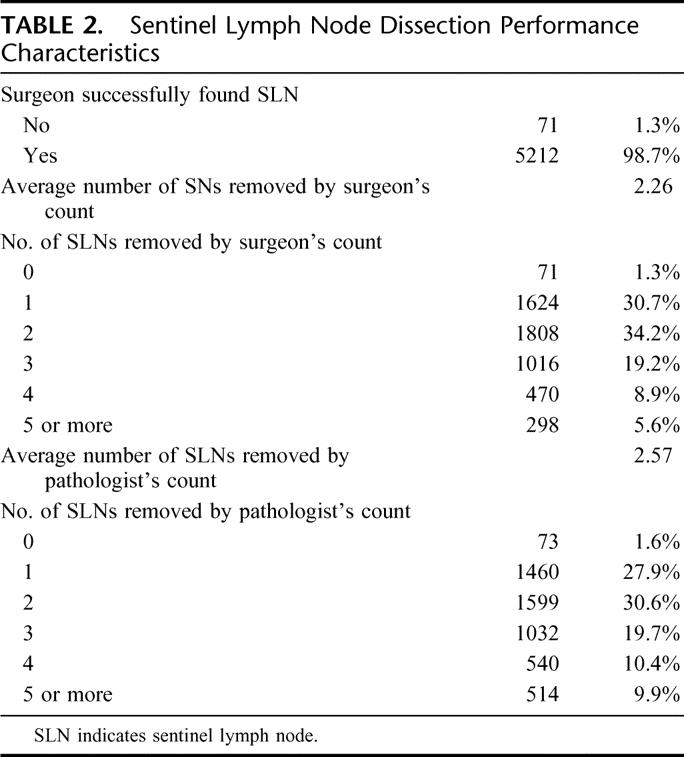
In the course of Z0010 participation, surgeons used a combination of blue dye and radiopharmaceutical in 79.4% of cases, blue dye alone in 14.8% of cases and radiopharmaceutical alone in 5.7% of cases. In 98.7% of evaluable cases (5283 patients), a sentinel lymph node was successfully identified, corresponding to a failure rate of 1.3% or only 71 patients (see Table 2). The average number of sentinel nodes removed, for successful SLND cases, was 2.26 lymph nodes by surgeon count and 2.57 lymph nodes by final pathology count. Of those patients who underwent successful SLND (N = 5212), 24% (N = 1255) were found to have at least one tumor-involved lymph node by H&E staining on pathologic evaluation.
TABLE 2. Sentinel Lymph Node Dissection Performance Characteristics
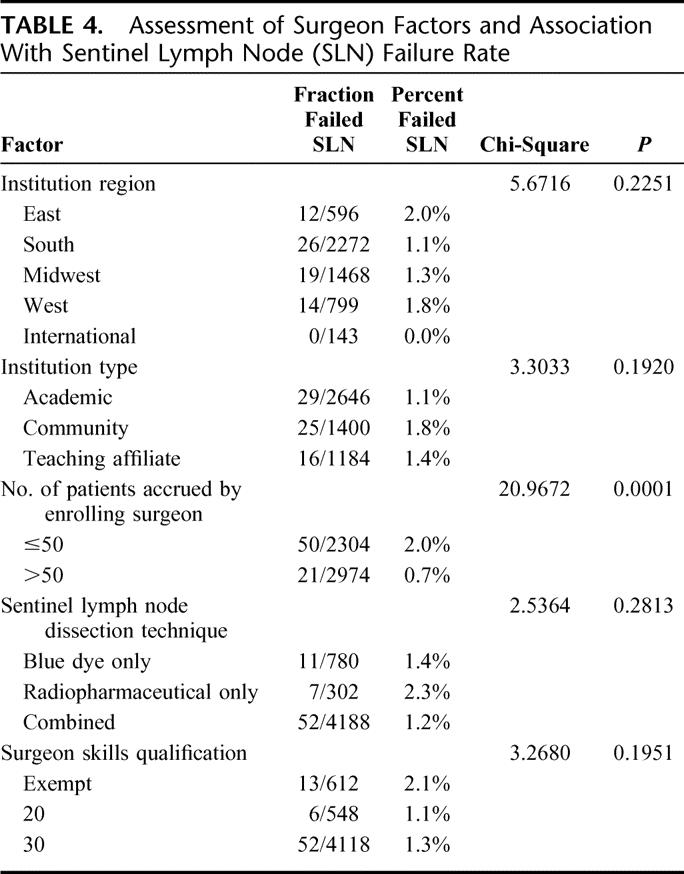
Using chi-squared analysis, patient factors that were associated with failure to identify a SLN included increased BMI (P = 0.0001) and age (P = 0.0004), whereas the presence of nodal metastases, pathologic tumor stage, location of tumor, tumor histology, and type of biopsy were not significant (Table 3). Surgeon accrual of 50 or fewer patients was associated with an increased likelihood of failed SLND (P ≤ 0.0001), whereas SLND technique, specific surgeon skill qualification, and type of institution were not (see Table 4). On multivariate logistic regression, fewer patients accrued, increased patient age, and increased patient BMI remained significant factors that were associated with SLND failure. These factors were significant as continuous variables as well.
TABLE 3. Patient Factors and Association With Sentinel Lymph Node (SLN) Failure Rate
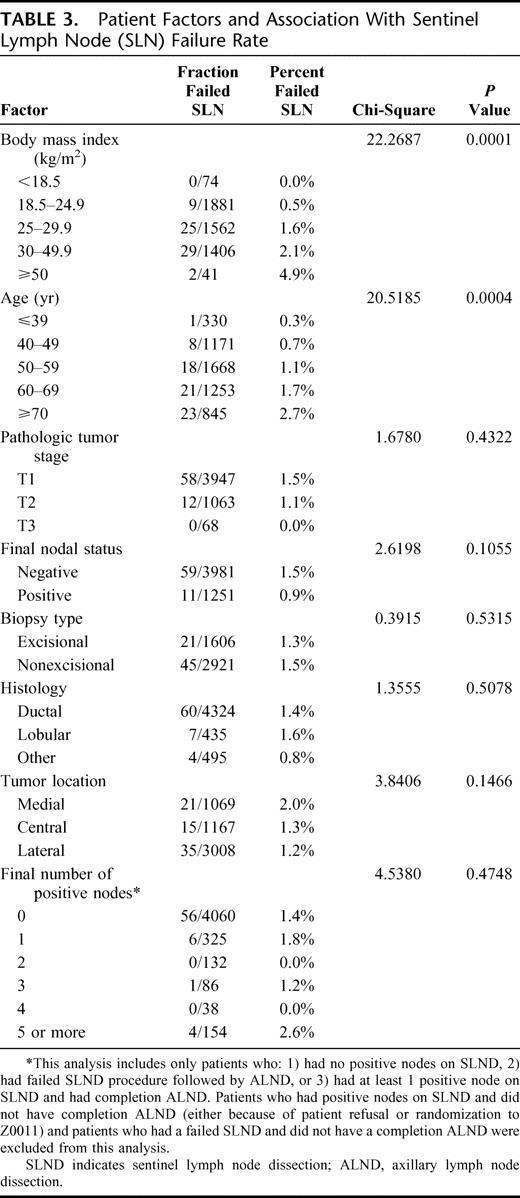
TABLE 4. Assessment of Surgeon Factors and Association With Sentinel Lymph Node (SLN) Failure Rate
With a median follow up of 31 months, only 16 patients (0.3%) were found to have regional recurrence. Three of these 16 patients had one positive SLN and did not undergo completion ALND at that time. One patient had 3 positive SLNs and underwent completion ALND. The remaining 12 (0.2%) patients with a reported axillary recurrence had a negative SLND and did not undergo ALND. The median time to axillary recurrence for the 16 reported cases was 19.1 months (range, 4.2–40.1 months). None of the patients who had a failed SLND have had a reported recurrence to date.
DISCUSSION
Adequate surgeon education has been identified as a critical factor in the successful application of new surgical procedures. Early studies of SLND advocated a training set of 60 to 80 cases to achieve acceptable SLN identification rates and to minimize false-negative events.18 Subsequent studies advocated performance of 25 to 30 consecutive SLND + ALND and defined a minimal success rate of 85% for the identification of SLNs based on observed learning curves at pioneering institutions.19–23 As the application of SLND technology has become more widespread in the surgical community, consensus statements have been released to provide performance guidelines based on a combination of expert opinions and panel reviews of existing literature.
The Institute for Clinical Systemic Improvement (ICSI) Technology Assessment Committee concluded that SLND should only be used in clinical settings by an experienced surgeon, defining acceptable identification and false-negative rates as ≥85% and ≤5%, respectively.24 At an international consensus conference in Philadelphia, expert opinion leaders agreed that 20 to 30 cases of SLND with ALND would reliably yield failure and false-negative rates less than 5%.4 The American Society of Breast Surgeons SLND consensus statement supports performing 20 cases of SLND + ALND and states that the “use of mentoring, proctored cases and formal training in accredited continuing medical education courses is thought to reduce the personal case experience necessary to achieve optimal results, but this effect has yet to be quantified.”25
Other multicenter trials have incorporated SLND training or required documentation of SLND validation cases before allowing study participation. In the Department of Defense (DOD) Multicenter Breast Lymphatic Mapping Trial, participating surgeons attended a training course and then divided into 2 groups: one completing a validation set of 20 to 25 cases of SLND + ALND and the other performing ALND only after a positive SLND. Participating academic and community surgeons achieved an identification rate of 85% and a false-negative rate of 4% (protocol 1). In protocol 2, there were no cases of axillary recurrence after a negative SLND at a median follow up of 16 months.26
In the recently reported NSABP B-32 study, participating surgeons were required to complete an on-site evaluation program and review of 5 cases of SLND with completion ALND before entering patients in the study. With 233 surgeons enrolling 5611 patients, the NSABP reported a false-negative rate of 9.7%.27 In the Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial, 13 surgeons completed an initial validation phase consisting of a proctored training program followed by 40 consecutive SLND + ALND. Each of the 13 surgeons achieved an identification rate of at least 90% and a false-negative rate of 5% or less.28 In the ACOSOG Z0010 trial, surgeons with a wide range of previous SLND experience qualified with 20 to 30 cases of SLND + ALND, but neither the number of required cases nor the type of institution significantly influenced the SLN identification rates. ACOSOG did not train surgeons to perform SLND before participation, and the number of validation cases did not necessarily reflect the volume of individual surgeon experience.
Although Cody and others have observed that false-negative events occur early in an individual surgeon's experience, Tanis et al suggest that initial SLND success does not prohibit future false-negative events.29,30 To assure with reasonable confidence that the false-negative and failure rates will both be less than 5%, the required number of SLND cases may exceed 150. Given the widespread popularity of this procedure and the number of patients who have undergone SLND in prospective trials to date, this number of cases is not a reasonable or practical requirement. In addition, because biologic factors such as multifocality and high S-phase fraction have been demonstrated to impact the false-negative rate, the experienced surgeon may experience a false-negative case at any time in his or her practice.31
The selection, timing, and location of injection of the mapping agent have all been identified as important variables influencing SLN identification.31 However, the Z0010 study did not limit surgeons to specific SLND injection strategies; rather, participants were permitted to perform SLND using the mapping agent(s) and injection technique of their choice. Performance of SLND before breast surgery and use of lymphoscintigraphy have also been demonstrated to improve SLN detection rates.32 Morrow et al randomized patients to SLN localization with blue dye alone, compared with blue dye plus radiopharmaceutical, and identified no significant advantage to the combination technique even in the setting of early SLND skill acquisition.33 In the Z0010 trial, we did not identify that there was a significantly decreased risk of failure for cases using the combined injection technique. Perhaps early in the learning curve, this strategy allows for easier skill acquisition and may be recommended as part of the training process, but in experienced centers, any proven technique may be used.
Consistent with previous reports in the literature, patient age and BMI were significant factors influencing SLN identification in this study.34,35 Surgeons must be aware of these factors as they explain the possibility of a failed SLND procedure to individual patients and the consequential increased possibility of proceeding to a complete ALND in this setting. When surgeons apply the SLND technique in their clinical practice, they may be more or less likely to experience failed SLN procedures, depending on the characteristics of their specific patient population. It is also noted that the patient population in this study was younger than the breast cancer population in general with a mean age of 56 years. This is consistent with several studies that have examined patient factors influencing clinical trial participation. Younger patients with cancer are more likely to participate in randomized and cooperative group trials, whereas older patients are less likely to be offered the opportunity to participate in breast cancer trials.36–39
We observed a significantly lower SLND failure rate for surgeons who accrued over 50 patients to our study, suggesting that surgeons who perform the procedure more frequently are more likely to have an increased SLN identification rate. Whether these are higher-volume practices with surgeons performing SLND more frequently or individual surgeons with more SLND experience before Z0010 participation or merely surgeons more inclined to participate in a clinical trial is unknown. Cox et al reported that surgeons who performed more than 6 SLNDs per month had lower failure rates than surgeons who performed fewer SLNDs.40 Therefore, the number of introductory credentialing cases, as well as ongoing SLND experience, may influence performance.
In addition to performing SLND with a low failure rate, surgeons must also prove SLND to be equivalent to ALND recurrence and survival. It is highly unlikely that removal of tumor-free nodes will improve survival. However, false-negative SLNDs may diminish survival by understaging patients, resulting in undertreatment and the risk of developing clinically apparent disease in the future. The gold standard for assessment of SLND accuracy is completion ALND. Although completion ALND was not performed on all patients in this trial, a reasonable surrogate for accuracy is clinical axillary recurrence. In general, SLND accuracy increases as SLN identification rate increases with surgeon experience. With at least 18 months of follow up for the majority of patients on the Z0010 trial, there have only been 12 (0.3%) reported cases of axillary recurrence in SLN-negative patients (median follow up of 31 months). This corresponds to an estimated clinical accuracy rate of 99.7% and a false-negative rate of 0.3%. Coupled with a 1.3% SLND failure rate, surgeons participating in Z0010 were well qualified to be performing SLND based on the protocol requirements of 20 to 30 documented cases or an adequate residency, fellowship, or institutional training program.
The purpose of consensus statements, guidelines, training courses, credentialing requirements, and institutional standards is to minimize the risk to the patient. For SLND, tumor characteristics, lymphatic variation, and surgical skill contribute to the successful performance, as do the contributions of the nuclear medicine and pathology staff.41 The importance of forming a collaborative team of adequately trained surgeons, nuclear medicine specialists, pathologists, and support staff cannot be overstated.42 The use of previous experience and expert opinions in the literature enabled adequate protocol guidelines and requirements to be defined, such that participating surgeons reliably identified SLNs during study Z0010. Twenty to 30 cases of SLND with completion ALND enabled surgeons in Z0010 to identify SLN with a high accuracy rate and low clinical regional recurrence rate.
SUMMARY
This report provides new data from a large multicenter trial to support standard SLND skill requirements for early-stage breast cancer and reinforces results from smaller, single-institution studies. In the setting of an international cooperative group study, surgeons from a wide variety of institutions and training backgrounds performed SLND with an acceptably low failure rate and a low axillary recurrence rate. Both academic and community surgical practices will be encouraged to adopt this minimum standard for SLND performance at their institutions. In addition, individual surgeons and their institutions are encouraged to maintain identification rates of at least 95% and false-negative rates of no more than 5%, and to monitor long-term outcomes of SLN-negative patients who do not undergo ALND.
Despite the wide and growing body of literature on this subject, a recent survey of participants in a SLND training course identified a surprisingly high number of surgeons who reported that they were performing SLND routinely at their institution without obtaining the specific approval of their Institutional Review Board and without completing a validation phase.43 Although factors such as patient awareness and the enticement of new technology may compel surgeons to yield to this temptation, the potential pitfalls of this type of practice are staggering and may impede the diligence of those participating in multiinstitutional registries and validation trials such as the ACOSOG Z0010 trial.
The American College of Surgeons Committee on Emerging Surgical Technology and Education (CESTE) has addressed the acquisition of new skills in the surgical management of patients.44 For surgeons to implement new technologies in a safe manner, they must complete adequate training in the new methodology, demonstrate specific experience in the surgical management of the particular disease, be formally recommended to their institution by another experienced surgeon, and maintain these new skills by ongoing practice and regular review of credentials. The “see one, do one, teach one” paradigm cannot be upheld in the face of the growing body of evidence to support the establishment of institutional standards and credentialing requirements. The routine application of SLND technology to appropriate patients, and the ultimate decision to discontinue routine ALND, must be a joint decision between the members of the breast care team with local institutional support.45
As the data from large multicenter trials such as ACOSOG Z0010 and NSABP B-32 continue to mature, it is anticipated that proponents and performers of SLND will acquire additional validation of this technique in experienced centers as the standard of care for women with clinically node-negative, early-stage breast cancer.
ACKNOWLEDGMENTS
The authors thank the surgeons and patients who participated in ACOSOG Z0010 supported by a grant from the National Cancer Institute U10-CA76001-09. The authors also thank the American College of Surgeons for its ongoing support of ACOSOG trials.
Discussions
Dr. Blake Cady (Providence, Rhode Island): In the interest of full disclosure, I must report that I have been trying to get rid of routine axillary dissection since 1974 after publishing a paper entitled “Lymph Node Metastases: Indicators, Not Governors of Survival.” Therefore, I have been heavily biased, indeed enthusiastic, about sentinel lymph node biopsy in breast cancer enabling the elimination of routine axillary dissection. It pleases me to comment on this manuscript that the author sent me a week ago.
This is a remarkable project in which 198 surgeons in 126 institutions operated on 5327 patients and achieved a 98.7% identification rate of sentinel nodes by following well-defined criteria for skill acquisition in the ACOSOG Z-10 trial headed by Dr. Giuliano. This success rate will clearly reinforce any conclusions from the trial.
They report that there were only 2 patient factors that led to a significantly increased failure rate by multivariate analysis, increased BMI and increased age. They also report only 1 significant surgeon factor that increased success rate, an individual experience with more than 50 cases. Surprisingly, none of the usual features that we worry about made a difference, such as location, size, stage, number of nodes, histology, or institution. With an overall success rate of 98.7%, however, none of the features studied made big clinical differences in outcome.
For instance, BMI below 25 had a 0.5% failure rate and a BMI over 30 had a 2.2% failure rate. Patients younger than 50 had a 0.6% failure rate while those over 60 had a 2.1% failure rate. Similarly, surgeons with more than 50 cases had a 0.7% failure rate while surgeons with 50 or fewer had a 2.2% failure rate. Because of the large number of patients these are statistically significant differences but clinically almost irrelevant since the results are still well within the range of acceptability. It reminds me of the saying, “Perfection is the enemy of good.”
All of the concerns about quality, process and outcome measurements discussed at this meeting were accomplished in this trial by these surgeons. In particular, Charles Cox's description of the “Paradox of Late Failure” was apparently avoided in that in his study some surgeons were good initially but later began to do much worse. I attributed this phenomenon to indicate that as some surgeons become more experienced they begin to rush the procedure or become careless through overconfidence. His description of this paradox should be a cautionary note to all of us.
I have several questions to the authors.
Why is the median age only 56 when the median age of all breast cancer patients in this country is about 65 and did this affect the overall success rate?
Why were intraparenchymal injections utilized at all since intradermal and subareolar routes have been documented to be superior? How did your surgeons obtain such success with an inferior technique?
Sentinel lymph node biopsy is clearly more accurate than axillary dissection since multiple sections are taken from the nodes, and immunohistochemical staining is frequently done, yet only 24% of patients had a positive node, a remarkable demonstration of earlier disease presentation in this era of extensive mammographic screening. Can you tell us the histologic and immunologic techniques utilized and the proportion of nodes that were micrometastases and the positive node rates in mammographically or clinically discovered cancers?
Can you tell us the median number of nodes in the axillary dissections to convince us that these were adequate procedures? What was the positive node rate in patients with failed sentinel node biopsy and immediate axillary dissection? Did they fail because they were positive?
In the report the authors state that we should show a survival equivalence between sentinel lymph node biopsies and dissection, a thesis that I dispute since lymph node involvement bears a statistical, not a causal, relationship to survival. Lymph node dissections do not improve survival, but relate to the biology of the cancer. Why did the authors make this resolution of this restriction on their results?
How soon do you think we will utilize microarray genetic analysis which already has been shown to supersede the effects of node metastases, size, grade, so on, in predicting outcome and promises to allow us to get rid of the morbidity of axillary dissection?
Dr. Armando E. Giuliano (Santa Monica, California): The points you made about the statistical significance and the clinical irrelevancy are absolutely correct. In my view, the low failure rates point out the wide applicability of this procedure in patients with breast cancer. There is no reason not to perform it on patients with excisional biopsies, obesity, the elderly, and most patients with breast cancer. I cannot comment why the median age was 56 and not more. That is what it was.
Intraparenchymal injection and the variations in injection technique arguably produce no different results. Perhaps techniques other than peritumoral are easier to do for those beginning this operation, but they rarely reveal nonaxillary drainage. Many of the world's most experienced investigators use intraparenchymal peritumoral injection techniques, and the highest success rates are reported with this technique. No specific technique was mandated in this study. So many of the cases may have had skin injection or subareolar injection. The operative technique was left to the discretion of the operating surgeon.
I am not prepared to comment on micrometastases. That will be the subject of a complete analysis of the study that will be available hopefully in the near future.
Failed sentinel node dissection was not due to the number of positive lymph nodes. I agree with you that survival equivalency is unlikely to be affected certainly by the removal of negative lymph nodes, but currently axillary dissection is and has been the gold standard. We must prove that a new procedure is as good as the old procedure. None of us wish to introduce new technology at the risk of patient safety or adverse outcome.
Someday we will be doing microarray analysis, Dr. Cady, and other things that I am not yet aware of, but rightnow this is what we have and I think this is the best we cando.
Dr. Anthony E. Meyer (Chapel Hill, North Carolina): First I would like to commend you and recognize you. I know there are many other people that helped push this along to try to get actually lymph node dissection as the uncommon rather than the common way to treat this disease, but I think nobody more than you in this country has led this effort.
Over the period of time that this has happened, and in your own personal experience, are you seeing differences in either success or the utilization of both the combined radioisotope and blue dye versus just one of the others in people's techniques now for doing sentinel lymph node biopsy?
Dr. Armando E. Giuliano (Santa Monica, California): This trial showed overwhelmingly that the combination of radioisotope and blue dye is how most investigators learn to do the procedure. There is no advantage in my mind of 1 technique over the other in experienced hands except that the preoperative lymphoscintigram may identify extra axillary drainage. The only randomized trial comparing the 2 methods showed no advantage of one technique over the other. There is a common misunderstanding that an excisional biopsy adversely affects the success rate of this procedure, and I think that is just not the case. This study showed no diminution of success rater after excisional biopsy.
Dr. Harry D. Bear (Richmond, Virginia): As you concluded, I think the Z-10 trial and the NSABP B-32 trial clearly can be expected to establish definitively the role of sentinel lymph node biopsy in the management of breast cancer patients. The recent initial report of the B-32 trial, as you know, also demonstrated increasing success rates with increasing experience of the surgeons in a trial of over 5600 patients.
But failure to map is really not a danger to the patient. The real danger to the patient is a false negative biopsy, particularly with a successful mapping procedure, because if you are unsuccessful you can always resort to axillary node dissection.
The real question is: What is the appropriate prior experience of the surgeon? How should that be defined not only in the success rate, but should there be a false negative rate defined as a threshold for being able to perform this procedure?
I would also like you to comment on the false negative rate. The clinical false negative rate you reported was very low, compared to the nearly 10% false negative rate in the 2800-plus patients in our trial who had a sentinel node biopsy and an axillary node dissection. Why do you think these are so different? In our trial we also showed a very dramatic increase, not in the failure rate but in the false negative rate, with the use of surgical biopsy versus needle biopsy, from about 8% to about 15% in patients who had incisional or excisional biopsy, although it did not influence the success rate.
Dr. Armando E. Giuliano (Santa Monica, California): I, too, was surprised by the low axillary recurrence rate and would argue that what is important to the patient is the axillary recurrence rate rather than the histologic false negative rate. So a histologic false negative rate of 9% in B-32 could be a reflection of surgical training or skill with this technique or in reality the differences between the studies may be explained by the biology of breast cancer. We know from another NSABP study, B-04, that not all patients with positive axillary nodes develop clinical recurrences. So there are biologic issues that we do not understand or have yet to appreciate that determine clinical recurrence.
The failure rate with respect to the biopsy type in the literature, as you know, is confusing. I appreciate what the NSABP found. Our initial studies years ago showed no difference in success rates with different biopsy types. With 35% of the patients in this study having excisional biopsies and a failure rate of only 3.4%, I think we can conclude that the biopsy type did not affect the results in this trial. There were differences in surgeon qualification between the NSABP trial and our trial which may affect the outcomes.
A failure to map is not the biggest problem. Those patients will have an axillary dissection. The false negative rate is the biggest problem. But what is the best measure of false negative rate: clinical or histologic? I think the outcome will be seen in the NSABP randomized trial in terms of effect on survival. The prior NSABP B-04 trial showed no effect on survival by removing lymph nodes and that was in an era of no adjuvant systemic treatment. It is unlikely that there will be a difference in the current NSABP B-32 study.
Dr. Nicholas J. Petrelli (Newark, Delaware): Dr. Giuliano, congratulations. It is so important, as you know, for surgeons to put patients on ACOSOG trials. I say that as the chair of the Colorectal Committee of the NSABP, and I would say that even if Norman Wolmark was present here at our Association meeting. I have a very naive question: Why do you think age is a predictor of the problem with sentinel lymph node?
Dr. Armando E. Giuliano (Santa Monica, California): I think age affects the vigor with which the lymphatics function. The very elderly patient has a much longer transit time. I have overcome the problem by waiting 10, 12, or even 15 minutes after injection prior to making my axillary incision. I usually do it at 4 to 5 minutes in younger women. In the elderly, I wait 10 or 15. And I think also in the obese patient if you wait longer, you can overcome the disadvantages that are probably related to the biology of the lymphatic system.
Dr. Daniel G. Coit (New York, New York): Dr. Giuliano, you have taught me something that I didn't know about any other solid tumor and I wonder if you would comment on it. It goes back to this issue of nodal recurrence. This is the only tumor system I know of in which the presence of a positive sentinel node is not a predictor of nodal recurrence. I wonder if you can expand on that, because that is unique to breast cancer, at least in this data set, and we have not seen it in any other data set where sentinel node is used commonly.
Dr. Armando E. Giuliano (Santa Monica, California): There is clearly a difference between the melanoma and breast sentinel node data. There are actually other tumors – for example, thyroid cancer – which have a high prevalence of nodal metastases that do not become clinically relevant. In breast cancer, this is not the first study to show this. This low recurrence rate may reflect the biology of the disease. Again I refer to the NSABP B-04 study published initially about 30 years ago and more recently with a 25-year follow-up. This study showed that the presence of axillary metastases did not necessarily result in axillary recurrence. The arm in the study that had no axillary treatment had half the expected number of axillary recurrences as there were metastases in the arm with axillary dissection. I cannot explain the results except that there must be biologic factors that lead to tumor growth or no growth in the axilla.
Dr. Daniel G. Coit (New York, New York): Yet another reason not to do an axillary dissection once you find the positive sentinel node.
Dr. Carlos A. Pellegrini (Seattle, Washington): In this day and age in trying to look at quality of surgery and performance of surgery by surgeons, what should be an accepted failure rate to identify, since this is the core of the study, as I understand it. What should be the acceptable failure rate today? You had hoped for a 5% and you found a 1.3% failure rate on the surgeon. What should an institution ask surgeons or what should surgeons ask of themselves when they are trying to figure out whether they should do this technique or obtain further training?
Dr. Armando E. Giuliano (Santa Monica, California): The technique has evolved. When we started doing this study, there were only a few centers in the United States doint it in any significant volume. Today the number to achieve proficiency is probably fewer than the 20 or 30 cases we initially believed. I can see the learning curve when I train my Fellows. I think somewhere between 5 or 10 cases and the Fellows are really quite proficient at the procedure.
The problem is, how do you assess proficiency? You must perform a sentinel node biopsy and axillary dissection in the same patient; so you are subjecting patients to an unnecessary operation in order to train the surgeon. We need other ways to teach surgeons, and I think now it is in Fellowship training, residency, and postgraduate courses.
To really assess a surgeon's skill requires a lot of patients. The false negative rate depends on the number of cases with positive lymph nodes. We asked for only 1 or less false negative case out of 20 procedures which might, on the average, be a 20–30% false negative rate because there might only be 3 or 4 node-positive patients. This is unacceptable. But, in reality, once you have done 10 of these procedures, if you are a decent surgeon, you are probably pretty good at it.
This is something we never acknowledge from the podium, but let me say it, Mr. President: Not all surgeons are created equal. I have seen surgeons do this procedure who may never be able to do it correctly. So the number varies, and I do not know what the real answer is. It, too, probably varies.
Footnotes
Supported by a grant from the National Cancer Institute: U10-CA76001-09.
Reprints: Armando E. Giuliano, MD, John Wayne Cancer Institute, 2200 Santa Monica Boulevard, Suite 113, Santa Monica, CA 90404. E-mail: giulianoa@jwci.org.
REFERENCES
- 1.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- 3.Dees EC, Shulman LN, Souba WW, et al. Does information from axillary dissection change treatment in clinically node-negative patients with breast cancer? Ann Surg. 1997;226:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz GF, Giuliano AE, Veronesi U, et al. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19–22, 2001, Philadelphia, Pennsylvania. Cancer. 2002;94:2542–2551. [DOI] [PubMed] [Google Scholar]
- 5.Krag DN, Weaver DL, Alex JC, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–339. [DOI] [PubMed] [Google Scholar]
- 6.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliano AE. Sentinel lymphadenectomy in primary breast carcinoma: an alternative to routine axillary dissection. J Surg Oncol. 1996;62:75–77. [DOI] [PubMed] [Google Scholar]
- 9.Krag DN, Ashikaga T, Harlow SP, et al. Development of sentinel node targeting technique in breast cancer patients. Breast J. 1998;42:67–74. [Google Scholar]
- 10.Cox CE, Bass SS, Boulware D, et al. Implementation of new surgical technology: outcome measures for lymphatic mapping of breast carcinoma. Ann Surg Oncol. 1999;6:553–561. [DOI] [PubMed] [Google Scholar]
- 11.McMasters KM, Tuttle TM, Calrson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560–2566. [DOI] [PubMed] [Google Scholar]
- 12.McMasters KM, Wong SL, Chao C, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques. Ann Surg. 2001;234:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voitk AJ, Tsao SG, Ignatius S. The tail of the learning curve for laparoscopic cholecystectomy. Am J Surg. 2001;182:250–253. [DOI] [PubMed] [Google Scholar]
- 14.Conover CJ, Sloan FA, Provenzale D, et al. Hospital credentialing for laparoscopic cholecystectomy: Is stricter better? Clinical Performance & Quality Health Care. 1998;6:155–162. [PubMed] [Google Scholar]
- 15.Sequeira R, Weinbaum F, Satterfield J, et al. Credentialing physicians for new technology: the physician's learning curve must not harm the patient. Am Surg. 1994;60:821–833. [PubMed] [Google Scholar]
- 16.Gates EA. New surgical procedures: can our patients benefit while we learn? Am J Obstet Gynecol. 1997;176:1293–1298. [DOI] [PubMed] [Google Scholar]
- 17.Wilke LG, McCall LM, Posther KE, et al. Surgical Complications Associated With Sentinel Lymph Node Biopsy: Results From a Prospective International Cooperative Group Trial. Presented at the Society of Surgical Oncology; March 2005. [DOI] [PubMed]
- 18.Morton DL. Intraoperative lymphatic mapping and sentinel lymphadenectomy: community standard care or clinical investigation? Cancer J Sci Am. 1997;3:328–330. [PubMed] [Google Scholar]
- 19.Giuliano AE. See one, do twenty-five, teach one: the implementation of sentinel node dissection in breast cancer. Ann Surg Oncol. 1999;6:520–521. [DOI] [PubMed] [Google Scholar]
- 20.Cox CE, Bass SS, Reintgen DS. Techniques for lymphatic mapping in breast carcinoma. Surg Oncol Clin North Am. 1999;8:447–468. [PubMed] [Google Scholar]
- 21.Bass SS, Cox CE, Reintgen DS. Learning curves and certification for breast cancer lymphatic mapping. Surg Oncol Clin North Am. 1999;8:497–509. [PubMed] [Google Scholar]
- 22.Dauway EL, Giuliano R, Haddad F, et al. Lymphatic mapping in breast cancer. Hematol Oncol Clin North Am. 1999;13:349–371. [DOI] [PubMed] [Google Scholar]
- 23.Morton DL, Giuliano AE, Reintgen DS, et al. Symposium: lymphatic mapping and sentinel node biopsy in patients with breast cancer and melanoma—part 1. Contemporary Surgery. 1998;53:281–288. [Google Scholar]
- 24.Institute for Clinical Systems Improvement Technology Assessment Update TA#045. Lymphatic mapping with sentinel node biopsy for breast cancer. Available at: www.isci.org. July 2002.
- 25.American Society of Breast Surgeons Consensus Statement on Guidelines for Performance of Sentinel Lymphadenectomy for Breast Cancer. Available at: www.breastsurgeons.org. Accessed March 1, 2005.
- 26.Shivers S, Cox C, Leight G, et al. Department of Defense Breast Lymphatic Mapping Investigators. Final results of the Department of Defense multicenter breast lymphatic mapping trial. Ann Surg Oncol. 2002;9:248–255. [DOI] [PubMed] [Google Scholar]
- 27.Julian TB, Krag D, Brown A, et al. Preliminary Technical Results of NSABP B-32, a Randomized Phase III Clinical Trials to Compare Sentinel Node Resection to Conventional Axillary Dissection in Clinically Node-Negative Breast Cancer Patients. Presented at the 28th Annual San Antonio Breast Cancer Symposium; San Antonio, TX; December 2004.
- 28.Clarke D, Newcombe RG, Mansel RE. The learning curve in sentinel node biopsy: the ALMANAC experience. Ann Surg Oncol. 2004;11:211S–215S. [DOI] [PubMed] [Google Scholar]
- 29.Cody HS III, Hill AD, Tran KN, et al. Credentialing for breast lymphatic mapping: how many cases are enough? Ann Surg. 1999;229:723–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanis PJ, Nieweg OE, Hart AAM, et al. The illusion of a learning phase for lymphatic mapping. Ann Surg Oncol. 2002;9:142–147. [DOI] [PubMed] [Google Scholar]
- 31.Bergkvist L, Frisell J, Liljegren G, et al. Multicentre study of detection and false-negative rates in sentinel node biopsy for breast cancer. Br J Surg. 2001;88:1644–1648. [DOI] [PubMed] [Google Scholar]
- 32.Kuehn T, Vogl FD, Helms G, et al. Sentinel-node biopsy for axillary staging in breast cancer: results from a large prospective German multi-institutional trial. Eur J Surg Oncol. 2004;30:252–259. [DOI] [PubMed] [Google Scholar]
- 33.Morrow M, Rademaker AW, Bethke KP, et al. Learning sentinel node biopsy: results of a prospective randomized trial of two techniques. Surgery. 1999;126:714–720. [PubMed] [Google Scholar]
- 34.Cox CE, Dupont E, Whitehead GF, et al. Age and body mass index may increase the chance of failure in sentinel lymph node biopsy for women with breast cancer. Breast J. 2002;8:88–91. [DOI] [PubMed] [Google Scholar]
- 35.Derossis AM, Fey JV, Cody HS 3rd, et al. Obesity influences outcome of sentinel lymph node biopsy in early-stage breast cancer. J Am Coll Surg. 2003;197:896–901. [DOI] [PubMed] [Google Scholar]
- 36.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. [DOI] [PubMed] [Google Scholar]
- 37.Ellis PM, Butow PN, Tattersall MH, et al. Randomized clinical trials in oncology: understanding and attitudes predict willingness to participate. J Clin Oncol. 2001;19:3554–3561. [DOI] [PubMed] [Google Scholar]
- 38.Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268–2275. [DOI] [PubMed] [Google Scholar]
- 39.Siminoff LA, Zhang A, Colabianchi N, et al. Factors that predict the referral of breast cancer patients onto clinical trials by their surgeons and medical oncologists. J Clin Oncol. 2000;18:1203–1211. [DOI] [PubMed] [Google Scholar]
- 40.Cox CE, Salud CJ, Cantor A, et al. Learning curves for breast cancer sentinel lymph node mapping based on surgical volume analysis. J Am Coll Surg. 2001;193:593–600. [DOI] [PubMed] [Google Scholar]
- 41.Tanis PJ, Nieweg AE, Merkus JJ, et al. False negative sentinel node procedure established through palpation of the biopsy wound. Eur J Surg Oncol. 2000;26:714–715. [DOI] [PubMed] [Google Scholar]
- 42.Nieweg OE, Rutgers EJT, Jansen L, et al. Is lymphatic mapping in breast cancer adequate and safe? World J Surg. 2001;25:780–788. [DOI] [PubMed] [Google Scholar]
- 43.Zervos EE, Saha S, Hoshaw-Woodard S, et al. Localizing the sentinel node outside of the specialty center: Success of a lymphatic mapping course in disseminating new technology. Ann Surg Oncol. 2001;8:7–12. [DOI] [PubMed] [Google Scholar]
- 44.Available at: www.facs.org. Accessed March 31, 2005.
- 45.Reintgen D, Modarelli C, Cox C. The training of surgeons in America. Ann Surg Oncol. 2001;8:1–2. [DOI] [PubMed] [Google Scholar]


