Abstract
PACAP peptides are expressed and regulated in sensory afferents of the micturition pathway. Although these studies have implicated PACAP in bladder control, the physiological significance of these observations has not been firmly established. To clarify these issues, the roles of PACAP and PACAP signaling in micturition and cystitis were examined in receptor characterization and physiological assays. PACAP receptors were identified in various tissues of the micturition pathway including bladder detrusor smooth muscle and urothelium. Bladder smooth muscle expressed heterogeneously PAC1null, PAC1HOP1 and VPAC2 receptors; the urothelium was more restricted in expressing preferentially the PAC1 receptor subtype only. Immunocytochemical studies for PAC1 receptors were consistent with these tissue distributions. Furthermore, the addition of 50 – 100 nM PACAP27 or PACAP38 to isolated bladder strips elicited transient contractions and sustained increases in the amplitude of spontaneous phasic contractions. Treatment of the bladder strips with tetrodotoxin (1 μM) did not alter the spontaneous phasic contractions suggesting direct PACAP effects on bladder smooth muscle. PACAP also increased the amplitude of nerve-evoked contractions. By contrast, VIP had no direct effects on bladder smooth muscle. In a rat cyclophosphamide (CYP)-induced cystitis paradigm, intrathecal or intravesical administration of PAC1 receptor antagonist, PACAP6-38, reduced cystitis-induced bladder overactivity. In sum, these studies support roles for PACAP in micturition and suggest that inflammation-induced plasticity in PACAP expression in peripheral and central micturition pathways contribute to bladder dysfunction with cystitis.
Keywords: neuropeptides, urinary bladder, bladder overactivity, dorsal root ganglia, spinal cord, inflammation
Introduction
The storage and periodic elimination of urine requires a complex neural control system that coordinates the activities of the smooth muscle of the urinary bladder and the smooth and striated muscle of the urethral sphincters (13, 32, 33). Coordination between these organs is mediated by a complex neural control system located in the brain, spinal cord and peripheral ganglia (12). Experiments with a chemically (cyclophosphamide, CYP)-induced bladder inflammation (11, 34, 38) rodent model have demonstrated alterations in neurochemical (52, 53, 66, 69), electrophysiological (29, 72), organizational (65, 68) and functional properties of the micturition reflex (24, 38, 39) suggesting dramatic reorganization of the micturition reflex pathways. Alterations in peripheral bladder afferent (sensory)/efferent (autonomic and motor) and central interneuronal pathway functions may underlie detrusor overactivity that accompany CYP-induced cystitis.
Pituitary adenylate cyclase activating polypeptide (PACAP) peptides have diverse functions in the endocrine, nervous, gastrointestinal and cardiovascular systems (1, 6) through PAC1, VPAC1 and VPAC2 G protein-coupled receptors. High levels of PACAP and vasoactive intestinal polypeptide (VIP) expression have been identified in many CNS neurons and in sensory and autonomic ganglia (1, 2, 7, 42, 43, 62). Both PACAP- and VIP-immunoreactivity have been identified in urinary bladder (17, 41). Widespread PACAP-immunoreactivity exists in nerve fibers in rat lower urinary tract (LUT) (18). The majority of the PACAP nerve fibers are derived from sensory neurons (18, 80).
Although PACAP- and VIP-immunoreactive fibers have been identified in bladder wall and suburothelial plexus, and PACAP peptides have been measured in bladder tissues using combined HPLC/RIA methodologies (17), which of the PACAP/VIP receptor subtypes and isoforms are expressed in bladder and other LUT tissues has not been fully assessed. As PACAP and VIP share receptor subtypes coupled to different intracellular effectors, the identification of specific PACAP/VIP receptors can be important in revealing the relevant peptides and their signaling mechanisms in bladder physiology. Only PACAP peptides exhibit high affinity for the PAC1 receptor, whereas VIP and PACAP have similar high affinities for the VPAC1 and VPAC2 receptors (1, 58, 64). While VPAC receptors appear to be coupled solely to adenylyl cyclase, PAC1 receptor isoforms display unique patterns of adenylyl cyclase and phospholipase C activation that differ for the alternatively processed PACAP27 and PACAP38 peptides (1, 58, 59, 64). Isoforms are produced by alternative splicing of the PAC1 receptor transcript regions encoding the amino-terminal extracellular domain and third cytoplasmic loop. Variants resulting from the presence or absence of a 21-residue insert into the amino-terminal extracellular domain (short and very short variants, respectively), affect PACAP38 and PACAP27 potency (50). Other variants arising from the alternative splicing of two 84 bp HIP and HOP cassettes in the region encoding the third cytoplasmic loop exhibit differential patterns of adenylyl cyclase and phospholipase C activation by PACAP27 and PACAP38 (59). There are no apparent variants for either VPAC1 or VPAC2 receptors.
PACAP expression in sensory neurons following nerve injury is known to be changed (35, 42, 75, 76); however, few studies have examined PACAP expression following inflammation (70, 77). We have demonstrated an upregulation of PACAP levels in micturition pathways following CYP-induced cystitis (69). CYP-induced cystitis is characterized by increased frequency of voiding in rats (24, 34, 36, 38). PACAP also facilitates spontaneous bladder contractions in control animals (27). Thus, PACAP may contribute to detrusor overactivity in CYP-induced cystitis; however, the function of PACAP and PAC1 receptor signaling in the LUT is unclear.
These studies were designed to determine: (1) specific PAC1 receptor molecular forms in LUT tissues, (2) effects of PACAP on isolated detrusor smooth muscle and (3) effects of PACAP selective PAC1 antagonist, PACAP6-38 on bladder function in CYP-induced cystitis. Preliminary results have been published in abstract form (8).
Materials and Methods
Cyclophosphamide (CYP)-induced cystitis
Chemical cystitis was induced in adult female Wistar rats by cyclophosphamide treatment (CYP; Sigma ImmunoChemicals, St. Louis, MO) as previously described. CYP is metabolized to acrolein, an irritant eliminated in the urine (11). CYP was administered in two different protocols (52, 53): 1) 48 hr (n = 12; 150 mg/kg; i.p.) for intermediate inflammation or 2) administered every third day for 10 days (n = 13; 75 mg/kg; i.p) to elicit chronic inflammation. All injections of CYP were performed under 2% isoflurane anesthesia. Control animals (n= 28) were gender matched to the experimental groups and received a corresponding volume of 0.9% saline or distilled water injected under 2% isoflurane anesthesia. No differences were observed between control groups and data were therefore pooled and presented as control. Animals were euthanized by 4% isoflurane anesthesia plus thoracotomy at the indicated time points and the urinary bladder was harvested and weighed. The University of Vermont IACUC approved all experimental procedures involving animal use. Animal care was under the supervision of the University of Vermont’s Office of Animal Care in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines.
RNA Extraction, Reverse Transcription and Polymerase Chain Reaction (PCR)
S1 spinal cord segments, S1 dorsal root ganglia (DRG) and urinary bladders were dissected from female rats; the urothelium and suburothelial structures were removed from the underlying bladder smooth muscle with the aid of fine forceps and a dissecting microscope and all tissues were snap-frozen on dry ice prior to processing. In this manuscript, use of the word urothelium refers to the urothelium and suburothelial structures. Total RNA from the different tissues was extracted using the STAT-60 total RNA/mRNA isolation reagent (Tel-Test “B”, Friendswood, TX) as previously described (5, 22). The quality and quantity of the resulting RNA was assessed spectrometry at 260/280nm and 2 μg of total RNA from each sample was used to synthesize first strand cDNA using SuperScript II reverse transcriptase with the SuperScript II Preamplification System (Invitrogen, Carlsbad, CA) in a 20 μl final reaction volume. Following digestion with RNase H to remove residual RNA, the cDNA was amplified with 200 μM dNTP, 0.2 M primers and 1.25 U AmpliTaq Gold DNA Polymerase with the following parameters; initial denaturation, 94 °C, 5 min, denaturation 94 °C for 45 sec; annealing, primer-specific annealing temperature for 30 sec; extension 72 °C for 45 sec (30 – 35 cycles); final extension, 72 °C for 5 min. The oligonucleotide primers for VPAC1, VPAC2 and the different PAC1 receptor variants were used as before (5). The amplified products were resolved on 1.6% agarose gels, stained with ethidium bromide, and visualized under UV illumination. Complementary DNA synthesis in the absence of RNA template or reverse transcriptase, or amplification without template, primers or DNA polymerase was used as controls.
Immunohistochemistry
Bladder cryosections (5 μm) from control (n = 5) and cystitis animals (n = 5 each for intermediate and chronic CYP-induced cystitis) were prepared for immunocytochemistry using an on-slide processing technique. Bladders were evaluated from rats not implanted with an intravesical catheter (see below). Tissues from control and experimental animals were processed simultaneously to minimize staining variability. For whole-mount preparations, the urinary bladder was dissected and placed in Kreb’s solution (in mM): (119 NaCl, 4.7 KCl, 24.0 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4.7H2O, 11.0 glucose, 2.5 CaCl2). The bladder was cut open through the urethra in the midline and pinned flat on a sylgard-coated dish. After maximal stretch of the tissue, the bladder was incubated for 1.5 h at room temperature in cold fixative (2% paraformaldehyde + 0.2% picric acid) and the urothelium was removed (81). Urothelium and bladder musculature were processed separately using a free-floating technique. For immunocytochemical processing, the cryosections or whole-mounts were incubated overnight at room temperature with PAC1 antibody (1:500, Novus Biologicals, Littleton, CO) diluted in 0.1M potassium phosphate buffered saline (KPBS) containing 1% goat serum. After washing, the preparations were incubated with a Cy3-conjugated species-specific secondary antibody (1:500) for 2 h at room temperature, rinsed and mounted with antifade medium (Citifluor Ltd., London) for fluorescent microscopy. Control preparations were incubated in the absence of primary or secondary antibody, processed and evaluated for specificity or background staining levels. No staining was observed in any of the staining controls.
Bladder sections or whole-mounts from control and experimental animal (6 – 10 preparations per animal) were examined under an Olympus fluorescence photomicroscope with Cy3 filter sets (560–596 nm excitation; 610–655 nm emission). Staining observed in experimental tissue was compared to that from matched controls. Digital images were obtained using a CCD camera (MagnaFire SP; Optronics; Optical Analysis Corp., Nashua, NH) and LG-3 frame grabber (Scion Corp; Frederick, MD). Exposure times, brightness and contrast were held constant when acquiring images from experimental or control animals processed and analyzed on the same day. Images were imported into Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA) assemblage and labeling.
Intrathecal Catheter Placement
Rats (n = 8 each for control and CYP-treated) were anesthetized with isoflurane anesthesia (2–3%), the occipital crest of the skull exposed and the atlanto-occiptal membrane was incised along the midline with an 18-gauge needle. Polyethylene tubing (PE-10, Clay Adams, Parsippany, NJ) was inserted through the slit and passed caudally to the lumbosacral spinal cord (L6 - S1) under the membrane. The volume of fluid in the intrathecal catheter was kept constant at 9 μl in all experiments. Correct catheter placement was confirmed by a laminectomy at time of euthanasia. The intrathecal catheter was secured in place with a small drop of dental cement, the catheter placed subcutaneously, externalized at the back of the neck and the incision was closed in two layers. At the time of study, 9 μl of the PAC1 selective antagonist PACAP6-38, dissolved in artificial cerebrospinal fluid (ACF) (28), was injected through the intrathecal catheter followed by a 9 μl ACF flush. Control animals received ACF alone. The concentrations of PACAP6-38 selected for intrathecal or intravesical administration and in vitro application were selected as a result of pilot studies and published work in rat sympathetic neurons (3).
Intravesical Catheter Placement
A lower midline abdominal incision was performed during animal anesthesia (n = 30). Polyethylene tubing (PE-50) with the end flared by heat was inserted into the dome of the bladder and secured in place with a 6-0 nylon purse string suture (79). The distal end of the tubing was sealed, tunneled subcutaneously and externalized at the back of the neck. Abdominal and neck incisions were closed with 4-0 nylon sutures. Animals received the analgesic, buprenorphine (0.05 mg/kg, s.c.), every twelve hours for 48 h after surgery; the animals were maintained for 72 h after survival surgery to ensure complete recovery. For intravesical administration of the PAC1 receptor antagonist, rats were anesthetized with 2% isoflurane, and 0.5 ml PACAP6-38 was injected through the bladder catheter; the animals were maintained under anesthesia to prevent expulsion of PACAP6-38 through a voiding reflex. In this procedure, the antagonist remained in the bladder for 30 min at which time, the antagonist was drained and animals recovered from anesthesia for 20 minutes prior to experimentation.
Cystometry in Conscious Animals
Control rats (n = 15) and CYP-treated animals (intermediate, 48 h; n = 7; chronic, n = 8) were treated with a PAC1 receptor antagonist (PACAP6-38; Bachem, Torrance, CA) for cystometry. The effectiveness of an intrathecal administration of PACAP6-38 (10 or 50 nM) was evaluated in chronic CYP-treated animals; the effectiveness of intravesical PACAP6-38 (300 nM) administration of was evaluated in rats 48 h (intermediate group) after a single injection of CYP (150 mg/kg, i.p.). These experiments were performed in the same CYP-treated rats before and after treatment with PACAP6-38. For cystometry in conscious rats, an unrestrained animal was placed in a plexiglass cage with a wire-bottom. Prior to the start of the recording, the bladder was emptied and the catheter was connected via a T-tube to a pressure transducer (Grass Model PT300, West Warwick, RI) and microinjection pump (Harvard Apparatus 22, South Natick, MA). For intravesical drug administration studies, a Small Animal Cystometry Lab Station (MED Associates, Inc., St. Albans, VT) was used for urodynamic measurements. Saline solution was infused at room temperature into the bladder at a rate of 10 ml/h to elicit repetitive bladder contractions. Intravesical pressure was recorded continuously using a Neurodata Acquisition System (Grass Model 15, Astro-Med, Inc, West Warwick, RI) (79). At least four reproducible micturition cycles were recorded after the initial stabilization period of 25 – 30 minutes. The following cystometric parameters were recorded in each animal: filling pressure (pressure at the beginning of the bladder filling), threshold pressure (bladder pressure immediately prior to micturition), micturition pressure (the maximal bladder pressure during micturition), micturition interval (time between micturition events), bladder capacity, void volume, presence and amplitude of non-voiding bladder contractions (NVC). For the present study, NVCs were defined as increases in bladder pressure of at least 7 cm H2O without release of urine. Numbers of NVCs were summed over seven micturition cycles defined as a single voiding event. At the conclusion of the experiment, the animal was euthanized (4% isoflurane plus thoracotomy) and placement of intravesical and intrathecal catheter confirmed.
Isometric Tension Recording
Rats (n = 8) were euthanized by isoflurane (3–4%) and thoracotomy and the urinary bladders dissected. The isolated bladder was stored in ice-cold 10 mM Ca2+-free HEPES-buffered saline solution (Ca2+-free HEPES, pH 8.3) containing (in mM): 80 Na-glutamate monosodium salt, 55 NaCl, 6 KCl, 2 MgCl2, and10 glucose. The bladder was opened with a longitudinal cut, the urothelial surface rinsed several times with fresh Ca2+-free HEPES to remove traces of urine and the urothelium was removed. Small strips (1–1.5 mm wide and 4–5 mm long) of detrusor were cut from the bladder wall and loops of silk thread were attached to each end. The strips were then transferred to ice-cold physiological saline solution (PSS) containing (in mM): 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 0.023 EDTA and 11 glucose. After equilibrating for 30 min, the muscle strips were mounted in a tissue chamber of a MyoMED Myograph System (MED Associates Inc., Georgia, Vermont) containing 6 ml of PSS aerated with 95% O2/5% CO2, pH 7.4 and warmed to 37 °C. Initially, a load of 10 mN was applied to each strip and after equilibration with PSS for 60 min (3 changes PSS every 20 min) and then nerve-mediated contractions were elicited by electrical stimulation by a pair of electrodes parallel to either side of the strip. Electrical field stimulation (EFS) for 2 sec was delivered with increasing frequency at 0.5 – 50Hz. Stimulations were delivered with a 20 V amplitude, alternating polarity between pulses, 0.2 ms stimulation width and at 3 min periods. These conditions evoke urinary bladder smooth muscle (UBSM) contractions that were completely eliminated by blocking neuronal sodium channels with 1 μM tetrodotoxin (TTX)(23). After the first round of stimulation, peptides (PACAP27, PACAP38 or VIP; 50 – 100 nM) were added directly to the bath. Timed control EFS experiments were performed to which the peptide responses were compared. After an incubation period of 15 min, a second frequency-response curve was generated. In some experiments, spontaneous phasic UBSM contractions were observed after peptide application. These experiments were repeated in UBSM strips pretreated with TTX (1 μM). Analysis of the contractions evoked by the EFS was performed using Mini Analysis software (Synaptosoft Inc., Decatur, GA) and graphs were created using Prism software (GraphPad Software, Inc., San Diego, CA).
Statistics
All values represent mean ± S.E.M. Data were compared using Student’s t-test and one-way analysis of variance, where appropriate. When F ratios exceeded the critical value (p ≤ 0.05), the Dunnett’s post-hoc test was used to compare the control mean with each experimental mean.
Results
Urinary bladder smooth muscle and urothelium express PACAP/VIP receptor subtypes and isoforms
From reverse transcription-PCR analyses, both urinary bladder detrusor smooth muscle and urothelium demonstrated similar patterns of PAC1 receptor transcript expression (Figure 1A, Table 1). Using primers flanking sequences encoding the third cytoplasmic loop of the PAC1 receptor to assess alternative HIP and/or HOP exon usage, the smooth muscle and urothelial layers expressed both the PAC1null (neither HIP nor HOP) and the one-cassette (HIP or HOP) insert variants. The null receptor isoform appeared to be more abundant. Since the HIP versus HOP receptor variants could not be distinguished solely from amplified product size, direct sequencing of the isolated 387 bp cDNA fragments revealed that the one-cassette insert represented uniquely the HOP1 receptor in both detrusor and urothelium. Comparable PCR analyses of the same cDNA templates demonstrated that both bladder tissues expressed predominantly the PAC1(short) receptor transcript variant containing exons 4/5, encoding the 21 amino acid insert in the amino terminus of the receptor; the expression of the PAC1(very short) variant was minor in detrusor (Figure 1, Table 1). Similar to smooth muscle from other organs, the detrusor also expressed VPAC2 receptor transcripts; by contrast, VPAC2 mRNA was not detected in urothelium (Figure 1B, Table 1). VPAC1 receptor transcripts were not apparent in either bladder smooth muscle or urothelium (data not shown). These results suggested that bladder urothelium may be uniquely responsive to PACAP peptides through PAC1 receptor coupling to multiple signaling cascades. The detrusor, by contrast, has the potential of responding to both PACAP and VIP signaling.
Figure 1.
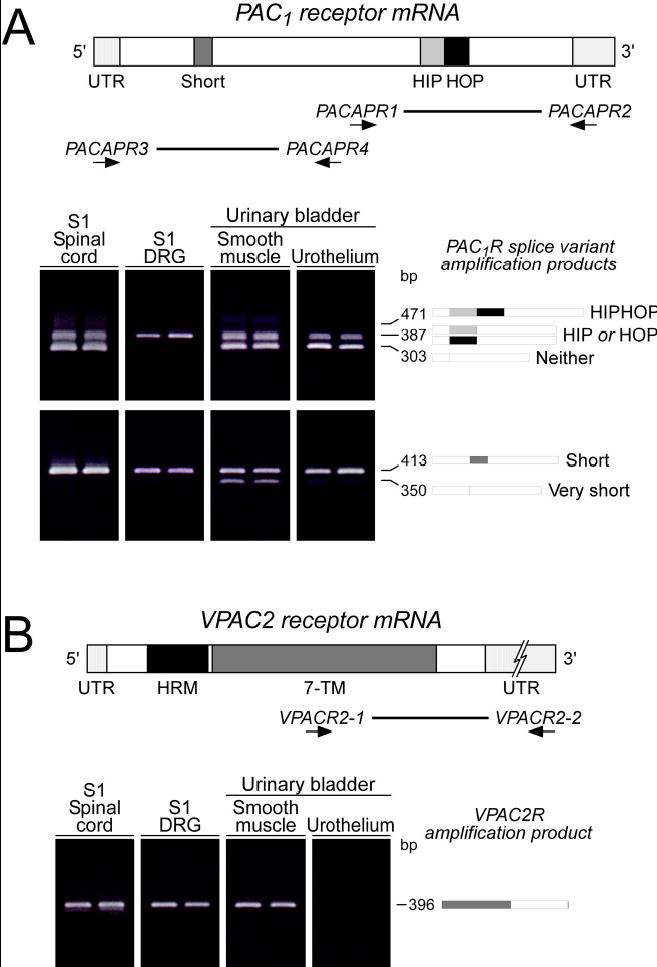
A. Lower urinary tract (LUT) tissues express PAC1 receptor variants. Complementary DNA templates were prepared from rat S1 spinal cord, S1 dorsal root ganglia (DRG) and bladder detrusor and urothelium total RNA. The region spanning the alternative splice site for the HIP and HOP exons within the third cytoplasmic loop was amplified using PACAPR1/2 oligonucleotide primers. Six third cytoplasmic loop isoform fragments containing neither, one or both HIP and HOP cassettes can potentially be amplified with these primers. LUT tissues express PAC1 receptor isoforms in a tissue-specific manner. S1 DRG express predominantly the one cassette isoform; other tissues possess both the null and the one cassette variant. Schematic shading: Dark grey, short region containing exons 4 and 5; light grey, HIP exon cassette; black, HOP cassette. Thick line, region amplified using PACAPR1/2 primers. Lower urinary tract (LUT) tissue expression of PAC1 receptor isoforms also result from alternative splicing in amino-terminal extracellular domain. Complementary DNA templates from LUT samples described above were amplified using primers PACAPR3/4 which flank the amino-terminal extracellular domain splice site. The amplified fragments of indicated sizes represent isoforms with both (short) or neither (very short) exons 4 and 5. All LUT tissues express the short variant; urinary detrusor smooth muscle also demonstrates very short PAC1 receptor expression. Shading in schematic denotes alternatively spliced exons. Thick line, region amplified using primers PACAR3/4. B. VPAC2 receptor expression in LUT tissues. cDNA from LUT tissues were prepared as described for amplification using oligonucleotide primers VPACR1/2 that span the carboxy-terminal domain of the 7 transmembrane (7-TM) receptor. All LUT tissues except bladder urothelium express VPACR2 receptor transcripts. Dark grey, 7 transmembrane domain; black, hormone receptor domain (HRM). Thick line, region amplified using primers VPACR1/2.
Table 1.
Summary of PACAP receptor isoforms and tissue distribution in the lower urinary tract pathways. ++, high expression; +, moderate expression; - no expression.
| Receptor subtypes
|
|||||
|---|---|---|---|---|---|
| PAC1 receptor
|
|||||
| Tissue | Null | HIP | HOP | VPAC1 receptor | VPAC2 receptor |
| Urinary bladder smooth muscle | ++ | − | ++ | − | + |
| Urinary bladder urothelium | ++ | − | + | − | − |
| DRG (S1) | − | − | + | − | + |
| Spinal cord (S1) | ++ | − | ++ | − | + |
To assess whether other LUT tissues may respond to PACAP/VIP signaling, RNA from S1 spinal cord and dorsal root ganglia (DRG) were also processed for PCR receptor transcript analyses. As in bladder, transcripts for PAC1 and VPAC2 receptor subtypes were present in these tissues (Figure 1A, B). The results were diagnostic for preferential PAC1(short)HOP1 receptor isoform expression in DRG, similar to other peripheral neuronal systems; the S1 cord segment expressed both PAC1(short)null and PAC1(short)HOP1 receptor transcripts. Tissue VPAC1 receptor mRNA was not evident under these assay parameters.
Immunocytochemical localization of PAC1 receptor in bladder tissues
PAC1 receptor immunoreactivity in LUT closely paralleled the tissue distribution of receptor transcripts. In control bladder whole-mounts (Figure 2A) and tissue sections (Figure 2B–D), PAC1 receptor immunoreactivity was prominent throughout the suburothelial plexus (Figure 2A, C, D) and detrusor smooth muscle where the staining was frequently localized to punctate structures (Figure 2B). Staining was moderate in urothelium but present in all urothelial layers (Figure 2D). No nerve fiber staining was observed within the urothelium only in the suburothelial plexus. Within suburothelial structures, PAC1 receptor immunoreactivity was present in structures other than nerve fibers but the cell/tissue types exhibiting PAC1 receptor immunoreactivity were not confirmed.
Figure 2.
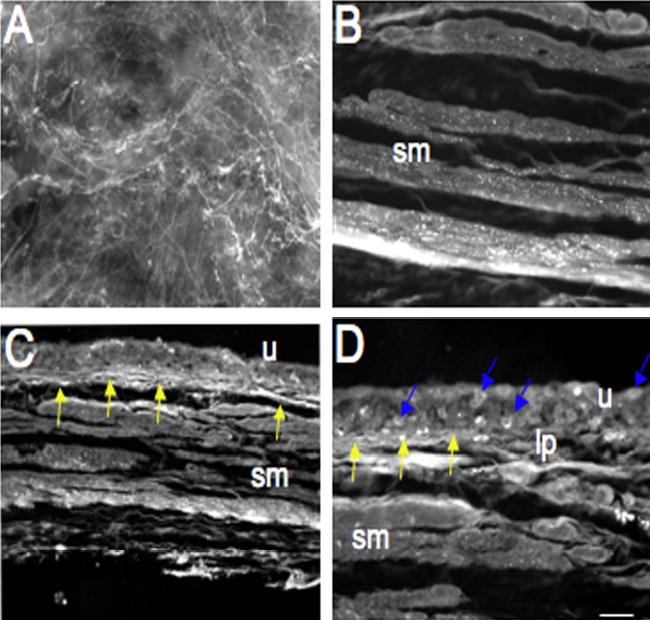
Fluorescence photographs of PAC1 receptor immunoreactivity (IR) in urinary bladder in control (A–D) rats. PAC1-IR was present in control bladder in the urothelium (u), suburothelial plexus (A, C, D, yellow arrows) and detrusor smooth muscle (B, sm). Individual urothelial cells express PAC1-IR (D, blue arrows). PAC1-IR in the suburothelial plexus was also examined in whole mount preparations (A). Calibration bar represents 100 μm in A and 80 μm in B–D.
PACAP peptides increase bladder smooth muscle EFS-induced contractions and tone
To evaluate the roles of PACAP and VIP in bladder contractility, rat bladder smooth muscle strips were denuded of urothelium and placed in myograph chambers for EFS-studies. A response curve, describing contraction amplitude as a function of increasing frequency, was produced before a second frequency response profile was generated in the presence of peptides. All contractions were abolished by the voltage gated sodium channel blocker tetrodotoxin demonstrating the neuronal basis for the EFS-induced contractions. PACAP27 or PACAP38 (80 nM) potentiated (p ≤ 0.001) the amplitude of the nerve-evoked contractions at all frequencies tested, with the largest fractional changes in contraction amplitudes occurring at lower stimulation frequencies (Figure 3C). By contrast, no difference in EFS-induced contraction amplitude was observed between time control and VIP-treated detrusor strips (Figure 3C). From these measurements, the PACAP peptides increased the sensitivity of the bladder smooth muscle to EFS. When EFS-induced contraction amplitudes under control and peptide treatments were normalized to the maximum response during each frequency response curve, the data fitted to the Boltzmann equation demonstrated that the frequency yielding 50% of the maximal response (F50) decreased from 12.7 Hz in controls to 5.8 and 5.5 Hz in the PACAP27- and PACAP38-treated samples, respectively; the F50 for VIP was 12.5 Hz and not different from controls.
Figure 3.
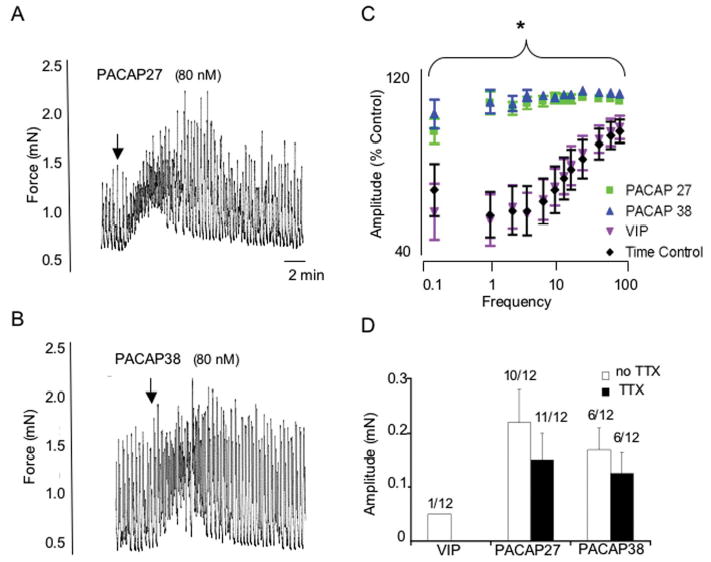
PACAP27 (80 nM; A) and PACAP 38 (80 nM; B) increased detrusor smooth muscle tone whereas VIP only rarely resulted in changes in tone (D). These changes in detrusor tone were not blocked by tetrodotoxin (1 μM) suggesting a direct effect of PACAP on detrusor smooth muscle (D). PACAP27 or PACAP 38 (80 nM) potentiated the amplitude of nerve-evoked contractions at all frequencies tested (C) whereas no difference in electric field stimulation induced contraction amplitude was observed between time control and VIP-treated detrusor strips (C). *, p ≤ 0.001.
In the absence of EFS, both PACAP27 and PACAP38 peptides (80 nM) also increased detrusor smooth muscle tone (Figure 3A, B) in 50% and 92% of the strips tested, respectively, which contrasted sharply with the rare VIP-mediated changes in tone (8% of the muscle strips). The PACAP-mediated increases in tone were superimposed on spontaneous muscle contractions (Figure 3A, B) and not blocked by 1 μM tetrodotoxin suggesting a direct effect of PACAP on detrusor smooth muscle (Figure 3D).
Intrathecal administration of PAC1 receptor antagonist reduces detrusor overactivity in CYP-induced cystitis
Consistent with previous studies, chronic CYP-induced cystitis decreased bladder capacity (66%) and intercontraction interval between voiding events (Figure 4A, B). Chronic CYP-induced cystitis also induced the appearance of non-voiding bladder contractions (i.e., increased bladder tension not associated with urine release) not seen in control rats, and increased threshold pressure without affecting either filling or micturition pressure (Table 1). PACAP peptide expression has been identified in different central and peripheral neuronal elements along the sensory pathway and has been shown to be upregulated in chemically-induced cystitis paradigms (69). To evaluate whether increased PACAP expression and signaling in these pathways participated in cystitis-associated urinary overactivity, PACAP6-38, a PAC1 receptor antagonist was administered intrathecally. Compared to cystitis animals presented with vehicle, intrathecal administration of 10 or 50 nM PACAP6-38 increased bladder capacity in CYP-treated rats 1.5-fold (p ≤ 0.001), and reduced (p ≤ 0.001) the number and amplitude of non-voiding bladder contractions approximately 50% (Figures 4A, B and 5). The duration of effect observed with intrathecal PACAP6-38 administration ranged from 1 to 2.5 hours with some animals not demonstrating any recovery before the experiment was stopped. Onset of effects with intrathecal administration of PACAP6-38 occurred within minutes, typically less than 5 minutes. There were no apparent differences between the two peptide doses tested; the concomitant addition of 50 nM PACAP27 with the receptor antagonist blunted the PACAP6-38 amelioration response in the CYP-animals and again diminished bladder capacity. Intrathecal PACAP6-38 did not affect filling, threshold or micturition pressure in the CYP-treated animals (Table 2). In aggregate, these studies appeared consistent with previous work demonstrating the facilitatory effects of PACAP27 on micturition reflex pathways (27).
Figure 4.
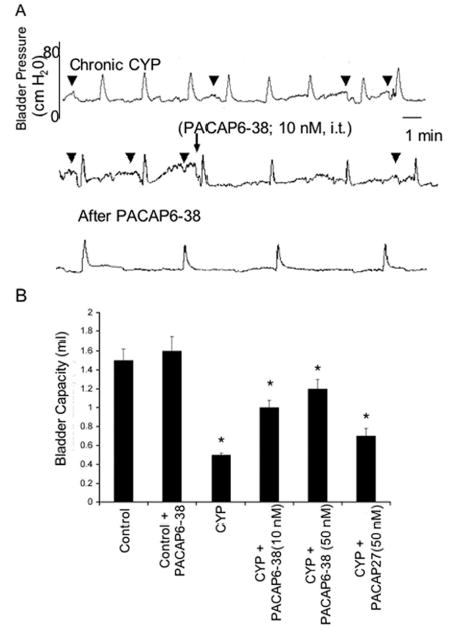
Intrathecal (i.t.) administration of PAC1 receptor antagonist, PACAP6-38 (10 nM) reduces voiding frequency (increased bladder capacity) after chronic cyclophosphamide (CYP)-induced cystitis (A). A. Continuous cystometrogram recording from the same rat treated chronically with CYP and then administered PACAP6-38 (10 nM; i.t.) with recording continuing after drug treatment. Intrathecal PACAP6-38 also reduced the number and amplitude of nonvoiding bladder contractions (NVCs) induced after CYP treatment. B. Summary histogram of bladder capacity in control, CYP-treated or CYP-treated with PACAP6-38 (i.t., 10 or 50 nM). CYP treatment significantly (p ≤ 0.001) reduced bladder capacity but this was significantly (p ≤ 0.001) increased after i.t. PACAP6-38 (10 or 50 nM) and again reduced after i.t. administration of agonist, PACAP27 (50 nM). PACAP6-38 (i.t., 10 or 50 nM) was without effect on bladder capacity in control rats.
Figure 5.
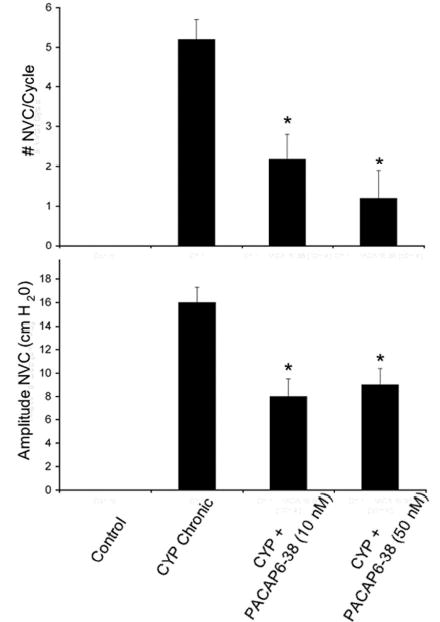
A. Summary histogram of non-voiding bladder contractions (NVCs) induced by chronic cyclophosphamide (CYP) treatment that were significantly (*, p ≤ 0.001) reduced with intrathecal PACAP6-38 (10 nM and 50 nM). B. Summary histogram of amplitude of NVCs induced by chronic CYP treatment that were significantly (*, p ≤ 0.001) reduced in amplitude with intrathecal PACAP6-38 (10 nM and 50 nM) treatment.
Table 2.
Intravesical pressures (cm H2O) during continuous filling cystometry in control, CYP-treated and CYP+PACAP6-38 (10, 50 nM; intrathecal) treated rats.
| Filling Pressure | Threshold Pressure | Micturition Pressure | |
|---|---|---|---|
| Control (n = 8) | 17.2 ± 3.5 | 17.5 ± 2.0 | 72.0 ± 4.5 |
| CYP chronic (n = 8) | 22.3 ± 2.0 | 32.5 ± 2.8* | 88.5 ± 5.9 |
| CYP + PACAP6-38 (10 nM; n = 8) | 18.6 ± 3.2 | 28.2 ± 2.2* | 78.6 ± 6.2 |
| CYP + PACAP6-38 (50 nM; n = 6) | 17.5 ± 4.5 | 30.2 ± 4.5* | 80.2 ± 4.2 |
, P ≤ 0.05 vs. control (one-way ANOVA)
Intravesical administration of PACAP6-38 reduces CYP-induced bladder overactivity
Given the presence of PAC1-IR fibers, the expression of PAC1/VPAC2 receptor expression in bladder tissues, and the abilities of PACAP to facilitate detrusor contractility, whether cystitis-induced PACAP expression and direct actions on detrusor smooth muscle contributed to bladder overactivity was investigated. Rats treated with CYP for 48 h decreased bladder capacity approximately 33% (Figure 6A, B), and increased threshold and micturition pressure compared to untreated control animals (Table 2). Intravesical administration of 300 nM PACAP6-38 significantly (p ≤ 0.01) increased bladder capacity in these same CYP-treated animals to control levels (Figure 6A, B); PACAP6-38 had no effects on untreated control rats (data not shown). Intravesical administration of PACAP6-38 did not affect bladder pressures in CYP-treated animals (Table 3). Effects of intravesical PACAP6-38 on non-voiding bladder contractions were not evaluated in these rats treated 48 h previously with CYP because the appearance of such contractions are inconsistent and when present did not meet our established criteria (≥ 7 cm H2O) for inclusion. The duration of effect observed with intravesical PACAP6-38 administration was less than that observed with intrathecal administration and ranged from 1 to 1.5 hours with most animals demonstrating recovery before the experiment was stopped. Onset of effects with intravesical administration of PACAP6-38 was not determined due to the waiting time associated with intravesical administration and the non-continuous nature of the bladder function testing.
Figure 6.
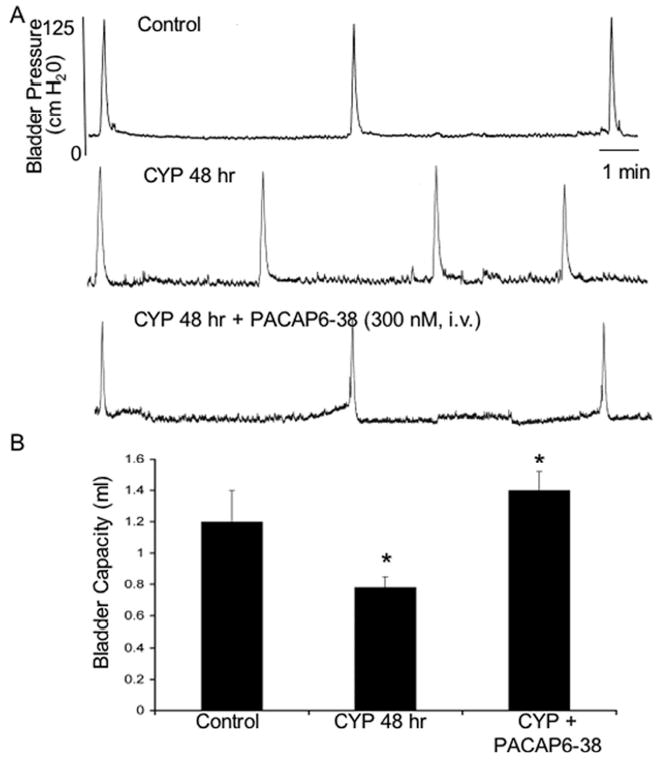
A. Bladder pressure recordings in control rats and in those treated with cyclophosphamide (CYP; 48 hr). CYP treatment (48 hr) significantly (p ≤ 0.01) decreased bladder capacity (increased voiding frequency) (A). Intravesical PACAP6-38 (300 nM) significantly (p ≤ 0.01) increased bladder capacity compared to the CYP treatment group alone (A, B). i.v., intravesical.
Table 3.
Intravesical pressures (cm H2O) during continuous filling cystometry in control, CYP-treated and CYP+PACAP6-38 (300 nM; intravesical) treated rats.
| Filling Pressure | Threshold Pressure | Micturition Pressure | |
|---|---|---|---|
| Control (n = 7) | 17.1 ± 3.0 | 17.5 ± 2.0 | 70.0 ± 4.0 |
| CYP 48 hr (n = 7) | 19.3 ± 2.0 | 32.5 ± 2.8* | 98.5 ± 5.9* |
| CYP + PACAP6-38 (n = 7) | 16.0 ± 1.1 | 28.2 ± 2.2* | 93.6 ± 6.2* |
, P ≤ 0.05 vs. control (one-way ANOVA)
Discussion
PACAP is a sensory peptide (44, 45); both PACAP immunoreactivity and mRNA has been identified in large proportions of small, nociceptive cells in DRG particularly those in L1, L2, L6 and S1 DRGs that innervate the LUT (14, 31). Dense PACAP immunoreactive sensory fibers have been found in spinal cord dorsal horn laminae I-II, and in the lateral collateral pathway of Lissauer. In the bladder, PACAP peptides and fibers have been localized to nerve fibers in the suburothelial plexus and bladder detrusor wall, in good agreement with previous studies suggesting that PACAP peptides may not only regulate urothelium solute permeability but also directly control bladder contractility and activity. The PACAP-IR fibers in bladder coexpressed CGRP and demonstrated capsaicin sensitivity demonstrating that they were sensory in origin (17, 74). By contrast, bladder VIP-IR fibers exhibited distribution patterns that were different from PACAP and were not sensitive to capsaicin suggesting that they may have been derived from pelvic autonomic ganglia (17). There is good evidence that PACAP and VIP peptides regulate smooth muscle function, either directly or by facilitating cholinergic and nitric oxide mechanisms, in a tissue- and species-specific manner (20, 40, 48, 57, 78). PACAP and VIP peptides have been shown to be relaxants in bronchi, gut, oviduct and vascular smooth muscle human, rat and porcine tissues (19, 37, 56, 60). By contrast, PACAP causes basal gall bladder contraction in guinea pig ileum and gall bladder that may be dependent on PAC1 receptor activation of protein kinase C (49). Related studies have further implicated PACAP in bladder function. Similar to previous studies for CGRP and substance P (41), CYP-induced cystitis altered the expression of PACAP in micturition pathways. Acute and chronic CYP-treatment in rats increased PACAP staining in lumbosacral DRG neurons and their projection fibers in the dorsal horn of corresponding spinal cord segments (69). Recent studies also demonstrate upregulation of PACAP in lower urinary tract tissues after chronic spinal cord injury (80). These observations were consistent with neurophenotypic changes in DRG following injury and inflammation (30, 76, 77), and suggested that among other sensory peptides, increased PACAP expression in the micturition pathways may participate in the physiological pathologies associated with interstitial cystitis, (IC).
There were a number of unknowns with respect to PACAP in micturition pathways including (1) the expression, distribution and identities of the PACAP/VIP receptor subtypes in bladder tissues that may underlie function, (2) the abilities for PACAP peptides to regulate bladder contractility and (3) the demonstration that PACAP signaling is involved in CYP-induced cystitis. Accordingly, the current studies were performed to address some of these principle issues. Notably, we showed that PAC1 and VPAC2 receptor transcripts and immunoreactivity were expressed in bladder urothelium and detrusor tissues. Although the signaling pathways for PAC1/VPAC2 receptor isoform function in LUT tissues require more detailed study, the prevalent expression of the PAC1HOP1 receptor variant transcripts in bladder may be significant in a physiological context. As described in many cellular systems, the HOP receptor isoform allows signaling diversity, including the potent and efficacious coupling to adenylyl cyclase and phospholipase C. Since phospholipase C generation of diacyl glycerol for protein kinase C signaling has been suggested to mediate smooth muscle contractions (49, 71), the expression of the PAC1HOP1 receptor variant in bladder may represent an important signaling mechanism controlling smooth muscle tone and contractility. While transcripts for the PAC1 null and HOP1 receptor variants were identified similarly in urothelial tissues to also implicate receptor coupling multiple intracellular cascades, the function of PACAP signaling in urothelium is under investigation.
To assess whether PACAP and/or VIP peptides had direct effects on bladder smooth muscle contractility, bladder detrusor smooth muscle strips, stripped of the urothelial layer, were placed in a myograph for isometric tension recordings. Both PACAP27 and PACAP38 increased bladder smooth muscle tone and potentiated EFS-induced contractions. The former was superimposed on spontaneous muscle contractions and tetrodotoxin-insensitive suggesting that the responses were direct detrusor smooth muscle effects. This suggestion is consistent with increased PAC1 receptor expression in the detrusor smooth muscle after CYP-induced cystitis. Surprisingly, VIP had no apparent effects on either bladder tone or EFS-stimulated contractions despite VPAC2 receptor transcript expression in detrusor. The reasons are unclear but may reflect low levels of VPAC2 receptor protein in bladder tissues that were not evident from semiquantitative PCR measures of transcript expression. However, as the number of VIP-IR fibers is scarce compared to that for PACAP, these results may be in keeping with suggestions that PACAP and PAC1 signaling are more prominent regulators of rat bladder physiology. These VIP results are consistent with previous studies that demonstrate that VIP application to detrusor smooth muscle had not effect on spontaneous or carbachol-induced bladder contractions despite facilitation of micturition when VIP was administered intrathecally or intraarterially close to the rat bladder (26).
A number of peptides have been demonstrated in the LUT and have demonstrated roles in regulating the micturition reflex. The facilitatory effects of PACAP observed in the present study are consistent with the action of other neuropeptides on LUT tissues including substance P (9), VIP (26), and cocaine and amphetamine-regulated transcript peptide (CARTp) (82) but contrast with the inhibitory effects of calcitonin gene-related peptide (21). In the present study, PACAP elicits a transient contraction, a sustained increase in the amplitude of spontaneous phasic contractions, and significantly increases the amplitude of nerve-mediated contractions in rat urinary bladder smooth muscle strips. Excitatory effects of PACAP on the micturition reflex pathway have also been shown to be enhanced after 2–4 weeks of spinal cord injury in the rat (73). In addition to changes in bladder function with altered expression of neuropeptides, changes in bladder function have also been demonstrated with disruption receptor types in the urinary bladder. Upregulation of P2X3 receptors has been demonstrated in cultured urothelial cells from patients with IC during in vitro stretch (61). Upregulation of P2X2 receptors in detrusor smooth muscle associated with idiopathic detrusor instability (47), IC (63) and feline IC (4) has also been demonstrated. P2X3 receptor knockout mice exhibit bladder hyporeflexia on cystometry with decreased voiding frequency and increased bladder capacity and voided volume but normal bladder pressures (10). Thus, neurophenotypic changes in neuropeptide expression or LUT receptors can contribute to altered bladder function in disease states.
IC is a chronic inflammatory bladder disease syndrome characterized by urinary frequency, urgency, suprapubic and pelvic pain. Although the etiology and pathogenesis of IC are unknown, numerous theories including; infection, autoimmune disorder, toxic urinary agents, deficiency in bladder wall lining and neurogenic causes have been proposed (15, 16, 54, 55). Consistent with previous studies, acute or chronic CYP-administration to rats results in bladder inflammation, and reduced bladder capacity and intercontraction intervals. In experimental inflammation paradigms, the expression of several sensory peptides including PACAP is increased while VIP levels, by contrast are unchanged. In congruence, PACAP expression is upregulated in spinal cord and DRG (69)(Figure 7) with CYP-induced cystitis and the PAC1 receptor immunoreactivity is upregulated in the urinary bladder with CYP-induced cystitis (data not shown). As the VIP peptidergic system appeared immutable with respect to regulation and function, two related experiments were performed to assess whether augmented PACAP signaling contributes to the disorders in cystitis. Firstly, the PACAP receptor antagonist PACAP6-38 was administered intrathecally at the level of the L6-S1 spinal cord to CYP-treated animals to block receptor signaling in the lumbosacral spinal cord and DRG. Intrathecal administration of PACAP6-38 can then act at both the spinal cord and dorsal root ganglia. Secondly, PACAP6-38 was presented intravesically to CYP-inflamed bladders. In both instances, PACAP6-38 ameliorated significantly many of the CYP-induced symptoms; compared to cystitis animals, PACAP6-38 treatments increased bladder capacity and diminished the appearance and amplitude of non-voiding bladder contractions. With respect to non-voiding bladder contractions, intraabdominal pressure recordings were not performed so it cannot be excluded that these pressure changes might represent straining as opposed to contractions of the urinary bladder. In the case of intravesical PACAP6-38 administration, the cystitis-induced reductions in bladder capacity were returned to control levels.
Figure 7.
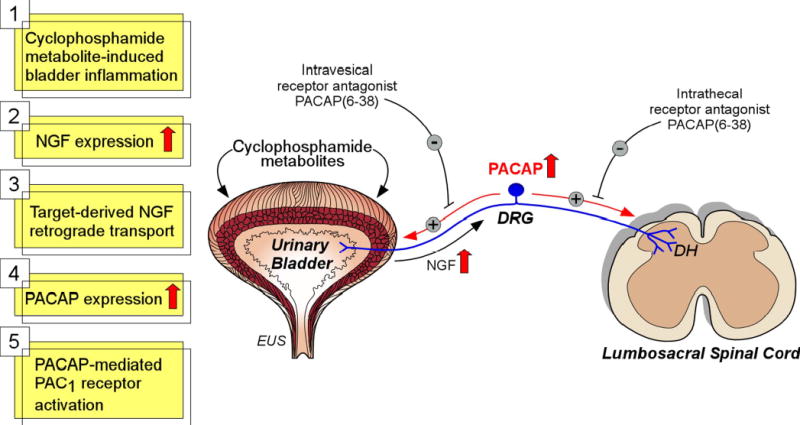
Potential mechanism underlying PACAP’s role in detrusor overactivity with CYP-induced bladder inflammation. Previous studies have demonstrated increases in nerve growth factor (NGF) in the urinary bladder with CYP-induced cystitis (25, 46, 67). NGF is retrogradely transported from the urinary bladder to dorsal root ganglia (DRG). NGF can upregulate PACAP expression in DRG and spinal cord with CYP-induced cystitis (69). The PAC1 receptor antagonist, PACAP6-38, was effective in reducing bladder overactivity with both intrathecal and intravesical administration. PACAP6-38 may act at the urinary bladder, DRG and spinal cord to reduce detrusor overactivity induced by CYP. MPG, major pelvic ganglion; DH, dorsal horn; SPN, sacral parasympathetic nucleus; INT, interneurons; PGN, preganglionic neurons; EUS, external urethral sphincter.
Because both routes (intrathecal and intravesical) of PACAP antagonist delivery were effective in reducing bladder overactivity, both central (spinal cord) and peripheral (DRG, urinary bladder) PACAP sources may contribute to bladder overactivity following CYP-induced cystitis (Figure 7). With respect to the site of action of PACAP6-38 after intrathecal administration, we suggest that the PAC1 receptor antagonist acts on superficial dorsal horn neurons that express the PAC1 receptor to block enhanced release of PACAP from C-fiber bladder afferents with CYP-induced cystitis (69). With respect to the site of action of PACAP6-38 after intravesical administration, we cannot, at this time, rule out effects of the PAC1 receptor antagonist on multiple cell or tissue types including urothelial cells, suburothelial structures and detrusor smooth muscle. With CYP-induced cystitis, erosion of the urothelium occurs so intravesical administration of PACAP6-38 can access the suburothelial structures and detrusor smooth muscle more easily than in control rats. PACAP6-38 has been widely used to block PAC1 receptor activation although recent data cannot preclude actions at VPAC2 receptor also. While more detailed studies with the PAC1 receptor-selective maxD.4 antagonist may clarify the mechanisms, the lack of direct VIP actions on detrusor suggests that PACAP38 may be acting predominantly on smooth muscle PAC1 receptors in the intravesical paradigms. The improvements to the CYP-induced cystitis symptoms after PACAP6-38 administration appeared striking and suggest that changes in PACAP signaling along the micturition pathway can have profound effects on bladder activity.
Inflammation has been well shown to increase tissue growth factor expression. In aggregate, these results suggest that cystitis-induced bladder inflammation elicits an increase in tissue-derived growth factors and regulators, including nerve growth factor (NGF)(25, 46, 67). Enhanced target-derived NGF availability has been shown to augment DRG PACAP expression in small nociceptive neurons, and the increase in PACAP production in DRG phenotypic plasticity may result in heightened PACAP signaling at PAC1 and/or VPAC2 receptors at central or peripheral tissue sites to exacerbate the functional defects in cystitis (Figure 7). As a corollary, NGF does not increase DRG VIP expression that may be consistent with the target tissue neurotrophic model to selectively augment DRG PACAP expression (V. May and K. Braas, unpublished observations). The exact sites or mechanisms of PACAP actions in cystitis are not determined from receptor antagonist studies but may include central spinal cord pathways, sensory afferents and bladder smooth muscle. From these studies, all of these targets appear likely to present the complete profile of altered responses in cystitis. While our work is consistent with previous studies demonstrating the facilitatory effects of PACAP in micturition (27, 73), our results also contrast with other data showing little or no direct effects of PACAP on bladder muscle in the isometric contraction assays (27). The reasons for the apparent differences are unclear and may be related to urothelium removal from the smooth muscle strips and the stimulation parameters used in the current studies. Accordingly, while we concur that PACAP signaling in spinal cord and/or autonomic pathways represents important mechanisms in micturition and cystitis, our isometric tension and cystometry studies also suggest that bladder smooth muscle is a pertinent target of that regulation. Many studies have shown secretion of sensory peptides at the periphery. The increase in DRG sensory neuron PACAP expression from CYP-induced bladder inflammation, may not only augment PACAP release at central spinal cord sites in the micturition pathway but also at peripheral bladder targets contributing to heightened reflex physiology (Figure 7).
Acknowledgments
Supported by NIH DK051369, DK060481, DK065989, NS04079, HD27468, NS37179
References
- 1.Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- 2.Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 3.Beaudet MM, Parsons RL, Braas KM, May V. Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons. J Neurosci. 2000;20:7353–7361. doi: 10.1523/JNEUROSCI.20-19-07353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- 5.Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274:27702–27710. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- 6.Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms. Ann N Y Acad Sci. 1996;805:204–216. doi: 10.1111/j.1749-6632.1996.tb17484.x. discussion 217-208. [DOI] [PubMed] [Google Scholar]
- 7.Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci. 1998;18:9766–9779. doi: 10.1523/JNEUROSCI.18-23-09766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT. Role for pituitary adenylate cyclase activating polypeptide (PACAP) in cystitis-induced plasticity of micturition reflexes. Regul Pept. 2005;130:157–158. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien CT, Yu HJ, Lin TB, Lai MK, Hsu SM. Substance P via NK1 receptor facilitates hyperactive bladder afferent signaling via action of ROS. Am J Physiol Renal Physiol. 2003;284:F840–F851. doi: 10.1152/ajprenal.00187.2002. [DOI] [PubMed] [Google Scholar]
- 10.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford APDW. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X(3)-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 11.Cox PJ. Cyclophosphamide cystitis--identification of acrolein as the causative agent. Biochem Pharmacol. 1979;28:2045. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- 12.de Groat WC, Booth AM, and Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: The Autonomic Nervous System, edited by Maggi CA. London: Harwood Academic Publishers, 1993, p. 227–290.
- 13.de Groat WC and Steers WD. Autonomic regulation of the urinary bladder and sex organs. In: Central Regulation of Autonomic Functions, edited by Loewy AD and Spyer KM. London: Oxford University Press, 1990, p. 310–333.
- 14.Donovan MK, Winternitz SR, Wyss JM. An analysis of the sensory innervation of the urinary system of the rat. Brain Res Bull. 1983;11:321–324. doi: 10.1016/0361-9230(83)90168-5. [DOI] [PubMed] [Google Scholar]
- 15.Erickson DR. Interstitial cystitis: Update on etiologies and therapeutic options. J Women's Health Gender-Based Med. 1999;8:745–758. doi: 10.1089/152460999319075. [DOI] [PubMed] [Google Scholar]
- 16.Erickson DR, Davies MF. Interstitial cystitis. Int Urogynecol J. 1998;9:174–183. doi: 10.1007/BF02001088. [DOI] [PubMed] [Google Scholar]
- 17.Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience. 1998;83:1261–1272. doi: 10.1016/s0306-4522(97)00474-0. [DOI] [PubMed] [Google Scholar]
- 18.Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience. 1998;83:1261–1272. doi: 10.1016/s0306-4522(97)00474-0. [DOI] [PubMed] [Google Scholar]
- 19.Fizanne L, Sigaudo-Roussel D, Saumet JL, Fromy B. Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. J Physiol. 2004;554:519–528. doi: 10.1113/jphysiol.2003.053835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox-Threlkeld JA, McDonald TJ, Woskowska Z, Iesaki K, Daniel EE. Pituitary adenylate cyclase-activating peptide as a neurotransmitter in the canine ileal circular muscle. J Pharmacol Exp Therap. 1999;290:66–75. [PubMed] [Google Scholar]
- 21.Gillespie JI. Inhibitory actions of calcitonin gene-related peptide and capsaicin: evidence for local axonal reflexes in the bladder wall. BJU Int. 2005;95:149–156. doi: 10.1111/j.1464-410X.2005.05268.x. [DOI] [PubMed] [Google Scholar]
- 22.Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept. 2002;109:89–101. doi: 10.1016/s0167-0115(02)00191-x. [DOI] [PubMed] [Google Scholar]
- 23.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279:R60–68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- 24.Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;284:R574–R585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- 25.Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol. 2005;173:1016–1021. doi: 10.1097/01.ju.0000155170.15023.e5. [DOI] [PubMed] [Google Scholar]
- 26.Igawa Y, Persson K, Andersson KE, Uvelius B, Mattiasson A. Facilitatory effect of vasoactive intestinal polypeptide on spinal and peripheral micturition reflex pathways in conscious rats with and without detrusor instability. J Urol. 1993;149:884–889. doi: 10.1016/s0022-5347(17)36252-3. [DOI] [PubMed] [Google Scholar]
- 27.Ishizuka O, Alm P, Larsson B, Mattiasson A, Andersson KE. Facilitatory effect of pituitary adenylate cyclase-activating polypeptide on micturition in normal, conscious rats. Neuroscience. 1995;66:1009–1014. doi: 10.1016/0306-4522(95)00038-k. [DOI] [PubMed] [Google Scholar]
- 28.Izquierdo I, Medina JH. Correlation between the pharmacology of long-term potentiation and the pharmacology of memory. Neurobiol Learn Mem. 1995;63:19–32. doi: 10.1006/nlme.1995.1002. [DOI] [PubMed] [Google Scholar]
- 29.Jennings LJ, Vizzard MA. Cyclophosphamide-induced inflammation of the urinary bladder alters electrical properties of small diameter afferent neurons from dorsal root ganglia. FASEB J. 1999;13:A57. [Google Scholar]
- 30.Jongsma Wallin H, Pettersson LM, Verge VM, Danielsen N. Effect of anti-nerve growth factor treatment on pituitary adenylate cyclase activating polypeptide expression in adult sensory neurons exposed to adjuvant induced inflammation. Neuroscience. 2003;120:325–331. doi: 10.1016/s0306-4522(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 31.Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- 32.Klück P. The autonomic innervation of the human urinary bladder neck and urethra: A histochemical study. Anat Rec. 1980;198:439–447. doi: 10.1002/ar.1091980306. [DOI] [PubMed] [Google Scholar]
- 33.Kuru M. Nervous control of micturition. Physiol Rev. 1965;45:425–494. doi: 10.1152/physrev.1965.45.3.425. [DOI] [PubMed] [Google Scholar]
- 34.Lantéri-Minet M, Bon K, de Pommery J, Michiels JF, Menétrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involves as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res. 1995;105:220–232. doi: 10.1007/BF00240958. [DOI] [PubMed] [Google Scholar]
- 35.Larsen JO, Hannibal J, Knudsen SM, Fahrenkrug J. Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) in the mesencephalic trigeminal nucleus of the rat after transection of the masseteric nerve. Mol Brain Res. 1997;46:109–117. doi: 10.1016/s0169-328x(96)00279-3. [DOI] [PubMed] [Google Scholar]
- 36.Lecci A, Giulani S, Santiciolo P, Maggi CA. Involvement of spinal tachykinin NK1 and NK2 receptors in detrusor hyperreflexia during chemical cystitis in anaesthetized rats. Eur J Pharmacol. 1994;259:129–135. doi: 10.1016/0014-2999(94)90501-0. [DOI] [PubMed] [Google Scholar]
- 37.Linden A, Cardell LO, Yoshihara S, Nadel JA. Bronchodilation by pituitary adenylate cyclase-activating peptide and related peptides. Eur Respir J. 1999;14:443–451. doi: 10.1034/j.1399-3003.1999.14b34.x. [DOI] [PubMed] [Google Scholar]
- 38.Maggi CA, Lecci A, Santiciolo P, Del Biance E, Giuliani S. Cyclophosphamide cystitis in rats: involvement of capsaicin-sensitive primary afferents. J Autonom Nerv Syst. 1992;38:201–208. doi: 10.1016/0165-1838(92)90031-b. [DOI] [PubMed] [Google Scholar]
- 39.Maggi CA, Lecci A, Santiciolo P, Del Bianco E, Giuliani S. Cyclophosphamide-induced cystitis in rats: involvement of capsaicin-sensitive primary afferents. Agents Actions. 1993;38:C28–C30. doi: 10.1007/BF01991127. [DOI] [PubMed] [Google Scholar]
- 40.Mizumoto A, Fujimura M, Ohtawa M, Ueki S, Hayashi N, Itoh Z, Fujino M, Arimura A. Pituitary adenylate cyclase activating polypeptide stimulates gallbladder motility in conscious dogs. Regul Pept. 1992;42:39–50. doi: 10.1016/0167-0115(92)90022-m. [DOI] [PubMed] [Google Scholar]
- 41.Mohammed H, Hannibal J, Fahrenkrug J, Santer R. Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: effects of age. Urol Res. 2002;30:248–255. doi: 10.1007/s00240-002-0261-6. [DOI] [PubMed] [Google Scholar]
- 42.Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M, Sundler F. The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res. 1997;775:166–182. doi: 10.1016/s0006-8993(97)00923-2. [DOI] [PubMed] [Google Scholar]
- 43.Moller K, Reimer M, Hannibal J, Fahrenkrug J, Sundler F, Kanje M. Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Res. 1997;775:156–165. doi: 10.1016/s0006-8993(97)00937-2. [DOI] [PubMed] [Google Scholar]
- 44.Moller K, Zhang YZ, Hakanson R, Luts A, Sjolund B, Uddman R, Sundler F. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57:725–732. doi: 10.1016/0306-4522(93)90018-b. [DOI] [PubMed] [Google Scholar]
- 45.Mulder H, Uddman R, Moller K, Zhang Y-Z, Ekblad E, Alumets J, Sundler F. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994;63:307–312. doi: 10.1016/0306-4522(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 46.Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol. 2004;172:2434–2439. doi: 10.1097/01.ju.0000143549.29867.4e. [DOI] [PubMed] [Google Scholar]
- 47.O'Reilly BA, Kosaka AH, Knight GF, Chang TK, Ford AP, Rymer JM, Popert R, Burnstock G, McMahon SB. P2X receptors and their role in female idiopathic detrusor instability. J Urol. 2002;167:157–164. [PubMed] [Google Scholar]
- 48.Onaga T, Harada Y, Okamoto K. Pituitary adenylate cyclase-activating polypeptide (PACAP) induces duodenal phasic contractions via the vagal cholinergic nerves in sheep. Regul Pept. 1998;77:69–76. doi: 10.1016/s0167-0115(98)00046-9. [DOI] [PubMed] [Google Scholar]
- 49.Pang PK, Kline LW. Protein kinase C mediates the contractile actions of pituitary adenylate cyclase activating polypeptide in guinea pig gallbladder strips. Regul Pept. 1998;77:63–67. doi: 10.1016/s0167-0115(98)00042-1. [DOI] [PubMed] [Google Scholar]
- 50.Pantaloni C, Brabet P, Bilanges B, Dumuis A, Houssami S, Spengler D, Bockaert J, Journot L. Alternative splicing in the N-terminal extracellular domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor modulates receptor selectivity and relative potencies of PACAP-27 and PACAP-38 in phospholipase C activation. J Biol Chem. 1996;271:22146–22151. doi: 10.1074/jbc.271.36.22146. [DOI] [PubMed] [Google Scholar]
- 51.Petrone RL, Agha AH, Roy JB, Hurst RE. Urodynamic findings in patients with interstitial cystitis. J Urol. 1995;153:290A. [Google Scholar]
- 52.Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol. 2002;454:200–211. doi: 10.1002/cne.10447. [DOI] [PubMed] [Google Scholar]
- 53.Qiao LY, Vizzard MA. Up-regulation of phosphorylated CREB but not c-Jun in bladder afferent neurons in dorsal root ganglia after cystitis. J Comp Neurol. 2004;469:262–274. doi: 10.1002/cne.11009. [DOI] [PubMed] [Google Scholar]
- 54.Rosamilia A, Dwyera PL. Pathophysiology of interstitial cystitis. Cur Opin Obst Gynecol. 2000;12:405–410. doi: 10.1097/00001703-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Sant GR, Theoharides TC. Interstitial cystitis. Cur Opin Urol. 1999;9:297–302. doi: 10.1097/00042307-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Schworer H, Clemens A, Katsoulis S, Kohler H, Creutzfeldt W, Schmidt WE. Pituitary adenylate cyclase-activating peptide is a potent modulator of human colonic motility. Scand J Gastroenterol. 1993;28:625–632. doi: 10.3109/00365529309096101. [DOI] [PubMed] [Google Scholar]
- 57.Seebeck J, Lowe M, Kruse ML, Schmidt WE, Mehdorn HM, Ziegler A, Hempelmann RG. The vasorelaxant effect of pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in isolated rat basilar arteries is partially mediated by activation of nitrergic neurons. Regul Pept. 2002;107:115–123. doi: 10.1016/s0167-0115(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 58.Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- 59.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 60.Steenstrup BR, Ottesen B, Jorgensen M, Jorgensen JC. Pituitary adenylate cyclase activating polypeptide induces vascular relaxation and inhibits non-vascular smooth muscle activity in the rabbit female genital tract. Acta Physiologica Scandinavica. 1994;152:129–136. doi: 10.1111/j.1748-1716.1994.tb09792.x. [DOI] [PubMed] [Google Scholar]
- 61.Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol. 2004;171:448–452. doi: 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- 62.Sundler F, Ekblad E, Hannibal J, Moller K, Zhang YZ, Mulder H, Elsas T, Grunditz T, Danielsen N, Fahrenkrug J, Uddman R. Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann NY Acad Sci. 1996;805:410–426. doi: 10.1111/j.1749-6632.1996.tb17501.x. [DOI] [PubMed] [Google Scholar]
- 63.Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int. 2004;93:1344–1348. doi: 10.1111/j.1464-410X.2004.04858.x. [DOI] [PubMed] [Google Scholar]
- 64.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- 65.Vizzard MA. Alterations in growth-associated protein (GAP-43) expression in lower urinary tract pathways following chronic spinal cord injury. Somatosen Motor Res. 1999;16:369–381. doi: 10.1080/08990229970429. [DOI] [PubMed] [Google Scholar]
- 66.Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 67.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- 68.Vizzard MA. Increased expression of spinal Fos protein in lower urinary tract pathways induced by bladder distension following chronic cystitis. Am J Physiol. 2000;279:R295–R305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- 69.Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000;420:335–348. [PubMed] [Google Scholar]
- 70.Wang Z-Y, Alm P, Hakanson R. PACAP occurs in sensory nerve fibers and participates in ocular inflammation in the rabbit. Ann NY Acad Sci. 1996;805:779–783. doi: 10.1111/j.1749-6632.1996.tb17556.x. [DOI] [PubMed] [Google Scholar]
- 71.Yoshida M, Nishi K, Machida J, Sakiyama H, Ikeda K, Ueda S. Effects of phorbol ester on lower urinary tract smooth muscles in rabbits. Eur J Pharmacol. 1992;222:205–211. doi: 10.1016/0014-2999(92)90856-y. [DOI] [PubMed] [Google Scholar]
- 72.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder following chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshiyama M, de Groat WC. Effects of intrathecal administration of pituitary adenylate cyclase-activating polypeptide (PACAP) on the lower urinary tract in the rat. Soc Neurosci Abstr. 1997;23:1523. [Google Scholar]
- 74.Zhang Q, Shi T-J, Ji R-R, Zhang Y-T, Sundler F, Hannibal J, Fahrenkrug J, Hokfelt T. Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res. 1995;705:149–158. doi: 10.1016/0006-8993(95)01150-1. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Q, Shi TJ, Ji RR, Zhang YZ, Sundler F, Hannibal J, Fahrenkrug J, Hokfelt T. Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res. 1995;705:149–158. doi: 10.1016/0006-8993(95)01150-1. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y-Z, Hannibal J, Zhao Q, Moller K, Danielsen N, Fahrenkrug J, Sundler F. Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury. Neuroscience. 1996;74:1099–1110. doi: 10.1016/0306-4522(96)00168-6. [DOI] [PubMed] [Google Scholar]
- 77.Zhang YZ, Danielsen N, Sundler F, Mulder H. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport. 1998;9:2833–2836. doi: 10.1097/00001756-199808240-00027. [DOI] [PubMed] [Google Scholar]
- 78.Zizzo MG, Mule F, Serio R. Interplay between PACAP and NO in mouse ileum. Neuropharmacology. 2004;46:449–455. doi: 10.1016/j.neuropharm.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 79.Zvara P, Kliment J, De Ross AL, Irwin BH, Malley S, Plant MK, Vizzard MA. Differential Expression of Urinary Bladder Neurotrophic Factor mRNA Expression in Male and Female Rat After Bladder Outflow Obstruction. J Urol. 2002;168:2682–2688. doi: 10.1016/S0022-5347(05)64244-9. [DOI] [PubMed] [Google Scholar]
- 80.Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol. 2005;192:46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 81.Zvarova K, Murray E, Vizzard MA. Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J Comp Neurol. 2004;475:590–603. doi: 10.1002/cne.20195. [DOI] [PubMed] [Google Scholar]
- 82.Zvarova K, Vizzard MA. Ontogeny of cocaine-and amphetamine-regulated transcript peptide (CARTp) in urinary bladder and lumbosacral spinal cord of neonatal rat. J Comp Neurol. 2005;489:501–517. doi: 10.1002/cne.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]


