In collaboration with the Canadian Critical Care Society, the Canadian Association of Transplantation and the Canadian Society of Transplantation, the Canadian Council for Donation and Transplantation (CCDT) sponsored a forum entitled “Medical Management to Optimize Donor Organ Potential,” 23–25 Feb. 2004, to develop guidelines and recommendations for organ donor management in Canada. Discussions were restricted to the interval of care that begins with neurological determination of death (NDD), commonly called “brain death,” and consent to organ donation, and culminates in surgical organ procurement. This period presents a significant opportunity to enhance multi-organ function and improve organ utilization.
The forum was the first structured, cooperative assembly of health professionals in the fields of critical care and transplantation and can be viewed as a landmark event in Canada. End-of-life care in the intensive care unit (ICU) includes all efforts to actualize the desire and opportunity to donate organs. The evolving collaboration to establish best donor management practices in the ICU and operating room must be accompanied by efforts to ensure optimum organ utilization. In turn, improving the use of organs must be linked with improving transplant graft and patient outcomes.
Forum overview
Therapies to protect organs rely on expert clinical management to increase the number of eligible donors and optimize organ function for the purposes of transplantation. Experts involved in this forum focused on 2 key areas:
• How to achieve optimum donor organ physiology
• How to increase the number of potential donors whose organs are suitable for donation, increase the number of organs transplanted per donor, improve graft function, graft survival and patient survival and identify and explore logistic challenges in the period from NDD to organ procurement.
This initiative did not address related challenges, such as the consent process, post-procurement organ perfusion and preservation or surgical retrieval team logistics.
The objectives of the forum were
• To review and benchmark existing national and international practices, guidelines and policies related to donor organ management, including organ-protective therapies (sources included articles and reports in the basic science and clinical literature; regional, national and international donor management guidelines; and material from related conferences and workshops)
• To develop expert consensus recommendations for organ-protective therapies in the ICU and intraoperative management of the organ donor
• To develop expert consensus recommendations for the CCDT, Canadian Society of Transplantation, Canadian Critical Care Society, Canadian Association of Transplantation and other relevant organizations and groups
• To develop a mechanism to involve both critical care and transplant communities in reviewing and updating the expert consensus recommendations as therapies evolve
• To disseminate the forum findings based on current understanding of knowledge transfer in Canada
• To develop recommendations for future research in this evolving field.
The participants were health care professionals from 27 organizations, including specialists in adult and pediatric critical care, physician and surgeon specialists in adult and pediatric organ-specific transplantation, neurologists, neurosurgeons, anesthetists, emergency medicine physicians, nurses and nurse practitioners. A working group of health administrators, policymakers and donation–transplant agencies also provided input on logistic barriers and supports and knowledge transfer in support of effective medical management of organ donors.
Discussions focused on collaborative, consensus-based decision-making at a national, strategic level.
These guidelines are aimed principally at physicians (or their delegates) who are involved in the management of organ donors in the ICU and operating room setting. The following 5 areas were addressed:
• Multisystem management of multi-organ donors
• Organ-specific considerations for hearts, lungs and intra-abdominal organs
• Other systemic challenges
• Potential questions for a national research agenda to optimize donor organ management
• Logistic and knowledge transfer considerations related to disseminating and implementing the forum's recommendations.
Process
Substantive background documents, provided by the steering committee in advance of the forum included comprehensive literature reviews and related practice surveys. During the forum, each of the 5 areas was addressed using the following process. Presentations by experts were followed by open plenary discussions. Participants then worked in small groups guided by worksheets that provided a description of current, well-accepted care in the Canadian context (based on a review of donor management guidelines in effect in Canadian health care facilities1); a summary of existing scientific evidence; key considerations; a summary of national and international donor management guidelines; and a list of references.
The small-group discussions focused on specific questions related to the processes of care. The Forum Recommendations Group (FRG) and the Pediatric Recommendations Group (PRG) met to review the results of the small-group and plenary discussions and develop recommendations for adults and children, which were then presented for discussion in a plenary session. Participants' input related to research questions was gathered and summarized (Appendix 1). The Logistics and Knowledge Transfer (LKT) Group considered issues related to the logistics and knowledge transfer that were identified during the forum (Appendix 2).
Overarching themes
Several overarching themes were identified during discussions:
• Prospective research is needed to improve the basis for levels of evidence used by experts to develop consensus on standards of care. Most existing evidence in the area of donor management is based on retrospective, uncontrolled and observational studies.
• As a result of uncontrolled intracranial hypertension and herniation, the organ donor has a distinct pathophysiology.
• Temporal changes in multi-organ function after NDD demand flexibility in identifying the optimal time of procurement. Participants recognized that:
• resuscitation of the cardiopulmonary system benefits function of all organs
• it is important to take time in the ICU to optimize multi-organ function to improve transplant outcomes
• reversible organ dysfunction may be addressed with aggressive resuscitation and frequent re-evaluation
• once organ function is optimized, surgical procurement should be arranged emergently.
• A 4-centre Canadian review of heart and lung utilization2 identified potential deficits in the process of obtaining consent for donation of individual organs, the offering of organs and the utilization of offered organs. The forum recommends that there be no predefined demographic factor or organ dysfunction that precludes provision of consent for individual organs or the offering of organs for transplantation.
• Final decisions about transplantability rest with the individual transplant programs represented by the organ-specific transplant doctors.
• Any initiatives aimed at improving donor organ potential should be evaluated not only in terms of increases in organ utilization, but also in terms of corresponding transplant outcomes.
• ICU-transplant collaboration in this field involves ensuring reciprocal accountability by procurement and transplant services if available organs are not ultimately used.
Multisystem management of the multi-organ donor
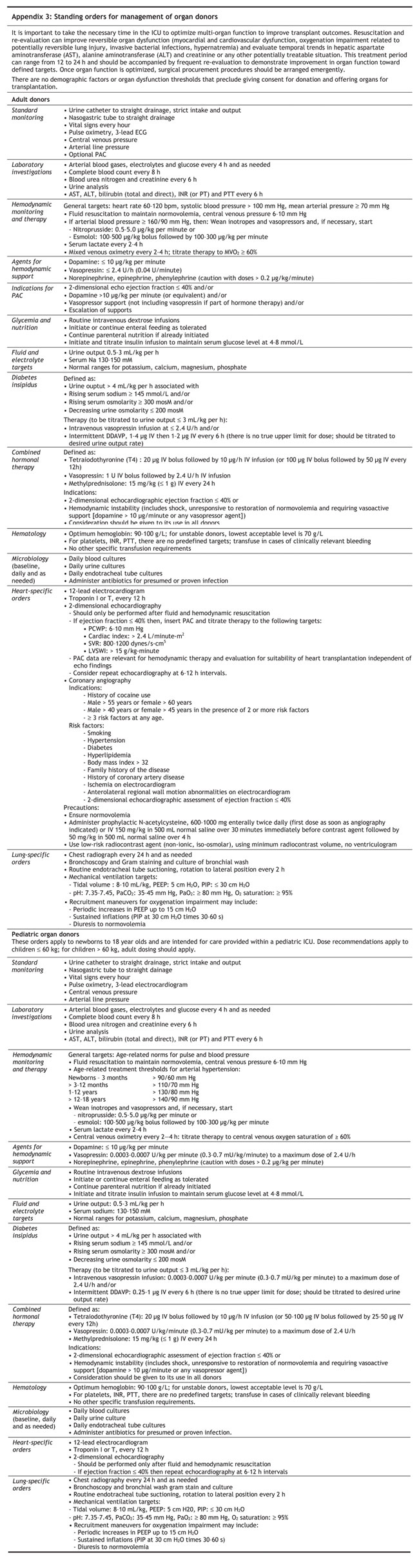
Standing orders for donor management are summarized in Appendix 3.
1. Systemic arterial hypertension related to intracranial pressure
Recommendation 1.1: Thresholds and preferred therapy
We recommend that arterial hypertension after neurological determination of death (NDD) be treated according to the following:
Thresholds:
• Systolic blood pressure > 160 mm Hg and/or
• Mean arterial pressure > 90 mm Hg
Preferred therapy:
• Nitroprusside, 0.5–5.0 μg/kg per minute and/or
• Esmolol, 100–500 μg/kg bolus followed by 100–300 μg/kg per minute
Infusions should be titrated until the desired clinical effect is achieved.
Existing Canadian practice
Significant variation in practice.
Key considerations
• There is a need to distinguish between acute intracranial pressure-related autonomic storm and arterial hypertension that may occur during herniation but before NDD. This period of care was not considered by this forum.
• Given evolving changes and risk of deterioration in cardiovascular function after NDD, short-acting agents are preferable.
• Alternative agents include:
• nitroglycerin (e.g., to reduce the potential risk of coronary steal compared with nitroprusside)
• labetolol is more commonly available and used in clinical practice than esmolol; however, there are concerns regarding its prolonged biologic half-life (4–6 h).
• Hypertension in the setting of vasopressor use or inotropic support is an indication for decreasing support rather than initiating antihypertensives.
2. Cardiovascular performance, monitoring and hemodynamic support
Overall considerations
The deterioration of cardiovascular function associated with intracranial hypertension, characterized by the sympathetic storm resulting in neurogenic myocardial dysfunction3 and intense vasoconstriction followed by sympathetic depletion, and vasodilatation4 will vary with
• Rapidity of rise of intracranial pressure5
• Time after herniation
• Etiology of brain injury (e.g., traumatic myocardial contusion, ischemia after cardiac arrest or shock, hypoxemia).
It is recognized that intensivists titrate cardiovascular therapy to clinical, biochemical and hemodynamic end points that ensure restoration of intravascular volume status without hypervolemia and provide appropriate support of the myo-cardium and vascular system to ensure optimal cardiac output for organ perfusion.
The initiation of cardiovascular support assumes that patients will have been restored to normovolemia.
Evaluation of cardiocirculatory status requires assessment of multiple variables. No single measurement or one value in isolation should determine therapy.
Escalation of support should be accompanied by escalation of hemodynamic monitoring.
Although these targets serve as guidelines for therapy, rigid adherence to numbers should be balanced by the overall evaluation of cardiovascular status by experienced clinicians.
Cardiovascular support should be based on rational physiology. Pure vasopressors (vasopressin, phenylephrine) should be distinguished from vasopressors with beta-agonist inotropic action (norepinephrine, epinephrine). Beta-agonist therapy should be used with caution in potential heart donors, given concerns about myocardial adenosine triphosphate (ATP) depletion and downregulation of beta-receptors.6 If the heart is being considered for donation, dopamine or its equivalent should not be administered at a rate higher than 10 μg/kg per minute.
Existing Canadian practice
The following were identified as areas of well-accepted practice and endorsed a priori:
• Standard monitoring: arterial line, central venous line, 12-lead electrocardiogram
• Hemodynamic targets include:
• mean arterial pressure ≥ 70 mm Hg
• systolic blood pressure ≥ 100 mm Hg
• heart rate 60–120 beats per minute
central venous pressure 6–10 mm Hg (normovolemia).
• Standard inotropic support includes dopamine ≤ 10 μg/kg per minute (or equivalent).
Recommendation 2.1: Monitoring of central or mixed venous oxygen saturation
Monitoring of mixed venous oxygen saturation is indicated in patients with ongoing hemodynamic instability. Hemodynamic therapy should be tailored to reach a target of ≥ 60% saturation.
Existing Canadian practice
Data not available.
Key considerations
• Serial trends are more useful than single measurements.
• Mixed venous oximetry may be determined by sampling intermittently from the pulmonary artery or continuously via oximetric catheters.
• Tissue oxygen extraction has not been well studied in patients declared neurologically dead. Low values may reflect reduced oxygen delivery; however, high values in the face of arrested neurological function or arrested brain circulation may not be interpreted reliably.
• Central venous oximetry is not well studied in patients declared neurologically dead.
Recommendation 2.2: Lactate monitoring
We recommend that serial lactate measurements be performed in all patients. In the presence of elevated or rising lactate levels, we recommend investigations to determine etiology.
Existing Canadian practice
Data not available.
Key consideration
Decreasing lactate levels reflect improvements in oxygen delivery.
Recommendation 2.3: Indications for pulmonary artery catheterization
We recommend pulmonary artery catheterization (PAC) when:
• 2-dimensional echocardiographic assessment of ejection fraction is ≤ 40% or
• Patients require
• dopamine (> 10 μg/kg per minute) or equivalent
• vasopressor support (where vasopressin is not included as part of hormone therapy) and/or
• an escalation of supports.
PAC hemodynamic targets are: pulmonary capillary wedge pressure (PCWP) 6–10 mm Hg; cardiac index > 2.4 L/minute-m2; systemic vascular resistance (SVR): 800–1200 dynes/s-cm5; left ventricular stroke work index (LVSWI) > 15 g/kg-minute.
Existing Canadian practice
Significant variation in practice.
Key considerations
• “Vasopressor” refers to a vasoconstricting agent.
• Although use of PAC in adult intensive care practice is decreasing, the organ donor has a distinct pathophysiology.
• Justifications for PAC are not limited to the precise titration of hemodynamic support but are also required for the evaluation of suitability for heart and lung transplantation.7
• Two-dimensional echocardiography is primarily indicated to evaluate cardiac function and the suitability of the heart for transplantation. The role of single or serial echocardiography in the assessment of cardiac function as a guide to hemodynamic therapy in the unstable organ donor is not well established.
Recommendation 2.4: First-line agents for hemodynamic support — vasopressin
We recommend that vasopressin be used for hemodynamic support when vasopressor agents are indicated. The maximum dose should be 2.4 U/h (0.04 U/minute).
Existing Canadian practice
Significant variation in practice.
Key considerations
• Vasopressin is a special agent because it can be used in a variety of applications, i.e., hemodynamic vasopressor support, diabetes insipidus therapy and hormonal therapy.
• Standardization of dosing units is required.
• Weaning of catecholamine support is the first approach to treating arterial hypertension in patients on vasopressin.
Recommendation 2.5: Second-line agents for hemodynamic support — norepinephrine, epinephrine and phenylephrine
We recommend the use of norepinephrine, epinephrine and/or phenylephrine for hemodynamic support. Doses should be titrated to achieve clinical effect, with no predetermined upper limit.
Existing Canadian practice
Significant variation in practice.
Key consideration
Escalation of doses of catecholamines should be guided by PAC data. Doses beyond 0.2 μg/kg per minute of any of these agents should be used with caution.
3. Glycemia and nutrition
Recommendation 3.1: Glycemic control
We recommend glucose control with insulin infusions titrated to achieve a blood glucose level of 4–8 mmol/L.
Existing Canadian practice
Significant variation in practice.
Key consideration
The use of insulin should not be misinterpreted as a form of insulin dependence that might preclude islet cell transplantation. If clarification is required, hemoglobin (HgB) A1C levels should be measured under these circumstances.
Recommendation 3.2: Nutrition
• Intravenous (IV) dextrose infusions should be given routinely.
• Routine enteral feeding should be initiated or continued as tolerated and discontinued on call to the operating room.
• Parenteral nutrition should not be initiated; however, where it has been initiated, it should be continued.
Existing Canadian practice
Data not available.
Key considerations
None.
4. Diabetes insipidus and hypernatremia
Existing Canadian practice
The following were identified as areas of well-accepted practice and endorsed a priori:
• Serum sodium target range is 130–150 mmol/L.
• Urine output target range is 0.5–3 mL/kg per h (in adults and children).
• Diabetes insipidus can be defined as urine ouptut > 4 mL/kg per h in adults and children
• Associated with rising serum sodium (≥ 145 mmol/L)
• Associated with rising serum osmolarity (≥ 300 mosM) and decreasing urine osmolarity (≤ 200 mosM).
• Doses of 1-desamino-D-arginine vasopressin (DDAVP) for diabetes insipidus
• adults: 1–4 μg IV then 1–2 μg IV every 6 h to achieve urine output < 4 mL/kg/h
• children: 0.25–1 μg IV every 6 h to achieve urine output < 4 mL/kg/h.
Recommendation 4.1: Diabetes insipidus
• Diabetes insipidus in isolation can be treated with continuous IV vasopressin infusion ( ≤ 2.4 U/h) or intermittent IV DDAVP
• Under the following circumstances, vasopressin infusion should be the first choice:
• hemodynamic support with vasospressin required
• combination hormonal therapy implemented
• If required, DDAVP should be used as a supplement to vasopressin
• DDAVP does not have to be discontinued before proceeding to the operating room.
Key consideration
DDAVP is an analog of AVP with a relatively pure antidiuretic effect and negligible vasopressor activity.8 Upper limits of DDAVP dosing are empirical. There is no clear upper limit for DDAVP dose, as it should be titrated to achieve the desired effect of reducing urine output.
Recommendation 4.2: Hypernatremia
We recommend that hypernatremia be treated in all donors if serum sodium levels are greater than 150 mmol/L.
Key consideration
Hypernatremia (serum sodium > 155 mmol/L) is independently associated with hepatic dysfunction and graft loss.9 In addition to sodium control, calcium, phosphate, potassium and magnesium levels should be empirically normalized.
5. Combined hormonal therapy
Recommendation 5.1: Thyroid hormone, vasopressin and methylprednisolone
Combined hormonal therapy is defined as administration of
• Thyroid hormone (tetraiodothyronine or T4), 20 μg IV bolus followed by 10 μg/h IV infusion
• Vasopressin, 1 U IV bolus followed by 2.4 U/h IV infusion
• Methylprednisolone, 15 mg/kg IV every 24 h.
We recommend that combined hormonal therapy be used in donors with an ejection fraction ≤ 40%, based on 2-dimensional echocardiographic assessment, or hemodynamic instability. Consideration should be given to its use in all donors.
Existing Canadian practice
Significant variation in practice.
Key considerations
• Hemodynamic instability includes shock unresponsive to attempts to restore normovolemia and requiring vasoactive support (dopamine > 10 μg/minute) or any vasopressor.
• Weight of currently available evidence in a large retrospective cohort study by the United Network for Organ Sharing (UNOS)10 in the United States suggests a substantial benefit of triple hormone therapy with minimal risk. A multivariate logistic regression analysis of 18 726 brain-dead donors showed significant increases in kidney, liver and heart utilization from donors receiving 3 hormonal therapies. Significant improvements in 1-year kidney graft survival and heart transplant patient survival were also demonstrated. A prospective randomized trial has not been performed.
• As peripheral tissue conversion of T4 into triiodothryonine (T3) may be impaired in organ donors and in those on corticosteroids, intravenous T3 may be preferred but is not commercially available in Canada at this time. Intravenous T4 and enteral T3 are currently available.
• In the UNOS patients receiving hormone therapy,10 T4 was used in 93% and T3 in 6.9% of cases, but numbers were insufficient to discriminate any benefit of T3 over T4.
• When administered by continuous infusion, the bioavailability of intravenous T4 may be affected by its stability in solution and potential adherence to plastic tubing resulting from its hydrophobic nature. The quantitative impact of this pharmacologic concern is unclear. Alternatively, intravenous T4 may be given as follows: 100 ug IV bolus followed by 50 ug IV q12h. Should future data show a benefit of intravenous T3 over T4, the Canadian Health Protection Branch should be lobbied to make intravenous T3 therapy available in Canada for this indication.
• Absorption and pharmacokinetic data on enteral T3 in organ donors is required before its use can be recommended.
• Vasopressin should be initiated at a fixed rate. If arterial hypertension ensues, catecholamines should be weaned before decreasing the vasopressin infusion rate.
Recommendation 5.2: Corticosteroids and lung protection
We recommend that methylprednisolone be administered intravenously to all donors at a dose of 15 mg/kg every 24 h; this is to be initiated following NDD.
Existing Canadian practice
Methylprednisolone is administered to potential lung donors at the dose of 15 mg/kg IV (maximum dose 1 g).
Key consideration
Corticosteroid therapy is currently indicated as the immune modulating therapy for potential lung donors11 but protocols for administration of corticosteroids are non-uniform.
6. Transfusion thresholds
Recommendation 6.1: Acceptable targets for hemoglobin, platelets and coagulation parameters
• A target hemoglobin level of 90–100 g/L is most appropriate to optimize cardiopulmonary function in the face of hemodynamic instability. A level of 70 g/L is the lowest acceptable limit for management of stable donors in the ICU.
• There are no defined targets for platelet concentration, international normalized ratio or partial thromboplastin time. Platelet or plasma factor replacement is indicated for clinical bleeding only.
• Blood drawing for donor serology and tissue typing should occur before transfusions to minimize the risk of false results related to hemodilution.
• No special transfusion precautions are required in organ donors.
Existing Canadian practice
Significant variation in practice.
Key considerations
• Red blood cell transfusions can be associated with inflammatory activation related to the age of the blood.
• Consider the crystalloid sparing effect of red cell transfusions in potential lung donors with alveolar-capillary leak.
• Invasive procedures associated with bleeding risk may require correction of thrombocytopenia and coagulation status.
• Intraoperative transfusion of red blood cells, platelets and plasma factors by anesthetists and the transplant team should be individually tailored.
• In Canada, blood is routinely depleted of leukocytes and the risk of transmission of cytomegalovirus (CMV) is negligible. It is not necessary to give CMV-negative blood to CMV-negative donors.
7. Invasive bacterial infections
Recommendation 7.1: Daily blood cultures
• An initial baseline blood culture should be carried out for all donors and repeated after 24 h and on an as-needed basis.
• Positive blood cultures or confirmed infections are not contraindications to organ donation
• Antibiotic therapy should be initiated in cases of proven or presumed infection. Duration of therapy depends on the virulence of the organism and decisions should be made in consultation with the transplant team and infectious disease services.
• No minimum duration of therapy before organ procurement can be defined at this time.
Existing Canadian practice
Standard care includes urine and endothracheal tube aspirate cultures.
Key considerations
None.
Recommendation 7.2: Broad-spectrum antibiotics
• Empiric broad-spectrum antibiotics are not indicated during ICU care of the organ donor.
• Decisions regarding use of perioperative antibiotics should be at the discretion of the surgical team.
Existing Canadian practice
Data not available.
Key considerations
None.
Organ-specific considerations: heart, lungs and intra-abdominal organs
8. Heart
Existing Canadian practice
The following was identified as well-accepted practice and endorsed a priori.
• Potential cardiac donors undergo routine screening by electrocardiogram and 2-dimensional echocardiography.
Recommendation 8.1: Initial evaluation of cardiac function
If the initial evaluation of cardiac function reveals an ejection fraction of ≤ 40% based on 2-dimensional echocardiographic assessment, the optimum course is to insert a pulmonary artery catheter and institute therapy to achieve the PAC hemodynamic targets listed in Recommendation 2.3.
Key considerations
• Achieving PAC targets has been linked to favourable transplant outcomes.7,12
• Initial echocardiography for heart donor evaluation should be performed only after hemodynamic resuscitation. Repeat echocardiography should be considered after 6 h. There is a need for prospective study of the utility of serial echocardiography.
• Justifications for PAC are not limited to precision of hemodynamic support, but are also required for the evaluation of suitability for heart and lung transplantation. Transplant decision-making should reflect these recommendations. An abnormal 2-dimensional echocardiogram followed by favourable hemodynamic data by PAC is an acceptable assessment and does not require follow-up echocardiography.
• PAC is recommended when appropriate technical and interpretive expertise is available.
Recommendation 8.2: Troponin levels
We recommend the measurement of troponin (either I or T) levels every 12 h as standard monitoring to obtain both clinical and prognostic information.
Key consideration
Troponin levels should not be used in isolation as the basis to reject hearts for transplantation.
Recommendation 8.3: Coronary angiography
• We recommend that the following donor characteristics be indications for coronary angiography:
• male > 55 years of age or female > 60 years
• male > 40 years of age or female > 45 years in the presence of 2 risk factors (see “Key considerations”)
• presence of 3 or more risk factors at any age
• history of cocaine use.
• If the hospital has angiography facilities and indications for angiography are present, an angiogram should always be performed to document coronary anatomy to assist in decision-making.
• There should be no absolute threshold for coronary luminal obstruction; decisions should be made in the context of the recipient status, heart function and the potential to intervene through coronary artery bypass grafting or percutaneous coronary intervention (e.g., stent).
• The inability to perform an angiogram should not preclude transplantation. Where coronary angiography is not available, cardiac donor organ suitability should still be considered in the following circumstances:
• ejection fraction of > 40% on 2-dimensional echocardiographic assessment or
• hemodynamic stability or
• surgical inspection at the time of procurement.
• The option of patient transfer to a procurement hospital with angiogram capabilities should be considered on a case-by-case basis with full consent of the donor family.
Key considerations
• Cardiovascular risk factors for coronary artery disease that have an impact on transplant outcomes include13,14
• smoking
• hypertension
• diabetes
• hyperlipidemia
• body mass index > 32
• family history of the disease
• prior history of coronary artery disease
• ischemia on electrocardiogram
• anterolateral regional wall motion abnormalities on echocardiogram
• 2-dimensional echocardiographic assessment of ejection fraction of ≤ 40%.
• To minimize the risk of contrast nephropathy:
• normovolemia should be ensured
• N-acetylcysteine should be given prophylactically at doses of 600–1000 mg enterally twice daily, with the first dose administered as soon as it is recognized that angiography is indicated. Alternatively N-acetylcysteine may be administered intravenously at 150 mg/kg in 500 mL normal saline over 30 minutes immediately before contrast agent, followed by 50 mg/kg in 500 mL of normal saline over 4 h.
• angiograms should be performed with a low-risk radiocontrast agent (non-ionic, iso-osmolar), using minimum radiocontrast volume and without a ventriculogram.
9. Lungs
For recommendations related to corticosteroids and lung protection, see Recommendation 5.2.
Existing Canadian practice
The following were identified as areas of well-accepted practice and endorsed a priori:
• Pulse oximetry, serial arterial blood gas monitoring, endotracheal tube suctioning, chest radiography, bronchoscopy and bronchoalveolar lavage.
• Mechanical ventilation with the following targets:
• fraction of inspired oxygen (Fio2) titrated to keep oxygen saturation ≥ 95% and partial pressure of arterial oxygen (PaO2) ≥ 80 mm Hg
• pH: 7.35–7.45, PaCO2: 35–45 mm Hg
• Positive end expiratory pressure (PEEP): 5 cm H2O.
Recommendation 9.1: Oxygenation impairment
In cases where the partial pressure of arterial oxygen/fraction of inspired oxygen (P/F) ratio is < 300, we recommend that
• Positional rotation therapy should be routine and defined as rotation to a lateral position every 2 h.
• Routine suctioning and physiotherapy should be standard care.
• A positive end expiratory pressure (PEEP) of 5 cm H2O is recommended, but periodic increases of PEEP up to 15 cm H2O is an acceptable form of alveolar recruitment.
• Sustained inflations (peak inspiratory pressure of 30 cm H2O for 30–60 s) is an acceptable form of alveolar recruitment.
• Diuresis to normovolemia should be initiated when indicated.
Key considerations
• Evaluation of P/F ratio is performed when PEEP is 5 cm H2O and Fio2 is 1.0.
• Recruitment maneuvers should be used periodically in all donors regardless of P/F ratio and should continue through the intraoperative period.
• Prone positioning is not recommended for either adult or pediatric donors.
Recommendation 9.2: Lower limits for the P/F ratio
• There should be no predefined lower limit for the P/F ratio that precludes transplantation.
• Timing of evaluation, temporal changes, response to alveolar recruitment and recipient status should be considered.
• In cases of unilateral lung injury, pulmonary venous PO2 during intraoperative assessment is required to evaluate contralateral lung function reliably.
Key considerations
None.
Recommendation 9.3: Optimum targets for tidal volume and peak inspiratory pressure
We recommend that:
• Tidal volume be 8–10 mL/kg.
• The upper limit of peak inspiratory pressure be ≤ 30 cm H2O.
Key consideration
Lung protective strategies are currently used in patients with, or at risk of, acute respiratory distress syndrome, where pressure-limited ventilation is defined by a peak inspiratory pressure < 35 cm H2O and tidal volume of 6–8 mL/kg.15 Benefits of these strategies apply to acute respiratory distress syndrome patients and corresponding data for organ donors are not available.
Recommendation 9.4: Bronchoscopy and antimicrobial therapy
• Bronchoscopy can be performed by the local hospital expert and reported to the responsible transplant surgeon.
• Antimicrobial therapy should be based on the results of Gram staining or culture or suspected or confirmed bronchopneumonia.
• Empiric broad-spectrum antibiotics are not routinely indicated, but may be used in donors at high risk of bronchopneumonia.
• Length of stay in the ICU is not an independent indication for antimicrobial therapy.
Key considerations
• Technology should be developed to enable:
• remote review of bronchoscopy and chest radiographic images
• 3-way communication among ICUs, organ procurement organizations and transplant surgeons
• Nephrotoxic antimicrobials should be avoided when possible.
10. Liver
Existing Canadian practice
The following was identified as an area of well-accepted practice and endorsed a priori:
• Potential liver donors are assessed for
• history of jaundice, hepatitis, excessive alcohol ingestion
• hepatic aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin (direct and indirect where available) and international normalized ratio (INR) or prothrombin time (PT), repeated every 6 h
• serum electrolytes, creatinine, urea hepatitis B surface antigen (HBsAg),
hepatitis B core antibody (HBcAb), hepatitis C virus antibody (HCVAb).
Recommendation 10.1: Upper limits of hepatic aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
We recommend that there be no upper limits for hepatic AST and ALT that preclude liver transplantability. All livers should be offered; decisions related to transplantability depend on organ status, trends in liver function over time and recipient status.
Key considerations
None.
Recommendation 10.2: Hepatic ultrasound
We recommend that there be no requirement for prospective liver donors to undergo hepatic ultrasound.
Key considerations
None.
Recommendation 10.3: Indications for liver biopsy
We recommend the following indications for ultrasound-guided percutaneous liver biopsy in the ICU before procurement, in consultation with the liver transplant team, to enable decisions about transplantability:
• Weight > 100 kg or body mass index > 30 or HCVAb positive donor and
• Distant procurement, i.e., when a procurement team is not immediately available.
Intraoperative biopsy by the liver retrieval team is recommended in all other instances where liver biopsy is indicated.
If the biopsy cannot be done in the ICU and indications for biopsy exist, the liver should be offered and transplantation should be at the discretion of the liver transplant team.
Key considerations
None.
11. Kidney
Existing Canadian practice
The following were identified as areas of well-accepted practice and endorsed a priori:
• A normal creatinine clearance rate (> 80 mL/minute/1.73 m2) defines the optimum function threshold for transplantation. However, an abnormal serum creatinine level or calculated creatinine clearance rate in a donor does not necessarily preclude use of the donor kidneys.
• Urinalysis is essential to rule out kidney abnormalities.
• Creatinine and serum urea (blood urea nitrogen) are measured every 6 h.
Recommendation 11.1: Creatinine clearance rate
We recommend that measurement of creatinine clearance rate be based on the Cockroft–Gault equation; urine collection to measure creatinine clearance is not indicated.
Key consideration
There is no absolute contraindication to kidney donation based on a serum creatinine level or creatinine clearance rate alone.
Recommendation 11.2: Renal ultrasound
We recommend that renal ultrasound be performed on a case-by-case basis, taking into account factors such as a history of renal disease.
Key consideration
In general, there are no firm indications for renal ultrasound; this investigation tends to yield little information.
Recommendation 11.3: Indications for kidney biopsy
We recommend that the following variables be considered in determining the need for intraoperative kidney biopsy at the time of procurement to enable decisions about transplantability:
• Age > 65 years or a younger age with a history of any of the following:
• creatinine level > 133 μmol/L
• hypertension
• diabetes
• abnormal urinalysis.
Key consideration
Histologic evaluation and assessing for glomerulosclerosis or vasculopathy or both is required before excluding kidneys. The biopsy should be performed intraoperatively at the time of procurement, rather than in the ICU.
Other systemic challenges
12. Optimum time for organ procurement and decisions regarding transplantability
Recommendation 12.1: Optimum time for organ procurement
It is important to take the necessary time in the ICU to optimize multi-organ function to improve transplant outcomes. Reversible organ dysfunction can be improved with resuscitation and re-evaluation. The treatment period can range from 12 to 24 h and should be accompanied by frequent re-evaluation to demonstrate improvement in organ function toward defined targets. Once organ function is optimized, surgical procurement procedures should be arranged emergently.
Existing Canadian practice
In general, after neurological death has been declared and consent to organ donation has been given, efforts are made to complete donation logistics and initiate procurement as quickly as possible.
Key considerations
• The existing paradigm of care should be adjusted in view of the following situations that may be correctable or may benefit from resuscitation and re-evaluation:
• myocardial or cardiovascular dysfunction
• oxygenation impairment related to potentially reversible lung injury
• invasive bacterial infections
• hypernatremia
• temporal trends in AST and ALT
• temporal trends in creatinine level
• any other potentially treatable situation.
• Extending the interval of donor care in the ICU to optimize transplant outcomes should be factored into donation consent discussions and should be consistent with the wishes of the family or surrogate decision-maker.
Recommendation 12.2: Decisions regarding transplantability
We recommend that there be no predefined demographic factor or organ dysfunction thresholds that preclude providing consent for individual organs or offering organs for transplantation and that:
• Consent be requested for all organs
• Within the context of existing legal and regulatory frameworks, all organs be offered
• Ultimate decisions about transplantability rest with the individual transplant programs represented by the organ-specific transplant doctors.
Existing Canadian practice
Significant variation in practice.
Key considerations
• Accountability for nonutilization of organs is required from procurement and transplant services. The limited data currently provided to the Canadian Organ Replacement Register recording reasons for nonutilization of organs are inadequate.
• Utilization of organs should be linked with corresponding transplant graft and patient outcomes.
• Issues related to transmissible viruses or malignancy should comply with existing Canadian standards and guidelines.16
13. Pediatric age-related adjustments
Recommendation 13: Pediatric age-related adjustments
We recommend that Recommendations 1–12 be applied to infants, children and adolescents with the following qualifications. (The numbers are those of the corresponding adult recommendations.)
Overarching
• The pediatric organ donor is defined as:
• newborn to 18 years and
• care provided within a pediatric ICU.
• Dosing recommendations apply to children ≤ 60 kg; for children over 60 kg, adult dosing should apply.
1: Systemic arterial hypertension related to intracranial pressure
Thresholds for treating arterial hypertension after NDD are:
Newborns–3 months > 90/60 mm Hg
> 3–12 months > 110/70 mm Hg
1–12 years > 130/80 mm Hg
> 12–18 years > 140/90 mm Hg
Cardiovascular performance, monitoring and hemodynamic support
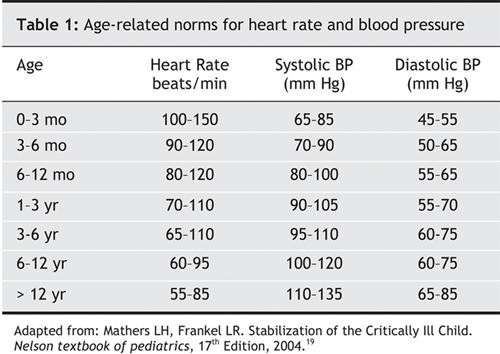
• Experienced pediatric intensive care practitioners adjust therapies to general, rather than specific, age-related targets. For information purposes, a guide to age-related norms for heart rate and blood pressure is provided under “Key considerations” below.
2.1: Monitoring of central or mixed venous oxygen saturation
• Central venous oximetry is currently used in many Canadian pediatric ICUs as a monitoring technique in patients with hemodynamic instability and is recommended for the pediatric donor. Therapy should be titrated to a central venous oxygen saturation of ≥ 60%.
2.3: Indications for PAC
• The use of PAC is limited in pediatric ICU practice and is not routinely recommended in pediatric donors. PAC may be used at the discretion of the pediatric ICU practitioner who is experienced with its application and interpretation.
• Serial echocardiography is the recommended method for re-evaluating myocardial function for transplantation. Its role as a potential tool to guide hemodynamic therapy should be individually tailored.
2.4: First-line agents for hemodynamic support — vasopressin
• The pediatric dose range for vasopressin is 0.0003–0.0007 U/kg per minute (0.3–0.7 mU/kg minute) to a maximum dose of 2.4 U/h.
2.5: Second-line agents for hemodynamic support — Norepinephrine, epinephrine and phenylephrine
• In the absence of PAC data, hemodynamic therapy should be titrated to clinical and biochemical targets.
4.1: Diabetes insipidus
• The pediatric dose range for vasopressin is 0.0003– 0.0007 U/kg per minute (0.3–0.7 mU/kg per minute) to a maximum dose of 2.4 U/h.
5.1: Thyroid hormone, vasopressin and methylprednisolone
• The pediatric dose range for vasopressin is 0.0003–0.0007 U/kg per minute (0.3–0.7 mU/kg per minute) to a maximum dose of 2.4 U/h.
• Intravenous T4: The precise dose range for IV T4 infusions is not known and its biological effect when given by infusion may be affected by its stability in solution and potential adherence to plastic tubing resulting from its hydrophobic nature. Adult practitioners have used up to 300–500 μg IV bolus for potential donors and this is standard dosing for myxedema coma. Given the wide dosing range cited in the literature for IV T4 and the low risk of toxicity for this current indication, the adult range — 20 μg IV bolus followed by 10 μg/h IV infusion — is also recommended for children.17 Alternatively, intravenous T4 may be given as follows: 50-100 μg IV bolus followed by 25–50 ug iv q12h.
8.1: Initial evaluation of cardiac function
• Serial echocardiography is recommended to evaluate myo-cardial function for the purposes of transplantation. The initial echocardiogram should be performed only after stabilization with adequate volume resuscitation.
• The echocardiogram should be repeated every 6–12 h under the following conditions:
• initial 2 dimensional echocardiogram reveals an ejection fraction ≤ 40% or
• escalation of supports as defined by dopamine > 10μg/kg per minute, the use of vasopressor agents or both.
11.1: Creatinine clearance rate
• For children > 1 year of age, a normal creatinine clearance rate is > 80 mL/minute/1.73 m2, as estimated by the Schwartz formula.18
11.3: Indications for kidney biopsy
• Creatinine level higher than normal for age.
Key considerations
See Table 1.
Table 1
Acknowledgments
These recommendations have been endorsed by the Canadian Critical Care Society, the Canadian Association of Transplantation, the Canadian Society of Transplantation and the Canadian Council for Donation and Transplantation.
Appendix 1.
Appendix 2.
Appendix 3.
Appendix 4.

Footnotes
See Appendix 4 for a complete list of forum participants.
This article has been peer reviewed.
We acknowledge the Canadian Council for Donation and Transplantation for financial support to prepare and organize the forum; the collaboration of the Canadian Critical Care Society, the Canadian Association of Transplantation and the Canadian Society of Transplantation in holding the forum; and the process consultation provided by Strachan-Tomlinson.
Competing interests: None declared for Sam Shemie, Heather Ross, Joe Pagliarello, Andrew Baker, Tracy Brand, Sandra Cockfield, Shaf Keshavjee, Peter Nickerson, Vivek Rao, Cameron Guest, Kimberly Young or Christopher Doig. Paul Greig has received speaker's fees and travel assistance from Hoffmann La-Roche and Fujisawa Canada Inc. to attend meetings.
Correspondence to: Dr. Sam D. Shemie, Division of Pediatric Critical Care, Montreal Children's Hospital, McGill University Health Centre, Montréal, QC H3H 1P3; sam.shemie@muhc.mcgill.ca
Reprint requests to: Ms. Kimberly Young, Canadian Council for Donation and Transplantation, 1702–8215 112 Street, Edmonton AB T6G 2C8; 780 409-5652; kimberly.young@ccdt.ca
REFERENCES
- 1.Hornby K, Shemie SD. Donor organ management: survey of guidelines and eligibility criteria. Edmonton: Canadian Council for Donation and Transplantation; 2004.
- 2.Hornby K, Ross H, Keshavjee S, et al. Factors contributing to non-utilization of heart and lungs after consent for donation: a Canadian multicentre study. Can J Anaesth 2006; in press. [DOI] [PubMed]
- 3.Tung P, Kopelnik A, Banki N, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke 2004; 35(2):548-51. [DOI] [PubMed]
- 4.Novitzky D. Detrimental effects of brain death on the potential organ donor. Transplant Proc 1997;29:3770-2. [DOI] [PubMed]
- 5.Shivalkar B, Van Loon J, Wieland W, et al. Variable effects of explosive or gradual increase of intracranial pressure on myocardial structure and function. Circulation 1993;87(1):230-9. [DOI] [PubMed]
- 6.D'Amico TA, Meyers CH, Koutlas TC, et al. Desensitization of myocardial beta-adrenergic receptors and deterioration of left ventricular function after brain death. J Thorac Cardiovasc Surg 1995;110(3):746-51. [DOI] [PubMed]
- 7.Wheeldon DR, Potter CD, Oduro A, et al. Transforming the “unacceptable” donor: outcomes from the adoption of a standardized donor management technique. J Heart Lung Transplant 1995;14(4):734-42. [PubMed]
- 8.Richardson DW, Robinson AG. Desmopressin. Ann Intern Med 1985;103(2):228-39. [DOI] [PubMed]
- 9.Totsuka E, Dodson F, Urakami A, et al. Influence of high donor serum sodium levels on early postoperative graft function in human liver transplantation: effect of correction of donor hypernatremia. Liver Transpl Surg 1999;5(5):421-8. [DOI] [PubMed]
- 10.Rosendale JD, Kauffman HM, McBride MA, et al. Hormonal resuscitation associated with more transplanted organs with no sacrifice in survival. Transplantation 2004;78(2) suppl 1:17.
- 11.Follette DM, Rudich SM, Babcock WD. Improved oxygenation and increased lung donor recovery with high-dose steroid administration after brain death. J Heart Lung Transplant 1998;17(4):423-9. [PubMed]
- 12.Hunt SA, Baldwin J, Baumgartner W, et al. Cardiovascular management of a potential heart donor: a statement from the Transplantation Committee of the American College of Cardiology. Crit Care Med 1996;24(9):1599-601. [DOI] [PubMed]
- 13.McGiffin DC, Savunen T, Kirklin JK, et al. A multivariable analysis of pretransplantation risk factors for disease development and morbid events. J Thorac Cardiovasc Surg 1995;109(6):1081-9. [DOI] [PubMed]
- 14.Zaroff JG, Rosengard BR, Armstrong WF, et al. Consensus conference report maximizing use of organs recovered from the cadaver donor: cardiac recommendations. Circulation 2002;106(7):836-41. [DOI] [PubMed]
- 15.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342(18):1301-8. [DOI] [PubMed]
- 16.Cells, tissues, and organs for transplantation and assisted reproduction: general requirements (Z900.1-03). Mississauga: Canadian Standards Association; 2003.
- 17.Rodriguez I, Fluiters E, Perez-Mendez LF, et al. Factors associated with mortality of patients with myxoedema coma: prospective study in 11 cases treated in a single institution. J Endocrinol 2004;180(2):347-50. [DOI] [PubMed]
- 18.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 1987;34(3):571-90. [DOI] [PubMed]
- 19.Mathers LH, Frankel LR. Stabilization of the critically ill child. In Behrman RE,Kliegman RM,Jenson HB (editors). Nelson textbook of pediatrics (17th ed.). Philadelphia: Saunders; 2004.
- 20.About knowledge transfer. Ottawa: Canadian Institutes of Health Research; 2005. Available: www.cihr-irsc.gc.ca/e/29418.html (accessed 1 Dec. 2005).


