The management of patients with severe brain injury falls within the disciplines of emergency medical services, trauma, critical care, neurology and neurosurgery. Consultation and collaboration between professionals in these disciplines and those involved in end-of-life care and organ donation and transplantation are required to standardize and optimize the management of severely brain-injured patients who progress to neurological death.
Brain death is better understood as brain arrest, or the final clinical expression of complete and irreversible neurological failure. Despite widespread national, international and legal acceptance of the concept of death as defined by neurological criteria, substantial variation exists in the standards and their application.1–5 In all Canadian provinces and territories, the legal definition of brain death is “according to accepted medical practice.” These practices are largely determined by individual hospitals or regions. Guidelines established by the Canadian Congress Committee on Brain Death in 19886 and the Canadian Neurocritical Care Group in 1997 initiated clarification of the criteria, but have not led to uniform practice.
Acknowledging this variation in the recognition, diagnosis and documentation of neurological death, the Canadian Council for Donation and Transplantation sponsored a national forum of experts to create a set of recommendations that will have significant implications for organ donation in Canada. Severe brain injury is a prerequisite for neurological determination of death (NDD); and NDD, commonly referred to as brain death, is a prerequisite for cadaveric organ donation. The right to entertain the option of organ and tissue donation is increasingly supported by society and will become legislated in some Canadian jurisdictions. Collaborative efforts are required to optimize the care of patients who may become eligible for donation and to ensure consistent and ethical conduct in care. This comprehensive national collaboration is the first of its kind in Canada in this domain.
Forum overview
The purpose of the forum “Severe Brain Injury to Neurological Determination of Death,” held in Vancouver from 9 to 11 April 2003, was to initiate the development of a national agreement on the processes of care, commencing with severe brain injury and culminating with NDD. A priori, the forum accepted brain death as a medical and legal concept of death in Canadian society and restricted the discussion to optimum practice in the field. Objectives were
• To review national and international legislation, policies and practices related to NDD
• To prepare a made-in-Canada definition of NDD for children and adults, to ensure consistency and reliability in its diagnosis, declaration, documentation and reporting
• To discuss and agree on policies and practices in relation to emergency department, neurological, neurosurgical and intensive care unit (ICU) management of critically injured patients with a poor neurological prognosis
• To develop recommendations for the Canadian Council for Donation and Transplantation and other interested organizations and groups on the dissemination of these definitions, policies and practices across Canada.
The forum was attended by 89 experts, including emergency, trauma and critical care physicians, neurologists, neurosurgeons, nurses and advanced nurse practitioners, as well as representatives of licensing colleges and donation–transplant agencies, health administrators, policy-makers, coroners, experts in end-of-life care and ethicists — a multidisciplinary group representing all regions of the country. Discussions focused on collaboration at a national level.
Each of the 3 main areas of focus — recommendations for a Canadian definition, criteria and minimum testing requirements for NDD; recommendations concerning the incidence and reporting of NDD and legal issues; and recommendations associated with the management of patients with severe brain injury from the emergency department to the intensive care unit — was addressed using the following process. Presentations by experts were followed by plenary discussions supported by fact sheets that summarized preceding American8 and Canadian guidelines7 and by substantial background papers9–11 and surveys12 provided by the planning committee in advance of the forum. Small-group discussions then focused on specific questions related to the processes of care. The Forum Recommendations Group (FRG) and the Pediatric Reference Group (PRG) reviewed the results of the small-group discussions, developed unanimous recommendations for adults and children and returned these for plenary discussion. A Neonatal Reference Group met subsequent to the forum to develop neonatal age-adjusted recommendations. (See Appendix 1 for a list of members of these groups.) Clinical checklists are included in Appendix 4.
Discussions at the forum were intense, rich in content and collegial. Members of the FRG and PRG panels unanimously agreed on recommendations that mark a significant advance on existing guidelines. Group members were invited to sit on these panels, both as representatives of their professional associations and as respected practitioners providing the benefit of their experience and expertise. Both panels were assisted by an external, objective facilitator.
Forum recommendations were developed for infants, children, adolescents and adults. Drs. Paul Byrne and Sam Shemie were members of both the FRG and PRG and provided consistent pediatric input during the development of guidelines in plenary sessions and at FRG and PRG meetings.
General considerations
During discussions, FRG and PRG members recognized that
• Recommendations must be in the best interests of patients with severe brain injury.
• Optimum end-of-life care is a priority for all patients who may die after severe brain injuries.
• The wishes of patients and their families are of paramount importance.
• There is a need to clarify and standardize terminology, e.g., ancillary and supplementary testing, brain death (NDD, neurologically determined death or death by neurological determination [see Appendix 2 and Appendix 3].)
• The current evidence base for existing NDD guidelines is inadequate.
• Clear medical standards for NDD and defining qualifications of physicians performing NDD augment the quality and rigour of the determination.
Overarching recommendations
In discussions related to Recommendations A.4: Apnea testing, A.5: Examination interval and A.7: Concept and definition of neurological death, FRG members identified the following overarching recommendations, which apply to all of the individual recommendations:
• We recommend that after NDD, the patient be declared dead.
• Existing provincial and territorial laws indicate that for the purposes of a post-mortem transplant, the fact of death shall be determined by at least 2 physicians in accordance with accepted medical practice. There is no clear medical basis for the law requiring a second physician to determine death before post-mortem transplantation.
• The first and second physicians' determinations, required by law, may be performed concurrently. However, if the determinations are performed at different times, a full clinical examination, including apnea testing, must be performed at each determination. No fixed interval of time is recommended for the second determination, except where age-related criteria apply.
A. Canadian medical standards for NDD: definition, criteria and minimum testing
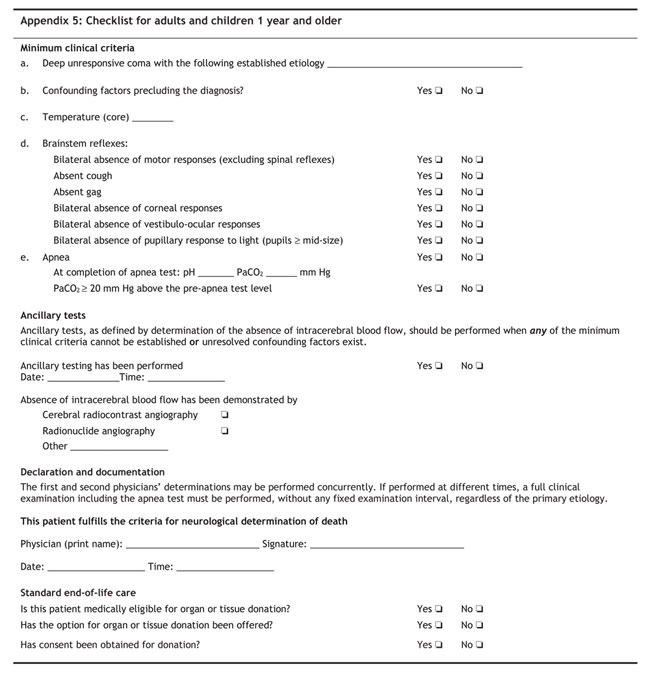
Recommendation A.1: Minimum clinical criteria for NDD
We recommend use of the following minimum clinical criteria as a Canadian medical standard for NDD:
• Established etiology capable of causing neurological death in the absence of reversible conditions capable of mimicking neurological death
• Deep unresponsive coma with bilateral absence of motor responses, excluding spinal reflexes
• Absent brain stem reflexes as defined by absent gag and cough reflexes and the bilateral absence of
• corneal responses
• pupillary responses to light, with pupils at mid-size or greater
• vestibulo-ocular responses
• Absent respiratory effort based on the apnea test
• Absent confounding factors
Key considerations
• A prerequisite for NDD is the absence of clinical neurological function with a known, proximate cause that is irreversible. There must be definite clinical or neuro-imaging evidence of an acute central nervous system (CNS) event consistent with the irreversible loss of neurological function.
• Deep unresponsive coma implies a lack of spontaneous movements as well as an absence of movement originating in the CNS, such as cranial nerve function, CNS-mediated motor response to pain in any distribution, seizures, decorticate and decerebrate responses. Spinal reflexes or motor responses confined to spinal distribution may persist.
• Minimum should not necessarily be understood as minimal. “Minimal” refers to the least possible that can be done and is an absolute value. “Minimum” refers to the lowest acceptable standard, which is a relative standard, often pitched above the minimal. The standard recommended by the forum sets minimum clinical criteria for NDD.
Recommendation A.2: Confounding factors
We recommend that, at the time of assessment for NDD, the following confounding factors preclude the clinical diagnosis:
• Unresuscitated shock
• Hypothermia (core temperature < 34°C)
• Severe metabolic disorders capable of causing a potentially reversible coma
• Severe metabolic abnormalities, including glucose, electrolytes (including phosphate, calcium and magnesium), inborn errors of metabolism, and liver and renal dysfunction may play a role in clinical presentation. If the primary etiology does not fully explain the clinical picture, and if in the treating physician's judgement the metabolic abnormality may play a role, it should be corrected.
• Peripheral nerve or muscle dysfunction or neuromuscular blockade potentially accounting for unresponsiveness
• Clinically significant drug intoxications (e.g., alcohol, barbiturates, sedatives, hypnotics); however, therapeutic levels or therapeutic dosing of anticonvulsants, sedatives and analgesics do not preclude the diagnosis.
Key considerations
• Neurological assessments may be unreliable in the acute post-resuscitation phase after cardiorespiratory arrest.13 In cases of acute hypoxic-ischemic brain injury, clinical evaluation for NDD should be delayed for 24 h subsequent to the cardiorespiratory arrest or an ancillary test could be performed (see Recommendation A.6).
• It is recognized that there are variations in confounding factors that may be associated with NDD; examiners are cautioned to review these confounding factors in the context of the primary etiology and examination. If physicians are confounded by data, either absolutely or by differing perspectives, they should not proceed with NDD. Clinical judgment is the deciding factor.
Recommendation A.3: Minimum temperature
The core body temperature required to apply the minimum clinical criteria (Recommendation A.1) should be ≥ 34°C.
Key considerations
• Core temperature should be obtained through central blood, rectal or esophageal–gastric measurement.
• The existing Canadian standard of 32.2°C was based on precedent.7 The relevance of the scientific evidence and the application of this standard in the context of severe brain injury is uncertain.
• Given that there is no evidence base, a decision was made to adopt 34°C as a rational, safe and attainable standard. This decision was based on the following rationale:
• ideally, temperature should be as close to normal as possible and this is the minimum temperature at which the test is valid
• raising a patient's temperature from 32.2°C to 34°C does not pose significant difficulty to the patient or treating physician.
Recommendation A.4: Apnea testing
We recommend that the thresholds at the completion of the apnea test be PaCO2 ≥ 60 mm Hg (and ≥ 20 mm Hg above the pre-apnea test level) and pH ≤ 7.28. These thresholds must be documented by arterial blood gas measurement.
To interpret an apnea test correctly, the certifying physician must continuously observe the patient for respiratory effort throughout the administration of the test.
Key considerations
• Optimum administration of the apnea test requires a period of preoxygenation followed by 100% oxygen delivered via the trachea upon disconnection from mechanical ventilation.
• The following codicil is required to address severe lung disease: Caution must be exercised in considering the validity of the apnea test if, in the physician's judgment, there is a history suggestive of chronic respiratory insufficiency and responsiveness to only supranormal levels of carbon dioxide, or if the patient is dependent on hypoxic drive. If the physician cannot be sure of the validity of the apnea test, an ancillary test should be administered.
Recommendation A.5: Examination interval
We recommend that when a second determination is performed, there should be no fixed examination interval, regardless of the primary mechanism of the brain injury.
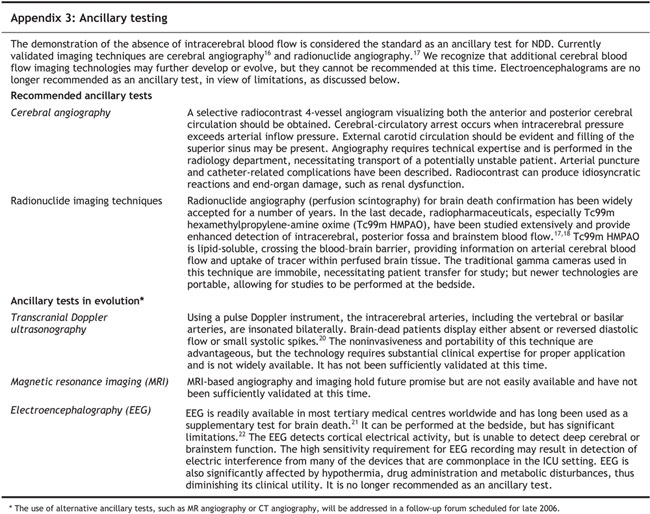
Recommendation A.6: Ancillary tests
We recommend that an ancillary test be performed when it is impossible to complete the minimum clinical criteria as defined in Recommendation A.1. At a minimum, 2 particular clinical criteria must be met before ancillary tests are performed:
• An established etiology capable of causing neurological death in the absence of reversible conditions capable of mimicking neurological death
• Deep unresponsive coma
We recommend that demonstration of the global absence of intracerebral blood flow be considered as the standard for NDD by ancillary testing.
Key considerations
• Before performing an ancillary test, unresuscitated shock and hypothermia must be corrected (see Recommendation A.2).
• The term “ancillary” should be understood to mean an alternative test to one that otherwise, for any reason, cannot be conducted. It replaces previous terminology such as “supplemental” (in addition to an already conducted test) or “confirmatory” (confirms a previously conducted test).
• Existing evidence, although not firmly established, suggests that for patients who fulfill minimum clinical criteria (see Recommendation A.1) under the circumstances of high-dose barbiturate therapy used for refractory intracranial hypertension to achieve deep coma or electrocerebral silence, NDD can be confirmed by the demonstration of absence of intracerebral blood flow.14
• A description of ancillary testing is provided in Appendix 3.
Recommendation A.7: Concept and definition of neurological death
We recommend that neurologically determined death be defined as the irreversible loss of the capacity for consciousness combined with the irreversible loss of all brain stem functions (as defined in Recommendation A.1), including the capacity to breathe.
Key consideration
Death determined by neurological criteria may occur as a consequence of intracranial hypertension or primary direct brain stem injury or both. In instances of intracranial hypertension, ancillary testing demonstrating absence of intracerebral blood flow confirms death when application of minimum clinical criteria (as defined in Recommendation A.1) cannot be completed, or if the interpretation of clinical criteria is confounded. There are currently no satisfactory ancillary tests for confirmation of neurologically determined death in instances of isolated primary brain stem injury.
Recommendation A.8: Physicians declaring neurological death
We recommend that the minimum level of physician qualification required to perform NDD be
• Full and current licensure for independent medical practice in the relevant Canadian jurisdiction
• Skill and knowledge in the management of patients with severe brain injury and in NDD.
In cases of NDD for the purposes of postmortem donation, we recommend that any physician who has had any association with the proposed recipient that might influence the physician's judgment shall not take any part in the declaration of death.
Key considerations
• For the purposes of this recommendation, a physician with “full and current licensure for independent practice in the relevant Canadian jurisdiction”
• is any physician licensed by the college of physicians and surgeons or licensing authority in that jurisdiction.
• excludes physicians who are only on an educational register.
• does not require a particular level of specialty certification; nonspecialists can declare NDD if they have the requisite skill and knowledge.
• The authority to perform NDD cannot be delegated.
Recommendation A.9: Age-related criteria
We recommend that recommendations A.1 to A.8 for NDD be applied to infants, children and adolescents, with the following qualifications.
NDD recommendations specific to children and adolescents
• For all children ≥ 1 year (corrected for gestational age), NDD standards established at the forum should apply. A second physician performing the NDD is required by law for the purposes of postmortem transplantation, with no fixed interval of time required, regardless of the primary mechanism of the brain injury (see Recommendation A.5).
• The minimum level of physician qualifications should be understood as specialists with skill and knowledge in the management of children and/or adolescents with severe brain injury and NDD (see Recommendation A.8).
NDD recommendations specific to infants aged 30 days to 1 year (corrected for gestational age)
• The minimum clinical criteria include the oculocephalic reflex, as this test may be more reliable than the vestibulo-ocular reflex in infants due to the unique anatomy of the external auditory canal (see Recommendation A.1).
• A repeat examination at a different time is recommended to ensure independent confirmation by another qualified physician, regardless of the primary mechanism of the brain injury. It is prudent to have an independent examination because of the lack of collective experience and research on brain death in this age group. There is no recommended minimum time interval between determinations. Should uncertainty or confounding issues arise that cannot be resolved, the time interval may be extended according to physician judgment, or an ancillary test demonstrating absence of intracerebral blood flow may be used.
• The minimum level of physician qualifications should be understood as specialists with skill and knowledge in the management of infants with severe brain injury and NDD (see Recommendation A.8).
Key considerations
• Studies should be undertaken to evaluate the necessity of this second examination relative to the risks (e.g., of repeating the apnea test, time delays with an impact on family stress and donor stability).
• Recommendations on NDD in newborns <30 days were addressed in a separate forum.
Neonatal recommendations
The Neonatal Reference Group recommends that all NDD standards established at the forum be adopted with the following adjustments and emphases:
NDD recommendations for term newborns aged < 30 days
• Standards apply to newborns aged > 36 weeks' gestation at the time of death.
• NDD is a clinical diagnosis, i.e., clinical criteria have primacy.
• Minimum clinical criteria include absence of oculocephalic reflex and suck reflex.
• Minimum temperature must be a core temperature ≥ 36°C.
• Minimum time from birth to first determination is 48 h.
• Two determinations are required, with a minimum interval of 24 h between examinations.
• Ancillary testing, as defined by demonstration of the absence of intracerebral blood flow, should be performed when any of the minimum clinical criteria cannot be established or confounding factors remain unresolved.
• “Minimum level of physician qualifications” should be understood as specialists with skill and knowledge in the management of newborns with brain injury and the determination of death based on neurological criteria.
Key considerations
• Accuracy of gestational age should be supported by clinical history (e.g., dates and prenatal ultrasound) and physical examination. Inability to confirm a gestational age > 36 weeks should preclude NDD.
• The higher recommended temperature thresholds reflect uncertainty about hypothermic effects on neurological function in the newborn and the fact that normothermia is an easily attainable standard.
• The 48-h recommendation from injury to first determination reflects a reduced certainty of neurological prognostication before the first 48 h of life.
• Prospective research should be done to confirm the necessity of the recommended 24-h interval between determinations.
B. Representation of NDD: incidence, reporting and legal issues
Recommendation B.1: Legal timing of death
We recommend that the legal time of death be marked by the first determination of death.
Recommendation B.2: Reporting
We recommend that NDD be reported when determined.
Key consideration
Currently, there are no mechanisms to report the incidence of NDD in Canada. Given that NDD is a prerequisite for cadaveric organ donation, there is a need to record this information for use in the analysis of statistics on organ donation.
Recommendation B.3: Reporting mechanisms
We recommend that the mechanism for reporting NDD be through the medical certificate of death and that hospitals be responsible for directing completed information to the appropriate agencies, such as the Canadian Institute for Health Information.
Key considerations
• Physicians should be required to report NDD through a single mechanism.
• Specific provisions for reporting NDD should be included on the medical certificate of death. If the NDD portion of the certificate is not completed, it should be returned to the physician for completion.
Recommendation B.4: Legal issues
We recommend that Canadian medical requirements for NDD (determined at this forum) be embodied in medical standards and clinical practice guidelines.
Key consideration
Hospital practices related to NDD vary across the country. There is a need to align them (e.g., accreditation) with medical standards and clinical practice guidelines related to NDD.
C. Severe brain injury: from emergency department to ICU
Recommendation C.1: Recognition of NDD
We recommend that all patients who are suspected of being brain dead be assessed for NDD unless this has no implications for prognostication or management, including end-of-life care (see Recommendation C.3).
Recommendation C.2: Emergency department to ICU triage — evolving neuroprotective therapies
We recommend that all patients with severe brain injury who may benefit from treatment, prognostication or optimal end-of-life care within an ICU have access to these services.
Key considerations
• Patient and family wishes must be considered, e.g., wishes made known during clinician consultations, in advance directives, on organ donor cards and to an organ donor registry.
• ICU is defined as care provided in an ICU, not critical care offered in an emergency department.
• Access to ICU services for patients with severe brain injury should be in addition to preserving access to ICU for other critically ill patients.
• Resource and societal issues require consideration.
• Clinicians need to have some flexibility in decision-making.
Recommendation C.3: End-of-life care
We recommend that for patients who die as a result of severe brain injury, standard postmortem care should include the option of organ and tissue donation for eligible patients.
[See also Appendix 5: Checklist for adults and children 1 year and older]
[See also Appendix 6: Checklist for infants less than 1 year old and term newborns (36 weeks gestation)]
Acknowledgments
These recommendations have been endorsed by the Canadian Critical Care Society, Canadian Association of Emergency Physicians, Canadian Neurological Society, Canadian Neurosurgical Society (recommendations A and B), Canadian Neurocritical Care Group, Conference of Chief Coroners and Medical Examiners of Canada, Canadian Association of Critical Care Nurses, Canadian Association of Transplantation, Canadian Society of Transplantation, Quebec Transplant, Trillium Gift of Life Network and its ICU Advisory Group, Alberta HOPE Programs, Newfoundland OPEN Program, Transplant Atlantic, New Brunswick Transplant and the Canadian Council for Donation and Transplantation.
Appendix 1.

Appendix 2.

Appendix 3.
Appendix 4.

Appendix 5.
Appendix 6.

Footnotes
See Appendix 1 for a complete list of forum participants.
This article has been peer reviewed.
The authors wish to acknowledge the financial support of the Canadian Council for Donation and Transplantation and the process consultation provided by Strachan-Tomlinson and Associates.
Competing interests: None declared.
Correspondence to: Dr. Sam D. Shemie, Division of Pediatric Critical Care, Montreal Children's Hospital, McGill University Health Centre, Montréal QC H3H 1P3; sam.shemie@muhc.mcgill.ca
Reprint requests to: Ms. Kimberly Young, Canadian Council for Donation and Transplantation, 1702–8215 112 St., Edmonton AB T6G 2C8; 780 409-5652; kimberly.young@ccdt.ca
REFERENCES
- 1.Wijdicks EF. Brain death worldwide: accepted fact but no global consensus in diagnostic criteria. Neurology 2002;58(1):20-5. [DOI] [PubMed]
- 2.Shemie SD. Variability of brain death practices. Crit Care Med 2004;32(12):2564-5. [DOI] [PubMed]
- 3.Powner DJ, Hernandez M, Rives TE. Variability among hospital policies for determining brain death in adults. Crit Care Med 2004;32(6):1284-8. [DOI] [PubMed]
- 4.Mejia RE, Pollack MM. Variability in brain death determination practices in children. JAMA 1995; 274(7):550-3. [PubMed]
- 5.Chang MY, McBride LA, Ferguson MA. Variability in brain death declaration practices in pediatric head trauma patients. Pediatr Neurosurg 2003;39(1):7-9. [DOI] [PubMed]
- 6.Canadian Congress Committee on Brain Death. Death and brain death: a new formulation for Canadian medicine. CMAJ 1988;138(5):405-6. [PMC free article] [PubMed]
- 7.Canadian Neurocritical Care Group. Guidelines for the diagnosis of brain death. Can J Neurol Sci 1999;26(1):64-6. [PubMed]
- 8.Quality Standards Subcommittee of the American Academy of Neurology. Practice parameters for determining brain death in adults [summary statement]. Neurology 1995;45(5):1012-24. [DOI] [PubMed]
- 9.Burke K. Legal foundations for the neurological determination of death. Edmonton: Canadian Council for Donation and Transplantation; 2003.
- 10.Barron LB, Shemie SD, Doig C. A review of literature on the neurological determination of death. Edmonton: Canadian Council for Donation and Transplantation; 2003.
- 11.Hornby KH, Shemie SD. Survey and analysis of hospital-based brain death guidelines in Canada. Edmonton: Canadian Council for Donation and Transplantation; 2003.
- 12.Decima Research. Survey of Canadian critical care and neuroscience physicians on severe brain injury, brain death and organ donation. Edmonton: Canadian Council for Donation and Transplantation; 2003.
- 13.Booth CM, Boone RH, Tomlinson G, et al. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 2004;291(7):870-9. [DOI] [PubMed]
- 14.López-Navidad A, Caballero F, Domingo P, et al. Early diagnosis of brain death in patients treated with central nervous system depressant drugs. Transplantation 2000;70(1):131-5. [PubMed]
- 15.Guidelines for the determination of death. Report of the medical consultants on the diagnosis of death to the President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. JAMA 1981;246(19): 2184-6. [PubMed]
- 16.Wilkening M, Lowvier N, D'Athis P, et al. Validity of cerebral angiography via venous route in the diagnosis of brain death. Bull Acad Natl Med 1995;179(1):41-8. French. [PubMed]
- 17.Wieler H, Marohl K, Kaiser KP, et al. Tc-99m HMPAO cerebral scitigraphy. A reliable, noninvasive method for determination of brain death. Clin Nucl Med 1993;18(2):104-9. [DOI] [PubMed]
- 18.Spieth ME, Ansari AN, Kawada TK, et al. Direct comparison of Tc-99m DPTA and Tc-99m HMPAO for evaluating brain death. Clin Nucl Med 1994;19(10):867-72. [DOI] [PubMed]
- 19.Bonetti MG, Ciritella P, Valle V, et al. 99mTc HM-PAO brain perfusion SPECT in brain death. Neuroradiology 1995;37(5):365-9. [DOI] [PubMed]
- 20.Ducrocq X, Braun M, Debouverie M,et al. Brain death and transcranial Doppler: experience in 130 cases of brain dead patients. J Neurol Sci 1998;160(1):41-6. [DOI] [PubMed]
- 21.A definition of irreversible coma: report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain death. JAMA 1968;205(6):337-40 [PubMed]
- 22.Young GB, Shemie SD, Doig C, et al. The role of ancillary tests in the neurological determination of death. Can J Anaesth 2006; in press. [DOI] [PubMed]


