Abstract
PTP-BL is a highly modular protein tyrosine phosphatase of unknown function. It consists of an N-terminal FERM domain, five PDZ domains, and a C-terminally located tyrosine phosphatase domain. Here we show that PTP-BL is involved in the regulation of cytokinesis. We demonstrate localization of endogenous PTP-BL at the centrosomes during inter- and metaphase and at the spindle midzone during anaphase. Finally PTP-BL is concentrated at the midbody in cytokinesis. We show that PTP-BL is targeted to the midbody and centrosome by a specific splicing variant of the N-terminus characterized by an insertion of 182 amino acids. Moreover, we demonstrate that the FERM domain of PTP-BL is associated with the contractile ring and can be cosedimented with filamentous actin, whereas the N-terminus can be cosedimented with microtubules. We demonstrate that elevating the expression level of wild-type PTP-BL or expression of PTP-BL with an inactive tyrosine phosphatase domain leads to defects in cytokinesis and to the generation of multinucleate cells. We suggest that PTP-BL plays a role in the regulation of cytokinesis.
INTRODUCTION
Cytokinesis is the last step of mitosis dividing the cell into two parts after chromosome segregation. This process is highly regulated both spatially and temporally and involves the reorganization of microtubules and actin filaments. In mammalian cells an actin-myosin ring is established immediately beneath the plasma membrane, accomplishing the division of the cytoplasm by contraction. A second main structure developed in late anaphase is the spindle midzone also known as central spindle or stem body (for review see Straight and Field, 2000; Robinson and Spudich, 2000; Glotzer, 2001). The spindle midzone is formed by interdigitating microtubules and has been shown to play an important role in the regulation of cytokinesis (Cao and Wang, 1996; Wheatley and Wang, 1996). In particular many proteins important for cytokinesis have been shown to accumulate at the spindle midzone. Among these are a number of kinesin-like proteins, for example, CHO1/MKLP1, CENP-E in mammalians, XKlp1, Eg5 in Xenopus, and KLP3A, Pavarotti-KLP in Drosophila (Nislow et al., 1992; Sawin and Mitchison, 1995; Vernos et al., 1995; Adams et al., 1998; Powers et al., 1998; Raich et al., 1998).
Some of these proteins regulate bundling and density of the spindle midzone and have been shown to be required for the proper formation of the spindle midzone (Williams et al., 1995; Adams et al., 1998; Powers et al., 1998; Raich et al., 1998). However, other proteins important for microtubule bundling and located at the spindle midzone are not related to kinesins as has recently been shown for the PRC1 protein (Mollinari et al., 2002). In addition so-called chromosome passenger proteins (such as INCENP or the aurora kinases) are initially located on the chromosomes and relocate to the spindle midzone during anaphase (Adams et al., 2000, 2001). Finally, a third class of proteins concentrated at the spindle midzone is involved in the rho signal transduction pathway and includes citron kinase, the rho exchange factor pebble/ECT-2 or the rhoGAP protein CYK-4 (Madaule et al., 1998; Prokopenko et al., 1999; Tatsumoto et al., 1999; Jantsch-Plunger et al., 2000). Rho signal transduction plays an important role in the regulation of the contractile ring. Inactivation of rhoA leads to profound defects in cytokinesis in many organisms, and inactivation of the rho exchange factor ECT-2 leads to improper contraction of the contractile ring. (Kishi et al., 1993; Drechsel et al., 1997; Tatsumoto et al., 1999). Interestingly, there is evidence for an interdependence between the spindle midzone and the contractile ring. For example, in rat kidney cells a physical barrier between spindle midzone and cortex inhibits actin ring formation and partially disrupts the organization of midzone microtubules (Cao and Wang, 1996). Furthermore, in Drosophila mutant for the kinesin-like protein Pavarotti the spindle midzone appears abnormal, containing fewer bundles of microtubules, and there is a severe defect in the contractile ring assembly. Moreover, RNAi experiments for ZEN-4 in Caenorhabditis elegans lead to defects in the spindle midzone and to a defect in cleavage furrow ingression (Adams et al., 1998; Raich et al., 1998).
Here we show that the protein tyrosine phosphatase PTP-BL accumulates at the spindle midzone during anaphase and is able to interact with the spindle midzone as well as with the contractile ring in a domain-specific manner. PTP-BL has been implicated in the regulation of the actin as well as the tubulin cytoskeleton (Saras et al., 1997; Cuppen et al., 1998). PTP-BL (the human homolog is PTP-BAS/PTPl1/hPTP1E/FAP-1; Saras et al., 1994; Banville et al., 1994; Maekawa et al., 1994) is a large nontransmembrane protein tyrosine phosphatase consisting of an N-terminal FERM domain (band four point one, ezrin, radixin, moesin homology domain) followed by five different PDZ (postsynaptic density protein-95, discs large, zonula occludens) domains and a C-terminally located tyrosine phosphatase domain (Hendriks et al., 1995). Recently, we have shown that PTP-BL plays a role in the dephosphorylation of ephrinB, a transmembrane receptor regulating axonal guidance of neurons during development (Palmer et al., 2002). Here we investigate the endogenous localization of PTP-BL in mitotic cells during the cell cycle. On the basis of the results of our domain-specific localization analysis of PTP-BL and on our finding that successful cleavage furrow ingression is inhibited after the expression of PTP-BL or PTP-BL lacking a functional tyrosine phosphatase domain, we suggest that PTP-BL plays a role in the regulation of cytokinesis.
MATERIALS AND METHODS
Generation of PTP-BL Expression Vectors
Cloning of full-length PTP-BL has been described previously (Erdmann et al., 2000). S-N- and L-N-EGFP-constructs were generated by PCR using primers vNdown (5′-GTTGCGGCCGCCGATAATATGCATGTGTCACTGG-3′) and vNup (5′-CCCACCGGTCCTTCTTCCTCTTGACGCCTTAGC-3′), the amplified fragments were ligated into pEGFP-NI (Clontech, Palo Alto, CA) by NotI- and AgeI-restriction sites. pcEGFP3 was constructed by amplifying the coding region for EGFP using primers EGFPd (5′-GGGAAGCTTTCGCCACCATGGTGAGCAAG-3′) and EGFPu (5′-CCCGGTACCCTTGTACAGCTCG-TCCATGCC-3′). The amplified fragment was subcloned into pcDNA3 (Invitrogen, Karlsruhe, Germany) via HindIII- and Acc65I-sites.
The FERM-domain was amplified by primers Fermd (5′-GGGGGTACCGCTTCCATGCTCGACATCTCTAG-3′) and Fermu (5′-CCC-GAATTCTCAAAGCTTTGGGAGTCCTGCCAG-3′) and cloned into pcEGFP3 via Acc65I and EcoRI sites. The PDZ1–5-construct was generated by the digestion of full-length PTP-BL with BglII and SmaI. Sticky ends were converted to blunt ends using Klenow fragment. This fragment was subcloned into linearized pcEGFP3 (digestion with BamHI and ApaI followed by filling the ends with Klenow fragment). The phosphatase domain was amplified by Phosd (5′-GCCGGTACCAGCTTAACTGCGGCATCACAAG-3′) and Phosu (5′-TGAGAATTCTCACTGTGGGAGCCCTGGCTGTGC-3′), restricted with Acc65I and phosphorylated. The fragment was cloned into pcEGFP3 that had been digested with BamHI, filled in by Klenow fragment and subsequently restricted with Acc65I (phos-EGFP).
For the generation of BL-EGFP expression vector the stop codon of full-length PTP-BL in pcDNA3 was removed by PCR using the primer BLoS (5′-CGGCTCGAGCACCTCCACCTCCCTGTGGGAG-CCCTGGCTGTGC-3′). The coding sequence for full-length EGFP was amplified with primers EGFPXd (5′-CGGCTCGAGGTGAGCAAGGGCGAGGAG-3′) and EGFPXu (5′-CGGCTCGAGTTACTTGTACAGCTCGTCCATGCC-3′) and subcloned into pcDNA3-PTP-BL via the XhoI-site. All PCR-generated fragments were sequenced by automated sequencing on an ALF-Express (Pharmacia Amersham Biotech, Freiburg, Germany).
Isolation of Microtubules
Microtubules were isolated from HeLa cell extracts by douncing sedimented cells in twice the volume 0.8× PEM (80 mM PIPES, 2 mM EGTA, 1 mM MgCl2, pH 6.9, supplemented with complete protease inhibitor cocktail; Roche, Mannheim, Germany). After centrifugation at 100,000 × g for 90 min at 4°C the supernatants (S100 extracts) were supplemented to 0.5 mM MgGTP, 2 mM LiATP, and 5 μM taxol (Sigma, Taufkirchen, Germany). After 2–3 min at room temperature, taxol was added to a final concentration of 20 μM; extracts were incubated at 33°C for 20 min, and polymerized microtubules were spun through a sucrose-cushion (1 M sucrose in 0.8× PEM, 10 μM taxol) at 100,000 × g at 25°C for 30 min. The supernatant and cushion were removed and carefully washed with warm 0.8× PEM plus 10 μM taxol three times. Purified microtubules were resuspended in appropriate S100 extracts.
Cell Culture and Transfection
HeLa cells were maintained in DMEM supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin in a 10% CO2 atmosphere at 37°C. For video microscopy cells were kept in L15 medium plus 10% FCS. Transfection of HeLa cells was performed using the Ca3(PO4)2-method (Chen and Okayama, 1987) or Polyfect (Qiagen, Hilden, Germany).
Immunoblot Analysis
Forty-eight hours after transfection cells were lysed in Boehringer lysis buffer (50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 40 mM NaF, 5 mM EDTA, 5 mM EGTA, 1 mM Na3VO3, 1% vol/vol Nonidet P-40, 0.1% Na-desoxycholate, 0.1% sodiumdodecylsulfate [SDS], complete protease inhibitor cocktail; Roche, Mannheim, Germany). After centrifugation at 20,000 × g for 30 min at 4°C the supernatant was separated by 6% or 8% SDS-PAGE. Proteins were transferred to nitrocellulose and probed with the indicated antibodies. Blots were developed using the ECL system (Pharmacia Amersham Biotech).
Microtubules Spin-down Assay
To test microtubule binding, taxol-stabilized microtubules from 800 μl HeLa cell extracts were resuspended in 600 μl S100 extracts of transfected HeLa cells in 0.5× PEM (50 mM PIPES, 2 mM EGTA, 1 mM MgCl2, pH 7.4, 20 μM taxol, supplemented with complete protease inhibitor cocktail [Roche], 0.5 mM MgGTP, 3 mM MgAMP-PNP [5′-Adenylylimidodiphosphate, Sigma, Taufkirchen, Germany], 15 U/ml hexokinase [Sigma], and 20 mM glucose). After incubation at 33°C for 20 min, microtubules were spun through a sucrose-cushion (1 M sucrose in 0.5× PEM, 10 μM taxol) at 100,000 × g at 25°C for 30 min. The supernatant and cushion were removed, and the pellet was carefully washed three times with warm 0.5× PEM plus 10 μM taxol. Samples were separated by 8% SDS-PAGE, transferred to nitrocellulose, and probed with the indicated antibodies.
F-actin Spin-down Assay
Nonmuscle actin (Cytoskeleton, Denver, CO) was used for F-actin binding experiments. G-actin was dissolved in actin buffer (5 mM Tris/HCl, pH 8.0, 0.2 mM CaCl2, 0.2 mM ATP) and incubated on ice for 30 min. After the addition of 1/10 volume polymerization buffer (500 mM KCl, 20 mM MgCl2, 50 mM ATP) actin was polymerized at 24°C for 1 h. F-actin was sedimented by centrifugation at 150,000 × g at 24°C for 90 min. Forty-eight hours after transfection sedimented HeLa cells were dounced in 0.5× PEM on ice and centrifuged for 30 min at 20,000 × g at 4°C. Supernatants were dialyzed against F-actin buffer (5 mM Tris-HCl, pH 8.0, 0.2 mM CaCl2, 50 mM KCL, 2 mM MgCl2) and centrifuged at 150,000 × g at 24°C for 90 min. Supernatants were supplemented with 1 mM ATP and used for spin-down experiments. Fifty micrograms of F-actin were resuspended in 50 μl lysate and incubated at 24°C for 1 h. Suspensions were layered on a 100 μl glycerol cushion (10% glycerol in F-actin buffer) and centrifuged at 150,000 × g at 24°C for 90 min. After carefully removing the cushion, the pellets were dissolved in 1× Laemmli buffer. Proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with the indicated antibodies.
Antibodies and Immunocytochemistry
HeLa cells were cultured on poly-ornithine–coated coverslips. The cells were fixed with 4% paraformaldehyde containing 4% sucrose for 15 min on ice or in 100% methanol for 15 min at −20°C. After incubation with 0.25% Triton X-100 and 1% bovine serum albumin in TBS (10 mM Tris/HCl, 150 mM NaCl, pH 7.4) for 15 min at room temperature, cells were washed extensively with TBS. Cells were incubated for 1 h with primary antibodies in TBS at room temperature followed by incubation with secondary antibodies under the same conditions. Monoclonal mouse anti–γ-tubulin (dilution 1:200) and anti–β-tubulin (dilution 1:200 for immunocytochemistry, 1:500 for immunoblotting) were purchased from Sigma, a polyclonal rabbit anti-GFP (dilution 1:100) from Clontech (Palo Alto, CA), AlexaFluor488-conjugated anti-rabbit (dilution 1:1000) and AlexaFluor546-conjugated anti-mouse antibody (dilution 1:1000) were from Molecular Probes (Eugene, OR). Generation, purification and specificity of anti-PTP-BL antibodies has been described (Erdmann et al., 2000). Syto60 (5 μM, Molecular Probes) or Hoechst-dye 33342 (1 μg/ml, Sigma) was used for DNA staining. Phalloidin-TRITC (50 μg/ml, Sigma) was used for F-actin staining. Coverslips were mounted with Immunofluor (ICN Biomedicals, Eschwege, Germany) to prevent bleaching. Confocal images were taken on a TCS 4D confocal laser scanning microscope (Leica, Bensheim, Germany), processed with Adobe Photoshop (Adobe, San Jose, CA). Transfected cells were followed by time-lapse videomicroscopy on an Axiovert 30 (Zeiss, Thornwood, NY) equipped with a cooled digital Coolsnap (fx) camera, and videos were taken using Metaview software (Universal Imaging Corporation, Downingtown, PA).
RESULTS
Subcellular Localization of Endogenous PTP-BL in HeLa Cells during Mitosis
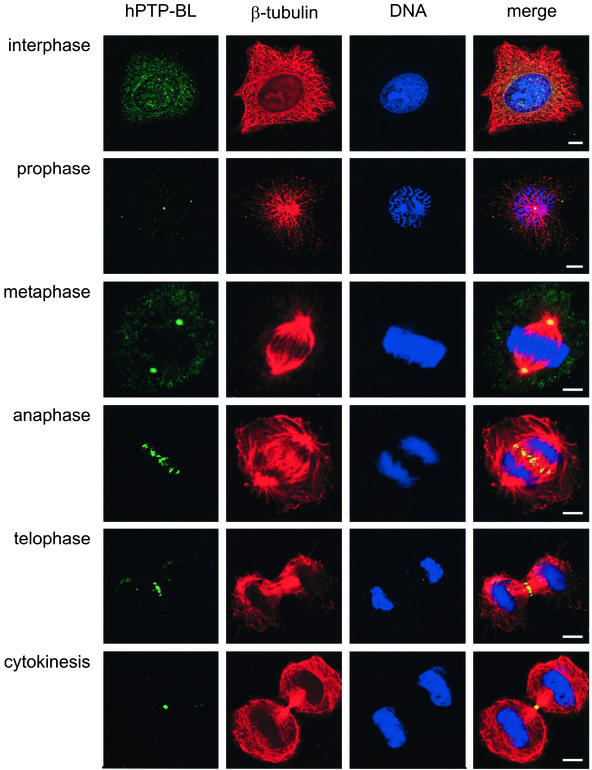
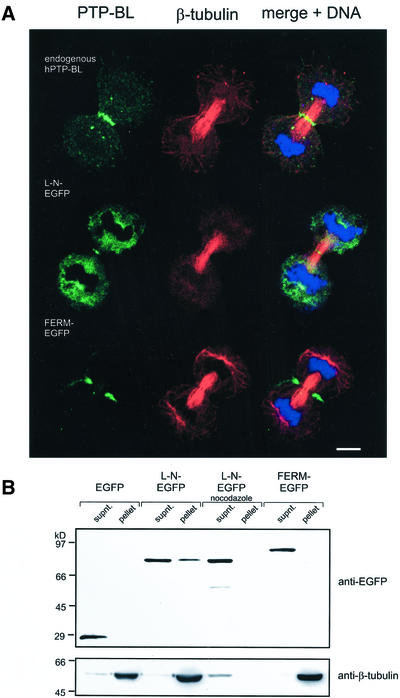
To obtain first insights into a function of PTP-BL, we analyzed the subcellular localization of endogenous human PTP-BL during cell division using confocal immunofluorescence laser scanning microscopy. During interphase we found a punctuate staining of PTP-BL in the cytosol and in the nucleus (Figure 1); however, there was also staining of PTP-BL at the centrosome (see Figure 3). In prophase punctuate staining was visible in the cytosol and at the centrosome. In metaphase PTP-BL distribution did not change, but PTP-BL was more concentrated at the centrosomes. A dramatic change in the localization of PTP-BL took place during anaphase, where PTP-BL was concentrated at the spindle midzone. There, PTP-BL is concentrated in regions of overlapping microtubules at the midzone. During telophase PTP-BL was accumulated into a small band at the midzone and finally became concentrated in the very center of the midbody in cytokinesis. Similar staining could be demonstrated when using an antibody recognizing a different epitope (PDZ1) of PTP-BL or using Madine Carnine Kidney (MDCK) cells (unpublished data).
Figure 1.
Localization of endogenous human PTP-BL in HeLa cells during the cell cycle. HeLa cells were stained with an anti–PTP-BL antibody (green), an anti–β-tubulin antibody (red) or with the dye syto60 (blue). Bars, 5 μm.
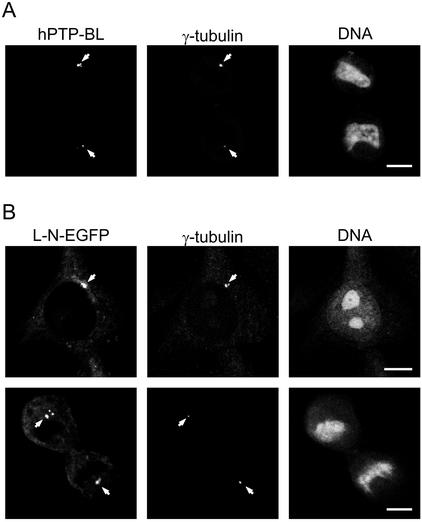
Figure 3.
(A) Centrosomal localization of human PTP-BL in HeLa cells (arrows). HeLa cells were stained for endogenous human PTP-BL using an anti-PTP-BL antibody and for γ-tubulin using a monoclonal anti-γ-tubulin antibody. (B) The long N-terminus of PTP-BL is sufficient to target EGFP to the centrosomes (arrows). HeLa cells were transiently transfected with an expression vector for L-N-EGFP and stained for γ-tubulin as above. EGFP derived fluorescence and γ-tubulin immunofluorescence is shown. Bars, 5 μm.
Domain-specific Localization of PTP-BL
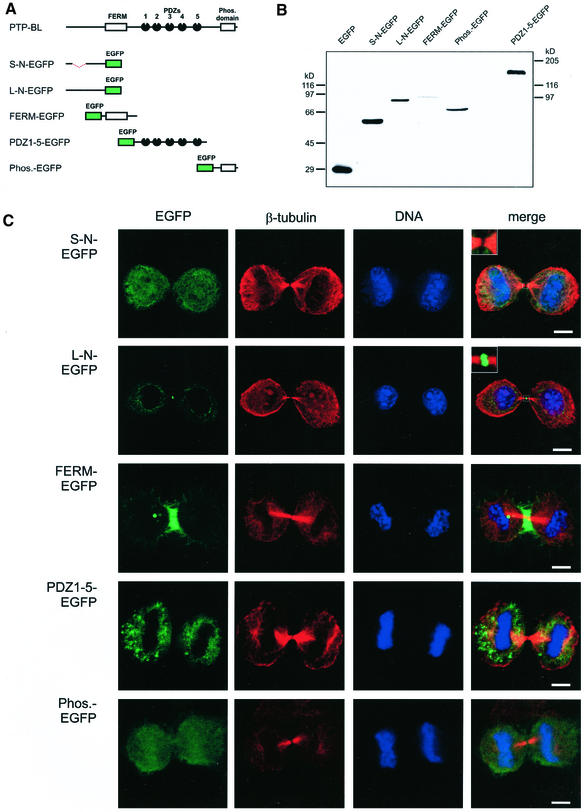
PTP-BL is a highly modular protein and furthermore is subject to alternative splicing at the N-terminus (Chida et al., 1995). To analyze which domain of this protein mediates midbody localization, we fused each of the various domains of PTP-BL to EGFP, i.e., the two alternative splicing variants of the N-terminus, the FERM domain, the five PDZ domains, and the phosphatase domain (Figure 2A). Expression of these constructs was verified by Western blotting lysates of transfected HeLa cells using an anti-EGFP and anti–PTP-BL antibody (Figure 2B). Subcellular localization of each construct was identified by detecting the EGFP-derived fluorescence of transfected HeLa cells. To determine the location of these constructs relative to the position of the midbody, we stained the cells for β-tubulin and DNA using an anti–β-tubulin antibody and the dye syto 60, respectively (Figure 2C). The short N-terminus fused to EGFP showed no specific localization and was evenly distributed in the cytoplasm and the nucleus. Surprisingly the long N-terminal splicing variant (L-N-EGFP) differing from the short version by the insertion of 182 amino acids showed a clear enrichment at the midbody with some additional staining in the cytoplasm. Interestingly, the FERM domain showed a specific localization around the midzone microtubules (verified by confocal X/Z view, unpublished data), suggesting colocalization with the contractile ring, but no colocalization with the midbody was detectable. PDZ1–5-EGFP showed a cytosolic distribution with some punctuate structures. Finally, the phosphatase domain could be detected in the nucleus as well as in the cytosol. We conclude that the long N-terminus of PTP-BL is sufficient for midbody localization.
Figure 2.
(A) Schematic representation of PTP-BL and EGFP-tagged PTP-BL constructs. (B) Expression of PTP-BL-EGFP domain-specific fusion proteins. Equal amounts of lysates from HeLa cells transiently transfected with the indicated expression vectors were separated by SDS-PAGE, proteins were transferred to nitrocellulose and detected using an anti-EGFP antibody, and PDZ1–5-EGFP was detected using the anti–PTP-BL antibody. (C) Subcellular localization of the PTP-BL-EGFP constructs in HeLa cells at late telophase. Confocal images were taken from transiently transfected HeLa cells expressing the indicated EGFP fusion proteins (green). For reference of the stage of cell division tubulin was stained using an anti–β-tubulin antibody (red); DNA was stained using the dye syto60 (blue). Bars, 5 μm.
L-N-EGFP Colocalizes with γ-Tubulin at Centrosomes
Endogenous human PTP-BL is partially localized at centrosomes in interphase cells as identified by colocalization with γ-tubulin, which is a specific marker for centrosomes (Figure 3A). During our analysis of the domain-specific localization of PTP-BL, we often observed one to two major spots strongly enriched by L-N-EGFP, suggesting that L-N-EGFP might be associated with the centrosome. To verify that L-N-EGFP also shows a prominent centrosomal localization, we performed immunocytochemistry on L-N-EGFP–transfected cells using a specific anti–γ-tubulin antibody. The γ-tubulin immunofluorescence completely matched the L-N-EGFP fluorescence, but in addition we often observed some smaller L-N-EGFP spots in the vicinity of the γ-tubulin staining, which did not collocate with γ-tubulin (Figure 3B). Thus, we conclude that the long N-terminus of PTP-BL contains a targeting signal for centrosomal localization, yet we do not rule out the existence of additional targeting sites.
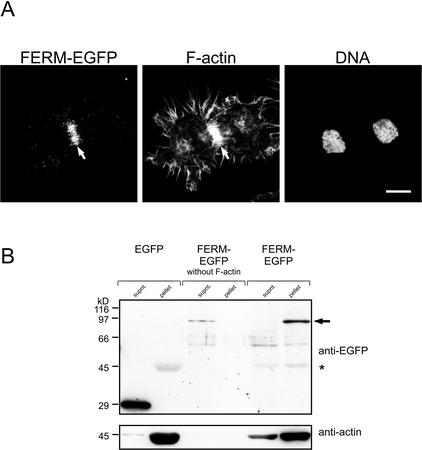
FERM-EGFP Colocalized with F-actin at the Contractile Ring
The localization of FERM-EGFP resembles the localization of the contractile ring, which is an F-actin/myosin–based structure developing along the equator of the elongated spindle after chromosome segregation. Thus, to provide evidence for colocalization of FERM-EGFP with the contractile ring, we identified F-actin in FERM-EGFP–transfected HeLa cells using TRITC-labeled phalloidin. The FERM-EGFP–derived fluorescence exactly matched the localization of F-actin at the contractile ring of dividing HeLa cells, thus indicating association of FERM-EGFP with the contractile ring (Figure 4A). To establish biochemical evidence for an association of the FERM domain of PTP-BL with F-actin, we applied an F-actin spin-down assay. F-actin was incubated with cytosol from HeLa cells transiently transfected with expression vectors for FERM-EGFP or EGFP. Subsequently F-actin was spun through a glycerol cushion, and associated proteins were identified by Western blotting with an anti-EGFP antibody. Although FERM-EGFP could easily be detected in the pellet and was quantitatively removed from the supernatant, EGFP alone was not able to cosediment with F-actin, suggesting specific cosedimentation of the FERM domain with F-actin. Moreover, no FERM-EGFP could be detected in the pellet without previous addition of F-actin (Figure 4B).
Figure 4.
FERM-EGFP localizes to the contractile ring and can be cosedimented with F-actin in a spin-down assay. (A) HeLa cells transiently transfected with an expression vector for FERM-EGFP were stained for filamentous actin (F-actin) using phalloidin-TRITC and for DNA using the dye syto 60. The contractile ring is marked by an arrow (B). In vitro–polymerized F-actin was incubated with cell lysates of HeLa cells transiently expressing EGFP or FERM-EGFP. F-actin was spun down by ultracentrifugation, and aliquots of the supernatant and pellet were separated by SDS-PAGE and transferred to nitrocellulose. The blot was developed using an anti-EGFP antibody. The arrow indicates the position of FERM-EGFP, position of actin is marked by an asterisk (top panel). For verification of actin sedimentation the blot was stripped and relabeled using an anti-actin antibody (bottom panel).
Complementary Localization of FERM-EGFP at the Cleavage Furrow and L-N-EGFP at the Midzone Microtubules during Telophase
Because of the dynamic localization of endogenous PTP-BL during cell division, we decided to analyze subcellular localization of L-N-EGFP and FERM-EGFP also in early telophase, when the ingression of the cleavage furrow is still incomplete (Figure 5A). At that point in time the L-N-EGFP construct was already enriched at the midzone, but we observed additional prominent staining in the cytosol. FERM-EGFP clearly localized in direct vicinity of but not directly at the midzone microtubules compatible with an association with the contractile ring during cleavage furrow ingression. These results suggest that the accumulation of endogenous PTP-BL at the very center of the midzone in telophase is a consequence of the targeting by the FERM domain as well as the long N-terminus.
Figure 5.
(A) Subcellular localization of L-N-EGFP and FERM-EGFP in HeLa cells at early telophase. Confocal images were taken from transiently transfected HeLa cells expressing the indicated EGFP-fusion proteins (green). For reference of the stage of cell division tubulin was stained using an anti–β-tubulin antibody (red); DNA was stained using the dye syto60 (blue). Bars, 5 μm. For comparison the top row shows the localization of endogenous human PTP-BL, which was identified by using an anti–PTP-BL antibody (green). (B) L-N-EGFP can be cosedimented with microtubules. Taxol-stabilized microtubules were incubated with lysates of HeLa cells transiently expressing the indicated PTP-BL-EGFP constructs. For the negative control experiment nocodazole was added to prevent microtubule assembly. Microtubules and associated proteins were sedimented by ultracentrifugation, and aliquots of the supernatant and pellet were separated by SDS-PAGE. Proteins were transferred to nitrocellulose, and the blot was developed using an anti-EGFP antibody (top panel). The blot was stripped and relabeled using an anti–β-tubulin antibody (bottom panel).
L-N-EGFP Is Able to Bind to Microtubules
The localization of the L-N-EGFP construct in early telophase as well as its presence in the midbody suggests that L-N-EGFP could be attached, directly or indirectly, to microtubules. To test this hypothesis, we performed microtubule cosedimentation assays. Lysates of HeLa cells expressing FERM-EGFP or L-N-EGFP or EGFP were incubated with taxol-stabilized microtubules. Microtubules were spun through a sucrose cushion, and cosedimented proteins were separated by SDS-PAGE, transferred to nitrocellulose and probed with an anti-EGFP antibody. Only L-N-EGFP could be cosedimented with taxol-stabilized microtubules, whereas no cosedimentation of FERM-EGFP or EGFP with microtubules could be detected (Figure 5B). In a control experiment where tubulin polymerization was inhibited by the addition of nocodazole, L-N-EGFP could not be sedimented, thus indicating that cosedimentation of L-N-EGFP occurs via microtubules.
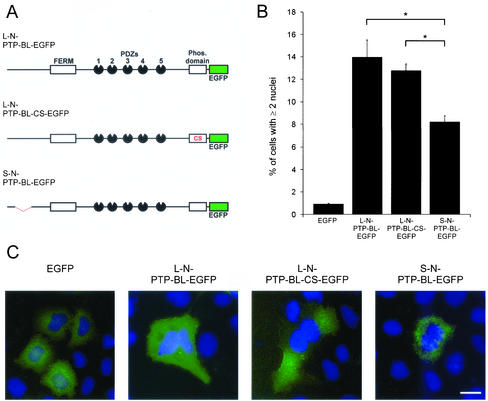
Expression of PTP-BL Constructs Leads to the Generation of Multinucleate Cells
To analyze the role of PTP-BL in cytokinesis, we transfected HeLa cells with splice variant specific expression constructs for full-length PTP-BL (Figure 6A). The first construct contained the long N-terminus (L-N-PTP-BL-EGFP), the second construct contained the short N-terminus (S-N-PTP-BL-EGFP). After 72 h cells were fixed and analyzed for the presence of more than one nucleus. We observed a prominent increase in the number of multinucleate cells after the expression of L-N-PTP-BL-EGFP (14 ± 1.5%) compared with control transfected HeLa cells expressing EGFP (0.9 ± 0.1%). A significantly lower increase in the number of multinucleate cells was observed after expressing S-L-PTP-BL-EGFP (8.2 ± 0.5%). To also address the function of tyrosine phosphatase activity of PTP-BL in the regulation of cytokinesis, we transfected HeLa cells with a variant of L-N-PTP-BL-EGFP devoid of a catalytically active tyrosine phosphatase domain (L-N-PTP-BL-CS-EGFP; Erdmann et al., 2000). We observed no difference in the capability of generating multinucleate cells (12.8 ± 0.6%) compared with L-N-PTP-BL-EGFP harboring a functional phosphatase domain. Unfortunately, probably because of the protein's long half-life, we were not able to deplete endogenous PTP-BL using siRNA. Thus, we were prevented from analyzing a null phenotype of PTP-BL.
Figure 6.
(A) Schematic diagram of the transfected full-length PTP-BL constructs. (B) Expression of PTP-BL-EGFP constructs lead to the generation of multinucleate cells. HeLa cells were transiently transfected with expression vectors for the indicated PTP-BL-EGFP constructs. Seventy-two hours after transfection cells were fixed and stained for DNA, transfected cells were identified by the presence of EGFP-derived fluorescence, and the number of transfected cells having more than one nucleus was determined. Error bars represent SDs; significant differences between L-N-PTP-BL-EGFP/L-N-PTP-BL-CS-EGFP and S-N-PTP-BL-EGFP transfected HeLa cells are marked by an asterisk, *p ≤ 0.01. (C) Representative pictures of HeLa cells transiently transfected with the indicated expression vectors are shown. EGFP-derived fluorescence is shown in green; DNA is shown in blue. Bar, 10 μm.
Overexpression of PTP-BL-EGFP Leads to Incomplete Cytokinesis
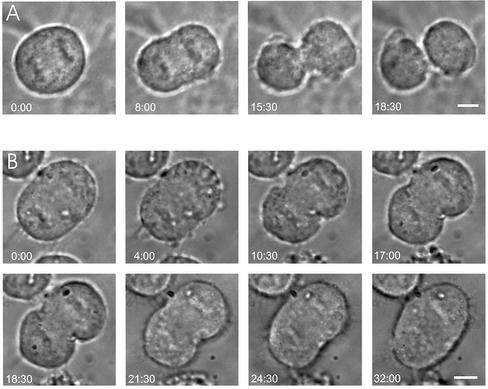
The generation of multinucleate cells after elevating the expression level of endogenous PTP-BL or after the expression of PTP-BL lacking a functional tyrosine phosphatase activity clearly indicates an interference with the progression of cytokinesis. To evaluate at which stage of cytokinesis this defect appears, we applied videomicroscopy. HeLa cells were transfected with expression vectors for EGFP or L-N-PTP-BL-EGFP; 16 h after transfection, cells were inspected for the presence of EGFP fluorescence, and transfected cells were selected for videomicroscopy. Figure 7 shows examples of HeLa cells during cytokinesis overexpressing EGFP (A) or L-N-PTP-BL-EGFP (B). Cells transfected with L-N-PTP-BL-EGFP were able to establish a cleavage furrow, and the cleavage furrow started to ingress. This ingression proceeded for some time, then stopped, and was followed by a complete regression of the cleavage furrow. This defect was observed in 4 of 21 cells analyzed. In contrast, an analysis of 41 control cells expressing EGFP did not show any cytokinesis defects. Thus, expression of L-N-PTP-BL-EGFP interferes with the ingression of the cleavage furrow and/or with the completion of cytokinesis.
Figure 7.
Expression of L-N-PTP-BL-EGFP leads to incomplete cleavage furrow ingression followed by complete regression of the cleavage furrow. HeLa cells were transiently transfected with an expression vector for EGFP (A) or L-N-PTP-BL-EGFP (B). Sixteen hours after transfection cells were monitored for EGFP fluorescence, and transfected cells were analyzed using videomicroscopy. Time is indicated in min:sec. Bar, 5 μm.
DISCUSSION
In an attempt to identify a function of PTP-BL in mitotic cells, we first characterized its subcellular localization during the cell cycle. We were able to show that endogenous human PTP-BL localizes mainly in a punctuate pattern in the cytosol, with clear enrichment at the centrosomes and spindle poles. We observed a striking accumulation of PTP-BL at the spindle midzone during anaphase and finally a concentration of PTP-BL at the midbody in cytokinesis. The dynamic behavior of PTP-BL localization during the cell cycle is similar to the localization of a number of proteins known to be involved in cytokinesis and chromosome segregation like the rho exchange factor ECT2 (Tatsumoto et al., 1999), the citron kinase (Madaule et al., 1998), INCENP (Savoian et al., 1999), or the kinesin Rab6-KIFL (Hill et al., 2000), suggesting a role of PTP-BL in the regulation of cytokinesis/chromosome segregation. However, using two different antibodies, we were not able to identify PTP-BL at the kinetochores (unpublished data).
We were able to identify the targeting signal for midbody localization in a specific splicing variant of the N-terminus of PTP-BL. This splicing variant differs from the short variant, which showed no specific localization, by the presence of additional 182 amino acids (Chida et al., 1995). In addition only the long N-terminus was sufficient for localization at the centrosome, as demonstrated by its colocalization with γ-tubulin. However, in distinct contrast the FERM domain colocalized with the contractile ring and could be cosedimented with F-actin. The FERM domain is a widespread protein module of ∼300-amino acid length that is involved in the localization of proteins to the plasma membrane (Chishti et al., 1998). Our observation that the FERM domain of PTP-BL is associated with the contractile ring, which itself is attached to the plasma membrane by a currently unknown mechanism, is compatible with this previously described membrane anchoring function. In addition it has recently been shown that the FERM domain of PTP-BL is sufficient for apical plasma membrane localization in transfected polarized MDCK cells (Cuppen et al., 1999), and similar apical localization has been reported for a truncated version of PTP-BL containing the FERM domain in vivo (Thomas et al., 1998). Two founding members of the protein family containing the FERM domain, radixin and the band 4.1 protein, have also been reported to localize at the cleavage furrow, indicating that FERM-containing proteins might play an important role in cytokinesis (Sato et al., 1991; Krauss et al., 1997).
Overexpression of PTP-BL did not disturb chromosome segregation or spindle assembly (unpublished data). Thus we analyzed the role of PTP-BL in the regulation of cytokinesis. Several proteins known to be involved in the regulation of cytokinesis interfere with the progression of cytokinesis after overexpression. This indicates that a delicate balance of their expression level is important for the regulation of this process (Tatsuka et al., 1998; Hirose et al., 2001). Indeed, elevating the expression level of full-length L-N-PTP-BL led to a clear increase in the number of multinucleate cells (L-N-PTP-BL-EGFP 14 ± 1.5% vs. EGFP control 0.9 ± 0.1%). In addition, overexpression of L-N-PTP-BL with a point mutation deleting the catalytic activity of PTP-BL tyrosine phosphatase led to a comparable increase of multinucleate cells (12.8 ± 0.6%). Thus we could not find a requirement of the phosphatase activity of PTP-BL in the regulation of cytokinesis. This either means that the phosphatase activity serves another function of L-N-PTP-BL and/or has only modulatory effects on the cytokinesis process. However, it has recently been demonstrated for (−/−) fibroblast of the protein tyrosine phosphatase PTP-PEST that the regulation of protein tyrosine phosphorylation may in principle play a role in cytokinesis (Angers-Loustau et al., 1999).
Videomicroscopy performed here on transfected HeLa cells suggests that PTP-BL probably plays a role in cleavage furrow ingression and/or completion of cytokinesis. In early telophase, when cleavage furrow ingression is still incomplete, we observed an accumulation of the long N-terminus of PTP-BL at the spindle midzone and a localization of the FERM domain in direct vicinity of, but not directly at the midzone microtubules. Moreover, using biochemical assays, we could show that the long N-terminus is able to cosediment with microtubules, whereas the FERM domain showed no binding to microtubules but was able to cosediment with F-actin. Given the fact that PTP-BL is in principle able to interact with components of the contractile ring via the FERM domain and with midzone microtubules via its long N-terminus, PTP-BL could play a role in connecting the spindle midzone with the contractile ring. This might be important for furrow ingression and completion of cytokinesis. There is an important functional interplay in cytokinesis between the spindle midzone microtubules and the actin-based contractile ring in different organisms (Cao and Wang, 1996; Fishkind et al., 1996; Adams et al., 1998; Giansanti et al., 1998; Swan et al., 1998). Although it has not yet been determined if the cell cortex is directly bound to midzone microtubules during cytokinesis, there are several other proteins known to play a role in cytokinesis that are able to bind, directly or indirectly, to both actin filaments and microtubules (Sisson et al., 2000). Recently, it has been shown that the kinesin-like protein CHO1, which accumulates at the spindle midzone between antiparallel microtubules similar to PTP-BL, is able to bind to F-actin and that blocking this interaction by antibody injections leads to defects in the terminal phase of cytokinesis and to regression of the cleavage furrow (Nislow et al., 1992; Kuriyama et al., 2002).
Moreover, rho signal transduction has been shown to play an important role in the regulation of the contractile ring (for review, see Prokopenko et al., 2000). Recently a novel complex (centralspindlin) composed of the kinesin-like protein ZEN-4 and the rhoGAP protein CYK-4 has been identified (Mishima et al., 2002). Both of these proteins have previously been implicated in the regulation of cytokinesis (Raich et al., 1998; Jantsch-Plunger et al., 2000). Centralspindlin is localized at the spindle midzone and appears to be required for bundling of midzone microtubules and for the completion of cytokinesis. Furthermore, the existence of a similar complex composed of HsCYK-4 and MKLP-1 has also been demonstrated in humans (Mishima et al., 2002). Interestingly, PTP-BL is known to interact with the rho GTPase-activating protein PARG via its PDZ4 domain (Saras et al., 1997) and with the rho-dependent kinase, PRK2, via its PDZ3 domain (Gross et al. 2001), implicating PTP-BL in a rho signal transduction pathway relevant to cytokinesis. However, PTP-BL expression in the early embryo is ubiquitous and becomes restricted to neurons and epithelial cells later in development (Hendriks et al., 1995; Thomas et al., 1998). This restricted expression pattern suggests that a possible regulatory role of PTP-BL in cytokinesis might be limited to the early stages of development and/or might be cell type specific, playing a role mainly for epithelial cells. Cell type–specific regulation of cytokinesis has been described recently for a subset of embryonic neuronal precursor cells, which require functional citron kinase for cytokinesis. Consequently, knock out mice deficient for citron kinase show a severe reduction of neurons in the CNS (Di Cunto et al., 2000; Sarkisian et al., 2002).
In summary, we have demonstrated that the localization of PTP-BL is regulated in a cell cycle-dependent manner. PTP-BL is capable of interacting with the contractile ring and with the spindle midzone microtubules. Together with the specific localization of endogenous PTP-BL and the defects of cytokinesis observed after overexpression of PTP-BL, these findings suggest that PTP-BL plays a regulatory role in cytokinesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rolf Heumann for continuous support. We thank Anette Tolle and Sabine Laerbusch for excellent technical assistance and Uta Wolfe and Hans-Joachim Herrmann for critical reading of the manuscript. We are grateful to Karl S. Zaenker for the access to the confocal microscope. This work is supported by a grant from the Deutsche Forschungsgemeinschaft (SFB452) to K.S.E.
Footnotes
Online version of this article contains video material for Figure 7. Online version is available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0191. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0191.
REFERENCES
- Adams RR, Carmena M, Earnshaw WC. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 2001;11:49–54. doi: 10.1016/s0962-8924(00)01880-8. [DOI] [PubMed] [Google Scholar]
- Adams RR, Tavares AA, Salzberg A, Bellen HJ, Glover DM. pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 1998;12:1483–1494. doi: 10.1101/gad.12.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, Gerloff DL, Earnshaw WC. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10:1075–1078. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- Angers-Loustau A, Cote JF, Charest A, Dowbenko D, Spencer S, Lasky LA, Tremblay ML. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J Cell Biol. 1999;144:1019–1031. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banville D, Ahmad S, Stocco R, Shen SH. A novel protein-tyrosine phosphatase with homology to both the cytoskeletal proteins of the band 4.1 family and junction-associated guanylate kinases. J Biol Chem. 1994;269:22320–22327. [PubMed] [Google Scholar]
- Cao LG, Wang YL. Signals from the spindle midzone are required for the stimulation of cytokinesis in cultured epithelial cells. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida D, Kume T, Mukouyama Y, Tabata S, Nomura N, Thomas ML, Watanabe T, Oishi M. Characterization of a protein tyrosine phosphatase (RIP) expressed at a very early stage of differentiation in both mouse erythroleukemia and embryonal carcinoma cells. FEBS Lett. 1995;358:233–239. doi: 10.1016/0014-5793(94)01432-z. [DOI] [PubMed] [Google Scholar]
- Chishti AH, et al. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci. 1998;23:281–282. doi: 10.1016/s0968-0004(98)01237-7. [DOI] [PubMed] [Google Scholar]
- Cuppen E, Gerrits H, Pepers B, Wieringa B, Hendriks W. PDZ motifs in PTP-BL and RIL bind to internal protein segments in the LIM domain protein RIL. Mol Biol Cell. 1998;9:671–683. doi: 10.1091/mbc.9.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppen E, Wijers M, Schepens J, Fransen J, Wieringa B, Hendriks W. A FERM domain governs apical confinement of PTP-BL in epithelial cells. J Cell Sci. 1999;112:3299–3308. doi: 10.1242/jcs.112.19.3299. [DOI] [PubMed] [Google Scholar]
- Di Cunto F, et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 2000;28:115–127. doi: 10.1016/s0896-6273(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- Erdmann KS, Kuhlmann J, Lessmann V, Herrmann L, Eulenburg V, Muller O, Heumann R. The Adenomatous Polyposis Coli-protein (APC) interacts with the protein tyrosine phosphatase PTP-BL via an alternatively spliced PDZ domain. Oncogene. 2000;19:3894–3901. doi: 10.1038/sj.onc.1203725. [DOI] [PubMed] [Google Scholar]
- Fishkind DJ, Silverman JD, Wang YL. Function of spindle microtubules in directing cortical movement and actin filament organization in dividing cultured cells. J Cell Sci. 1996;109:2041–2051. doi: 10.1242/jcs.109.8.2041. [DOI] [PubMed] [Google Scholar]
- Giansanti MG, Bonaccorsi S, Williams B, Williams EV, Santolamazza C, Goldberg ML, Gatti M. Cooperative interactions between the central spindle and the contractile ring during Drosophila cytokinesis. Genes Dev. 1998;12:396–410. doi: 10.1101/gad.12.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. Animal cell cytokinesis. Annu Rev Cell Dev Biol. 2001;17:351–386. doi: 10.1146/annurev.cellbio.17.1.351. [DOI] [PubMed] [Google Scholar]
- Gross C, Heumann R, Erdmann KS. The Protein Kinase C-related kinase PRK2 interacts with the Protein Tyrosine Phosphatase PTP-BL via a novel PDZ domain binding motif. FEBS Lett. 2001;496:101–104. doi: 10.1016/s0014-5793(01)02401-2. [DOI] [PubMed] [Google Scholar]
- Hendriks W, Schepens J, Bachner D, Rijss J, Zeeuwen P, Zechner U, Hameister H, Wieringa B. Molecular cloning of a mouse epithelial protein-tyrosine phosphatase with similarities to submembranous proteins. J Cell Biochem. 1995;59:418–430. doi: 10.1002/jcb.240590403. [DOI] [PubMed] [Google Scholar]
- Hill E, Clarke M, Barr FA. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 2000;19:5711–5719. doi: 10.1093/emboj/19.21.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Kawashima T, Iwamoto I, Nosaka T, Kitamura T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J Biol Chem. 2001;276:5821–5828. doi: 10.1074/jbc.M007252200. [DOI] [PubMed] [Google Scholar]
- Jantsch-Plunger V, Gonczy P, Romano A, Schnabel H, Hamill D, Schnabel R, Hyman AA, Glotzer M. CYK-4: a Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss SW, Larabell CA, Lockett S, Gascard P, Penman S, Mohandas N, Chasis JA. Structural protein 4.1 in the nucleus of human cells: dynamic rearrangements during cell division. J Cell Biol. 1997;137:275–289. doi: 10.1083/jcb.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Gustus C, Terada Y, Uetake Y, Matuliene J. CHO1, a mammalian kinesin-like protein, interacts with F-actin and is involved in the terminal phase of cytokinesis. J Cell Biol. 2002;156:783–790. doi: 10.1083/jcb.200109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394:491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- Maekawa K, Imagawa N, Nagamatsu M, Harada S. Molecular cloning of a novel protein-tyrosine phosphatase containing a membrane-binding domain and GLGF repeats. FEBS Lett. 1994;337:200–206. doi: 10.1016/0014-5793(94)80273-4. [DOI] [PubMed] [Google Scholar]
- Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- Mollinari C, Kleman JP, Jiang W, Schoehn G, Hunter T, Margolis RL. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J Cell Biol. 2002;157:1175–1186. doi: 10.1083/jcb.200111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature. 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell. 2002;9:725–737. doi: 10.1016/s1097-2765(02)00488-4. [DOI] [PubMed] [Google Scholar]
- Powers J, Bossinger O, Rose D, Strome S, Saxton W. A nematode kinesin required for cleavage furrow advancement. Curr Biol. 1998;8:1133–1136. doi: 10.1016/s0960-9822(98)70470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko SN, Brumby A, O'Keefe L, Prior L, He Y, Saint R, Bellen HJ. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko SN, Saint R, Bellen HJ. Untying the Gordian knot of cytokinesis. Role of small G proteins and their regulators. J Cell Biol. 2000;148:843–848. doi: 10.1083/jcb.148.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich WB, Moran AN, Rothman JH, Hardin J. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol Biol Cell. 1998;9:2037–2049. doi: 10.1091/mbc.9.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DN, Spudich JA. Towards a molecular understanding of cytokinesis. Trends Cell Biol. 2000;10:228–237. doi: 10.1016/s0962-8924(00)01747-5. [DOI] [PubMed] [Google Scholar]
- Saras J, Claesson-Welsh L, Heldin CH, Gonez LJ. Cloning and characterization of PTPL1, a protein tyrosine phosphatase with similarities to cytoskeletal-associated proteins. J Biol Chem. 1994;269:24082–24089. [PubMed] [Google Scholar]
- Saras J, Franzen P, Aspenstrom P, Hellman U, Gonez LJ, Heldin CH. A novel GTPase-activating protein for Rho interacts with a PDZ domain of the protein-tyrosine phosphatase PTPL1. J Biol Chem. 1997;272:24333–24338. doi: 10.1074/jbc.272.39.24333. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR, Li W, Di Cunto F, D'Mello SR, LoTurco JJ. Citron-kinase, a protein essential to cytokinesis in neuronal progenitors, is deleted in the flathead mutant rat. J Neurosci. 2002;22:RC217. doi: 10.1523/JNEUROSCI.22-08-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Yonemura S, Obinata T, Tsukita S. Radixin, a barbed end-capping actin-modulating protein, is concentrated at the cleavage furrow during cytokinesis. J Cell Biol. 1991;113:321–330. doi: 10.1083/jcb.113.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoian MS, Earnshaw WC, Khodjakov A, Rieder CL. Cleavage furrows formed between centrosomes lacking an intervening spindle and chromosomes contain microtubule bundles, INCENP, and CHO1 but not CENP-E. Mol Biol Cell. 1999;10:297–311. doi: 10.1091/mbc.10.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, Mitchison TJ. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc Natl Acad Sci USA. 1995;92:4289–4293. doi: 10.1073/pnas.92.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight AF, Field CM. Microtubules, membranes and cytokinesis. Curr Biol. 2000;10:R760–R770. doi: 10.1016/s0960-9822(00)00746-6. [DOI] [PubMed] [Google Scholar]
- Swan KA, Severson AF, Carter JC, Martin PR, Schnabel H, Schnabel R, Bowerman B. cyk-1: a C. elegans FH gene required for a late step in embryonic cytokinesis. J Cell Sci. 1998;111:2017–2027. doi: 10.1242/jcs.111.14.2017. [DOI] [PubMed] [Google Scholar]
- Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, Terada Y. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998;58:4811–4816. [PubMed] [Google Scholar]
- Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol. 1999;147:921–928. doi: 10.1083/jcb.147.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Voss AK, Gruss P. Distribution of a murine protein tyrosine phosphatase BL-beta-galactosidase fusion protein suggests a role in neurite outgrowth. Dev Dyn. 1998;212:250–257. doi: 10.1002/(SICI)1097-0177(199806)212:2<250::AID-AJA9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vernos I, Raats J, Hirano T, Heasman J, Karsenti E, Wylie C. Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell. 1995;81:117–127. doi: 10.1016/0092-8674(95)90376-3. [DOI] [PubMed] [Google Scholar]
- Wheatley SP, Wang Y. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BC, Riedy MF, Williams EV, Gatti M, Goldberg ML. The Drosophila kinesin-like protein KLP3A is a midbody component required for central spindle assembly and initiation of cytokinesis. J Cell Biol. 1995;129:709–723. doi: 10.1083/jcb.129.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.