Abstract
The organization of endoplasmic reticulum (ER) was examined in mouse eggs undergoing fertilization and in embryos during the first cell cycle. The ER in meiosis II (MII)-arrested mouse eggs is characterized by accumulations (clusters) that are restricted to the cortex of the vegetal hemisphere of the egg. Monitoring ER structure with DiI18 after egg activation has demonstrated that ER clusters disappear at the completion of meiosis II. The ER clusters can be maintained by inhibiting the decrease in cdk1-cyclin B activity by using the proteasome inhibitor MG132, or by microinjecting excess cyclin B. A role for cdk1-cyclin B in ER organization is further suggested by the finding that the cdk inhibitor roscovitine causes the loss of ER clusters in MII eggs. Cortical clusters are specific to meiosis as they do not return in the first mitotic division; rather, the ER aggregates around the mitotic spindle. Inositol 1,4,5-trisphosphate-induced Ca2+ release is also regulated in a cell cycle-dependent manner where it is increased in MII and in the first mitosis. The cell cycle dependent effects on ER structure and inositol 1,4,5-trisphosphate-induced Ca2+ release have implications for understanding meiotic and mitotic control of ER structure and inheritance, and of the mechanisms regulating mitotic Ca2+ signaling.
INTRODUCTION
In all species that have been studied, fertilization stimulates an increase in the concentration of cytosolic Ca2+ (Stricker, 1999). The increase in Ca2+ is responsible for stimulating cortical granule exocytosis and the resumption of the cell cycle (Kline and Kline, 1992; Swann and Ozil, 1994; Runft et al., 2002). In mammals, the fertilization Ca2+ signal takes the form of a series of Ca2+ transients that continue for ∼4 h, stopping close to the time that the embryo enters interphase of the first mitotic division (Jones et al., 1995). Ca2+ transients are also seen during mitosis, at nuclear envelope breakdown (NEBD) (Steinhardt and Alderton, 1988; Whitaker and Patel, 1990; Tombes et al., 1992; Kono et al., 1996; Day et al., 2000; Carroll, 2001; Whitaker and Larman, 2001) and the metaphase-anaphase transition (Steinhardt, 1990; Groigno and Whitaker, 1998). These meiotic and mitotic Ca2+ transients are stimulated by increases in the production of inositol 1,4,5-trisphosphate (InsP3) (Ciapa et al., 1994) that mobilize Ca2+ through the activation of InsP3 receptors in the endoplasmic reticulum (ER) (Berridge et al., 2000). Thus, the ER serves as the reservoir of Ca2+ that is used for the generation of Ca2+ transients that drive meiosis and mitosis.
The ER is a multifunctional organelle consisting of a network of membranous tubules that extends throughout the cell (Terasaki et al., 1984). The ER membranes contain Ca2+ channels (InsP3 and ryanodine receptors) and Ca2+ pumps for returning Ca2+ to the lumen of the ER, where high-capacity Ca2+-binding proteins are located (Berridge et al., 2000). In maturing oocytes, the ER undergoes changes in organization that are associated with the ability of the oocyte to be successfully fertilized (Campanella et al., 1988; Jaffe and Terasaki, 1994; Mehlmann et al., 1995; Shiraishi et al., 1995; Kume et al., 1997; Stricker et al., 1998; Kline, 2000; Terasaki et al., 2001). The changes in ER organization in hamster, mouse, and Xenopus oocytes consist of the development of cortical clusters of ER and their formation correlates with the ability of the maturing oocyte to generate Ca2+ transients in response to sperm and InsP3 (Kline, 2000). The presence of cortical ER clusters in mammalian oocytes has been proposed to explain the spatial organization of sperm-induced Ca2+ wave (Deguchi et al., 2000) and the reason why the cortex is more sensitive to sperm factors and InsP3 than the center of the egg (Oda et al., 1999). The similar distribution of InsP3 receptors (Insp3Rs) and the ER clusters (Mehlmann et al., 1996; Kume et al., 1997; Kline et al., 1999; Terasaki et al., 2001) further suggests that the ER clusters are specialized sites for the initiation and propagation of Ca2+ waves in oocytes and eggs.
Changes in ER structure also take place after fertilization (Kline, 2000). In starfish and sea urchins, this change consists of a transient Ca2+-induced loss of membrane continuity (Jaffe and Terasaki, 1994; Terasaki et al., 1996). A change in ER structure in the first minutes after egg activation also occurs in Xenopus eggs, although it is not clear whether this involves a loss of continuity, or simply a loss of ER clusters (Terasaki et al., 2001). In ascidians, nemerteans, and mammals there is no obvious change in ER structure immediately after fertilization (Speksnijder et al., 1993; Carroll et al., 1997; Stricker et al., 1998; Kline et al., 1999; Kline, 2000). These species differences in ER organization may be related to the pattern of Ca2+ signaling at fertilization, such that eggs from species that generate single Ca2+ transients at fertilization (sea urchins, starfish, and Xenopus) show ER fragmentation, whereas species that generate multiple transients (ascidians, nemerteans, and mammals) do not (Stricker, 1999; Kline, 2000). The functional significance of this relationship is not clear, but it has been suggested that fragmentation of the ER may inhibit the generation of multiple Ca2+ transients (Kline, 2000).
Although no loss of ER continuity has been reported in species that show multiple Ca2+ transients, changes in ER structure do take place over a longer time course. In ascidians the ER collects in the contraction pole in the vegetal cortex of the egg where it acts as a pacemaker sites for the Ca2+ oscillations at meiosis II (MII) (Speksnijder et al., 1993; Roegiers et al., 1995). In nemertean oocytes, the ER is distributed in clusters throughout the cytoplasm. These clusters have dispersed after ∼40–60 min, around the time that the Ca2+ oscillations stop (Stricker et al., 1998). This has led to the suggestion that the clusters may be necessary for the continuation of sperm-induced Ca2+ transients. In mammals, no changes in ER structure have been detected during the first seven Ca2+ oscillations after fertilization (Kline et al., 1999) but it is not known whether changes in the ER take place over the time course of the Ca2+ transients, and in particular, around the time Ca2+ oscillations stop.
A causal relationship between Ca2+ oscillations and ER clusters remains to be demonstrated, but it is now well established that there is a relationship between Ca2+ release and the state of the cell cycle (Nixon et al., 2000; Carroll, 2001). In ascidians, sperm-induced Ca2+ oscillations are closely related to the activity of cdk1-cyclin B (McDougall and Levasseur, 1998; Levasseur and McDougall, 2000; Nixon et al., 2000). In mammals, the correlation is not so clear. At fertilization of mouse eggs the transients stop close to the time of some 2 h after cdk1-cyclin B activity decreases. However, maintaining meiotic arrest leads to persistent Ca2+ oscillations (Jones et al., 1995). The cessation of Ca2+ oscillations at fertilization may be related to a decrease in the sensitivity of InsP3-induced Ca2+ release that has been detected after pronucleus formation (Jones and Whittingham, 1996; Brind et al., 2000). The mechanisms underlying the cell cycle-dependent changes in sperm and InsP3-induced Ca2+ release are not understood; one possibility is that changes in ER structure after fertilization may be involved. In this study, we investigate the relationship between the organization of the ER, the generation of Ca2+ transients and the activity of the cdk1-cyclin B.
MATERIALS AND METHODS
Oocytes
Mature (MII) oocytes were recovered from 21- to 24-d-old MF1mice previously administered 5 IU human chorionic gonadotrophin (hCG) 48 and 7 IU of pregnant mares serum gonadotrophin at a 48-h interval. Mice were culled by cervical dislocation and the oviducts removed 14–16 h post-hCG. Cumulus masses were released into HEPES-buffered KSOM (H-KSOM) (Lawitts and Biggers, 1993) containing 1 mg/ml bovine serum albumin by rupture of the oviduct with a 27-gauge needle. When it was necessary to remove the cumulus cells, hylauronidase (150 IU ml−1) was added to the H-KSOM. Cumulus-free oocytes were collected and washed in H-KSOM three times and placed in a drop of the same medium under mineral oil.
In Vitro Fertilization and Parthenogenetic Activation
For in vitro fertilization, epididimi were removed from an MF1 male mouse of proven fertility. The epididimi were placed in a 1 ml drop of fertilization media (Cook UK, Herts, United Kingdom), which had been preequilibrated under oil in an incubator at 37°C, 6% CO2 in air. After 20 min the sperm dispersed and 11 μl of the sperm suspension was diluted into a 100-μl drop of the same medium. The dilute sperm suspensions were incubated for 90–120 min to allow the sperm to capacitate after which the cumulus masses were added to the drops and incubated for a further 2 h. The cumulus-free oocytes were collected from the sperm suspension and washed three times in preequilibrated fertilization media. In vitro fertlization was performed 17–18 h after administration of hCG. Parthenogenetic embryos were produced by exposure of MII oocytes (18 h after hCG) to a 7% solution of ethanol in HEPES-KSOM for 7 min at 25% CO2. Cells were subsequently washed repeatedly in ethanol-free media.
Microinjection
Cells were pressure injected with a micropipette and Narishige manipulators mounted on an inverted microscope (Leica, Wetzlar, Germany). Oocytes were placed in a drop of HEPES-KSOM covered with mineral oil. A holding pipette was used to immobilize the oocyte and the injection pipette was pushed through the zona pellucida until it contacted with the oocyte plasma membrane. To penetrate the plasma membrane, a brief overcompensation of negative capacitance was applied. Microinjection was performed using a fixed pressure pulse delivered using a Picopump (WPI, Sarasota, FL) that was set up on any one day to deliver an injection that displaced a sphere of cytoplasm with a diameter of ∼4 μm. This ensured that the size of the oil droplet was similar in all cases. For cyclin injections, we estimated the injections were 8–10 pg of cyclin-GFP. To ensure oocytes and embryos received the same dose of InsP3 they were injected using the same pipette, at the same time, and using the same pulse parameters.
Measurement of Intracellular Ca2+ and Photolysis of Caged InsP3
Intracellular Ca2+ was measured using Fura red. Oocytes were loaded in H-KSOM containing 4 μM acetoxymethyl ester form of Fura red and 0.02 pluronic for 10 min at 37°C. After loading, oocytes were placed in a drop of H-KSOM under oil in a chamber with a coverslip. The chamber was placed in a heated stage on an Axiovert microscope (Zeiss, Welwyn Garden City, United Kingdom). Fura red was excited at 427 and 490 nm by using a monochromator and emission was collected using a 600-nm long-pass filter placed in front of a cooled charge-coupled device camera (MicroMax; Princeton Scientific Instruments, Monmouth Junction, NJ). Changes in intracellular calcium concentration are expressed as the change in ratio of emission collected at 427 nm and 490-nm excitation (Δ427/490).
Caged InsP3 was microinjected as described above to an estimated final concentration of 50 μM. Photorelease was performed 30–60 min after microinjection by brief timed exposures of injected oocytes to UV light (360 nm). To ensure that any comparisons between oocytes and embryos at different times after fertilization were treated similarly, comparisons were made by placing both the treatments being compared on the stage at the same time. Thus, comparisons of the sensitivity of InsP3-induced Ca2+ release were made between groups that were injected with the same pipette, loaded with Fura red, and exposed to UV light at the same time in the same conditions. The excitation wavelengths and camera exposure times were controlled using Metafluor software.
Labeling of Endoplasmic Reticulum
To label the endoplasmic reticulum, DiI18 (Molecular Probes, Eugene, OR) was microinjected as a saturated solution in soybean oil (Sigma Chemical, Poole, Dorset, United Kingdom) 30 min before imaging. Eggs and early embryos were placed on the stage of the microscope so that the first or second polar body was visible in the equatorial plane. This ensured we did not scan for ER in the granule-free domain close to the meiotic spindle. Imaging was performed using a μ-radiance confocal scan head (Bio-Rad, Hemel Hemstead, United Kingdom) mounted on an Axiovert microscope (Zeiss). DiI18 was excited using the 514-nm line of an argon laser and the emitted light collected using a 600-nm long-pass filter. Examination of oocytes was carried out on a heated microscope stage as described above at 37°C.
H1 Kinase Assays
Histone H1 kinase assays were performed to measure mitosis-promoting factor (MPF) activity. The protocol was similar to that described previously (Kubiak et al., 1993; Moos et al., 1995). Five eggs in 2 μl of H-KSOM were transferred in 3 μl of storing solution (10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 mM p-nitrophenyl phosphate, 20 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 5 mM EGTA) and immediately frozen on dry ice. After three thaw-freeze cycles, the samples were diluted twice by the addition of two times concentrated kinase buffer containing 60 μg/ml leupeptin, 60 μg/ml aprotinin, 24 mM p-nitrophenyl phosphate, 90 mM β-glycerophosphate, 4.6 mM sodium orthovanadate, 24 mM EGTA, 24 mM MgCl2, 0.2 mM EDTA, 4 mM NaF, 1.6 mM dithiothreitol, 2 mg/ml polyvinyl alcohol, 40 mM 3-(N-morpholino)propanesulfonic acid, 0.6 mM ATP, 2 mg/ml histone H1 (HIII-S from calf thymus; Sigma Chemical), and 0.25 mCi/ml [32P]ATP. The samples were incubated at 30°C for 30 min and the reaction stopped by adding two times SDS-sample buffer (0.125 M Tris-HCl, 4% SDS, 20% glycerol, 10% mercaptoethanol, 0.002% bromphenol blue) and boiling for 3–5 min. The samples were then analyzed with SDS-PAGE followed by autoradiography. The autoradiographs were imaged using the Fuji Bas-1000 phosphorImager system and analyzed with TINA 2.0 software.
Data Analysis
Analysis of cortical clusters was carried out using MetaMorph imaging software. Clusters were counted in a confocal slice an estimated 5–7 μm from the surface of the oocyte. A cortical cluster was arbitrarily defined as any circular area of 1.5 μm in diameter with a mean pixel intensity of at least 1.5 times that of the entire cortical slice. All t tests were two tailed and based upon two-sample-equal variance. Error bars show the SE.
RESULTS
Reorganization of ER after Fertilization
To ensure that DiI18 labeling of the ER was consistent with previous studies we examined the ER in mature oocytes. Examination of DiI-injected MII oocytes revealed that the ER extended throughout the cytoplasm in a reticular organization (Figure 1A). There was no labeling in the area assumed to be the meiotic spindle (Figure 1A, top). In the cortex, there were accumulations of ER similar to those described previously (Kline, 2000) (Figure 1A, bottom). These cortical accumulations of ER (ER clusters) were typically no more than 2 μm in diameter and were present in 12 of 13 MII oocytes examined. These results confirm previous observations in mouse oocytes that the DiI-labeling technique reports the distribution of ER (reviewed in Kline, 2000). This technique has been extensively characterized in a variety of other cell types and found in all cases to be a faithful reporter of ER (Terasaki et al., 1984, 1994; Terasaki, 1989; Terasaki and Reese, 1992).
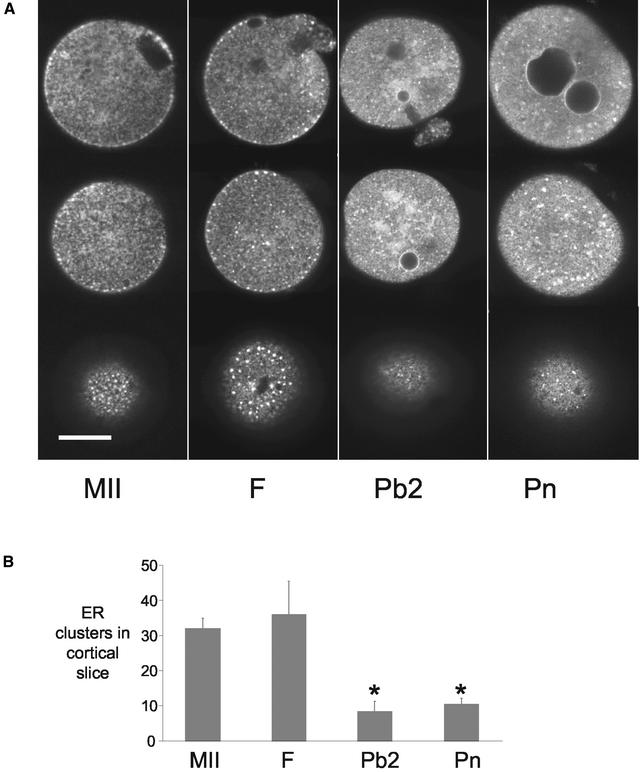
Figure 1.
ER reorganizes around the time of polar body formation. Unfertilized MII-arrested oocytes (MII), fertilized oocytes before polar body formation (F), fertilized oocytes after polar body formation (Pb2), and pronucleate stage (Pn) embryos were injected with DiI and examined using confocal microscopy (A). Equatorial sections (top row) and sections estimated to be 18–20 μm (middle row) and 5–7 μm (bottom row) from the coverslip are displayed. Note the ER clusters in the cortex of MII and F stages and their absence in Pb2 and Pn stages. In the equatorial section of MII oocytes the spindle apparatus can be seen as the ER-free region. Pronuclei at various stages of development are evident in the Pb2 and Pn stages and the ER can be seen to be continuous with the pronuclear membranes. The numbers of cortical clusters were quantified at the different stages of development according to the criteria set out in MATERIALS AND METHODS. (B). Only the cortical slices were used for analysis of ER clusters. There was a significant reduction in the number of cortical clusters in Pb2 and Pn stages. Asterisk indicates (P < 0.01). Data are from at least two different days examining 13 MII, 7 fertilized, 9 Pb2, and 10 Pn stage eggs.
To determine whether the ER organization changes after fertilization we compared the ER staining patterns and counted cortical clusters of ER (see MATERIALS AND METHODS) at different stages of fertilization (Figure 1). Stages were chosen that correspond to the main cell cycle transitions that take place after fertilization of mammalian oocytes; just before polar body extrusion 1–2 h after insemination (F), after extrusion of the second polar body 3–4 h after insemination (Pb2), and after pronucleus formation 6–8 h after fertilization (Pn). The mean number of clusters found in the cortex of the MII oocyte (n = 13) was similar to fertilized oocytes that had not extruded the second polar body (n = 7) (Figure 1). After polar body formation (Pb2) there was a dramatic and significant reduction in the number of ER clusters in the cortex (n = 9; P < 0.001), which remained low at the pronucleate (Pn) stage (n = 10). These data suggest that ER clusters disappear around the time of second polar body formation. Associated with the loss of ER clusters in Pb2 stage oocytes was the appearance of larger areas of fluorescence deeper in the cytoplasm that were not present before Pb2 formation (Figure 1A). Similar accumulations were present in Pn stage embryos. In addition, consistent with the continuity between ER and nuclear membranes, the pronuclear membranes were labeled with DiI18 (Figure 1A, top).
Reorganization of ER after Parthenogenetic Activation
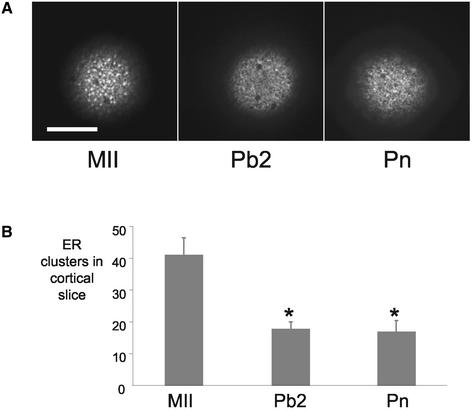
To investigate whether the ER reorganization was specific to fertilization, the ER was stained in parthenogenetic embryos at comparable stages of development. ER was examined in MII oocytes, in activated oocytes after Pb2 extrusion, and after pronucleus formation. We did not examine parthenogenetic embryos before polar body formation because it was not possible to determine which of the oocytes would be stimulated to extrude polar bodies. MII oocytes exhibited ER clusters in the pattern described above (n = 8). Similar to fertilized embryos, parthenogenetic embryos had significantly fewer cortical ER clusters after extrusion of the second polar body (n = 12; P < 0.01) and at the pronucleate stage (n = 13; P < 0.01) (Figure 2). Parthenogenetic and fertilized embryos had a similar distribution of ER at the different stages of development (Figure 2). These data show that the loss of ER clusters is independent of the method of activation and seems to be related to the timing of polar body formation.
Figure 2.
Reorganization of ER occurs independently of sperm and repetitive Ca2+ transients. Figure labels as for Figure 1. Parthenogenetic embryos were produced by incubating MII oocytes in 7% ethanol for 7 min. Representative cortical slices of oocytes at MII, Pb2, and Pn stages are shown in A. The number of cortical clusters is significantly reduced after egg activation (B). The asterisk indicates P < 0.01. The loss of ER clusters is dependent on egg activation and does not require fertilization and associated Ca2+ oscillations. Data are from two experiments on 8, 12, and 13 MII, Pb2 and Pn stages, respectively.
Maintenance of cdk1-Cyclin B Activity Prevents Loss of Cortical Clusters
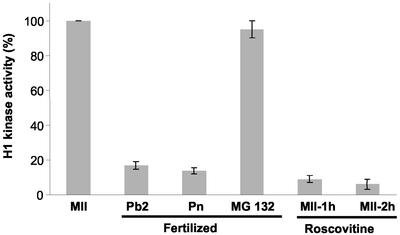
It is well established that the extrusion of the second polar body is a result of a decrease in the activity of cdk1-cyclin B (Verlhac et al., 1994; Schultz and Kopf, 1995). To investigate the relationship between the reorganization of the ER at fertilization and cdk1-cyclin B activity, we have used a number of approaches to manipulate the activity of cdk1-cyclin B during egg activation. First, by using an inhibitor of the proteasome, known to be responsible for cyclin destruction; second, by microinjecting excess cyclin B1-GFP (Levasseur and McDougall, 2000); and, third, by using the cdk1-cyclin B inhibitor roscovitine (Deng and Shen, 2000). In a previous study, we have shown that the proteasome inhibitor MG132 inhibits egg activation but does not affect the ability of the sperm to generate Ca2+ oscillations at fertilization (Brind et al., 2000). Similar results have been obtained in cyclin-GFP–injected eggs (Marangos and Carroll, unpublished data). We have confirmed that the treatments had the predicted effect on cdk1-cyclin B activity by measuring histone H1 kinase activity (Figure 3). The kinase activity in MII eggs was normalized to 100%, against which other groups were compared. As known from previous studies fertilization leads to a decrease in H1 kinase activity at the time of polar body extrusion. Treatment of fertilizing oocytes with MG132 (50 μM) inhibited egg activation and maintained H1 kinase activity to the levels found in MII oocytes (Figure 3). Roscovitine treatment (75 μM) for 1 or 2 h inhibited H1 kinase activity to levels similar to that of fertilized embryos (Figure 3).
Figure 3.
H1 kinase activity after fertilization, and treatment with MG132 or roscovitine. The presence of ER clusters in relation to cdk1-cyclin B activity, as measured by histone H1 kinase activity, requires manipulation of cdk1-cyclin B activity. The H1 kinase activity in MII oocytes has been set to 100% against which other treatments are compared. Fertilized one-cells at the Pb2 stage and Pn stage have reduced levels of H1 kinase activity, consistent with previous observations. Fertilized embryos treated with MG132 (50 μM) maintain an elevated level of H1 kinase activity. The cdk inhibitor roscovitine (75 μM) inhibits histone H1 kinase activity after incubation for 1 and 2 h. Data are from two experiments with two replicates on each day.
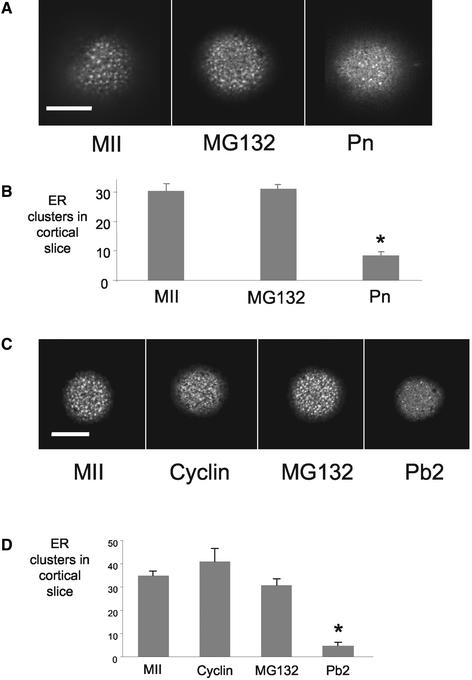
In the first series of experiments the effects of MG132 on the reorganization of ER at fertilization were examined. Oocytes were incubated in MG132 for 30 min before fertilization and then during fertilization to inhibit proteasome-mediated destruction of cyclin B. After 7 h, when all the control oocytes had formed pronuclei, DiI was injected into MG132-treated and unfertilized and fertilized controls (Figure 4A). The numbers of cortical clusters in unfertilized oocytes and pronucleate stage embryos was similar to that described above (n = 10 and 12, respectively; Figure 4B). Treatment with MG132 to maintain cdk1-cyclin B activity prevented the loss of cortical ER clusters (n = 12; Figure 4B). MG132-treated embryos showed similar numbers of ER clusters because unfertilized controls and significantly more than the pronucleate stage embryos (P < 0.01) (Figure 4B).
Figure 4.
Maintenance of M phase leads to persistent ER clusters. Confocal sections through the cortex of MII oocytes and fertilized oocytes treated with MG132 (A and C) or parthenogenetically activated oocytes injected with GFP-cyclin (C) are shown. Note that in the presence of MG132 and in cyclin-injected oocytes, ER clusters persist compared with fertilized control oocytes (A and C). This is confirmed in the quantification of ER clusters (B and D). See Figure 3 for H1 kinase assays after fertilization and treatment with MG132. Numbers of eggs examined for each treatment were MII, 6; GFP-cyclin, 7; MG132, 12; and Pb2, 10.
In the second series of experiments, oocytes were treated with MG132 as described above or were microinjected with 8–10 pg of cyclin B1-GFP before activation with ethanol. The numbers of cortical clusters were examined after 4 h when the controls had extruded second polar bodies. Maintenance of cdk1-cyclin B activity by using both approaches prevented the decrease in cortical clusters seen after parthenogenetic activation (Figure 4, C and D). The MG132-treated oocytes and cyclin-GFP-injected oocytes did not extrude polar bodies after exposure to ethanol (Figure 4, C and D). Together, these studies demonstrate that a decrease in cdk1-cyclin B activity is necessary for the loss of cortical ER clusters after egg activation.
Inhibition of cdk1-Cyclin B Activity Causes Premature Loss of ER Clusters in Unfertilized Eggs
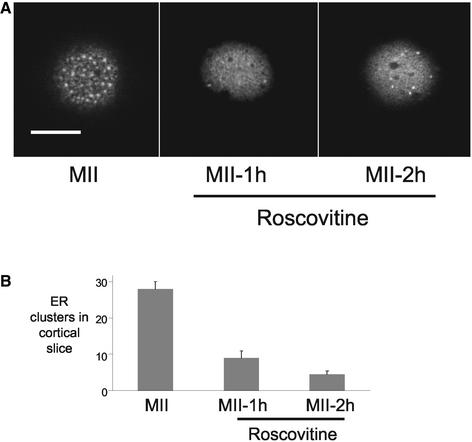
The experiments described above show that high cdk1-cyclin B activity is sufficient to maintain the presence of ER clusters at fertilization. To further investigate the relationship between MPF activity and ER reorganization, we have used an inhibitor of cdk1-cyclin B activity (Meijer et al., 1997). Unfertilized eggs were incubated in 75 μM roscovitine (as described above) and scored for signs of egg activation. After 1 h, none of the eggs had extruded a polar body but after 2 h, 7 of 11 eggs had undergone parthenogenetic activation as indicated by the presence of the second polar body. Roscovitine treatment had a dramatic effect on ER clusters, significantly reducing the number of cortical ER clusters at both 1-h (n = 9) and 2-h (n = 15) time points compared with controls (n = 10; P < 0.01) (Figure 5).
Figure 5.
Inhibition of cdk1-cyclin B activity leads to the loss of ER clusters. Confocal cortical slices of MII oocytes and of oocytes treated with roscovitine for 1 or 2 h are shown (A). Inhibition of cdk1-cyclin B activity causes the loss of ER clusters in the cortex of MII oocytes. (B) Effects of roscovitine on cdk1-cyclin B activity, as determined by H1 kinase assays, are shown in Figure 3. The numbers of eggs examined were 9, 15 and 10 for 1 h in roscovitine, and 2 h in roscovitine and controls, respectively
Cortical ER Clusters Do Not Return at Mitosis of First Mitotic Division
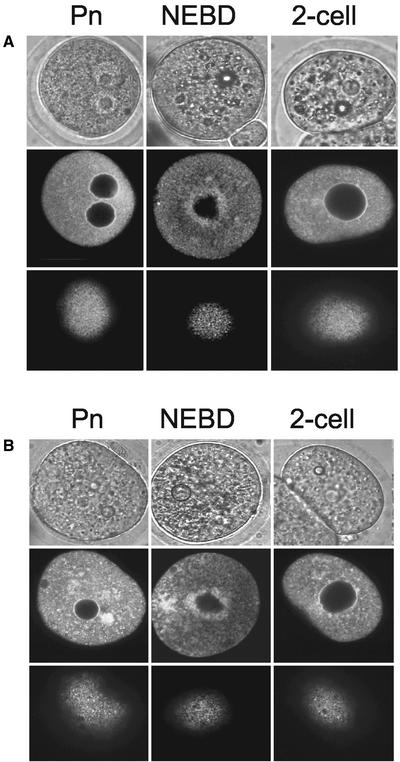
The relationship between ER clusters and cdk1-cyclin B activity during meiosis II raises the question of whether the clusters return when cdk1-cyclin B activity returns during mitosis of the first mitotic division. To examine ER structure during mitosis, fertilized (Figure 6A) and parthenogenetic embryos (Figure 6B) were injected with DiI ∼2–3 h before the expected time of NEBD, just after NEBD and after cleavage to the two-cell stage. A cortical (bottom row) and an equatorial slice (middle row) are displayed with a bright field image (top row) of each stage of mitosis (Figure 6). A number of differences were observed between ER organization in oocytes in meiosis II and in embryos at the first mitosis. First, no cortical clusters of ER were detected in embryos at any stage of the cell cycle (Figure 6, A and B, bottom row). Second, in mitotic one-cell embryos that had undergone NEBD, there was an accumulation of ER around the mitotic spindle in the center of the embryo (Figure 6, A and B, middle row). Thus, the presence of cortical ER clusters is specific to M phase of meiosis II.
Figure 6.
Cortical ER clusters are not present in mitotic one-cell embryos. Fertilized (A) and parthenogenetic (B) one-cell embryos were injected with DiI at different times of the first mitotic division. These stages were the Pn stage 2–4 h before the expected time of NEBD, in mitosis after NEBD, and after cleavage to the two-cell stage. Bright field (top row), confocal equatorial (middle row), and cortical (bottom row) sections are illustrated. Note that in the cortical sections there is no evidence of ER clusters. Pn and two-cell stages show a typical interphase ER organization with a diffuse cytoplasmic network that is continuous with the nuclear envelope. In the NEBD stage embryos there is an accumulation of ER around the mitotic spindle. Data are from at least seven embryos at each stage.
Sensitivity of InsP3-induced Ca2+ Release during Exit from Meiosis II
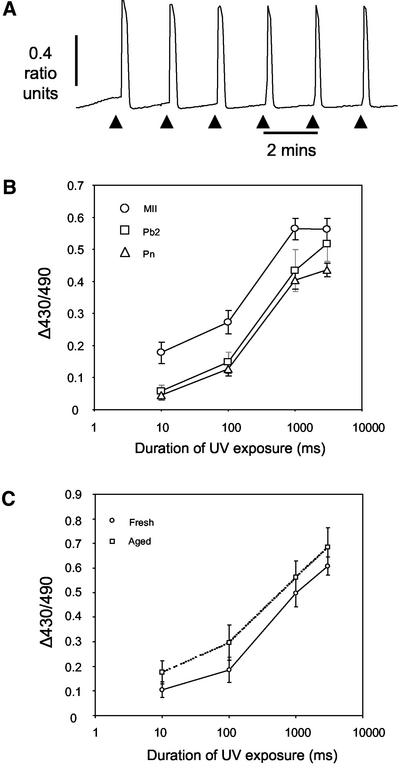
The finding that ER clusters disappear around the time of polar body formation suggests they are not necessary for fertilization-induced Ca2+ oscillations. To examine more closely a possible relationship between ER organization and the sensitivity of Ca2+ release we have examined the sensitivity of InsP3-induced Ca2+ release by photoreleasing caged InsP3 (cInsP3) at different times after parthenogenetic activation. It is known that there is a dramatic loss in the sensitivity of InsP3-induced Ca2+ release by the time the fertilized egg has formed pronuclei but it is not known when in the period from the initiation of egg activation to pronucleus formation this takes place. We have photoreleased InsP3 in MII oocytes, in activated eggs at the Pb2 or Pn stages, while monitoring intracellular Ca2+ with Fura red. To treat these different stages simultaneously we staggered the timing of the pregnant mares serum gonadotrophin and hCG so that cInsP3 could be released in activated eggs and oocytes simultaneously. A concentration-response relationship was established at each stage of development by using a series of exposures of UV light. We first verified that the cInsP3 (∼50 μM) provided a reservoir of InsP3 that was not significantly depleted by repeated photolysis events (Callamaras and Parker, 1994; Jones and Nixon, 2000). This was confirmed by carrying out a sequence of six consecutive 1000-ms exposures at 2-min intervals in MII eggs (Figure 7A). No significant difference in peak change in Fura red ratio is observed between the first (0.48 ± 0.025) and sixth transient (0.47 ± 0.026; n = 10; P > 0.7), suggesting that repeated photorelease in these conditions does not limit the availability of cInsP3.
Figure 7.
InsP3-induced Ca2+ release is reduced in oocytes that have extruded the second polar body. Oocytes were injected with 50 μM cInsP3 and loaded with Fura red to monitor Ca2+ in response to a series of InsP3 concentrations. Injection of 50 μM cInsP3 provides a reservoir of InsP3 that is not significantly depleted by repeated photolysis events of 1000 ms every 2 min (A). InsP3 was photoreleased in MII oocytes and ethanol-activated parthenogenetic embryos at the Pb2 and Pn stages. The amount of InsP3 released, as measured by the change in Fura red ratio, was controlled by changing the duration of UV light from 10 to 3000 ms. MII oocytes release more Ca2+ for a given dose of InsP3 than those that have been activated (B). Release of Ca2+ in aged and fresh oocytes is similar, suggesting time from ovulation does not unduly affect the amount of Ca2+ released (C). Difference in amount of Ca2+ released in response to InsP3 was not due to oocyte aging.
Intracellular Ca2+ concentration was monitored using Fura red in cInsP3-injected oocytes during exposure to UV light for 10, 100, 1000, and 3000 ms. The mean change in Fura red ratio was significantly greater in MII oocytes than Pn stage embryos at all four exposure times tested (P < 0.01) (Figure 7B). In comparison with eggs that had extruded the second polar body, a similar decrease in the peak Fura red ratio was seen in response to 10- and 100-ms exposures (P < 0.05), whereas no difference was detected at the highest exposure of 3000 ms (Figure 7B). Comparing the increases in Ca2+ seen in embryos at the Pb2 stage and the Pn stage reveals that similar amounts of Ca2+ are released at both stages for all amounts of InsP3 tested. Finally, to verify that the observed changes in Ca2+ release were due to activation of the oocyte, and not attributable to oocyte aging, we photoreleased cInsP3 in oocytes 18 h post-hCG and 24 h after hCG. No significant difference in Ca2+ release was seen between fresh and aged oocytes (Figure 7C). Thus, there is a decrease in the ability to release Ca2+ in response to InsP3 in embryos from as early as polar body formation; the time that cdk1-cyclin B activity decreases and ER clusters disappear.
Sensitivity of InsP3-induced Ca2+ Release during First Mitotic Cell Cycle
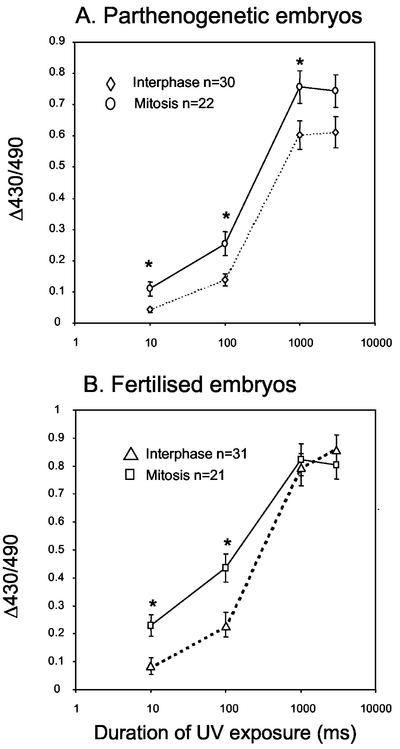
The loss of InsP3R sensitivity correlates with the loss of ER clusters and the decrease in cdk1-cyclin B activity. We have shown that in mitosis the cortical ER clusters do not return despite the presence of cdk1-cyclin B activity. We can therefore test the hypothesis that the increased sensitivity of Ca2+ release before polar body formation is a result of the presence of cortical ER clusters. If embryos undergoing mitosis also show an increase in Ca2+ release then the ER clusters in the cortex may not be solely responsible. To investigate this hypothesis we have photoreleased InsP3 (as described above) in fertilized and parthenogenetic embryos in interphase and in mitosis. For both fertilized and parthenogenetic embryos, experiments were designed by setting up two sets of activation or in vitro fertilization so as to obtain interphase and mitotic embryos at the same time. This design allowed photorelease of cInsP3 at both stages of the cell cycle under the same conditions and at the same time, thereby minimizing variation in batches of cInsP3, injection pipettes and UV treatment. For interphase embryos, photorelease of InsP3 was at 12–13 h and 17–18 h after exposure to ethanol or sperm, respectively. Mitotic embryos were treated when 50% had undergone NEBD at 17–18 h and 21–22 h after exposure to ethanol and sperm, respectively. The results show that in parthenogenetic embryos significantly more Ca2+ is released at the low exposures (10 and 100 ms) by mitotic embryos (n = 22) compared with embryos in interphase (n = 30; P < 0.01; Figure 8A). At higher levels of InsP3 (1000 and 30000 ms) similar increases in Ca2+ were seen (Figure 8A). A comparable response was seen in fertilized embryos. For UV exposures of 10, 100, and 1000 ms, mitotic (post-NEBD) embryos (n = 21) released significantly more Ca2+ than those in interphase (n = 31; P < 0.01; Figure 8B). These data show that InsP3-induced Ca2+ release is increased in mitotic embryos and that cortical clusters of ER are not necessary for the increase.
Figure 8.
InsP3-induced Ca2+ release increases during mitosis. Interphase and mitotic parthenogenetic (A) and fertilized (B) embryos were injected with cInsP3 and loaded with Fura red. In response to InsP3, mitotic embryos release significantly more Ca2+ than those in interphase (A and B). Significant differences are indicated by the asterisk (P < 0.01). Note the difference at low InsP3 levels seems greater in fertilized compared with parthenogenetic embryos.
DISCUSSION
ER Organization Is Cell Cycle Dependent
We have examined the ER after fertilization in mouse oocytes and discovered that it undergoes a reorganization that is dependent upon a decrease in the activity of cdk1-cyclin B. Our data confirm previous observations that in MII eggs, ER is characterized by the presence of clusters of 1–2 μm in diameter. We provide several lines of evidence that demonstrate that the presence of cortical clusters of ER seen in mammalian oocytes requires cdk1-cyclin B activity. First, the clusters disperse around the time that cdk1-cyclin B decreases when the second polar body is extruded. Second, the ER clusters persist if cdk1-cyclin B activity is maintained with the proteasome inhibitor MG132 or by injection of GFP-cyclin. Third, inhibition of cdk1-cyclin B activity with roscovitine, leads to the loss of ER clusters. In mitosis of the first mitotic division we found no evidence of cortical clusters of ER, rather the ER envelops the mitotic spindle. These DiI experiments show that aggregates of ER exist in both meiosis and mitosis but they take very different forms: cortical clusters and spindle accumulations, respectively. These data raise three main issues for discussion. First, the role of cdk1-cyclin B in regulating ER organization; second, the mechanisms underlying the different structures of ER in meiosis and mitosis; and third, the functional significance of the ER reorganization during meiosis and mitosis.
Cdk1-Cyclin B Activity Regulates ER Organization
We have demonstrated that cdk1-cyclin B activity regulates ER organization in meiosis II. In MII there are two main kinase activities that control cell cycle progression, cdk1-cyclin B and mitogen-activated protein (MAP) kinase (Verlhac et al., 1994; Schultz and Kopf, 1995; Moos et al., 1996). The timing of the disappearance of ER clusters after fertilization correlates with the decrease in cdk1-cyclin B activity at polar body formation rather than MAP kinase activity 2 h later when the pronuclei form. This observation, together with the observations described above demonstrates that cdk1-cyclin B activity (rather than MAP kinase) is necessary for the maintenance of cortical ER clusters in meiosis II. A contribution of MAP kinase to the formation of the clusters during oocyte maturation remains to be investigated. ER clusters also disappear after fertilization of eggs from Xenopus and nemerteans (Stricker et al., 1998; Terasaki et al., 2001). The role of cell cycle kinase activities and ER reorganization has not been examined in these species but the timing of the loss of clusters is consistent with a role for cdk1-cyclin B. In Xenopus, the clusters disappear in the first minutes after a Ca2+ wave (Terasaki et al., 2001), similar to the timing of MPF destruction (Beckhelling et al., 2000), and in nemerteans the clusters have disappeared by the time the second meiotic division is complete (Stricker et al., 1998), an indication that MPF has declined. In addition to the loss of ER clusters after fertilization, there has been some suggestion from previous studies that the formation of ER clusters during oocyte maturation is related to cdk1-cyclin B activity. In Xenopus oocytes, clusters first appear around the time of NEBD, decrease in number between MI and MII, before increasing again in MII (Terasaki et al., 2001). This tracks the changes in cdk1-cyclin B activity that take place during maturation. Further direct experiments are required to test whether the role of cdk1-cyclin B in ER organization is universal; early indications suggest it may be, at least in eggs that are fertilized at MI or MII.
The mechanism of cdk1-cyclin B-induced changes in ER organization is not known. It is well known that cdk1-cyclin B2, and to a lesser extent, cdk1-cyclin B1, are membrane associated, which puts the cdk1 activity in the appropriate compartment to directly affect ER organization (Draviam et al., 2001). Cdk1-dependent effects on endomembrane systems have been well characterized (Warren, 1993; Bergeland et al., 2001), the Golgi complex in particular (Shima et al., 1997; Lowe et al., 2000). Cdk1-dependent phosphorylation of the Golgi protein GM130 in the face of continuous budding leads to the fragmentation of the entire organelle (Lowe et al., 1998). Further work will be needed to determine whether cdk1 directly regulates specific ER proteins that lead to the changes in ER organization in meiosis and mitosis.
ER Behaves Differently in Meiosis and Mitosis
The formation of ER clusters in the cortex and a relatively ER-free spindle apparatus in meiosis II differs markedly from the lack of ER in the cortex and the spindle associated ER typical of mitosis. The spindle associated ER in mitosis is seen in most mitotic cells, including embryos and somatic cells (Terasaki et al., 1984; Terasaki, 2000). This organization of ER in mitotic cells indicates that the meiotic M phase uses additional mechanisms to regulate ER structure. One of the major differences between mitosis and meiosis II is that the spindles are located centrally and cortically, respectively. It may be that cortical ER clusters arise during meiosis because the cortical localization of the spindle allows the dispersal of ER from the spindle apparatus, possibly through interactions with the cortical cytoskeleton. Experiments manipulating the position of the spindle in meiosis II and mitosis will help to clarify this point.
There are a number of reasons why female meiosis may benefit from the dispersal of ER from the MII spindle to the cortex. First, it isolates the meiotic spindle from the source of intracellular Ca2+ that would otherwise provide a threat to a stable MII arrest and potentially lead to inappropriate stimulation of the metaphase to anaphase transition (Groigno and Whitaker, 1998). Second, it places the source of Ca2+ in the cortex, the site of sperm-egg fusion. This may prove important in the ability of the fertilized oocyte to generate Ca2+ release in response to limited amount of phospholipase C ζ introduced by the fertilizing sperm (Saunders et al., 2002). Third, the highly unequal nature of cell division in female meiosis would result in the polar body inheriting a significant proportion of the ER. Dispersing the ER before the meiotic divisions provides a means of retaining the ER for roles in Ca2+ release and egg activation (Kline, 2000).
ER Organization and Ca2+ Release: A Functional Link?
The obvious reason for specialized ER organization in MII mouse oocytes is that the cortical clusters of ER act as pacemaker sites for the generation of Ca2+ oscillations at fertilization (Dumollard et al., 2002). The cortex is more sensitive to InsP3 and Ca2+-releasing sperm extracts (Oda et al., 1999) and it is the vegetal cortex that acts as the Ca2+ wave pacemaker at fertilization (Deguchi et al., 2000; Dumollard et al., 2002), even after fertilization near the spindle (Kline et al., 1999). The localization of InsP3Rs to the ER clusters further suggests an important role in regulating the initiation of Ca2+ release (Kline et al., 1999; Terasaki et al., 2001). A recent mathematical model has demonstrated that the clustering of InsP3Rs increases the sensitivity of Ca2+ release such that coherent signals can be generated in response to low levels of stimuli, that otherwise would not elicit a response (Shuai and Jung, 2002). This may be particularly pertinent at fertilization in mammalian eggs where low InsP3 concentrations have been predicted (Jones and Nixon, 2000; Halet et al., 2002) and where the signaling pathway seems to involve the introduction of a phospholipase C from a very small cell (the sperm) into a very large cell (the egg) (Saunders et al., 2002). These observations suggest the cortical ER clusters play an important role in the initiation and spatial organization of Ca2+ signaling at fertilization. A similarly important role in the temporal organization of Ca2+ signaling is not as clear.
A relationship has been noted between the occurrence of ER reorganization at fertilization and the generation of long-lasting Ca2+ oscillations (Stricker, 1999; Kline, 2000). Previous studies have shown that species that show repetitive oscillations (ascidians, mouse, and nemerteans) do not undergo early changes in ER organization (Speksnijder et al., 1993; Stricker et al., 1998; Kline et al., 1999), whereas those that generate a single transient (starfish, sea urchins, and Xenopus) undergo a dramatic reorganization that in some cases involves ER fragmentation (Terasaki et al., 1996; Terasaki et al., 2001). A reasonable conclusion from this data is that reorganization of the ER may lead to the cessation of Ca2+ transients (Kline, 2000). However, our data show that such a correlation does not hold for mice where the ER reorganizes and clusters disperse ∼2 h before Ca2+ oscillations stop (Jones et al., 1995; Day et al., 2000). The continuation of Ca2+ oscillations in the absence of cortical ER clusters shows that the clusters are not critical for maintaining fertilization-induced Ca2+ oscillations.
It remains possible that the loss of ER clusters in mouse oocytes have more subtle affects on the Ca2+ oscillations, such as a decrease in frequency or rise time. A subtle effect on Ca2+ signaling that is insufficient to inhibit fertilization-induced Ca2+ oscillations is suggested by our experiments using caged InsP3. These experiments reveal that that InsP3-induced Ca2+ release is greater in MII oocytes compared with oocytes that have extruded the second polar body and do not have cortical ER clusters. Previously, it was thought that the decrease in sensitivity was associated with pronucleus formation or oocyte aging (Jones and Whittingham, 1996). The data described herein show that oocytes that have extruded a polar body have the same ability to generate Ca2+ transients in response to InsP3 as those that have formed pronuclei and entered interphase. It is not known precisely when after egg activation the sensitivity to InsP3 decreases but our data show that it has decreased by the time the ER clusters disperse. A causal link remains to be established and it may be that other mechanisms involving InsP3R down-regulation (Brind et al., 2000) or cell cycle-dependent changes in Ca2+ homeostasis also play a role.
Cell Cycle-dependent Changes in InsP3-induced Ca2+ Release
A cell cycle-dependent effect on Ca2+ release is suggested by the finding that InsP3-mediated Ca2+ release is increased in mitotic compared with interphase one-cell embryos. This result demonstrates that cortical ER clusters are not necessary for an increase in Ca2+ release. However, it is possible that ER accumulation around the spindle (rather than in the cortex) acts as a pacemaker and underlies the increased Ca2+ release in mitosis. In this case, we propose that the M-phase–specific ER organization is important in regulating Ca2+ release and that it is simply the location of the ER that is different in meiosis and mitosis. Alternatively, other ER-independent mechanisms may be at play to sensitize Ca2+ release during meiosis and mitosis. A number of components of the Ca2+ homeostatic machinery are regulated in a cell cycle-dependent manner (Machaca and Haun, 2002). Determining whether the increased InsP3-induced Ca2+ release in mitosis is a direct result of ER reorganization or other mechanisms will require manipulation of ER structure independently of the cell cycle.
In conclusion, we have demonstrated that ER organization and InsP3-induced Ca2+ release are regulated in a cell cycle-dependent manner. The reorganization of the ER does not lead to dramatic changes in sperm-induced Ca2+ oscillations but is associated with a decrease in sensitivity of InsP3-induced Ca2+ release. These results will have implications for understanding of meiotic and mitotic organization and inheritance of the endoplasmic reticulum and for the regulation of intracellular Ca2+ during mitosis.
ACKNOWLEDGMENTS
We thank Jon Pines for the gift the GFP-cyclin B1; Mark Larman, Karl Swann, and Remi Dumollard for comments and helpful discussions during the course of this work; and Charles Rodeck and Paul Serhal for support and encouragement. This research was supported by a Medical Research Council Career Establishment Grant (to J.C.). G.F. is supported by a Reproductive Medicine Studentship from the Department of Obstetrics and Gynaecology and the Assisted Conception Unit (University College London).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–07–0431. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–07–0431.
REFERENCES
- Beckhelling C, Perez-Mongiovi D, Houliston E. Localized MPF regulation in eggs. Biol Cell. 2000;92:245–253. doi: 10.1016/s0248-4900(00)01067-4. [DOI] [PubMed] [Google Scholar]
- Bergeland T, Widerberg J, Bakke O, Nordeng TW. Mitotic partitioning of endosomes and lysosomes. Curr Biol. 2001;11:644–651. doi: 10.1016/s0960-9822(01)00177-4. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Brind S, Swann K, Carroll J. Inositol 1,4,5-trisphosphate receptors are downregulated in mouse oocytes in response to sperm or adenophostin A but not to increases in intracellular Ca(2+) or egg activation. Dev Biol. 2000;223:251–265. doi: 10.1006/dbio.2000.9728. [DOI] [PubMed] [Google Scholar]
- Callamaras N, Parker I. Inositol 1,4,5-trisphosphate receptors in Xenopus laevis oocytes: localization and modulation by Ca2+ Cell Calcium. 1994;15:66–78. doi: 10.1016/0143-4160(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Campanella C, Talevi R, Kline D, Nuccitelli R. The cortical reaction in the egg of Discoglossus pictus: a study of the changes in the endoplasmic reticulum at activation. Dev Biol. 1988;130:108–119. doi: 10.1016/0012-1606(88)90418-6. [DOI] [PubMed] [Google Scholar]
- Carroll J. The initiation and regulation of Ca2+ signaling at fertilization in mammals. Semin Cell Dev Biol. 2001;12:37–43. doi: 10.1006/scdb.2000.0215. [DOI] [PubMed] [Google Scholar]
- Ciapa B, Pesando D, Wilding M, Whitaker M. Cell-cycle calcium transients driven by cyclic changes in inositol trisphosphate levels. Nature. 1994;368:875–878. doi: 10.1038/368875a0. [DOI] [PubMed] [Google Scholar]
- Day ML, McGuinness OM, Berridge MJ, Johnson MH. Regulation of fertilization-induced Ca(2+)spiking in the mouse zygote. Cell Calcium. 2000;28:47–54. doi: 10.1054/ceca.2000.0128. [DOI] [PubMed] [Google Scholar]
- Deguchi R, Shirakawa H, Oda S, Mohri T, Miyazaki S. Spatiotemporal analysis of Ca(2+) waves in relation to the sperm entry site and animal-vegetal axis during Ca(2+) oscillations in fertilized mouse eggs. Dev Biol. 2000;218:299–313. doi: 10.1006/dbio.1999.9573. [DOI] [PubMed] [Google Scholar]
- Deng MQ, Shen SS. A specific inhibitor of p34(cdc2)/cyclin B suppresses fertilization-induced calcium oscillations in mouse eggs. Biol Reprod. 2000;62:873–878. doi: 10.1095/biolreprod62.4.873. [DOI] [PubMed] [Google Scholar]
- Draviam VM, Orrechia S, Lowe M, Pardi R, Pines J. The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. J Cell Biol. 2001;152:945–958. doi: 10.1083/jcb.152.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumollard R, Carroll J, Dupont G, Sardet C. Calcium wave pacemakers in eggs. J Cell Sci. 2002;115:3557–3564. doi: 10.1242/jcs.00056. [DOI] [PubMed] [Google Scholar]
- Groigno L, Whitaker M. An anaphase calcium signal controls chromosome disjunction in early sea urchin embryos. Cell. 1998;92:193–204. doi: 10.1016/s0092-8674(00)80914-9. [DOI] [PubMed] [Google Scholar]
- Halet G, Tunwell R, Balla T, Swann K, Carroll J. The dynamics of plasma membrane PtdIns(4,5)P(2) at fertilization of mouse eggs. J Cell Sci. 2002;115:2139–2149. doi: 10.1242/jcs.115.10.2139. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Terasaki M. Structural changes in the endoplasmic reticulum of starfish oocytes during meiotic maturation and fertilization. Dev Biol. 1994;164:579–587. doi: 10.1006/dbio.1994.1225. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Merriman JA, Whittingham DG, Kono T. Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development. 1995;121:3259–3266. doi: 10.1242/dev.121.10.3259. [DOI] [PubMed] [Google Scholar]
- Jones KT, Nixon VL. Sperm-induced Ca(2+) oscillations in mouse oocytes and eggs can be mimicked by photolysis of caged inositol 1,4,5-trisphosphate: evidence to support a continuous low level production of inositol 1,4,5-trisphosphate during mammalian fertilization. Dev Biol. 2000;225:1–12. doi: 10.1006/dbio.2000.9826. [DOI] [PubMed] [Google Scholar]
- Jones KT, Whittingham DG. A comparison of sperm- and IP3-induced Ca2+ release in activated and aging mouse oocytes. Dev Biol. 1996;178:229–237. doi: 10.1006/dbio.1996.0214. [DOI] [PubMed] [Google Scholar]
- Kline D. Attributes and dynamics of the endoplasmic reticulum in mammalian eggs. Curr Top Dev Biol. 2000;50:125–154. doi: 10.1016/s0070-2153(00)50007-6. [DOI] [PubMed] [Google Scholar]
- Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992;149:80–89. doi: 10.1016/0012-1606(92)90265-i. [DOI] [PubMed] [Google Scholar]
- Kline D, Mehlmann L, Fox C, Terasaki M. The cortical endoplasmic reticulum (ER) of the mouse egg: localization of ER clusters in relation to the generation of repetitive calcium waves. Dev Biol. 1999;215:431–442. doi: 10.1006/dbio.1999.9445. [DOI] [PubMed] [Google Scholar]
- Kono T, Jones KT, Bos-Mikich A, Whittingham DG, Carroll J. A cell cycle-associated change in Ca2+ releasing activity leads to the generation of Ca2+ transients in mouse embryos during the first mitotic division. J Cell Biol. 1996;132:915–923. doi: 10.1083/jcb.132.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak JZ, Weber M, de Pennart H, Winston NJ, Maro B. The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. EMBO J. 1993;12:3773–3778. doi: 10.1002/j.1460-2075.1993.tb06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S, Yamamoto A, Inoue T, Muto A, Okano H, Mikoshiba K. Developmental expression of the inositol 1,4,5-trisphosphate receptor and structural changes in the endoplasmic reticulum during oogenesis and meiotic maturation of Xenopus laevis. Dev Biol. 1997;182:228–239. doi: 10.1006/dbio.1996.8479. [DOI] [PubMed] [Google Scholar]
- Lawitts JA, Biggers JD. Culture of preimplantation embryos. Methods Enzymol. 1993;225:153–164. doi: 10.1016/0076-6879(93)25012-q. [DOI] [PubMed] [Google Scholar]
- Levasseur M, McDougall A. Sperm-induced calcium oscillations at fertilisation in ascidians are controlled by cyclin B1-dependent kinase activity. Development. 2000;127:631–641. doi: 10.1242/dev.127.3.631. [DOI] [PubMed] [Google Scholar]
- Lowe M, Gonatas NK, Warren G. The mitotic phosphorylation cycle of the cis-Golgi matrix protein GM130. J Cell Biol. 2000;149:341–356. doi: 10.1083/jcb.149.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M, Nakamura N, Warren G. Golgi division and membrane traffic. Trends Cell Biol. 1998;8:40–44. doi: 10.1016/s0962-8924(97)01189-6. [DOI] [PubMed] [Google Scholar]
- Machaca K, Haun S. Induction of maturation-promoting factor during Xenopus oocyte maturation uncouples Ca(2+) store depletion from store-operated Ca(2+) entry. J Cell Biol. 2002;156:75–85. doi: 10.1083/jcb.200110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall A, Levasseur M. Sperm-triggered calcium oscillations during meiosis in ascidian oocytes first pause, restart, then stop: correlations with cell cycle kinase activity. Development. 1998;125:4451–4459. doi: 10.1242/dev.125.22.4451. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol. 1996;180:489–498. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Terasaki M, Jaffe LA, Kline D. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev Biol. 1995;170:607–615. doi: 10.1006/dbio.1995.1240. [DOI] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Moos J, Visconti PE, Moore GD, Schultz RM, Kopf GS. Potential role of mitogen-activated protein kinase in pronuclear envelope assembly and disassembly following fertilization of mouse eggs. Biol Reprod. 1995;53:692–699. doi: 10.1095/biolreprod53.3.692. [DOI] [PubMed] [Google Scholar]
- Moos J, Xu Z, Schultz RM, Kopf GS. Regulation of nuclear envelope assembly/disassembly by MAP kinase. Dev Biol. 1996;175:358–361. doi: 10.1006/dbio.1996.0121. [DOI] [PubMed] [Google Scholar]
- Nixon VL, McDougall A, Jones KT. Ca2+ oscillations and the cell cycle at fertilisation of mammalian and ascidian eggs. Biol Cell. 2000;92:187–196. doi: 10.1016/s0248-4900(00)01068-6. [DOI] [PubMed] [Google Scholar]
- Oda S, Deguchi R, Mohri T, Shikano T, Nakanishi S, Miyazaki S. Spatiotemporal dynamics of the [Ca2+]i rise induced by microinjection of sperm extract into mouse eggs: preferential induction of a Ca2+ wave from the cortex mediated by the inositol 1,4,5-trisphosphate receptor. Dev Biol. 1999;209:172–185. doi: 10.1006/dbio.1999.9233. [DOI] [PubMed] [Google Scholar]
- Roegiers F, McDougall A, Sardet C. The sperm entry point defines the orientation of the calcium-induced contraction wave that directs the first phase of cytoplasmic reorganization in the ascidian egg. Development. 1995;121:3457–3466. doi: 10.1242/dev.121.10.3457. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLCzeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. doi: 10.1016/s0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- Shima DT, Haldar K, Pepperkok R, Watson R, Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi K, Okada A, Shirakawa H, Nakanishi S, Mikoshiba K, Miyazaki S. Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5-trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes. Dev Biol. 1995;170:594–606. doi: 10.1006/dbio.1995.1239. [DOI] [PubMed] [Google Scholar]
- Shuai JW, Jung P. Optimal intracellular calcium signaling. Physiol Rev Lett. 2002;88:068102. doi: 10.1103/PhysRevLett.88.068102. [DOI] [PubMed] [Google Scholar]
- Speksnijder JE, Terasaki M, Hage WJ, Jaffe LF, Sardet C. Polarity and reorganization of the endoplasmic reticulum during fertilization and ooplasmic segregation in the ascidian egg. J Cell Biol. 1993;120:1337–1346. doi: 10.1083/jcb.120.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA. Intracellular free calcium and the first cell cycle of the sea-urchin embryo (Lytechinus pictus) J Reprod Fertil Suppl. 1990;42:191–197. [PubMed] [Google Scholar]
- Steinhardt RA, Alderton J. Intracellular free calcium rise triggers nuclear envelope breakdown in the sea urchin embryo. Nature. 1988;332:364–366. doi: 10.1038/332364a0. [DOI] [PubMed] [Google Scholar]
- Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Stricker SA, Silva R, Smythe T. Calcium and endoplasmic reticulum dynamics during oocyte maturation and fertilization in the marine worm Cerebratulus lacteus. Dev Biol. 1998;203:305–322. doi: 10.1006/dbio.1998.9058. [DOI] [PubMed] [Google Scholar]
- Swann K, Ozil JP. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol. 1994;152:183–222. doi: 10.1016/s0074-7696(08)62557-7. [DOI] [PubMed] [Google Scholar]
- Terasaki M. Fluorescent labeling of endoplasmic reticulum. Methods Cell Biol. 1989;29:125–135. doi: 10.1016/s0091-679x(08)60191-0. [DOI] [PubMed] [Google Scholar]
- Terasaki M. Dynamics of the endoplasmic reticulum and Golgi apparatus during early sea urchin development. Mol Biol Cell. 2000;11:897–914. doi: 10.1091/mbc.11.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Jaffe LA, Hunnicutt GR, Hammer JA., III Structural change of the endoplasmic reticulum during fertilization: evidence for loss of membrane continuity using the green fluorescent protein. Dev Biol. 1996;179:320–328. doi: 10.1006/dbio.1996.0263. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Reese TS. Characterization of endoplasmic reticulum by co-localization of BiP and dicarbocyanine dyes. J Cell Sci. 1992;101:315–322. doi: 10.1242/jcs.101.2.315. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Runft LL, Hand AR. Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation. Mol Biol Cell. 2001;12:1103–1116. doi: 10.1091/mbc.12.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Slater NT, Fein A, Schmidek A, Reese TS. Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc Natl Acad Sci USA. 1994;91:7510–7514. doi: 10.1073/pnas.91.16.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Song J, Wong JR, Weiss MJ, Chen LB. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell. 1984;38:101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- Tombes RM, Simerly C, Borisy GG, Schatten G. Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J Cell Biol. 1992;117:799–811. doi: 10.1083/jcb.117.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Clarke HJ, Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994;120:1017–1025. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- Warren G. Membrane partitioning during cell division. Annu Rev Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]
- Whitaker M, Larman MG. Calcium and mitosis. Semin Cell Dev Biol. 2001;12:53–58. doi: 10.1006/scdb.2000.0217. [DOI] [PubMed] [Google Scholar]
- Whitaker M, Patel R. Calcium and cell cycle control. Development. 1990;108:525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]