Abstract
The stimulation of vascular endothelial growth factor receptor-2 (VEGFR-2) by tumor-derived VEGF represents a key event in the initiation of angiogenesis. In this work, we report that VEGFR-2 is localized in endothelial caveolae, associated with caveolin-1, and that this complex is rapidly dissociated upon stimulation with VEGF. The kinetics of caveolin-1 dissociation correlated with those of VEGF-dependent VEGFR-2 tyrosine phosphorylation, suggesting that caveolin-1 acts as a negative regulator of VEGF R-2 activity. Interestingly, we observed that in an overexpression system in which VEGFR-2 is constitutively active, caveolin-1 overexpression inhibits VEGFR-2 activity but allows VEGFR-2 to undergo VEGF-dependent activation, suggesting that caveolin-1 can confer ligand dependency to a receptor system. Removal of caveolin and VEGFR-2 from caveolae by cholesterol depletion resulted in an increase in both basal and VEGF-induced phosphorylation of VEGFR-2, but led to the inhibition of VEGF-induced ERK activation and endothelial cell migration, suggesting that localization of VEGFR-2 to these domains is crucial for VEGF-mediated signaling. Dissociation of the VEGFR-2/caveolin-1 complex by VEGF or cyclodextrin led to a PP2-sensitive phosphorylation of caveolin-1 on tyrosine 14, suggesting the participation of Src family kinases in this process. Overall, these results suggest that caveolin-1 plays multiple roles in the VEGF-induced signaling cascade.
INTRODUCTION
Angiogenesis, the growth of novel capillaries from preexisting vessels, is essential for a number of physiological processes such as wound healing, the female reproductive cycle, embryonic development, organ formation, tissue regeneration, and tissue remodeling (Folkman, 1995). However, under pathological conditions, uncontrolled angiogenesis sustains the progression of many diseases, including diabetic retinopathy, psoriasis, rheumatoid arthritis, and tumor growth (Folkman, 1995). In the latter condition, numerous studies have provided evidence that tumor growth and metastasis are angiogenesis dependent (Hanahan and Folkman, 1996). On oxygen and nutrient deprivation, tumor cells promote neovascularization by inducing the expression of angiogenic cytokines such as the vascular endothelial growth factor (VEGF). VEGF is a potent and unique angiogenic protein that induces endothelial cell (EC) proliferation, EC migration, and vascular permeability, and acts as a crucial survival factor for endothelial cells (Gerbert et al., 1998). For these reasons, interfering with VEGF's biological effects to block tumor angiogenesis has received considerable attention in recent years (Schlaeppi and Wood, 1999), and a number of agents that interfere with VEGF-mediated biological events are currently being tested in clinical trials (Mendel et al., 2000).
At the endothelial cell surface, VEGF binds with high affinity to at least two distinct vascular endothelial growth factor receptors: VEGFR-1, also known as fms-like tyrosine kinase (Flt-1) and VEGFR-2, also known as fetal liver kinase (Flk-1/KDR; Thomas, 1996). In adult cells, the mitogenic and chemotactic effects of VEGF appear mainly to be mediated by VEGFR-2 (Waltenberger et al., 1994). The activation of VEGFR-2 induces dimerization and transphosphorylation of the receptors, followed by tyrosine phosphorylation and/or activation of several downstream substrates, including the second messenger-producing enzymes phospholipase Cγ and phosphatidylinositol 3-kinase (Guo et al., 1995; Xia et al., 1996), p120GAP (Guo et al., 1995), the adaptor proteins Nck, Grb2, and Shc (Guo et al., 1995; Kroll and Waltenberger, 1997), the tyrosine phosphatases SHP1 and SHP2 (Kroll and Waltenberger, 1997), and the cytoskeleton-associated proteins focal adhesion kinase (FAK) and paxillin (Abedi and Zachary, 1997), these events leading to the activation of the extracellular-signal regulated kinase and p38 stress-activated protein kinase pathways (Kroll and Waltenberger, 1997; Rousseau et al., 2000).
In spite of these advances, the mechanisms underlying the regulation of VEGFR-2 activity remain poorly understood. The EC response to VEGF is increased by cell attachment to vitronectin, a specific substrate for the αvβ3 integrin (Borges et al., 2000), a process possibly involving association of this integrin with VEGFR-2. Neuropilin-1, a coreceptor of VEGFR-2, also increases VEGF-induced signaling by transferring VEGF onto VEGFR-2 (Gitay-Goren et al., 1992). Recently, we reported that inhibition of RhoA activity markedly reduced VEGFR-2 activity and downstream signaling (Gingras et al., 2000). Interestingly, most of these proteins have been shown to be localized within small invaginations of plasma membrane (caveolae), suggesting a role for caveolae in the activation and regulation of the VEGF-dependent signaling pathway (Anderson, 1998).
Caveolae domains are found in most cell types, particularly in terminally differentiated cells such as adipocytes, muscle cells, and EC. They are rich in cholesterol, sphingomyelin, and glycosphingolipids, which contributes to their characteristic low buoyant density and insolubility in detergents (Anderson, 1998). The maintenance of cholesterol levels is essential for functional caveolae (Chang et al., 1992; Schnitzer et al., 1994) and depends, in part, on the interaction of cholesterol with caveolin-1, a major caveolae component (Smart et al., 1996). Caveolin-1 is also involved in the regulation of signaling activity via a direct interaction of its scaffolding domain, corresponding to amino acids 82 through 101, with a consensus sequence present in several signaling proteins, including EGF and PDGF receptors, the kinases Src and Fyn, and heterotrimeric G-proteins (Li et al., 1995, 1996; Couet et al., 1997b; Yamamoto et al., 1999). Two related caveolin-binding motifs (AXAXXXXA and AXXXXAXXA, where A is an aromatic amino acid Trp, Phe or Tyr) have been identified, and these motifs exist within most caveolae-associated proteins (Couet et al., 1997a).
Interestingly, the interaction of caveolin with signal transducing proteins that contain these motifs has been shown to inhibit the activity of these proteins. Given the role of these proteins in cell growth and mitogenesis, the negative regulatory activity of caveolin-1 suggests that it may act as a putative tumor suppressor (Galbiati et al., 1998; Wiechen et al., 2001). Consistent with this hypothesis, the caveolin-1 gene was localized to a suspected tumor suppressor locus in mice and humans (7q31.1/D7S522) that is deleted in a number of human cancers (Engelman et al., 1999). In addition, caveolin-1 expression is reduced or absent in NIH 3T3 cells transformed by activated oncogenes such as v-Abl, Bcr-Abl or H-Ras [G12V], and caveolae are absent from these transformed cells (Koleske et al., 1995). Moreover, antisense-mediated reduction of caveolin-1 levels in normal NIH 3T3 cells leads to hyperactivation of the p42/44 mitogen-activated protein kinase cascade, anchorage-independent growth, and tumor formation in nude mice (Galbiati et al., 1998). In addition, caveolin-1 expression in a metastatic adenocarcinoma cell line inhibits EGF-stimulated lamellipod extension and cell migration (Zhang et al., 2000).
In addition to its role in tumor cell biology, there is increasing evidence that caveolin-1 may also play an important role in angiogenesis. Incubation of endothelial cells with VEGF leads to a marked down-regulation of both caveolae and caveolin-1 levels (Liu et al., 1999), and overexpression of caveolin-1 blocks VEGF-dependent activation of Elk-1 promotor activity (Liu et al., 1999). Disruption of the caveolin-1 gene resulted in uncontrolled EC proliferation in vivo (Drab et al., 2001; Razani et al., 2001), as well as the suppression of capillary-like tube formation in vitro (Griffoni et al., 2000), further supporting the importance of caveolin-1 in EC function. However, the mechanisms by which caveolin-1 regulates VEGF-induced angiogenesis remain largely unknown. In this work, we present evidence that VEGF-induced signaling is initiated in low-density caveolar membrane domains and that caveolin-1 may play an important role in this pathway by acting both as a negative regulator of VEGFR-2 activity under resting conditions and as a substrate that is tyrosine phosphorylated under activating conditions. These results provide important information on the mechanisms involved in the regulation of angiogenesis by caveolae and caveolin-1.
MATERIALS AND METHODS
Reagents and Antibodies
Cell culture media were obtained from Life Technologies (Burlington, Ontario, Canada) and serum was purchased from Hyclone Laboratories (Logan, UT). Electrophoresis reagents were purchased from Bio-Rad (Mississauga, Ontario, Canada). mAb A3, directed against VEGFR-2, mAbs against Fyn (sc-434) and RhoA (sc-418), as well as the monoclonal antiphosphotyrosine antibody PY99 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). PAbs against neuropilin-1 (sc-7239), FAK (sc-557), RhoB (sc-180), pAbs N-998 (sc-505), and C-1158 (sc-504), directed against VEGFR-2, and agarose-conjugated pAbs against caveolin-1 (sc-894), VEGFR-2 (sc-504), and c-src (sc-19) were also purchased from Santa Cruz Biotechnology. mAbs against ACE and αv integrin, and pAb against β3 integrin were from Chemicon International (Temecula, CA). mAbs against eNOS (N30020), caveolin-1 (C37120), paxillin (P13520), phosphocaveolin (P-Tyr 14 and C91520), and pAb against caveolin (C13630) were from Transduction Laboratories (Lexington, KY). mAbs against Cbl (no. 05-44) and Src (no. 05-184) were from Upstate Biotechnology (Lake Placid, NY). A mAb against pan Ras (Ab3) was from Calbiochem (La Jolla, CA), and a mAb against β-cop (G2279) was from Sigma-Aldrich Canada (Oakville, Ontario, Canada). Polyclonal anti-Src [pY 529] (44-662Z) was obtained from BioSource International (Camarillo, CA). Anti-mouse and anti-rabbit immunoglobulin (Ig) G horseradish peroxidase-linked secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and enhanced chemiluminescence (ECL) reagents were from Amersham Pharmacia Biotech (Baie d'Urfé, Québec, Canada). Human recombinant VEGF was obtained from R&D Systems (Minneapolis, MN). PP2 was purchased from Calbiochem. Micro bicinchoninic acid protein assay reagents were from Pierce (Rockford, IL). All other reagents were from Sigma-Aldrich Canada.
Cell Culture
Bovine aortic endothelial cells (BAEC) were kindly provided by Dr. R. Sauvé (Université de Montréal). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with low glucose, containing 10% heat-inactivated calf serum (Hyclone Laboratories), 100 U/ml penicillin, and 100 μg/ml streptomycin and were used between passages 9 through 20. For experimental purposes, cells were plated in 100-mm plastic dishes at 5000 cells/cm2 and were grown to confluence in a humidified atmosphere containing 5% CO2 and 90% air at 37°C. The endothelial cell (EC) line ECV304 was purchased from the American Tissue Culture Collection (Manassas, VA) and the human embryonal kidney cell line 293T was kindly provided by Dr M. Park (McGill University, Montreal, PQ). ECV304 cells were maintained in medium M199 containing 5% heat-inactivated calf serum, and 293T cells were maintained in DMEM high glucose containing 10% fetal bovine serum.
Caveolae Isolation
Caveolae membranes were prepared by the method of Smart et al. (1995) with minor modifications. ECV304 EC grown to near confluence in 175-mm2 flasks were serum-starved by a 48-h incubation in serum-free M199 medium, scraped into 10 ml of homogenization buffer (Buffer A: 250 mM sucrose, 1 mM EDTA, and 20 mM Tricine, pH 7.4), and collected by low-speed centrifugation. The cells were resuspended in 2 ml of buffer A and homogenized with 20 stokes of a motor-driven Teflon-glass Potter homogenizer. After removal of cellular debris by low-speed centrifugation, the postnuclear supernatant was layered on top of 23 ml of 30% (vol/vol) Percoll in buffer A and centrifuged at 84,000g for 30 min. The plasma membrane fraction was collected and diluted to 7 ml with buffer A. The resulting membranes were sonicated (six bursts of 15 s at 50% maximal power) and mixed with 6.44 ml of 50% Optiprep in buffer B (0.25 M sucrose, 6 mM EDTA, and 120 mM Tricine, pH 7.4) and 0.56 ml of buffer A. The resulting mixture (23% final Optiprep concentration) was placed at the bottom of a centrifuge tube and a linear 20% to 10% Optiprep gradient was layered onto the membranes. After overnight centrifugation at 52,000g, the top 16 ml of the gradient were collected, mixed with 14 ml of 50% Optiprep in buffer B, and overlaid with 3.5 ml of 15% Optiprep and 1.75 ml of 5% Optiprep, both in buffer A. The mixture was centrifuged at 52,000g for 4 h and the material at the 5% interface was collected. The purified membranes were collected by ultracentrifugation, resuspended in 20 mM Tris-HCl buffer, pH 7.4, and stored at −80°C. Noncaveolae membranes were obtained after ultracentrifugation of nonpooled fractions of the linear and discontinued Optiprep gradients. Tyrosine phosphorylation activities of the membranes were not affected by the storage conditions (up to 1 month).
Caveolae were also purified using a hyperosmotic carbonate method described previously (Song et al., 1996). Briefly, confluent BAEC cells cultured in 100-mm2 dishes were treated as described above and were scraped into 2 ml of 0.5 M sodium carbonate (pH 11) and homogenized extensively using a Polytron (three 15-s bursts at medium speed) followed by sonication (five 15-s bursts at 50% of maximal power). The resulting homogenate was brought to 45% sucrose by the addition of 2 ml of 90% sucrose in Mes-buffered saline (MBS; 25 mM Mes, pH 6.5 and 150 mM NaCl) and overlaid with two layers of 35% and 5% sucrose in MBS containing 0.25 M carbonate (4 ml each). The gradient was then centrifuged at 200,000 g for 18 h using a rotor (SW41Ti; Beckman Instruments, Fullerton, CA). For analysis of the resulting gradient, 1-ml fractions were collected from the top to the bottom of the gradient. The light-density fractions were pooled, diluted in 10 mM Tris-HCl (pH 7.5), and centrifuged at 100,000g.
VEGF Stimulation of Caveolae Membranes
Caveolae membranes (250 ng protein) were preincubated at 4°C for 30 min in 1× MEM (pH 7.4) containing 250 μg/ml bovine serum albumin, 2 mM activated sodium orthovanadate, 1 mM zinc acetate, 10 mM NaF, 10 mM MgCl2, and a protease inhibitor cocktail (100 μg/ml leupeptin, 100 μg/ml pepstatin A, 20 μg/ml antipain, 10 mM benzamidine, and 1 mM Pefabloc), in the presence of 0 to 100 ng/ml human recombinant VEGF (R&D Systems). The preincubation mixture was placed at 30°C for 2 min and the reaction was started by the addition of ATP (1 mM final concentration). The mixtures were incubated at 30°C for 5 min and the reactions were stopped by the addition of fivefold concentrated Laemmli sample buffer.
VEGF Stimulation of EC
BAEC grown to confluence were serum-starved by an 18-h incubation in serum-free DMEM, followed by incubation for 1 to 30 min at 37°C in 2 ml of serum-free DMEM containing 50 ng/ml human recombinant VEGF. In some experiments, cells were treated for 2 h with PP2 or for 60 min with 10 mM β-cyclodextrin (CD) before stimulation with VEGF. Cells were replenished with cholesterol by incubation in the presence of 16 μg/ml cholesterol and 0.4% CD (Furuchi and Anderson, 1998). After VEGF treatment, cells were washed once with phosphate-buffered saline (PBS) containing 1 mM sodium orthovanadate and were incubated in the same medium for 1 h at 4°C. The cells were solubilized on ice in lysis buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.5% Nonidet P-40, 1% Triton X-100, and 60 mM n-octylglucoside) containing 1 mM sodium orthovanadate. The cells were then scraped from the culture dishes and the resulting lysates were clarified by centrifugation at 10,000g for 10 min. Protein concentrations were determined by the bicinchoninic acid method (Pierce).
Immunoprecipitation and Western Blotting
For the immunoprecipitation studies, identical amounts of proteins from each sample were clarified by a 1-h incubation at 4°C with a mixture of protein A/Protein G Sepharose beads. After removal of the Sepharose beads by low-speed centrifugation, the supernatants were transferred to fresh tubes and incubated in lysis buffer overnight at 4°C in the presence of 1 to 4 μg/ml of a specific antibody. With the exception of the agarose-conjugated anti-VEGFR-2 and anti-caveolin antibodies, the immune complexes were collected by incubating the mixtures with 25 μl (50% suspension) of Protein A (rabbit primary antibody) or Protein G (mouse primary antibody) Sepharose beads. Nonspecifically bound proteins were removed by washing the beads three times in 1 ml of lysis buffer containing 1 mM sodium orthovanadate, and bound material was solubilized in 25 μl of twofold concentrated Laemmli sample buffer, boiled for 5 min, and resolved by SDS-PAGE. The proteins were transferred onto polyvinylidene difluoride (PVDF) membranes, blocked overnight at 4°C with Tris-buffered saline/Tween 20 (147 mM NaCl, 20 mM Tris-HCl, pH 7.5, and 0.1% Tween 20) containing 2% bovine serum albumin and incubated with primary antibody for 2 h at room temperature. Immunoreactive bands were revealed after a 1-h incubation with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies, and the signals were visualized with an ECL detection system.
Migration Assay
Transwells (8-μm pore size; Costar, Cambridge, MA) were precoated with 0.5% gelatin/PBS by adding 600/100 μl in the lower/upper chambers for 24 h at 4°C. The transwells were then washed with PBS and assembled in 24-well plates. The upper chamber of each transwell was filled with 100 μl of BAEC (1.0 × 106 cells/ml) and cells were allowed to adhere for 1 h. Cells were then treated for 1 h by adding 100 μl of 2× drug solution prepared in serum-free DMEM in the upper chamber and 600 μl of 1× drug solution in the lower chamber. For cholesterol repletion, solution in the chambers was aspirated and replaced by the CD/cholesterol preparation for an additional 1 h. Migration was initiated by adding VEGF to the lower chamber at a final concentration of 10 ng/ml and the plate was placed at 37°C in 5% CO2/95% air for 2 h. Cells that had migrated to the lower surface of the filters were fixed with 10% formalin phosphate and stained with 0.1% Crystal Violet/20% (vol/vol) methanol before being counted.
Transfection
cDNAs encoding VEGFR-2 (Patterson et al., 1995) and caveolin-1, kindly provided by Dr S.-S. Yoon (Sung Kyon Kwan University, Korea), were used to study the regulation of VEGFR-2 activity in an heterologous system. 293T cells (6 × 105) were transiently transfected with 2 μg of cDNAs encoding VEGFR-2 and caveolin-1 using the calcium phosphate precipitation method (Wigler et al., 1979). Eighteen hours posttransfection, the culture medium was replaced with fresh complete medium and, 48 h posttransfection, the 293T cells were starved for 18 h with serum-free medium, harvested, and used for immunoprecipitation assays. In some experiments, transfected cells were stimulated with VEGF 50 ng/ml after starvation.
In Vitro Kinase Assay
Precleared cell lysates (500 μg protein) were incubated overnight with anti-VEGFR-2 antibodies, and the resulting immune complexes were collected with Protein A-Sepharose beads as described above. The beads were washed three times with lysis buffer, followed by one additional wash with PBS. The washed beads were then incubated for 1 h on ice with glutathione S-transferase (GST) or GST-caveolin (61-101), in 40 μl of kinase buffer (100 mM Tris-HCl, pH 7.0, 0.2% β-mercaptoethanol, 20 mM MgCl2, and 0.2 mM sodium orthovanadate). After a 2-min preincubation of the mixtures at 30°C, the reactions were initiated by the addition of 5 μCi of [γ-32P]ATP (ICN Biochemicals, Costa Mesa, CA) or by the addition of 1 mM nonradioactive ATP. Reactions were stopped after 15 min by the addition of fivefold concentrated Laemmli sample buffer. After electrophoresis on 7.5% acrylamide/bis-acrylamide gels, the gels were exposed to Fuji films (Tokyo, Japan) or transferred onto PVDF and subjected to Western blotting procedures.
Purification of GST-Caveolin(61-101)
The caveolin-1 scaffolding domain was constructed by polymerase chain reaction (PCR) using the full-length caveolin-1 cDNA as the template (gift of Dr. R. Venema) and primers annealing to the cDNA regions encoding amino acids 61 through 101. The PCR product was subcloned in the prokaryotic expression vector pGEX-2T and was transformed into Escherichia Coli strain BL21. The GST-caveolin-1 scaffolding domain fusion proteins were purified from isopropyl-β-d-thio-galactopyranoside-induced log phase bacterial cultures, followed by affinity chromatography on glutathione-agarose beads.
RESULTS
Cofractionation of VEGFR-2, Caveolin-1, and Signaling Proteins in Caveolae Membranes of ECV304 and BAE Cells
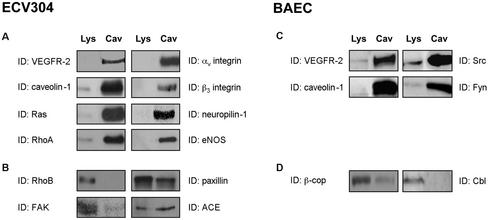
VEGFR-2 has been previously reported to localize in caveolae membranes of endothelial cells (Feng et al., 1999a, 1999b). To establish whether these microdomains may represent specialized signal transduction centers for VEGF-induced signaling, caveolae membranes were purified from ECV304 cells, a spontaneously immortalized human umbilical vein endothelial cell line (Figure 1, A and B), and from BAEC (Figure 1, C and D) using the detergent-free method developed by Smart et al. (1995). Although the endothelial origin of ECV304 cells has been recently questioned (Brown et al., 2000), recent data show that these cells express various endothelial characteristics not found in epithelial cells (Suda et al., 2001). However, given their controversial nature, ECV304 were used in this study only for the initial characterization of the presence of VEGFR-2 in endothelial caveolae.
Figure 1.
Colocalization of VEGFR-2 and numerous regulatory proteins in caveolae membranes isolated from endothelial cells. Caveolae membranes were isolated from ECV304 cells (A and B) or BAEC (C and D), after ultracentrifugation of plasma membranes on Optiprep gradients (Smart et al., 1995). Equivalent amounts of proteins from the lysates and purified membranes (5 μg) were separated by SDS-PAGE and were immunoblotted with the indicated antibodies.
Equal amounts of protein from whole cell lysates and from the isolated caveolae membranes were separated by SDS-PAGE electrophoresis, transferred onto PVDF membranes, and probed using specific antibodies. As shown in Figure 1, isolated membranes from both cell lines are characterized by a significant enrichment in caveolae markers, including caveolin-1, Ras, and the kinases Src and Fyn. Interestingly, we also observed an enrichment of VEGFR-2 in both preparations as well as of proteins involved in the regulation of VEGF-induced signaling pathways, such as αv and β3 integrin subunits (Borges et al., 2000), RhoA (Gingras et al., 2000), eNOS (Papapetropoulos et al., 1997; Parenti et al., 1998), and neuropilin-1 (Gitay-Goren et al., 1992; Figure 1). By contrast, some proteins are not enriched in caveolae membranes or are completely excluded from these domains, including RhoB, FAK, β-cop, and Cbl (Figure 1, B and D). The absence of these proteins from caveolae purified from other cell lines has been reported previously (Mastick and Saltiel, 1997; Gingras et al., 1998; Demeule et al., 2000; Oh and Schnitzer, 2001), and confirms the lack of contamination of our preparations by Golgi (β-cop), endosomal (rhoB), and cytoskeletal (FAK) components.
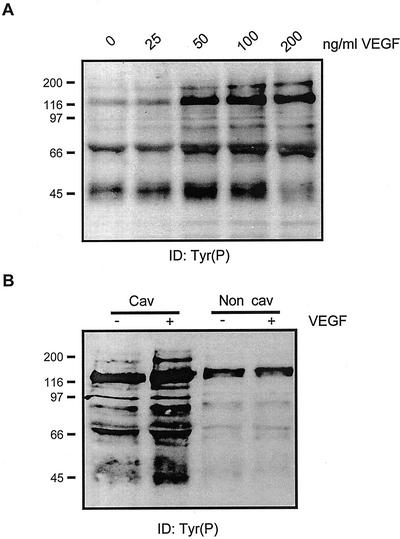
To determine whether these cholesterol-rich microdomains contain functional VEGFR-2, the caveolar membrane fraction isolated from ECV304 cells was stimulated in vitro with VEGF. Kinase activity has been already found in caveolae membranes isolated by this method after treatment with epidermal growth factor (EGF; Mineo et al., 1996) and platelet-derived growth factor (PDGF; Liu et al., 1997). Accordingly, stimulation of the caveolae membranes isolated from endothelial cells with VEGF leads to a concentration-dependent increase in the tyrosine phosphorylation of several membrane-associated proteins (Figure 2A), the stimulatory effect being maximal at 50 to 100 ng/ml, as observed in whole cells (Gingras et al., 2000). This stimulatory effect of VEGF seems to be associated exclusively with the caveolae membranes because no VEGF-induced phosphorylation of noncaveolar membrane proteins could be detected (Figure 2B). These results suggest that the initial steps of VEGF-induced signalization are localized within caveolae domains.
Figure 2.
VEGF stimulates tyrosine phosphorylation of caveolae-associated proteins. (A) Caveolae purified from ECV304 cells (250 ng) were stimulated 30 min on ice with the indicated concentrations of VEGF, in the presence of phosphatase inhibitors. The phosphorylation reaction was initiated by the addition of 1 mM ATP, and the mixtures were incubated at 30°C for 5 min. Reactions were stopped by the addition of fivefold concentrated Laemmli buffer and proteins were separated by SDS-PAGE electrophoresis followed by immunoblotting with a monoclonal anti-Tyr(P) antibody. (B) Caveolae or noncaveolae membranes (250 ng) were incubated with 50 ng/ml VEGF and the phosphorylation reaction was performed as described above. Results are representative of two experiments performed with distinct caveolae preparations.
Cholesterol Depletion Modulates VEGFR-2 Localization and Signaling Activity
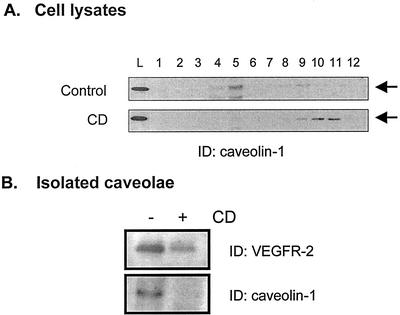
We next investigated whether the localization of VEGFR-2 to endothelial caveolae was essential for its signaling activity. Removal of cholesterol from cells has been shown to cause the disassembly of caveolae and thus represents an interesting tool for the study of caveolae-dependent processes (Chang et al., 1992; Rothberg et al., 1992). We first tested whether cholesterol depletion resulted in the removal of VEGFR-2 from caveolae. BAECs were treated with CD, a cholesterol-binding agent that extracts cholesterol from the plasma membrane (Rodal et al., 1999; Ushio-Fukai et al., 2001), and the resulting lysates were separated on sucrose gradients (Figure 3). Under these conditions, the light-density caveolae membranes sedimented at the 5%/35% sucrose interface (fractions 4 through 6). As shown in Figure 3A, CD treatment of the cells resulted in the displacement of caveolin-1 from caveolae to noncaveolae fractions (fractions 9 through 12; Figure 3A), this redistribution being also observed for other caveolae-resident proteins such as Ras and eNOS (L. Labrecque, D. Gringras, R. Beliveau, unpublished data). To detect whether VEGFR-2 was removed from caveolae, fractions corresponding to the light-density membranes were pooled, concentrated, and immunoblotted with anti-VEGFR-2 antibodies. As shown in Figure 4B, cholesterol depletion resulted in the removal of VEGFR-2 from the light-density membranes, supporting the disruption of the molecular organization of the plasma membrane by CD treatment.
Figure 3.
Cholesterol depletion of plasma membrane induces relocalization of caveolin-1 and VEGFR-2. Serum-starved BAEC were incubated for 1 h in the presence of 10 mM CD and subjected to sucrose gradient sedimentation as described in “Material and Methods.” (A) Gradients were fractionated by collecting 1-ml fractions from the top, and 20 μl of each fraction was loaded onto polyacrylamide gels for the detection of caveolin-1. (B) Fractions corresponding to caveolae-enriched membranes (fractions 4 through 6) were diluted in Tris-HCl 10 mM (pH 7.5) and centrifuged at 100,000g. Membranes were resuspended in lysis buffer, and equal quantities of proteins were separated by SDS-PAGE electrophoresis.
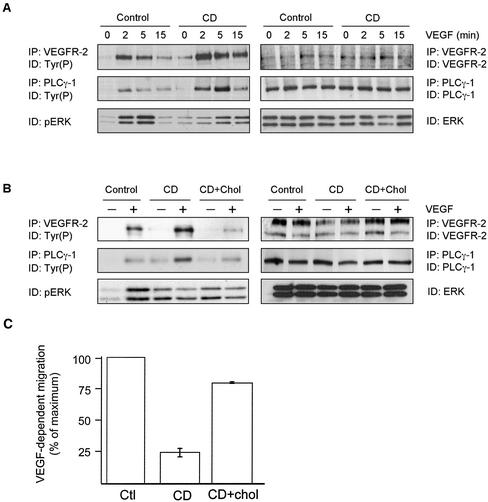
Figure 4.
Cholesterol depletion modulates VEGF-induced signaling. (A) Serum-starved BAEC were incubated for 1 h in the presence of 10 mM CD and were incubated in the presence of 50 ng/ml VEGF for the indicated times. Cells were lysed, subjected to immunoprecipitation with the indicated antibodies, and phosphorylation was monitored by Western blotting using a specific anti-Tyr(P) mAb. (B) After incubation in the presence of 10 mM CD, BAEC were treated for an additional 1 h with 16 μg/ml cholesterol and 0.4% CD. Cells were then stimulated with VEGF and subjected to immunoprecipitation with the indicated antibodies. (C) BAEC were allowed to adhere in transwells with gelatin-coated membranes. Cells were treated as indicated and migration was initiated by adding VEGF to the lower chamber to a final concentration of 10 ng/ml, and the plate was placed at 37°C in 5% CO2/95% air for 2 h. The number of cells that crossed the membrane were normalized to those from nontreated cells.
We next investigated the effects of cholesterol depletion on VEGF-induced signaling. We first observed that both basal and VEGF-induced phosphorylation of VEGFR-2 were markedly increased by CD treatment, and that the stimulatory effect of VEGF was maintained for a longer period of time, resulting in increased tyrosine phosphorylation of PLCγ-1, an important substrate that is tyrosine-phosphorylated by VEGFR-2 (Figure 4A, left panel; Guo et al., 1995). The expression levels of all proteins was unaffected by the treatment, supporting a specific alteration of kinase activity by CD (Figure 4A, right panel). However, despite the hyperphosphorylation of VEGFR-2 and PLCγ, activation of ERK was markedly reduced by CD, even although basal phosphorylation of the enzymes was increased (Figure 4A). Replenishment of cholesterol content, by incubation of the cells in the presence of 16 μg/ml cholesterol and 0.4% CD, inhibited most of the stimulatory effect of CD because it restored the initial low VEGF-induced VEGFR-2 and PLCγ phosphorylation (Figure 4B). However, cholesterol replenishment failed to restore VEGF-dependent activation of ERK, suggesting additional alterations in downstream components of the signaling pathway. These results demonstrate that cholesterol is essential for proper spatiotemporal VEGF-dependent cell signaling.
VEGF is known to be an important chemoattractant for EC (Waltenberger et al., 1994). To evaluate the importance of cholesterol-enriched domains for this biological activity, we induced CD-treated BAEC to migrate on transwells in the presence of VEGF as the chemoattractant. We observed a considerable inhibition of VEGF-dependent migration in the presence of CD, possibly reflecting the importance of plasma membrane cholesterol in the migration of EC, as was recently reported (Vincent et al., 2001). However, VEGF-induced migration was significantly restored after cholesterol repletion (Figure 4C). These results suggest that plasma membrane caveolae integrity is essential for the proper response of EC to VEGF.
Caveolin-1 Is a Negative Regulator of VEGFR-2 Activity
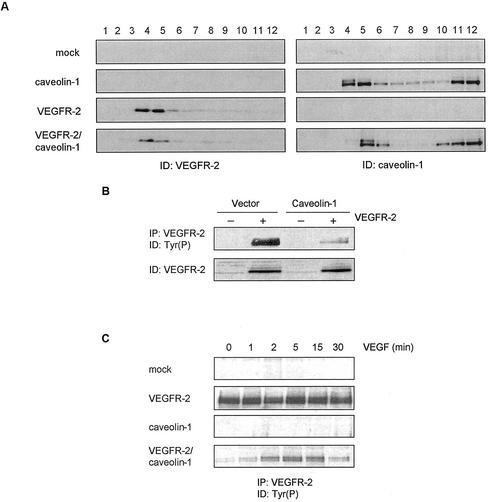
We further investigated the regulatory function of caveolae on VEGF-dependent signaling by examining the effect of caveolin-1 on VEGFR-2 activity. Caveolin-1 has emerged as a key caveolae-associated protein that regulates the activity of numerous signaling proteins (Schlegel et al., 2000b). VEGFR-2 and caveolin-1 were cotransfected into 293T cells, an human embryonic kidney cell line that expresses extremely low levels of endogenous caveolin-1 (Schlegel and Lisanti, 2000a) and no VEGFR-2, and the extent of VEGFR-2 phosphorylation was examined. As shown in Figure 5A, overexpression of VEGFR-2 in these cells, either in the presence or absence of caveolin-1, resulted in the localization of VEGFR-2 to low-density membrane domains, suggesting that the protein contains intrinsic structural motifs sufficient for targeting to these domains. Under these conditions a high and ligand-independent phosphorylation of the receptor was observed, possibly due to high protein expression levels that promote ligand-independent receptor dimerization (Fuh et al., 1998). Interestingly, coexpression of caveolin-1 and VEGFR-2 resulted in a marked inhibition of VEGFR-2 activity (Figure 5B) that correlated with the colocalization of both proteins to the low-density domains (Figure 5A). Addition of VEGF to cells expressing both caveolin-1 and VEGFR-2 induced a time-dependent increase in the tyrosine phosphorylation of VEGFR-2, suggesting that stimulation of the receptor with VEGF is able to circumvent the inhibitory action of caveolin.
Figure 5.
Cotransfection of VEGFR-2 and caveolin-1 in human embryonal kidney cells inhibits receptor autophosphorylation. 293T cells were transiently transfected with VEGFR-2 and caveolin-1 cDNAs, using the calcium phosphate precipitation procedure (Wigler et al., 1979). Forty-eight hours posttransfection, cells were serum-starved and lysed. (A) Caveolae membranes were purified using the hyperosmotic carbonate method (Song et al., 1996). Fractions were separated by SDS-PAGE electrophoresis, blotted, and tested for caveolin-1 or VEGFR-2, as indicated. (B and C) VEGFR-2 was immunoprecipitated from cell extracts (350 μg) and phosphorylation was monitored by immunoblotting with a monoclonal anti-Tyr(P) antibody. In C, cells were treated with 50 ng/ml VEGF for the periods indicated before lysis.
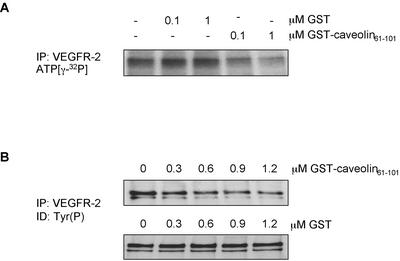
The inhibitory effect of caveolin-1 on the activity of several signal-transducing proteins is mediated by the scaffolding domain of caveolin-1, corresponding to residues 82 through 101 of the protein (Couet et al., 1997b; Engelman et al., 1998; Yamamoto et al., 1999). We investigated the effect of this domain, fused to GST, on VEGFR-2 activity using an in vitro kinase assay. VEGFR-2 was immunoprecipitated from cell lysates, and the immune complexes were preincubated in the presence of increasing amounts of GST-cav (61-101) or GST. The extent of VEGFR-2 activity was monitored either by incubating the complexes with [γ-32P]ATP (Figure 6A) or with 1 mM ATP, followed by antiphosphotyrosine detection (Figure 6B). As shown in Figure 6, the addition of the GST-cav(61-101) fusion protein caused a dose-dependent inhibition of VEGFR-2 autophosphorylation in both assays, whereas GST had no effect. This suggests that the caveolin-1 negative regulation of VEGFR-2 activity is likely to be mediated by the scaffolding domain of the protein.
Figure 6.
The caveolin-1 scaffolding domain inhibits the in vitro kinase activity of VEGFR-2. Immunoprecipitated VEGFR-2 from serum-starved BAEC was incubated for 1 h on ice in the presence of GST or GST-Caveolin-161–101 and submitted to an in vitro kinase assay in the presence of 5 μCi of ATP (γ-32P) (A) or in the presence of nonradioactive ATP at a final concentration of 1 mM (B). After 15 min at 30°C, reactions were stopped by the addition of fivefold concentrated sample buffer. After electrophoresis on 7.5% acrylamide/bis-acrylamide gels, the gels were exposed to Fuji films (A) or transferred onto PVDF and subjected to Western blotting (B).
VEGFR-2 Is Associated with Caveolin-1
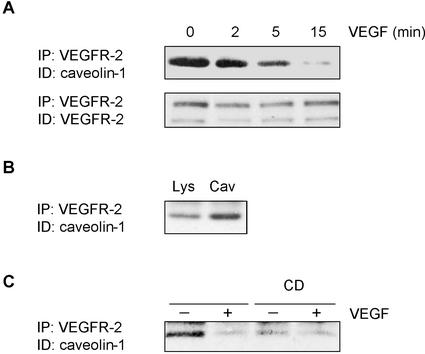
A consensus sequence for proteins that bind to caveolin has been identified in the kinase domains of several RTK associated with caveolae domains (Couet et al., 1997b). Close examination of the VEGFR-2 sequence indicates that the protein contains a putative caveolin-binding motif in its kinase domain (1091WSFGVLLWEIF1101). Based on the observed inhibition of VEGFR-2 activity by caveolin-1, we thus examined the possibility of an association between the proteins. VEGFR-2 from lysates isolated from unstimulated or from VEGF-stimulated BAEC were immunoprecipitated and the presence of caveolin-1 in the immune complexes was monitored by immunoblotting. In unstimulated BAEC, we observed the coprecipitation of caveolin-1 with VEGFR-2 (Figure 7A; 0 min). Interestingly, the stimulation of BAEC with VEGF induced a rapid, time-dependent dissociation of caveolin-1 from VEGFR-2, this dissociation being nearly complete 15 min after the addition of VEGF. The association of caveolin-1 with VEGFR-2 was enriched in isolated caveolae domains (Figure 7B), but was markedly reduced after cholesterol depletion, suggesting that the protein association requires the integrity of these domains (Figure 7C). These results indicate that under resting conditions, caveolin-1 may act as a negative regulator of VEGFR-2 activity and that stimulation of the receptor by VEGF may promote activation of this signaling pathway by inducing the dissociation of the receptor from the inhibitory action of caveolin-1.
Figure 7.
VEGF induces dissociation of caveolin-1 from VEGFR-2. (A) BAEC were serum-starved overnight and incubated in the presence of 50 ng/ml VEGF for the indicated times. After a 1 h sodium orthovanadate treatment, cells were lysed (700 μg) and subjected to immunoprecipitation with agarose-conjugated anti-VEGFR-2. Associated caveolin-1 was observed by Western blotting using a specific mAb. (B) After VEGF stimulation, caveolae membranes were prepared and fractions corresponding to caveolae-enriched membranes were diluted and concentrated by ultracentrifugation. Equal quantities of proteins were used for immunoprecipitation assays. (C) Before VEGF-stimulation, cells were incubated for 1 h in the presence of 10 mM CD.
Caveolin-1 Is Tyrosine Phosphorylated after Stimulation of BAEC with VEGF
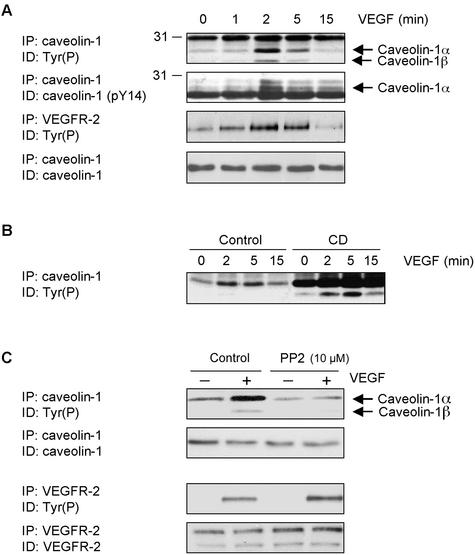
Caveolin-1 has been shown to be tyrosine phosphorylated in response to stimulation of cells with EGF and insulin (Mastick et al., 1995; Kim et al., 2000). Based on the presence of VEGFR-2 in caveolae and on the inhibitory effect of caveolin-1 on its activity, we next investigated the extent of caveolin-1 phosphorylation induced by VEGF stimulation. Addition of VEGF to BAEC leads to a marked increase in the tyrosine phosphorylation of caveolin-1, this effect being predominantly observed for the α isoform of caveolin-1 (caveolin-1α) as compared with the β isoform (caveolin-1β), which lacks the first 32 amino acids (Figure 8A). The phosphorylation of caveolin on tyrosine 14 shows kinetics similar to those of caveolin-1 phosphorylation, reaching a maximum after a 2-min incubation with VEGF, suggesting that caveolin-1 is phosphorylated at this residue (Figure 8A). Interestingly, caveolin-1 phosphorylation follows the kinetics of VEGFR-2 activation (Figure 8A), and the maximal induction of caveolin-1 and VEGFR-2 phosphorylation correlated with the observed VEGF-induced dissociation of caveolin-1 from VEGFR-2 (Figure 7A), suggesting that caveolin-1 is a substrate for VEGFR-2 or for a kinase activated by VEGFR-2. These results suggest that VEGFR-2 activation, after its dissociation from caveolin-1, leads to caveolin-1 phosphorylation. In this respect, we observed that CD treatment of BAEC, which removes VEGFR-2 from caveolae and induces its dissociation from caveolin-1, induced a massive tyrosine phosphorylation of caveolin-1 (Figure 8B).
Figure 8.
VEGF induces Src-dependent tyrosine phosphorylation of caveolin-1. (A) BAEC were serum-starved 18 h and incubated in the presence of 50 ng/ml VEGF for the indicated times. After a 1-h sodium orthovanadate treatment, cells were lysed and subjected to immunoprecipitation with agarose-conjugated anti-caveolin-1 (350 μg of cell lysate) or anti-VEGFR-2 (200 μg). Incubation of BAEC was also performed before VEGF stimulation in the presence of 10 mM CD (B) or 10 μM PP2 (C). Phosphorylation was monitored by Western blotting using a specific anti-Tyr(P) mAb.
Although caveolin-1 possesses several tyrosine residues that are known to be phosphorylated (Nomura and Fujimito, 1999), the main site of phosphorylation has been shown to occur on tyrosine 14 of the protein and is catalyzed by members of the Src family kinases (SFK; Li et al., 1996). We thus examined the effects of SFK inhibition on the VEGF-dependent caveolin-1 tyrosine phosphorylation. As shown in Figure 8C, incubation of BAEC with PP2, an inhibitor of SFK, completely abolished VEGF-induced caveolin-1 phosphorylation. This effect was not related to a non specific inhibitory effect on VEGFR-2 kinase activity because VEGF-dependent phosphorylation of the receptor was not inhibited under these conditions (Figure 8C). In fact, we consistently observed a slight increase in the phosphorylation of VEGFR-2 after treatment of the cells with PP2 (Figure 8C). Overall, these results suggest that caveolin-1 is phosphorylated after VEGF-dependent activation of VEGFR-2; this phosphorylation possibly occurs in the N-terminal region of the protein and involves activation of Src family kinases.
VEGF Induces the Association of Caveolin-1 with Src
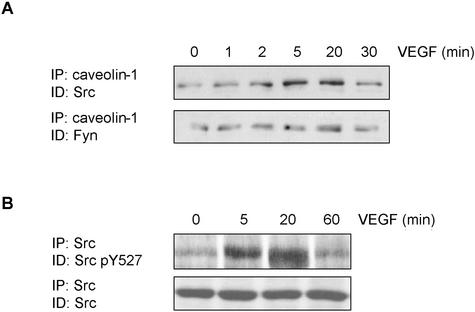
Src has recently been shown to play an essential role in the VEGF-dependent activation of EC, possibly through its phosphorylation of FAK (Abu-Ghazaleh and al., 2001), resulting in the coupling of this enzyme to the αvβ5 integrin (Eliceiri and al., 2002). However, this activation appears to be transient because phosphorylation of FAK is maximal at 2 to 5 min and rapidly declines afterward (Eliceiri et al., 2002). Because caveolin is known to interact preferentially with the inactive conformation of Src (Li et al., 1996), we were interested in determining whether the inhibition of Src activity during longer incubation times correlated with an association of the kinase with caveolin-1. As shown in Figure 9A, the stimulation of BAEC with VEGF promoted a time-dependent association of Src with caveolin-1, maximal association occurring at 20 min, which corresponds temporally with the down-regulation of its activity (Eliceiri et al., 2002). We further observed that 20 min after the addition of VEGF, Src is predominantly phosphorylated on Y527 (Figure 9B), which reflects the inactive status of the kinase (Brown and Copper, 1996). Another member of the Src kinases, Fyn, was also associated with caveolin-1 under resting conditions, but the degree of association was unaffected by VEGF. These results suggest that VEGF treatment induces a rapid but transient activation of Src, followed by inactivation of the enzyme and its association with caveolin-1.
Figure 9.
VEGF induces association of Src to caveolin-1. BAEC were serum-starved overnight and incubated in the presence of 50 ng/ml VEGF for the indicated times. After a 1-h sodium orthovanadate treatment, cells were lysed (700 μg) and subjected to immunoprecipitation with agarose-conjugated anti-caveolin-1. Associated caveolin-1 was observed by Western-blotting with a specific mAb. (B) After lysis, Src was immunoprecipitated and Western blotted using a specific antibody against the form of Src phosphorylated on tyrosine 527.
DISCUSSION
The targeting of VEGFR-2 activity as a mean of blocking angiogenesis and tumor growth has received considerable attention in recent years (Schlaeppi and Wood, 1999). Although the signaling pathways triggered by this important receptor are becoming increasingly understood (Matsumoto and Claesson-Welsh, 2001), there are still considerable gaps in our knowledge of the mechanisms involved in the regulation of the activity of VEGFR-2 as well as their relationship to tumor angiogenesis. In this respect, we were intrigued by recent reports showing the potential involvement of caveolae in the regulation of angiogenesis (Liu et al., 1999), which were subsequently strengthened by results showing that gene disruption of caveolin-1 results in uncontrolled EC proliferation in vivo (Razani et al., 2001), and that reduction of caveolin-1 levels by antisense oligonucleotides results in impaired angiogenesis in vitro (Griffoni et al., 2000). These considerations led us to investigate the localization and regulation of VEGFR-2 activity in endothelial caveolae. In this study, we report that endothelial caveolae-enriched membrane domains are highly enriched in both VEGFR-2 and in a number of proteins that have been shown to participate in the VEGF signal transduction pathways, such as αvβ3 integrin (Borges et al., 2000), eNOS (Papapetropoulos et al., 1997; Parenti et al., 1998), and RhoA (Gingras et al., 2000).
Such compartmentization has been shown to be crucial for several signaling pathways, such as those triggered by EGF (Mineo et al., 1996) and PDGF (Liu et al., 1996), possibly allowing efficient interactions between key signaling proteins that are required for both positive and negative regulation of these activities. Moreover, we observed that the isolated caveolae membranes undergo a significant increase in tyrosine phosphorylation when incubated in the presence of VEGF, demonstrating that the first steps of VEGF-induced signaling could be initiated in these domains. Interestingly, overexpression of VEGFR-2 in cells lacking caveolin-1 resulted in its targeting to low-density domains, suggesting that the protein contains intrinsic features that allow its targeting to these domains. This further emphasizes the important role of localization for the proper function of the receptor.
The integrity of caveolae structure seems to be important for accurate VEGF-induced signaling. Cholesterol depletion of EC by CD, which leads to the loss of caveolae structure as observed by transmission electron microscopy of the plasma membrane (Parpal et al., 2001), induced the relocalization of both caveolin-1 and VEGFR-2 to high-density membranes. This was accompanied by an increase in VEGFR-2 and PLCγ-1 phosphorylation, possibly due to reduced association between VEGFR-2 and caveolin-1 (Figure 7C). This is in agreement with the observed absence of caveolae in transformed cells (Koleske et al., 1995), which are also characterized by an increase in signaling events. Similar results were observed with vascular smooth muscle cells, where CD caused an increase in EGF-induced EGF receptor phosphorylation (Ushio-Fukai et al., 2001), but not in Rat-1 cells, in which EGF receptor phosphorylation was not affected by CD (Furuchi and Anderson, 1998), nor in the activation of the insulin receptor in 3T3-L1 adipocytes (Parpal et al., 2001). Thus, it seems that caveolae and caveolin-1 lead to different actions depending on the cell type and stimuli. However, our results show that despite hyperphosphorylation of upstream components of the signaling cascade, the disassembly of caveolae structures by cholesterol depletion inhibits VEGF-induced EC migration. It is likely that inadequate localization of the signaling machinery outside of caveolae could prevent proper continuation of the signal, a hypothesis supported by the observation that CD treatment results in reduced VEGF-dependent ERK activation (Figure 4A).
One important observation of this study is that, under resting conditions, caveolin-1 is associated with the inactive form of VEGFR-2 and undergoes rapid dissociation from the receptor upon stimulation with VEGF. This association of caveolin-1 is inhibitory to VEGFR-2 activity, based on the observation that coexpression of both proteins in a heterologous system results in the inhibition of receptor activity, as well as by the correlation between the kinetics of caveolin-1 dissociation and VEGFR-2 activation in response to VEGF. The inhibitory action of caveolin-1 likely involves interaction of its scaffolding domain with a putative consensus caveolin-1-binding motif located in the kinase domain of the receptor, as reflected by the inhibitory effect of this domain on the in vitro autophosphorylation activity of the receptor. It is tempting to speculate that the interaction of caveolin-1 with VEGFR-2 may be crucial for normal cell function based on the observation that disruption of the caveolin-1 gene leads to uncontrolled EC proliferation (Razani et al., 2001).
In addition to its inhibitory action on VEGFR-2 activity, our study also provides evidence that caveolin-1 may fulfill other functions in the signaling pathways triggered by VEGF. Stimulation of EC with VEGF resulted in a marked increase in the tyrosine phosphorylation of caveolin-1, which correlated with the kinetics of VEGFR-2 activation. A portion of the VEGF-dependent tyrosine phosphorylation of caveolin-1 occurred on tyrosine 14 of the protein, which is recognized as the principal residue phosphorylated by Src kinases (Li et al., 1996). Accordingly, VEGF-dependent phosphorylation of caveolin-1 was inhibited by pharmacological inhibitors of Src kinases, suggesting that these enzymes may be responsible for the caveolin-1 phosphorylation induced by activation of VEGFR-2. An important role for Src kinases in VEGF-induced signaling pathways was recently described (Eliceiri et al., 1999) and seems to be related to the phosphorylation of the focal adhesion kinase (Abu-Ghazaleh et al., 2001), possibly resulting in the interaction of this enzyme with the αvβ5 integrin (Eliceiri et al., 2002) Whether tyrosine phosphorylation of caveolin-1 by Src plays a role in these processes remains to be determined.
The role of caveolin-1 phosphorylation in cell function remains unclear but seems important. Under normal conditions, caveolin-1 has been shown to be phosphorylated in response to growth factors such as EGF and insulin (Kim et al., 2000). Inactivation of Src kinases, either by specific inhibitors such as PP1 (Lee et al., 2000) and PP2 (Li et al., 1996) or by transfection of dominant negative versions of the kinase (Volonte et al., 2001), inhibits caveolin-1 phosphorylation induced by EGF, insulin, or cellular stress, further emphasizing the important roles of these kinases in caveolin-1 phosphorylation. Tyrosine-phosphorylated caveolin-1 appears to be localized at focal adhesion sites, which are major sites of tyrosine kinase signaling in vivo, suggesting that it may exert important functions in signaling. Such an important role is supported by the specific interaction of phosphocaveolin with Grb7, during growth factor-induced cell migration (Lee et al., 2000), and with the low-molecular-weight protein-tyrosine phosphatase involved in the down-regulation of PDGF and insulin receptors (Caselli et al., 2001). However, caveolin-1 phosphorylation seems to be cell type- and stimulus-specific because it was not observed in adrenal cortex EC after VEGF stimulation (Esser et al., 1998) nor in BAEC under shear stress condition (Fujioka et al., 2000), even though this phosphorylation was similarly demonstrated in NIH 3T3 cells (Volonte et al., 2001).
The mechanisms involved in the VEGF-dependent phosphorylation of caveolin-1 by Src kinase remain obscure. Association of caveolin-1 with Src-family kinases has also been reported in the signaling pathway required for albumin endocytosis and transcytosis, where activation of an EC membrane albumin-binding protein (gp60) induced caveolin-1 phosphorylation and association with kinases Src and Fyn (Tiruppathi et al., 1997). Thus, VEGFR-2/caveolin-1 association could be mediated using Src as an intermediary, as was suggested for the insulin-stimulated tyrosine phosphorylation of caveolin-1 (Mastick and Saltiel, 1997). However, the VEGFR-2 protein lacks consensus binding sites for Src SH2 domains (Matsumoto and Claesson-Welsh, 2001) and we were unable to detect the association of Src with VEGFR-2 under our experimental conditions, although this has recently been suggested (He et al., 1999). Whether caveolin-1 phosphorylation involve its interaction with other components, such as integrin α subunits (Wary et al., 1998), is currently under investigation.
Cholesterol depletion also induced hyperphosphorylation of caveolin-1 (Figure 8B). In v-Src-expressing cells, caveolin-1 phosphorylation is associated with flattening and aggregation of caveolae (Nomura and Fujimoto, 1999). Thus, caveolin-1 phosphorylation could be involved in the internalization of caveolae in response to stimuli. This could explain the observed augmentation of VEGFR-2 phosphorylation after PP2 treatment (Figure 8C) where internalization, or down-regulation mechanisms, are inhibited and hence the receptor remains at the cell surface for a longer period of time, resulting in its hyperphosphorylation. The mechanisms leading to down-regulation of VEGFR-2 are not well understood. Although the clathrin-coated pits/vesicle system is still the best known system for the degradation and recycling of receptors, recent evidence has pointed out caveolae domains as an alternative internalization pathway involving fusion of caveolae with the early/sorting endocytic compartment, where the phosphorylation of Raf-1, Mek, and eventually MAPK could be achieved (Pol et al., 1998, 2000). In agreement with this model, EGF receptors rapidly move out of caveolae domains after EGF stimulation in quiescent fibroblasts (Mineo et al., 1999), and VEGF-stimulation induces nuclear translocation of VEGFR-2 in BAEC and in bovine retinal endothelial cells (Feng et al., 1999a).
In addition to its role as both a regulator of VEGFR-2 and as a substrate of VEGFR-2-activated Src kinase, our results also suggest that caveolin-1 interacts with inactive Src after stimulation of the cells with VEGF. Under the conditions used here, this interaction is a late event in this cascade because it reached a maximum after 20 min, well after the peaks of VEGFR-2 and caveolin-1 phosphorylation. The interaction of caveolin-1 with Src correlated with the VEGF-dependent tyrosine phosphorylation of Src on tyrosine 527, a major site of Src phosphorylation by C-terminal Src kinase (Csk) that results in inactivation of Src (Erpel and Courtneidge, 1995). However, under our experimental conditions, we were unable to determine whether caveolin-1-associated Src was phosphorylated on tyrosine 527, due to the low levels of Src in the immune complexes. Although the mechanisms involved in this interaction remain to be clarified, it is noteworthy that Csk was recently shown to interact preferentially with caveolin-1, which is phosphorylated on tyrosine 14, which may provide a mechanism for its recruitment to caveolae membranes and allow the subsequent inactivation of Src by phosphorylation (Cao et al., 2002).
In summary, our results show that caveolae domains and its resident protein caveolin-1 may play multiple roles in the regulation of VEGFR-2 activity and of angiogenesis. Caveolin-1 inhibits basal VEGFR-2 activity through the formation of a molecular complex that rapidly dissociates upon stimulation by VEGF, enabling caveolin-1 to serve as a substrate for Src kinases. Moreover, caveolin-1 may serve as an additional regulatory component of the pathway by sequestering Src in its inactive conformation. These results emphasize a complexity and versatility of caveolin-1 in the VEGF-induced signaling pathway that are consistent with recent results showing that caveolin-1 is down-regulated during EC proliferation (Liu et al., 1999) but is up-regulated during their differentiation into tubular networks in vitro (Liu et al., 2002). Thus, the recognition of caveolin-1 as both a negative and positive regulator of angiogenesis through its interaction with both upstream and downstream components of the VEGF-induced signaling cascade may provide interesting new tools for the study of the role of this protein in angiogenesis.
ACKNOWLEDGMENTS
We thank Dr. R. Sauvé and Dr. M. Park for providing the BAEC and 293T cells, respectively, and Dr. S.-S. Yoon for the generous gift of the caveolin-1 construct. This study was funded by a Cancer Research Society grant to R.B.
Abbreviations:
- BAEC
bovine aortic endothelial cell
- CD
β-cyclodextrin
- EGF
epidermal growth factor
- PDGF
platelet-derived growth factor
- Tyr(P)
phosphotyrosine
- VEGF
vascular endothelial growth factor
- VEGFR-2
vascular endothelial growth factor receptor-2
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–07–0379. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–07–0379.
This study was funded by a Cancer Research Society grant to R. B.
REFERENCES
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem, 1997;272:15443–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J. 2001;360:255–264. doi: 10.1042/0264-6021:3600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RGW. The caveolae membrane system. Annu Rev Biochem, 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Borges E, Jan Y, Ruoslahti E. PDGF-receptor-b and VEGF-receptor-2 bind to the β-3 integrin through its extracellular domain. J Biol Chem. 2000;275:39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- Brown J, Reading SJ, Jones S, Fitchett CJ, Howl J, Martin A, Longland CL, Michelangeli F, Dubrova YE, Brown CA. Critical evaluation of ECV304 as a human endothelial cell model defined by genetic analysis and functional responses: a comparison with the human bladder cancer derived epithelial cell line T24/83. Lab Invest. 2000;80:37–45. doi: 10.1038/labinvest.3780006. [DOI] [PubMed] [Google Scholar]
- Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochem Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine14: recruitment of C-terminal Src kinase. J Biol Chem. 2002;277:8771–8774. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- Caselli A, Taddei ML, Manao G, Camici G, Ramponi G. Tyrosine-phosphorylated caveolin is a physiological substrate of the low Mr protein-tyrosine phosphatase. J Biol Chem. 2001;276:18849–18854. doi: 10.1074/jbc.M100705200. [DOI] [PubMed] [Google Scholar]
- Chang W-J, Rothberg KG, Kamen BA, Anderson RGW. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J Cell Biol. 1992;118:63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997a;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997b;272:30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- Demeule M, Jodoin J, Gingras D, Béliveau R. P-Glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 2000;466:219–224. doi: 10.1016/s0014-5793(00)01087-5. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin αvβ5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP. Reciprocal regulation of neu tyrosine kinase activity and caveolin-1 protein expression in vivo and in vitro. Implications for human breast cancer. J Biol Chem. 1998;273:20448–20455. doi: 10.1074/jbc.273.32.20448. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Lisanti MP. Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5′ promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett. 1999;48:221–230. doi: 10.1016/s0014-5793(99)00365-8. [DOI] [PubMed] [Google Scholar]
- Erpel T, Courtneidge SA. Src family protein tyrosine kinases and cellular signal transduction pathways. Curr Opin Cell Biol. 1995;7:176–182. doi: 10.1016/0955-0674(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Venema VJ, Venema RC, Tsai N, Behzadian MA, Caldwell RB. VEGF-induced permeability increase is mediated by caveolae. Invest Ophthal Vis Sci. 1999b;40:157–167. [PubMed] [Google Scholar]
- Feng Y, Venema VJ, Venema RC, Tsai N, Caldwell RB. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem Biophys Res Commun. 1999a;256:192–197. doi: 10.1006/bbrc.1998.9790. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:149–153. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Fuh G, Li B, Crowley C, Cunningham B, Wells JA. Requirements for binding and signaling of the kinase domain receptor for vascular endothelial growth factor. J Biol Chem. 1998;273:11197–11204. doi: 10.1074/jbc.273.18.11197. [DOI] [PubMed] [Google Scholar]
- Fujioka K, Azuma N, Kito H, Gahtan V, Esato K, Sumpio BE. Role of caveolin in hemodynamic force-mediated endothelial changes. J Surg Res. 2000;92:7–10. doi: 10.1006/jsre.2000.5838. [DOI] [PubMed] [Google Scholar]
- Furuchi T, Anderson RGW. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK) J Biol Chem. 1998;273:21099–21104. doi: 10.1074/jbc.273.33.21099. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbert HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Gingras D, Gauthier F, Lamy S, Desrosiers RR, Béliveau R. Localization of RhoA GTPase to endothelial caveolae-enriched membrane domains. Biochem Biophys Res Commun. 1998;247:888–893. doi: 10.1006/bbrc.1998.8885. [DOI] [PubMed] [Google Scholar]
- Gingras D, Lamy S, Béliveau R. Tyrosine phosphorylation of the vascular endothelial-growth-factor receptor-2 (VEGFR-2) is modulated by Rho proteins. Biochem J. 2000;348:272–280. [PMC free article] [PubMed] [Google Scholar]
- Gitay-Goren H, Soker S, Vlodavsky I, Neufeld G. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J Biol Chem. 1992;267:6093–6098. [PubMed] [Google Scholar]
- Griffoni C, Spisni E, Santi S, Riccio M, Guarnieri T, Tomasi V. Knockdown of caveolin-1 by antisense oligonucleotides impairs angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2000;276:756–761. doi: 10.1006/bbrc.2000.3484. [DOI] [PubMed] [Google Scholar]
- Guo D, Jia Q, Song H-Y, Warren RS, Donner DB. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J Biol Chem. 1995;270:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin throughout Flk-1/KDR activation of c-src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- Kim YN, Wiepz GJ, Guadarrama AG, Bertics PJ. Epidermal growth factor-stimulated tyrosine phosphorylation of caveolin-1. Enhanced caveolin-1 tyrosine phosphorylation following aberrant epidermal growth factor receptor status. J Biol Chem. 2000;275:7481–7491. doi: 10.1074/jbc.275.11.7481. [DOI] [PubMed] [Google Scholar]
- Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The α-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271:3863–3868. [PubMed] [Google Scholar]
- Liu J, Razani B, Tang S, Terman BI, Ware JA, Lisanti MP. Angiogenesis activators and inhibitors differentially regulate caveolin-1 expression and caveolae formation in vascular endothelial cells. Angiogenesis inhibitors block vascular endothelial growth factor-induced down-regulation of caveolin-1. J Biol Chem. 1999;274:15781–15785. doi: 10.1074/jbc.274.22.15781. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang XB, Park DS, Lisanti MP. Caveolin-1 expression enhances endothelial capillary tubule formation. J Biol Chem. 2002;277:10661–10668. doi: 10.1074/jbc.M110354200. [DOI] [PubMed] [Google Scholar]
- Liu J, Ying Y, Anderson RGW. Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc Natl Acad Sci USA. 1997;94:13666–13670. doi: 10.1073/pnas.94.25.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Ying Y, Ko YG, Anderson RGW. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- Mastick CC, Brady MJ, Saltiel AR. Insulin stimulates the tyrosine phosphorylation of caveolin. J Cell Biol. 1995;129:1523–1531. doi: 10.1083/jcb.129.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastick CC, Saltiel AR. Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3–L1 cells. J Biol Chem. 1997;272:20706–20714. doi: 10.1074/jbc.272.33.20706. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:RE21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- Mendel DB, Laird AD, Smolich BD, Blake RA, Liang C, Hannah AL, Shaheen RM, Ellis LM, Weitman S, Shawver LK, Cherrington JM. Development of SU5416, a selective small molecule inhibitor of VEGF receptor tyrosine kinase activity, as an anti-angiogenesis agent. Anticancer Drug Des. 2000;15:29–41. [PubMed] [Google Scholar]
- Mineo C, Gill GN, Anderson RGW. Regulated migration of epidermal growth factor receptor from caveolae. J Biol Chem. 1999;274:30636–30643. doi: 10.1074/jbc.274.43.30636. [DOI] [PubMed] [Google Scholar]
- Mineo C, James GL, Smart EJ, Anderson RGW. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- Nomura R, Fujimoto T. Tyrosine-phosphorylated caveolin-1: immunolocalization and molecular characterization. Mol Biol Cell. 1999;10:975–986. doi: 10.1091/mbc.10.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, Schnitzer JE. Segregation of heterotrimeric G protein in cell surface microdomains. Mol Biol Cell. 2001;12:685–698. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos A, Garcîa-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angigenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JG, Granger HJ, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling formetabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem. 2001;276:9670–9678. doi: 10.1074/jbc.M007454200. [DOI] [PubMed] [Google Scholar]
- Patterson C, Perrella MA, Hsieh CM, Yoshizumi M, Lee ME, Haber E. Cloning and functional analysis of the promoter for KDR/flk-1, a receptor for vascular endothelial growth factor. J Biol Chem. 1995;270:23111–23118. doi: 10.1074/jbc.270.39.23111. [DOI] [PubMed] [Google Scholar]
- Pol A, Calvo M, Enrich C. Isolated endosomes from quiescent rat liver contain the signal transduction machinery. Differential distribution of activated Raf-1 and Mek in the endocytic compartment. FEBS Lett. 1998;441:34–38. doi: 10.1016/s0014-5793(98)01517-8. [DOI] [PubMed] [Google Scholar]
- Pol A, Lu A, Pons M, Peiro S, Enrich C. Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation. Role of cholesterol and actin cytoskeleton. J Biol Chem. 2000;275:30566–30572. doi: 10.1074/jbc.M001131200. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-b-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rousseau, S., Houle, F., Kotanides, H., Witte, L., Waltenberger, J., Landry, J., and Huot, J. (2000). Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J. Biol. Chem., 10661–10672. [DOI] [PubMed]
- Schlaeppi JM, Wood JM. Targeting vascular endothelial growth factor (VEGF) for anti-tumor therapy, by anti-VEGF neutralizing monoclonal antibodies or by VEGF receptor tyrosine-kinase inhibitors. Cancer Metastasis Rev. 1999;18:473–481. doi: 10.1023/a:1006358220123. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Lisanti MP. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 c-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem. 2000a;275:21605–21617. doi: 10.1074/jbc.M002558200. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Pestell RG, Lisanti MP. Caveolins in cholesterol trafficking and signal transduction: implications for human disease. Front Biosci. 2000b;5:D929–D937. doi: 10.2741/schlegel. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying Y, Donzell WC, Anderson RGW. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying Y-S, Mineo C, Anderson RGW. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Suda K, Rothen-Rutishausser B, Gunthert M, Wunderli-Allenspach H. Phenotypic characterization of human umbilical vein endothelial (ECV304) and urinary carcinoma (T24) cells: endothelial versus epithelial features. In Vitro Cell Dev Biol Anim. 2001;37:505–514. doi: 10.1290/1071-2690(2001)037<0505:PCOHUV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. JBiol Chem. 1996;271:603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272:25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, Alexander RW. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells. role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- Vincent L, Chen W, Hong L, Mirshahi F, Mishal Z, Mirshahi-Khorassani T, Vannier JP, Soria J, Soria C. Inhibition of endothelial cell migration by cerivastatin, an HMG-CoA reductase inhibitor: contribution to its anti-angiogenic effect. FEBS Lett. 2001;495:159–166. doi: 10.1016/s0014-5793(01)02337-7. [DOI] [PubMed] [Google Scholar]
- Volonte D, Galbiati F, Pestell RG, Lisanti MP. Cellular stress induces the tyrosine phosphorylation of caveolin-1(Tyr(14)) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J Biol Chem. 2001;276:8094–8103. doi: 10.1074/jbc.M009245200. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Wiechen K, Sers C, Agoulnik A, Arlt K, Dietel M, Schlag PM, Schneider U. Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am J Pathol. 2001;158:833–839. doi: 10.1016/S0002-9440(10)64031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M, Pellicer A, Silverstein R, Axel R, Urlaub G, Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci USA. 1979;76:1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Aiello LP, Ishii H, Jiang ZY, Park DJ, Robinson GS, Takagi H, Newsome WP, Jirousek MR, King GL. Characterization of vascular endothelial growth factor's effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest. 1996;98:2018–2026. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Toya Y, Jensen RA, Ishikawa Y. Caveolin is an inhibitor of platelet-derived growth factor receptor signaling. Exp Cell Res. 1999;247:380–388. doi: 10.1006/excr.1998.4379. [DOI] [PubMed] [Google Scholar]
- Zhang W, Razani B, Altschuler Y, Bouzahzah B, Mostov KE, Pestell RG, Lisanti MP. Caveolin-1 inhibits epidermal growth factor-stimulated lamellipod extension and cell migration in metastatic mammary adenocarcinoma cells (MTLn3). Transformation suppressor effects of adenovirus-mediated gene delivery of caveolin-1. J Biol Chem. 2000;275:20717–20725. doi: 10.1074/jbc.M909895199. [DOI] [PubMed] [Google Scholar]