Abstract
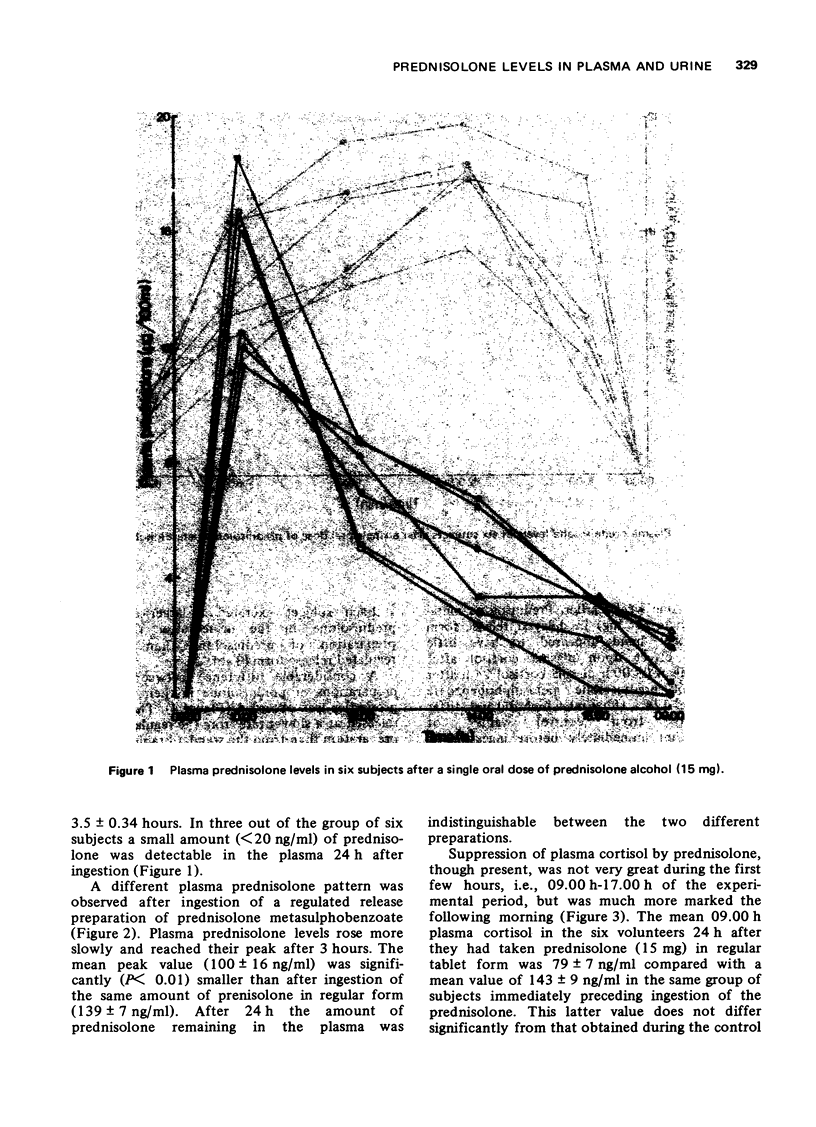
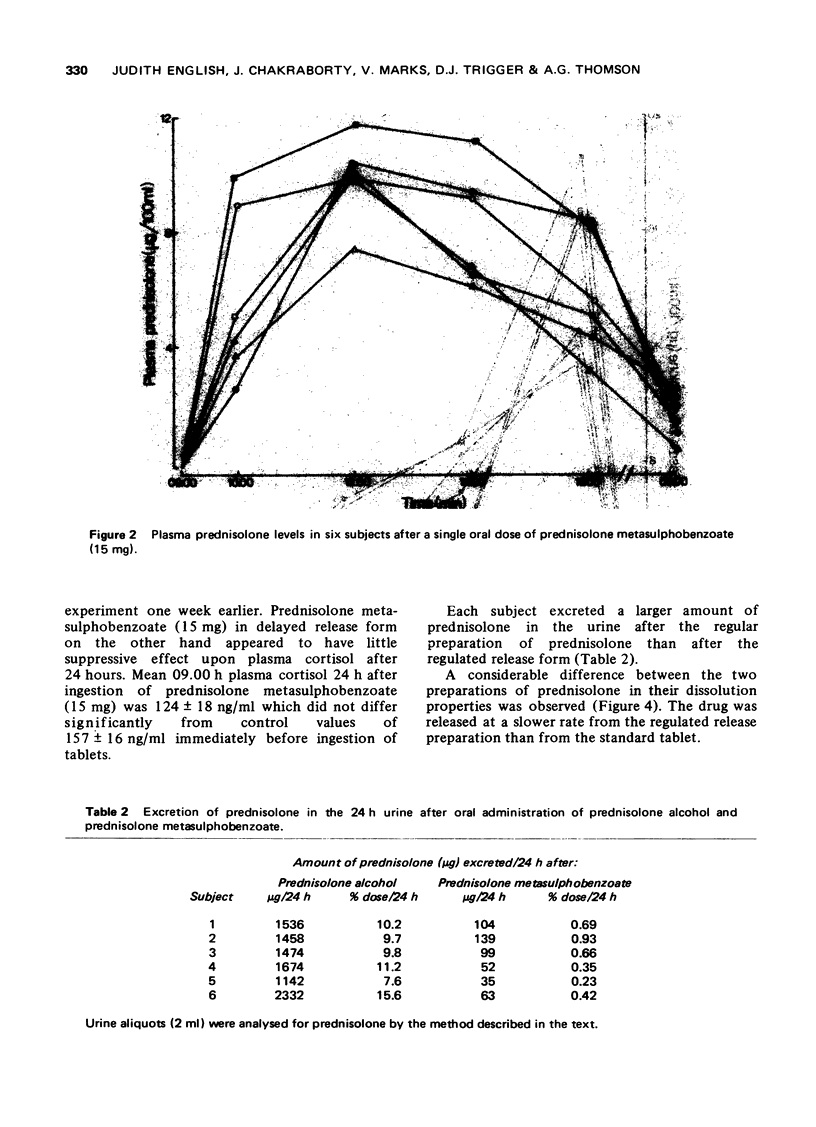
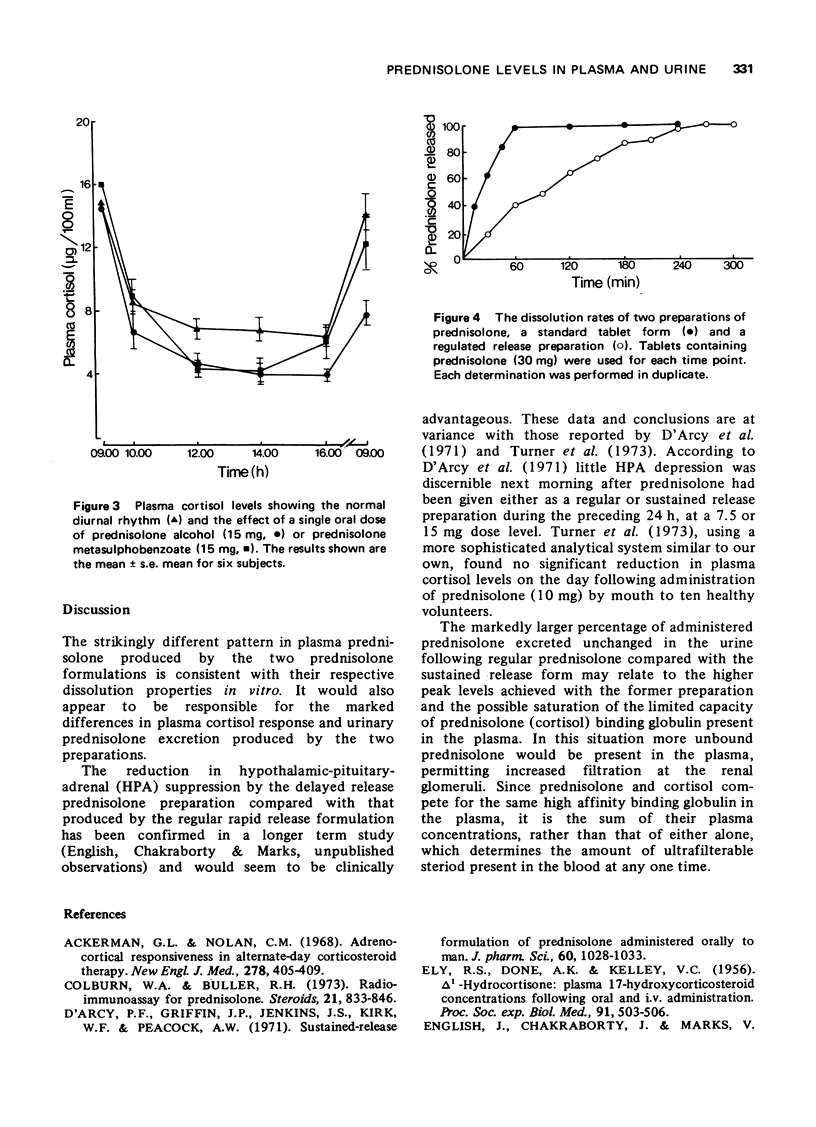
1. A competitive protein binding method was used to measure prednisolone and cortisol in blood and urine of volunteers given prednisolone by mouth (15 mg) in the standard tablet form and a fortnight later a regulated release formulation of prednisolone metasulphobenzoate containing an equivalent amount of prednisolone. 3. Plasma prednisolone levels rose rapidly after the standard tablet and more slowly after the regulated release form. The normal activity of the hypothalamic-pituitary-adrenal axis as measured by the 09.00 h plasma cortisol concentration was present 24 h after ingestion of the regulated release preparation. In contrast, the 09.00 h plasma cortisol level was reduced in subjects 24 h after receiving prednisolone in the standard tablet form.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman G. L., Nolsn C. M. Adrenocortical responsiveness after alternate-day corticosteroid therapy. N Engl J Med. 1968 Feb 22;278(8):405–409. doi: 10.1056/NEJM196802222780801. [DOI] [PubMed] [Google Scholar]
- Colburn W. A., Buller R. H. Radioimmunoassay for prednisolone. Steroids. 1973 Jun;21(6):833–846. doi: 10.1016/0039-128x(73)90124-4. [DOI] [PubMed] [Google Scholar]
- D'Arcy P. F., Griffin J. P., Jenkins J. S., Kirk W. F., Peacock A. W. Sustained-release formulation of prednisolone administered orally to man. J Pharm Sci. 1971 Jul;60(7):1028–1033. doi: 10.1002/jps.2600600705. [DOI] [PubMed] [Google Scholar]
- ELY R. S., DONE A. K., KELLEY V. C. Delta1-Hydrocortisone: plasma 17-hydroxycorticosteroid concentrations following oral and I.V. administration. Proc Soc Exp Biol Med. 1956 Mar;91(3):503–506. doi: 10.3181/00379727-91-22307. [DOI] [PubMed] [Google Scholar]
- Grant S. D., Forsham P. H., DiRaimondo V. C. Suppression of 17-hydroxycorticosteroids in plasma and urine by single and divided doses of triamcinolone. N Engl J Med. 1965 Nov 18;273(21):1115–1118. doi: 10.1056/NEJM196511182732101. [DOI] [PubMed] [Google Scholar]
- HARTER J. G., REDDY W. J., THORN G. W. STUDIES ON AN INTERMITTENT CORTICOSTEROID DOSAGE REGIMEN. N Engl J Med. 1963 Sep 19;269:591–596. doi: 10.1056/NEJM196309192691201. [DOI] [PubMed] [Google Scholar]
- Jenkins J. S., Sampson P. A. Conversion of cortisone to cortisol and prednisone to prednisolone. Br Med J. 1967 Apr 22;2(5546):205–207. doi: 10.1136/bmj.2.5546.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDBERG A. A., SLAUNWHITE W. R., Jr Differences in metabolism of prednisolone-C14 and cortisol-C14. J Clin Endocrinol Metab. 1957 Sep;17(9):1040–1050. doi: 10.1210/jcem-17-9-1040. [DOI] [PubMed] [Google Scholar]
- Sandberg D. H., Bacallao C. Z., Cleveland W. W. Measurement of plasma prednisolone by a competitive protein-binding assay. Biochem Med. 1970 Dec;4(5):383–390. doi: 10.1016/0006-2944(70)90066-9. [DOI] [PubMed] [Google Scholar]


