Abstract
Coronary vessels develop from a primary vascular network that differentiates in the subepicardium through a process of vasculogenesis, that is, self-assembly of mesenchymal vascular progenitors. Further growth of the subepicardial vascular plexus through a complex process of angiogenesis, vascular remodeling, and arterialization of specific branches gives rise to the definitive coronary system. This report is intended to summarize current knowledge on the origin of the coronary vascular progenitors and to provide new insights suggested by recent findings.
It has been established that the mesenchymal precursors of the vascular smooth muscle cells and the adventitial fibroblasts originate from an epithelial-mesenchymal transformation of the epicardial mesothelium. We report herein experimental evidence that the precursors of the coronary endothelium are also epicardium-derived cells (EPDCs). The evidence shown includes co-localization of mesothelial and endothelial molecular markers as well as cell lineage studies performed through direct labeling of the epicardial cells.
If this proposal is confirmed, the early EPDCs might be found to have a competence similar to that shown by the recently discovered bipotential vascular progenitor cells, which are able to differentiate into endothelium or smooth muscle depending on their exposure to VEGF or PDGF-BB. It is conceivable that the earliest EPDCs differentiate into endothelial cells in response to myocardially secreted VEGF, while subsequent EPDCs, recruited by the nascent capillaries via PDGFRβ signaling, differentiate into pericytes and smooth muscle cells. (Tex Heart Inst J 2002;29:243–9)
Key words: Cardiovascular system/embryology; cell differentiation/physiology; cell movement; chick embryo; coronary vessels/embryology; developmental biology; embryologic induction; endothelial growth factors; endothelium, vascular/cytology; fetal development; fibroblasts/cytology; muscle, smooth, vascular/cytology; platelet-derived growth factor; mesoderm/cytology; quail/embryology
The developing myocardium is working tissue that has to satisfy the metabolic needs of a fast-growing organism. The early myocardium is composed of a thin layer of cells that are in direct contact with the circulating blood, from which these myocardial cells obtain their basic metabolic supplies. As heart development progresses, the thickness of the myocardial layer increases and the circulation of venous blood through the trabecular spaces becomes insufficient for the metabolic demands of the myocardial cells. For that reason, a coronary vascular system develops to perfuse the cardiac wall. 1
For a long time, it was thought that the coronary arteries were outgrowths of the aortic root. Consequently, questioning the origin of the cellular precursors of the coronary vessels was pointless. Coronary endothelium and tunica media were considered to be mere derivatives of the aortic endothelial and smooth muscle cells. This view was challenged in the late eighties by the pioneering work of the Leiden group. 2 Descriptive and experimental findings showed that coronary endothelial precursors self-organize in the subepicardial space, forming a vascular plexus that only in later stages connects to the aorta. The presence of both endothelial and smooth muscle precursors of the coronary vessels in the proepicardium was soon demonstrated by retroviral labeling. 3,4
Therefore, early coronary development arose as a paradigm of vasculogenesis (primary formation of vessels by self-assembling of mesenchymal precursors). 5 Of course, angiogenesis (vascular growth by outgrowth of preexisting vessels) occurs in a 2nd phase of coronary development, wherein the final patterning of the coronary tree is established.
Since coronary vessels form by vasculogenesis, the precursors of the endothelium, smooth muscle, and fibroblasts of the coronary arteries must be found in the subepicardial mesenchyme, as stated above. We currently know that in avian and mammalian embryos there are 2 possible sources for this mesenchyme. The 1st is the migration of cells from extracardiac areas, either passively via transport inside the proepicardial matrix, or actively throughout the subepicardium from the area of the liver and transverse septum. In avian embryos, the attachment of the proepicardium to the myocardial wall involves the transfer of a number of mesenchymal cells that start the colonization of the subepicardial space. 6 In mammals, mesenchymal cells can also be transported in the matrix of the proepicardial vesicles that attach to the myocardial surface. 7
The 2nd possible source, which we will consider more deeply, is a localized transformation of the epicardial mesothelium into mesenchymal cells. 8–14 This epithelial-mesenchymal transition or transformation accounts for the major portion of the subepicardial mesenchymal cells, the epicardium-derived cells (EPDCs). It can be said, therefore, that the subepicardial mesenchyme is composed chiefly of EPDCs, without excluding the migration of cells from extracardiac areas.
Recent findings have demonstrated that the progenitors of coronary smooth muscle and fibroblasts are EPDCs. 12,14 For example, quail epicardial cells labeled in vitro and grafted into the pericardial cavity of chick embryos became coronary smooth muscle cells and perivascular fibroblasts. 13 When cultured in vitro, proepicardial cells expressed smooth muscle cell markers in a process that was stimulated by PDGF-BB and dependent on the expression of SRF (serum response factor). 15,16
The Origin of the Coronary Endothelium
The epicardial origin of the precursors of the coronary smooth muscle and the adventitial fibroblasts seems to be a well established fact. However, the origin of the endothelium is still controversial. Some authors have proposed that the primitive coronary endothelium differentiates from angioblasts that have migrated from the region of the liver and are already located in the proepicardial matrix. 17 hypothesis implies a migration of coronary angioblasts all over the developing heart, an occurrence that does not agree with recent studies about the in situ differentiation of splanchnic angioblasts. 18,19 We will present an alternative point of view that considers the coronary endothelial precursors, at least in part, as EPDCs, and we will show the evidence that supports this point of view.
We have stated that extracardiac mesenchymal cells can arrive at the subepicardium through the proepicardium. However, the proepicardium might also contain mesenchymal cells that have originated in the proepicardial mesothelium. Evidence in support of this origin has been reported by us elsewhere. 10,11 For example, most proepicardial mesenchyme is simultaneously immunoreactive to cytokeratin and vimentin. Cytokeratins are the proteins that constitute the intermediate filaments of the mesothelial and other epithelial cells. The presence of cytokeratin remnants in mesenchymal cells (whose intermediate filaments are made of vimentin) is a clear indication of their mesothelial origin. On the other hand, the proepicardial mesothelium of avian embryos also expresses markers of epithelial-mesenchymal transition, such as the transcription factor Slug 20 and the protein fibrillin-2. 11 Another transcription factor expressed by both proepicardial mesothelium and its mesenchymal derivatives is the protein WT1, 21 which can play an important role in the differentiation of the EPDCs, as we will discuss below.
Therefore, most of the subepicardial mesenchyme seems to originate from the transformation of mesothelial cells into mesenchymal cells, either in the proepicardium, before or during its attachment to the myocardial wall, or in the newly formed epicardium. The molecular regulation of this epithelial-mesenchymal transformation remains unclear. However, it seems certain that the epicardial transformation occurs in response to a signal produced by the myocardium. These phenotypical changes can be induced by members of the fibroblast-growth-factor family, as suggested by a number of in vitro assays, 22 without excluding the possibility of preliminary activation of the epicardial cells by BMPs. Downstream of the triggering mechanism, the zinc-finger transcription factors Slug and Snail, in avian and mammalian embryos respectively, seem to be main transcriptional regulators of the events of epithelio-mesenchymal transition. 23,24 Slug and Snail proteins repress expression of adhesion molecules such as desmoplakins, desmogleins, 25 and E-cadherin. 26,27 On the other hand, the Wilms' tumor protein WT1, widely expressed in the epicardium and subepicardium (Figs. 1G and H), is likely to be essential in keeping the EPDC population in an undifferentiated state. 21 Finally, FOG-2, a cofactor of the GATA transcription factors that is expressed in cardiac tissue, has been shown to be essential for the generation of coronary progenitors from the epicardium. 28
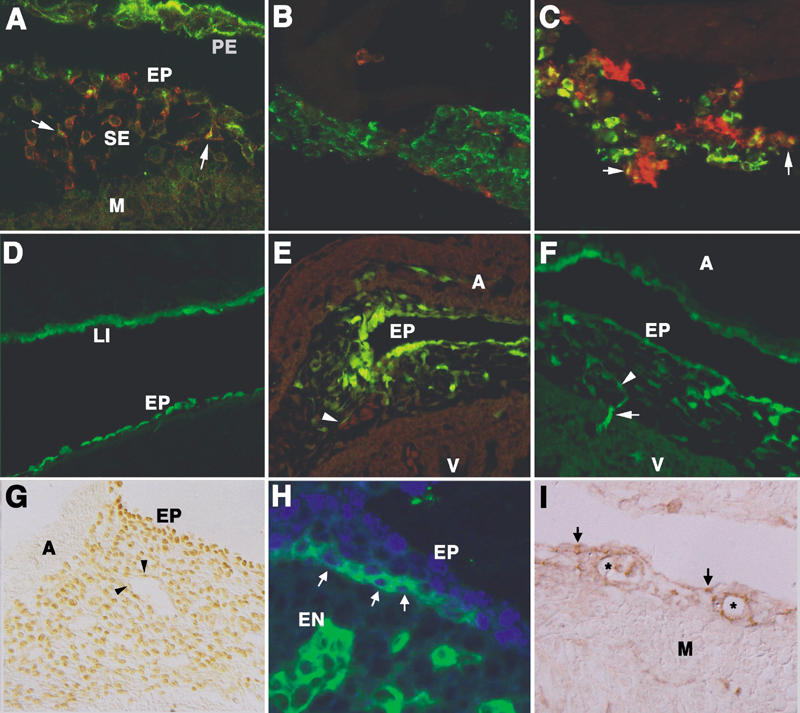
Fig. 1 Origin of the coronary endothelium from epicardium-derived cells and from circulating progenitor cells. A) Transplantation of a quail proepicardium into the pericardial cavity of a chick embryo generates a quail epicardium (EP) on the chick myocardium (M). Mesothelial cells (labeled in green due to cytokeratin immunostaining) are migrating into the subepicardium (SE). The endothelial marker QH1 (in red) co-localizes with cytokeratin (arrows). B, C) Quail proepicardial explants cultured on Matrigel™ for 3 h (B) and 24 h (C). Mesothelial (green) and vascular (red) cells can be identified by cytokeratin and QH1 staining, respectively. Note the abundant co-localization of the markers in the proepicardial tissue cultured for 24 h (arrows in C). D–F) Chick epicardial labeling with the carboxyfluorescein CCFSE. After 3 h, only mesothelial cells are labeled in the epicardium (EP) and the liver mesothelium (LI), as shown in D. However, after 48 h, epicardium-derived cells become fluorescent and form vascular structures (arrowheads in E and F). The image in E was also taken in the red channel (shown here) to reveal the presence of erythrocytes. In F, note the myocardial invasion by vascular cells (arrow). G) Wilms' tumor protein WT1 immunostaining shows the presence of this mesothelial marker in most of the subepicardial mesenchyme of an HH27 chick embryo. Note the faint labeling of some endothelial cells (arrowheads). H) Co-localization (arrows) of WT1 (blue) and the endothelial marker PECAM-1 (green) in a subepicardial cord-like structure of a mouse embryo (11.5 days post coitum). The epicardium (EP) and the endocardium (EN) express only WT1 and PECAM, respectively. I) Immunolocalization of PDGFRβ in the subepicardium of a mouse embryo (12.5 dpc). The developing vessels (asterisks), the subepicardial mesenchymal cells, and some mesothelial cells (arrows) are immunoreactive. The myocardium (M) is PDGFRβ-negative.
A = atrium; PE = pericardium; V = ventricle
Mesothelium-derived cells constitute most, if not all, of the subepicardial mesenchyme. Does this mean that the precursors of the coronary endothelium are derived from EPDCs? We will summarize the arguments that we have collected from our own work, as well as from the literature, to discuss this hypothesis in the wider context of the coelomic origin of the circulatory system in vertebrates:
1) We have localized the earliest subepicardial endothelial progenitors, identified by the endothelial markers QH1 in quail and VEGFR2 (Flk-1) in mice, and we have occasionally found in these cells some remains of cytokeratin, which suggests a mesothelial origin. 29 As we have said before, cytokeratin remnants persist for some time in mesenchymal cells derived from the coelomic epithelium. Moreover, we have transplanted quail proepicardia into the pericardial cavity of chick embryos, thereby giving rise to quail epicardium on the chick myocardial surface. This manipulation seems to accelerate the differentiation of EPDCs, and co-localization of QH1 and cytokeratin becomes usual in subepicardial cells of quail-chick chimeras (Fig. 1A).
2) Culture of quail proepicardial explants on Matrigel™ gives rise to conspicuous co-localization of cytokeratin and QH1 after 24 h of culture (Figs. 1B and C). Matrigel, a reconstituted basement membrane, promotes endothelial differentiation, a fact that can explain the quick expression of QH1 by mesothelial cells that can still be identified by their cytokeratin content.
3) We have specifically labeled the proepicardial mesothelium with retrovirus (avoiding the tagging of proepicardial mesenchyme). Chick proepicardia, infected in the surface by β-Gal transfecting retroviruses, were isolated and grafted into the pericardial cavities of other chick embryos. This technique precluded infection of cells other than donor mesothelial cells, as shown by control embryos reincubated for only 8 to 12 h. After reincubation to stages HH29-35, fibroblasts, vascular smooth muscle, and coronary endothelial cells were labeled (data not shown).
4) We have also labeled the epicardial mesothelium with the fluorescent carboxyfluorescein dye CCFSE, 30 which labels endothelial cells after 48 to 96 h (Figs. 1D–F). This experiment has been performed in ovo, through direct injection of the dye into the pericardial cavity, as well as in vitro, by culturing excised hearts of embryos that were labeled in toto. Control embryos or excised hearts incubated for shorter periods show that the fluorescent dye cannot cross the epicardial barrier.
5) The mesothelial marker WT1 is widely expressed by the EPDCs. Although it seems to be downregulated as EPDCs differentiate, it can still be found in some endothelial cells of the subepicardial capillary-like structures (Fig. 1G). These cells coexpress WT1 with vascular markers such as VEGFR2 or PECAM-1 (Fig. 1H).
There is wide experimental support for the hypothesis that EPDCs can differentiate not only into smooth muscle and fibroblasts, but also into endothelial cells. The development of the coronary endothelium could therefore be regarded as an example of type I vasculogenesis (differentiation of angioblasts in situ) instead of type II vasculogenesis, a process characterized by the migration of angioblasts into the developing organ from external sources. 31
The possibility of an alternative origin of cardiac angioblasts, from the liver primordium, cannot be discarded. However, it is important to remark that the liver is the only organ in which differentiation of mesothelium-derived cells into endothelial cells has been described in the literature. 32 The differentiation of the endothelium of the hepatic sinusoids must be a very fast process, due to the rapid growth of the liver. This accelerated differentiation would explain the transient presence of cytokeratin remains in the liver endothelial cells (co-localizing with QH1 in quail embryos). 10,33 This is consistent with the reported expression of WT1, a mesothelial marker, in the endothelial cells of the liver of mice embryos. 34 On the other hand, when we label the liver mesothelium with CCFSE, we find fluorescent sinusoidal cells after only 24 h. 33 We can then conclude that mesothelium-derived cells also differentiate into endothelial cells in the liver. Therefore, a migration of angioblasts from the liver to the subepicardium would be compatible with the hypothesis of a mesothelial origin of the primary coronary endothelium.
Are Epicardium-Derived Cells Bipotential Vascular Progenitors?
We think that epicardium-derived cells constitute a population of pluripotential cells able to differentiate into endothelium, smooth muscle, and fibroblasts. If this working hypothesis is confirmed, EPDCs would exhibit a developmental potential similar to that displayed by the stem-cell-derived bipotential vascular progenitor whose existence has recently been demonstrated. 35
According to the model of the bipotential vascular progenitor, 2 tyrosine kinase receptor-binding proteins, VEGF and PDGF-BB, are the most important regulators of this process. Culturing the bipotential progenitors in the presence of VEGF gives rise to endothelial cells. When the progenitors are exposed to PDGF-BB or to serum, they differentiate into smooth muscle cells. Exposure to both growth factors originates mixed cultures composed of endothelium and smooth muscle. 35
It is tempting to suggest a model (Fig. 2) in which myocardium-derived FGFs and BMPs induce the epicardial-mesenchymal transition, generating bipotential EPDC. In a 1st stage, myocardium- and epicardium-derived VEGF 36 would induce the differentiation of EPDCs into endothelial cell precursors. In turn, these angioblasts and endothelial cells would promote the differentiation of further EPDCs into the smooth-muscle-cell lineage through a PDGF-BB and PDGFRβ signaling mechanism. 37,38 In fact, the immature endothelium of the nascent capillaries expresses PDGFRβ 39 (Fig. 1I); this is consistent with the hypothesis of an origin from a bipotential progenitor (which would express receptors for both growth factors, PDGF-BB and VEGF). On the other hand, it is interesting that the over-expression of VEGF causes not only oversized epicardial vessels but also an under-development of the myocardium. 40 This observation suggests that the fine balance that might exist between 3 lineages derived from EPDC—that is, endothelium, smooth muscle and undifferentiated cells invading the myocardium—is broken due to an excessive differentiation into the endothelial cell type. The subsequent scarcity of cells invading the myocardium might preclude the formation of the compact ventricular layer. 21,41

Fig. 2 Diagram depicting the model suggested for the origin and differentiation of the coronary precursor cells. A) Epicardial cells transform into mesenchyme in response to signals secreted by the myocardium, which possibly belong to the families of fibroblast growth factors (FGF) and bone morphogenetic proteins (BMP). B) These early epicardium-derived cells, induced by the myocardially and epicardially secreted vascular endothelial growth factor (VEGF), differentiate into the endothelial cells of the primary vascular plexus. C) Platelet-derived growth factor-BB (PDGF-BB), released by this nascent endothelium, induces the recruitment of subsequent epicardium-derived cells, which differentiate into perivascular cells (smooth muscle and pericytes).
It is important to remark that, according to the properties of the bipotential vascular progenitors described by Yamashita and colleagues, 35 if we culture EPDCs with serum, in the absence of VEGF, we should expect a differentiation into smooth muscle cells. This can explain why the experiments performed with EPDCs in vitro have resulted only in smooth-muscle differentiation. 13,15
Origin of Endothelium from Coelomic Epithelium: A Phylogenetic Perspective
We think that the origin of vascular progenitors from the coelomic epithelium must be regarded in a phylogenetic and comparative context. It is important to emphasize that the endothelium is an exceptional cell type among animals. Most of the circulatory systems of the invertebrates are composed of hemal cavities located between basement membranes, which is to say that they are not lined by a cellular epithelium. The vascular basement membranes belong to endodermal or coelomic epithelia or, more frequently, to myoepithelial cells derived from the coelomic epithelium. Invertebrate microvessels are usually composed only of myoepithelial cells, which regulate the blood flow through a lumen lined by their basement membranes. Only the nemerteans, a group of marine worms, bear a system of epithelium-lined circulatory cavities, which were considered true blood vessels. However, detailed embryologic studies demonstrated that these cavities were coelomic derivatives. 42
The only case of endothelial-like cells in invertebrates is found among the cephalopods. The circulatory vessels of these mollusks are lined by cells that adhere to the basement membranes, forming a discontinuous cell layer. Interestingly, these cells are similar to the “amoebocytes,” which adhere to and migrate upon the vascular basement membranes of other invertebrates, such as the annelids. 43 Amoebocytes are circulating cells, probably derived from the coelomic epithelium. 44
With all of these comparative data in mind, we can speculate about the origin of the unique vertebrate endothelium. It is conceivable that circulating amoebocytes acquired, in the ancestor of vertebrates, specialized intercellular junctions, in this manner reacquiring an epithelial phenotype and lining all of the vascular basement membranes. The ability of these primitive endothelial cells 1) to transiently regain their ancestral migratory phenotype and 2) to produce their own basement membrane would have allowed them to invade all of the embryonic body through angiogenic growth. Note that smooth-muscle and blood cells, in this view, are phylogenetically older than endothelium.
This hypothesis may explain why smooth-muscle, endothelial, and blood cells seem to share cell progenitors, the “hemangioblast” and the bipotential vascular progenitor. 35,45 A number of striking features of endothelial cells (reviewed in Muñoz-Chápuli and associates 46) might also be explained in this way.
If the ability of the coelomic epithelium to give rise to hemangioblasts or bipotential vascular progenitor cells (or both) is confirmed, it will open new perspectives in biotechnology and tissue engineering. For tissue engineering, it will be crucial to determine definitively if this ability is retained or lost by the adult mesothelium.
References
Key to Abbreviations
BMP: Bone morphogenetic protein
CCFSE: 5,6 Carboxy 2′,7′ dichlorofluorescein diacetate succynimidil ester
EPDC: Epicardium-derived cells
FGF: Fibroblast growth factor
FOG-2: Friend of GATA (transcription factor)-2
GATA: Consensus motif of a family of transcription factors
HH: Hamburger & Hamilton stage
PDGF: Platelet-derived growth factor
PDGFR: Platelet-derived growth factor receptor
PECAM: Platelet endothelial cell adhesion molecule
QH1: Quail hemangioblast antigen (monoclonal antibody)
SRF: Serum response factor
VEGF: Vascular endothelial growth factor
VEGFR (Flk-1): Vascular endothelial growth factor receptor (also called fetal liver kinase-1)
WT1: Wilms' tumor associated protein
Footnotes
Address for reprints: Dr. Ramón Muñoz-Chápuli, Department of Animal Biology, Faculty of Science, University of Málaga, E-29071 Málaga, Spain
This paper has its basis in a presentation made at the symposium Coronary Artery Anomalies: Morphogenesis, Morphology, Pathophysiology, and Clinical Correlations, held on 28 Feb.–1 March 2002, at the Texas Heart® Institute, Houston, Texas.
The authors have provided a short key to abbreviations at the end of the paper.
This work was supported by grants PM98-0219 and 1FD97-0693 (Ministerio de Educación y Cultura, Spain). Mauricio González is the recipient of a fellowship from Ministerio de Educación y Cultura.
References
- 1.Tomanek RJ. Formation of the coronary vasculature: a brief review. Cardiovasc Res 1996;31:E46–51. [PubMed]
- 2.Bogers AJ, Gittenberger-de Groot AC, Poelmann RE, Peault BM, Huysmans HA. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat Embryol (Berl) 1989;180:437–41. [DOI] [PubMed]
- 3.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A 1992;89:9504–8. [DOI] [PMC free article] [PubMed]
- 4.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 1996;174:221–32. [DOI] [PubMed]
- 5.Poole TJ, Coffin JD. Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish embryonic vascular pattern. J Exp Zool 1989;251:224–31. [DOI] [PubMed]
- 6.Viragh S, Gittenberger-de Groot AC, Poelmann RE, Kalman F. Early development of quail heart epicardium and associated vascular and glandular structures. Anat Embryol (Berl) 1993;188:381–93. [DOI] [PubMed]
- 7.Van den Eijnde SM, Wenink AC, Vermeij-Keers C. Origin of subepicardial cells in rat embryos. Anat Rec 1995;242:96–102. [DOI] [PubMed]
- 8.Markwald R, Eisenberg C, Eisenberg L, Trusk T, Sugi Y. Epithelial-mesenchymal transformations in early avian heart development. Acta Anat (Basel) 1996;156:173–86. [DOI] [PubMed]
- 9.Munoz-Chapuli R, Macias D, Ramos C, Gallego A, Andres AV. Development of the subepicardial mesenchyme and the early cardiac vessels in the dogfish (Scyliorhinus canicula). J Exp Zool 1996;275:95–111.
- 10.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. Contribution of the primitive epicardium to the subepicardial mesenchyme in hamster and chick embryos. Dev Dyn 1997;210:96–105. [DOI] [PubMed]
- 11.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail-chick chimera study. Dev Biol 1998;200:57–68. [DOI] [PubMed]
- 12.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res 1998;82:1043–52. [DOI] [PubMed]
- 13.Dettman RW, Denetclaw W Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol 1998;193:169–81. [DOI] [PubMed]
- 14.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999;199:367–78. [DOI] [PubMed]
- 15.Landerholm TE, Dong XR, Lu J, Belaguli NS, Schwartz RJ, Majesky MW. A role for serum response factor in coronary smooth muscle differentiation from proepicardial cells. Development 1999;126:2053–62. [DOI] [PubMed]
- 16.Lu J, Landerholm TE, Wei JS, Dong XR, Wu SP, Liu X, et al. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Dev Biol 2001;240:404–18. [DOI] [PubMed]
- 17.Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bokenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res 1993;73:559–68. [DOI] [PubMed]
- 18.Drake CJ, Brandt SJ, Trusk TC, Little CD. TAL1/SCL is expressed in endothelial progenitor cells/angioblasts and defines a dorsal-to-ventral gradient of vasculogenesis. Dev Biol 1997;192:17–30. [DOI] [PubMed]
- 19.Cox CM, Poole TJ. Angioblast differentiation is influenced by the local environment: FGF-2 induces angioblasts and patterns vessel formation in the quail embryo. Dev Dyn 2000;218:371–82. [DOI] [PubMed]
- 20.Carmona R, Gonzalez-Iriarte M, Macias D, Perez-Pomares JM, Garcia-Garrido L, Munoz-Chapuli R. Immunolocalization of the transcription factor Slug in the developing avian heart. Anat Embryol 2000;201:103–9. [DOI] [PubMed]
- 21.Carmona R, Gonzalez-Iriarte M, Perez-Pomares JM, Munoz-Chapuli R. Localization of the Wilm's tumour protein WT1 in avian embryos. Cell Tiss Res 2001;303:173–86. [DOI] [PubMed]
- 22.Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev Biol 2001;234:204–15. [DOI] [PubMed]
- 23.Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 1994;264:835–9. [DOI] [PubMed]
- 24.Sefton M, Sanchez S, Nieto MA. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development 1998;125:3111–21. [DOI] [PubMed]
- 25.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol 1997;137:1403–19. [DOI] [PMC free article] [PubMed]
- 26.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000;2:76–83. [DOI] [PubMed]
- 27.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000;2:84–9. [DOI] [PubMed]
- 28.Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, et al. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of the coronary vessels from epicardium. Cell 2000;101:729–39. [DOI] [PubMed]
- 29.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. Immunolocalization of the vascular endothelial growth factor receptor-2 in the subepicardial mesenchyme of hamster embryos: identification of the coronary vessel precursors. Histochem J 1998;30:627–34. [DOI] [PubMed]
- 30.Sun D, Griffith CM, Hay ED. Carboxyfluorescein as a marker at both light and electron microscope levels to follow cell lineage in the embryo. In: Twan RS, Lo CW, editors. Methods in molecular biology, Vol 135: Developmental biology protocols, Vol. 1. Totowa (NJ): Humana Press Inc; 2000. p. 357–63. [DOI] [PubMed]
- 31.Coffin JD, Poole TJ. Endothelial cell origin and migration in embryonic heart and cranial blood vessel development. Anat Rec 1991;231:383–95. [DOI] [PubMed]
- 32.Douarin NM. An experimental analysis of liver development. Med Biol 1975;53:427–55. [PubMed]
- 33.Munoz-Chapuli R., Carmona R, Gonzalez-Iriarte M, Perez-Pomares JM, Macias D, Atencia G, Aranda MJ. Origin of endothelial cells from mesothelial-derived mesenchymal cells in the liver of avian embryos. Int J Dev Biol 2001;45(Suppl):S155–6.
- 34.Moore AW, Schedl A, McInnes L, Doyle M, Hecksher-Sorensen J, Hastie ND. YAC transgenic analysis reveals Wilms' tumour 1 gene activity in the proliferating coelomic epithelium, developing diaphragm and limb. Mech Dev 1998;79:169–84. [DOI] [PubMed]
- 35.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000;408:92–6. [DOI] [PubMed]
- 36.Tomanek RJ, Ratajska A, Kitten GT, Yue X, Sandra A. Vascular endothelial growth factor expression coincides with coronary vasculogenesis and angiogenesis. Dev Dyn 1999;215:54–61. [DOI] [PubMed]
- 37.Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell 1996;87:1153–5. [DOI] [PubMed]
- 38.Kaminski WE, Lindahl P, Lin NL, Broudy VC, Crosby JR, Hellstrom M, et al. Basis of hematopoietic defects in platelet-derived growth factor (PDGF)-B and PDGF beta-receptor null mice. Blood 2001;97:1990–8. [DOI] [PubMed]
- 39.Shinbrot E, Peters KG, Williams LT. Expression of the platelet-derived growth factor beta receptor during organogenesis and tissue differentiation in the mouse embryo. Dev Dyn 1994;199:169–75. [DOI] [PubMed]
- 40.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular growth factor gene expression. Development 2000;127:3941–6. [DOI] [PubMed]
- 41.Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R, Wessels A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (SPDCs). Dev Biol. In press. [DOI] [PubMed]
- 42.Turbeville JM. Nemertinea. In: Harrison FW, Bogitsh BJ, editors. Microscopic anatomy of invertebrates. Vol 3: Platyhelminthes and Nemertinae. New York: Wiley-Liss; 1991. p. 285–328.
- 43.Jamieson BG. Oligochaeta. In: Harrison FW, Gardiner SL, editors. Microscopic anatomy of invertebrates. Vol 7: Annelida. New York: Wiley-Liss; 1992. p. 217–322.
- 44.Bossche JP, Jangoux M. Epithelial origin of starfish coelomocytes. Nature 1976;261:227–8. [DOI] [PubMed]
- 45.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development 1998;125:725–32. [DOI] [PubMed]
- 46.Munoz-Chapuli R, Perez-Pomares JM, Macias D, Garcia-Garrido L, Carmona R, Gonzalez M. Differentiation of hemangioblasts from embryonic mesothelial cells? A model on the origin of the vertebrate cardiovascular system. Differentiation 1999;64:133–41. [DOI] [PubMed]


