Abstract
This communication briefly reviews the role of angiogenic growth factors in myocardial vessel formation during development. The earliest signs of vascularization are the migration and differentiation of angioblasts from the epicardium and subepicardium into the myocardium. A regulator of this process is vascular endothelial growth factor (VEGF), which is probably triggered by hypoxia. The subsequent formation of vascular tubes is regulated by multiple growth factors: VEGF family members, fibroblast growth factors (FGFs), and angiopoietins and their receptors. Our studies on explanted quail hearts reveal that these growth factors are interdependent. We also have shown that a harmonic interplay of growth factors characterizes early postnatal development in rats. Neutralizing antibodies to either basic FGF (bFGF) or VEGF inhibit capillary formation, whereas arteriolar growth is markedly inhibited by bFGF, but not VEGF, neutralizing antibodies. Arteriolar diameter is also increased when anti-bFGF and anti-VEGF are administered in combination. Thus, the hierarchical development of the arteriolar vasculature depends on both of these growth factors; however, the establishment of arterioles, as reflected by length density, is dependent on bFGF but not on VEGF.
Finally, stretch of cardiac myocytes and endothelial cells serves as a stimulus for increases in growth factor and receptor proteins. We have shown that cyclic stretch of either cell type increases VEGF, and that endothelial cells respond to stretch by up-regulation of VEGF receptor-2 (VEGFR-2), and Tie-2 receptor. These results indicate that both mechanical and metabolic factors are primary stimuli for coronary angiogenesis. (Tex Heart Inst J 2002;29:250–4)
Key words: Coronary angiogenesis; coronary vessels/embryology; endothelial growth factors/antagonists & inhibitors; fibroblast growth factors/antagonists & inhibitors; endothelium, vascular; growth factor signaling; heart/growth & development
During the last decade, significant progress has been made in understanding how the coronary vasculature is formed during the embryonic, fetal, and neonatal periods. 1,2 Myocardial vascularization begins with vasculogenesis, which involves the formation of vascular tubes from angioblasts that differentiate into endothelial cells. This process is followed by coalescence of the tubes and the formation of branches. The latter constitutes angiogenesis and occurs via sprouting or intussusception (splitting of the vascular channel by ingrowth of endothelial cells). Arterioles and arteries form only after a capillary plexus that encircles the base of the aorta penetrates the aorta to give rise to the roots of the 2 main coronary arteries.
Considerable interest has developed concerning the source of the cells that contribute to the coronary vasculature. Proepicardial tissue, consisting of precursor cells that differentiate into mesothelial, endothelial, smooth muscle, and fibroblast cells, forms a gland-like structure between the sinus horns and the liver primordium. 3–6 These precursor cells cover the developing myocardium and 1) form the mesothelial layer of the epicardium and 2) migrate to form vascular structures and connective tissue. 7–9
Herein, we focus on the role of growth factors in regulating the processes of proliferation, migration, tube formation, and further assembly of the vascular wall. An understanding of these processes is essential for gaining insight into congenital anomalies of the coronary arteries (see reference 10 for a review of anomalies).
Tube Formation in the Embryonic Heart
The migration of endothelial precursor cells into the myocardium is followed by their assembly into vascular tubes. As documented by our studies in rats, an epicardial-to-endocardial vascular gradient develops 11 and is closely related to the deposition of specific components of the extracellular matrix. 12 At this early point in myocardial vascularization, basic fibroblast growth factor (bFGF) levels are high, 11 and vascular endothelial growth factor (VEGF) expression is closely related to sites of vascular tube formation. 13 Both growth factors have been found to stimulate vascular growth in the embryonic heart. 14 Moreover, the rate and extent of vascularization is related to the rate of myocardial growth. 15
Interplay of Growth Factors in the Regulation of Tube Formation
A number of growth factors and receptors play a role in myocardial vascularization. We have used quail embryonic heart explants to study the effects of growth factors on vasculogenesis and angiogenesis. 16 We begin by placing small portions of the ventricular apex onto a collagen gel and incubating them. Vascular tubes form in the 3-dimensional collagen matrix, which we stain with a quail endothelial cell (QH-1) antibody (Fig. 1). We then quantify the aggregate tube by using image analysis.
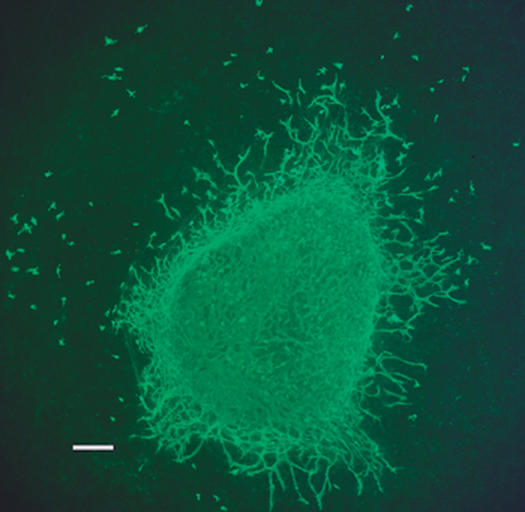
Fig. 1 Photomicrograph of an explanted quail heart (apical 1/3). Vascular tubes have formed in the collagen gel matrix and are seen here radiating outward from the heart. Some “free” endothelial cells are also visible in the gel matrix. A quail endothelial cell (QH-1) antibody was used to visualize these cells and tubes.
Bar = 200 μm
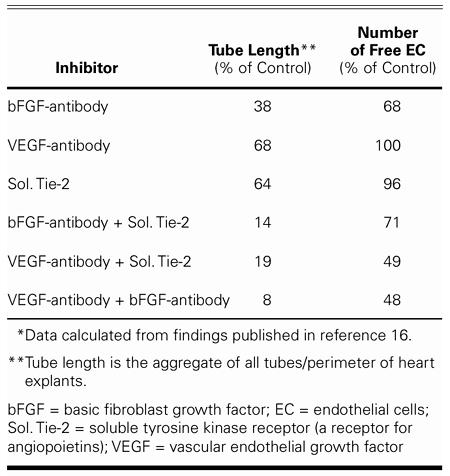
With this method, we were able to show that tube formation is inhibited by the addition of anti-bFGF or anti-VEGF antibodies, or by the soluble tyrosine kinase (Tie-2) receptor (a receptor for angiopoietins). A summary of these experiments is provided in Table I. Each of the 3 inhibitors attenuated tube formation; anti-bFGF had the most marked effect. When any 2 inhibitors were added to the explant cultures, tube formation was only 8% to 19% of the tube formation in the controls. This finding indicates that at least 3 growth factor families contribute to tube formation in the embryonic heart.
TABLE I. Inhibition of Coronary Vascularization in Explanted Quail Hearts* (n = 7–33 hearts per group)
As shown in Figure 2, we studied the stimulating effect of bFGF and VEGF by adding various doses of these growth factor proteins to the explant cultures. 16 Each enhanced tube formation; bFGF caused the greatest increase (6-fold). However, since effects of the 2 growth factors were not additive, we proceeded to study their interdependencies. The addition of anti-bFGF to the cultures prevented the increase associated with the addition of exogenous VEGF protein (Fig. 2). The addition of bFGF protein caused a 6-fold increase in tube formation, but the addition of VEGF neutralizing antibodies markedly attenuated tube formation. The tube formation induced by VEGF was also prevented by the addition of soluble Tie-2, the receptor for angiopoietins (Fig. 3). Thus, we were able to show that the effects of these growth factors on myocardial vascular tube formation are interdependent. These data strengthen the hypothesis that multiple growth factors regulate vascularization.
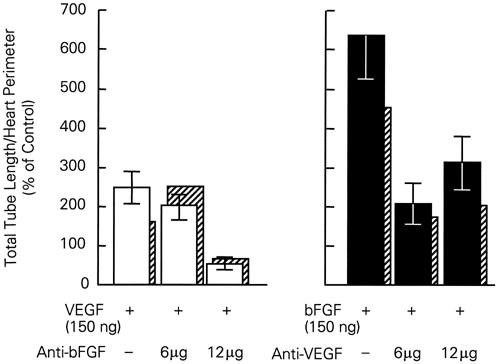
Fig. 2 Stimulation of tube formation in quail heart explants. Interdependency of VEGF and bFGF is documented. Neutralizing antibodies to bFGF prevent the increase in tube formation stimulated by VEGF. The increase in tube formation due to bFGF is markedly attenuated in the presence of anti-VEGF neutralizing antibodies. Data are means ± SEM and are calculated on the basis of 7 to 10 explants per group. The crosshatched bars are median values.
bFGF = basic fibroblast growth factor; VEGF = vascular endothelial growth factor
(From Tomanek RJ, Zheng W, Peters KG, Lin P, Holifield JS, Suvarna PR. Multiple growth factors regulate coronary embryonic vasculogenesis. Dev Dyn 2001;221:265–73. 16 Reproduced with permission from Developmental Dynamics © 2001, John Wiley & Sons, Inc.)
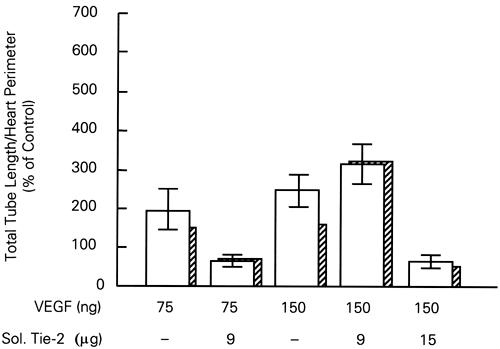
Fig. 3 Dependency of VEGF on angiopoietin. The 2- to 3-fold increase in quail heart explant tube formation associated with VEGF is prevented when soluble Tie-2 receptor, the receptor for angiopoietins, is added to the culture media. In each group, the mean ± SEM is calculated on the basis of 8 explants. The cross-hatched bars are median values.
Sol. Tie-2 = soluble tyrosine kinase receptor (a receptor for angiopoietins); VEGF = vascular endothelial growth factor
(From Tomanek RJ, Zheng W, Peters KG, Lin P, Holifield JS, Suvarna PR. Multiple growth factors regulate coronary embryonic vasculogenesis. Dev Dyn 2001;221:265–73. 16 Reproduced with permission from Developmental Dynamics © 2001, John Wiley & Sons, Inc.)
Our data lend further support to the idea that several angiogenic ligands and receptors contribute to myocardial vascularization. 17 We have found that at least 3 VEGF family members play a role in tube formation: VEGFs A, B, and C. Consistent with this finding, we have documented a role for VEGF receptors 1 and 2 (VEGFR-1 and VEGFR-2, or flt-1 and flk-1).
Early Postnatal Growth of the Coronary Vasculature
The postnatal period is characterized by rapid growth of the coronary vasculature. We tested the hypothesis that bFGF and VEGF interplay regulates the growth of the capillaries and arterioles. 18 During the 2nd week of postnatal life, neutralizing antibodies to 1) bFGF, 2) VEGF, or 3) a combination of the two were administered to rat pups. Each of the neutralizing growth factor antibodies attenuated capillary growth, as indicated by lower capillary length densities (data not shown). Arteriolar length density was not affected by anti-VEGF, but was markedly attenuated in the rats treated with anti-bFGF (Fig. 4).

Fig. 4 Effects of neutralizing antibodies on coronary arterioles. Arteriolar length density and diameters expressed as percent of control rat pups (vehicle-treated). Significant differences versus control (P ≤ 0.05) are indicated by (*).
bFGF = basic fibroblast growth factor; VEGF = vascular endothelial growth factor
(This figure was prepared from data published in reference 17.)
When the antibodies to VEGF and bFGF were given in combination, arteriolar length density was not affected, but the mean arteriolar diameter was significantly higher (Fig. 4). Examination of the diameter distributions revealed that the hierarchy of the vascular tree was altered in this group: there were fewer of the smallest diameter arterioles (10 μm and less) and a greater number of the largest class of arterioles (26–50 μm). As seen in Figure 5, treatment with the 2 neutralizing antibodies resulted in 81% fewer small-diameter arterioles and a 2.5-fold increase in the largest arterioles. Taken together, these findings illustrate at least 2 salient points. First, while both VEGF and bFGF modulate capillary growth, arteriolar angiogenesis, as indicated by the overall length of the arteriolar network, is controlled by bFGF. Therefore, bFGF plays a key role in the establishment of arteriolar channels. Second, the 2 growth factors demonstrate a harmonic interplay and thereby establish the hierarchy of the arteriolar tree. This conclusion is supported by data that show expansion in the diameters of both capillaries and arterioles when both growth factors are inhibited but not eliminated. Therefore, the presence of sufficient amounts of bFGF and VEGF is required to limit vessel size.
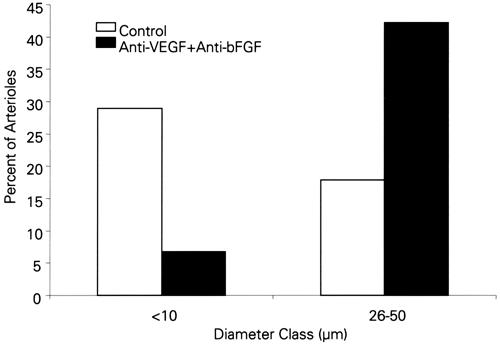
Fig. 5 Percent of small (10 μm and less) and large (26–50 μm) arterioles in hearts treated with a combination of anti-VEGF and anti-bFGF neutralizing antibodies.
bFGF = basic fibroblast growth factor; VEGF = vascular endothelial growth factor
(This figure was prepared from data published in reference 17.)
Metabolic Regulation of Coronary Vasculogenesis and Angiogenesis
The role of hypoxia in triggering vessel growth has long been recognized and has been studied aggressively in tumors and retinopathies. Hypoxia-inducible factor 1 activates VEGF. 19 In the embryo, hypoxia-inducible factor 1α and VEGF are spatiotemporally co-localized with possible hypoxic regions. 20 Endothelial cell proliferation and vessel formation in the hypoxic regions were documented in our laboratory. We tested the role of hypoxia on vascular tube formation in our quail embryonic heart explant model and found increased tube formation when 5% to 10% oxygen was provided. 21 Hypoxia up-regulated 3 splice variants of VEGF—specifically, VEGF122, VEGF166, and VEGF190—and increased the extent of tube formation. Consistent with these findings, hyperoxia had the opposite effect on the VEGF splice variants and on tube formation. As previously noted, 13 VEGF protein localization in the developing rat heart is closely related to sites of tube formation, which suggests that VEGF up-regulation occurs selectively in regions of relative hypoxia in the ventricle. Therefore, the epicardial-to-endocardial gradient of tube formation and VEGF localization supports the hypothesis of hypoxic regulation.
Stretch as a Stimulus for Growth Factors and Vascular Growth
Mechanical factors, such as shear stress, wall tension, and stretch have long been implicated in vascular growth. 22,23 Several mechanical forces in the myocardium are potential stimulators of vasculogenesis, angiogenesis, and arteriogenesis; these include shear stress and stretch associated with blood flow, and stretch due to diastolic filling. The stretch due to diastolic filling affects nonvascular as well as vascular cells and thus provides for both autocrine and paracrine signaling. During embryonic heart development, before formation of the coronary arteries, the major mechanical influences on the vascular tubes are most likely stretch imposed by diastolic filling and possibly stretch associated with the expanding myocardium. To study the role of growth factors that might be activated by stretch, we conducted in vitro experiments on cardiac myocytes and endothelial cells, in which these cells were subjected to cyclic stretch (30 cycles/min and 10% elongation) with use of a Flexercell® strain unit™ (Flexcell International Corp.; Hillsborough, NC). 24 When endothelial cells were cultured in conditioned media from cardiac myocytes that had been stretched, the endothelial cells showed increases in proliferation, migration, and tube formation. Both VEGF mRNA and protein were up-regulated in the stretched myocytes, implicating this growth factor in paracrine signaling.
Stretch of endothelial cells also induces growth factor and receptor up-regulation—a finding that supports the hypothesis that stretch of vessels results in autocrine signaling. The VEGF message for all 3 major splice variants, as shown by reverse transcription-polymerase chain reaction (RT-PCR), increases in these cells after cyclic stretch. 24 This increase is due to elevated transcription levels. Unpublished data from our laboratory also indicate that cyclic stretch of endothelial cells induces increases in flk-1 (VEGFR-2) and Tie-2.* These studies, taken together, support the concept of stretch as a trigger for angiogenic growth factors and receptors in both endothelial cells and cardiac myocytes. Therefore, stretch provides for both paracrine and autocrine signaling in the myocardium. We are currently attempting to identify the stretch response element in the VEGF promoter that may regulate VEGF transcription.
Mechanical and Metabolic Factors in Coronary Angiogenic Therapy
Mechanical and metabolic factors are candidates as therapeutic agents for heart diseases, because there is well documented evidence that they are activators of growth factors and, consequently, of coronary angiogenesis. The advantages of activating mechanical or metabolic factors are that multiple growth factors may be activated and that the therapy can be noninvasive. Therefore, this form of therapy may have advantages over gene or protein therapy, either of which usually involves the invasive delivery of a single growth factor. As noted in a document published by an expert panel concerning clinical trials in angiogenesis, 25 a number of problems exist relative to gene or protein therapy; for example, we know of no clear means of maximizing myocardial distribution and retention. Pharmacologic interventions that activate multiple, appropriate growth factors in the myocardium may prove to be the best choices for therapy.
Footnotes
Address for reprints: Robert J. Tomanek, PhD, Department of Anatomy and Cell Biology, Bowen Science Building, University of Iowa, Iowa City, IA 52242
This paper has its basis in a presentation made at the symposium. Coronary Artery Anomalies: Morphogenesis, Morphology, Pathophysiology, and Clinical Correlations, held on 28 Feb.–1 March 2002, at the Texas Heart Institute, Houston, Texas.
Supported by funds from NIH grants HL62178 and HL62587.
References
- 1.Tomanek RJ. Vascularization of the heart during prenatal and perinatal growth. In: Bittar EE, Rakusan K, editors. Advances in organ biology: coronary angiogenesis. Vol 7. Stamford (CT): Jai Press; 1999. p. 111–27.
- 2.Tomanek RJ, Yue X, Zheng W. Vascular development of the heart. In: Tomanek RJ, editor. Assembly of the vasculature and its regulation. Boston: Birkhauser; 2002. p. 133–55.
- 3.Viragh S, Gittenberger-de Groot AC, Poelmann RE, Kalman F. Early development of quail heart epicardium and associated vascular and glandular structures. Anat Embryol (Berl) 1993;188(4):381–93. [DOI] [PubMed]
- 4.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A 1992;89:9504–8. [DOI] [PMC free article] [PubMed]
- 5.Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bokenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res 1993;73:559–68. [DOI] [PubMed]
- 6.Kalman F, Viragh S, Modis L. Cell surface glycoconjugates and the extracellular matrix of the developing mouse embryo epicardium. Anat Embryol (Berl) 1995;191:451–64. [DOI] [PubMed]
- 7.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 1996;174:221–32. [DOI] [PubMed]
- 8.Dettman RW, Denetclaw W Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol 1998;193:169–81. [DOI] [PubMed]
- 9.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999;199:367–78. [DOI] [PubMed]
- 10.Boucek RJ, Morales AR, Romanelli R, Judkins MP. Embryology and congenital anomalies of the coronary arteries. In: Boucek RJ, et al., editors. Coronary artery disease, pathologic and clinical assessment. Baltimore: Williams and Wilkins; 1984. p. 38–65.
- 11.Tomanek RJ, Haung L, Suvarna PR, O'Brien LC, Ratajska A, Sandra A. Coronary vascularization during development in the rat and its relationship to basic fibroblast factor. Cardiovasc Res 1996;31:E116–26. [PubMed]
- 12.Rongish BJ, Hinchman G, Doty MK, Baldwin HS, Tomanek RJ. Relationship of the extracellular matrix to coronary neovascularization during development. J Mol Cell Cardiol 1996;28:2203–15. [DOI] [PubMed]
- 13.Tomanek RJ, Ratajska A, Kitten GT, Yue X, Sandra A. Vascular endothelial growth factor expression coincides with coronary vasculogenesis and angiogenesis. Dev Dyn 1999;215:54–61. [DOI] [PubMed]
- 14.Tomanek RJ, Lotun K, Clark EB, Suvarna PR, Hu N. VEGF and bFGF stimulate myocardial vascularization in embryonic chick. Am J Physiol 1998;274(5 Pt 2):H1620–6. [DOI] [PubMed]
- 15.Tomanek RJ, Hu N, Phan B, Clark EB. Rate of coronary vascularization during embryonic chicken development is influenced by the rate of myocardial growth. Cardiovasc Res 1999;41:663–71. [DOI] [PubMed]
- 16.Tomanek RJ, Zheng W, Peters KG, Lin P, Holifield JS, Suvarna PR. Multiple growth factors regulate coronary embryonic vasculogenesis. Dev Dyn 2001;221:265–73. [DOI] [PubMed]
- 17.Tomanek RJ, Holifield JS, Reiter RS, Sandra A, Lin JJ-C. Role of VEGF family members and receptors in coronary vessel formation. Dev Dyn. In press. [DOI] [PubMed]
- 18.Tomanek RJ, Sandra A, Zheng W, Brock T, Bjercke RJ, Holifield JS. Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res 2001;88:1135–41. [DOI] [PubMed]
- 19.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996;16:4604–13. [DOI] [PMC free article] [PubMed]
- 20.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, et al. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn 2001;220:175–86. [DOI] [PubMed]
- 21.Yue X, Tomanek RJ. Stimulation of coronary vasculogenesis/angiogenesis by hypoxia in cultured embryonic hearts. Dev Dyn 1999;216:28–36. [DOI] [PubMed]
- 22.Hudlicka O, Brown MD. Physical forces and angiogenesis. In: Rubanyi GM, editor. Mechanoreception by the vascular wall. Mount Kisko (NY): Futura Publishing Co., Inc.; 1993. p. 197–241.
- 23.Tomanek RJ. Angiogenesis in nonischemic myocardium. In: Ware JA, Simons M, editors. Angiogenesis and cardiovascular disease. New York: Oxford Univ. Press; 1999. p. 199–212.
- 24.Zheng W, Seftor EA, Meininger CJ, Hendrix MJ, Tomanek RJ. Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-beta [published erratum appears in Am J Physiol Heart Circ Physiol 2001;280:section H, following table of contents]. Am J Physiol 2001;280:H909–17. [DOI] [PubMed]
- 25.Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: An expert panel summary. Circulation 2000;102(11):E73–86. [DOI] [PubMed]


