Abstract
In the following review, we outline the cellular ontogeny and time course of coronary artery development within the vertebrate heart. Our eventual focus will be the potential role of arteriogenesis in the differentiation of a subset of specialized conduction cells in the chick heart. We begin by briefly outlining early heart formation, showing how the outermost layer of the looped, tube heart—the epicardium—is of extracardiac origin and provides the progenitor cells to the entire vascular bed. Subsequently, we summarize the events of coronary arterial development that follow epicardialization. Finally, we discuss work in the chick that indicates how arteries form pioneering, directional conduits through ventricular tissue, adjacent to which myocardial cells differentiate to form the most peripheral component of the avian conduction system—a network of periarterial Purkinje fibers. (Tex Heart Inst J 2002;29:262–70)
Key words: Animal, birds/embryology, cell differentiation, cell movement, chick embryo, conduction, coronary vessels/embryology/growth and development, heart/embryology, mouse, Purkinje fibers/cytology/embryology, quail, retrovirus, review
Before discussing the derivation and formation of coronary arteries, we will consider briefly the development of the heart from its earliest principles. Indicative of its pivotal function and importance, the heart is the 1st major organ to form in vertebrates. A substantial body of literature exists concerning the embryonic heart field—a region containing heart-cell progenitors. Cardiomyogenic cells can been detected at the onset of gastrulation as early as Hamburger and Hamilton stage 3 (HH3). 1–4 At stage HH5, these cells are found lying in 2 columns bilateral to the primitive streak. 2,5,6 By stage HH10 (equivalent to mouse embryonic day 8), these bilateral heart primordia fuse at the embryonic midline. 2 The resulting structure takes the form of a simple, linear vessel—the tube heart—that pumps blood in a caudal to rostral direction, triggered by a pacemaker. 8
Conventionally, the tube heart has been divided into 5 subcompartments or segments: progressing rostrally, one encounters the sinus venosus, atrium, ventricle, bulbous arteriosus, and truncus arteriosus. The process by which the tubular heart initiates its transition into the more complex 4-chamber structure found in the mature animal is termed looping. Here the right ventricular wall expands and bulges while the opposing side becomes concave, forming a “hub” for the looping process. Concurrently, the heart rotates and the atrium assumes its mature position above the ventricle. 8,9
For the purposes of this article, an appraisal of the cellular composition of the tubular heart is relevant. The early tubular heart is initially a bilaminar structure consisting of an internal layer, the endocardium, and a surrounding outer layer, the myocardium. 7 As development proceeds, a 3rd layer external to the myocardium, known as the epicardium, forms. The epicardium is derived from a highly proliferative and migratory cell population of extracardiac origin. 10–15 This small aggregation of cells, on the coelomic wall between the sinus venosus and the developing liver, is known as the proepicardial organ (PEO) and extends projections over the dorsal aspect of the heart from HH18. 10–12 The PEO is a transient structure, decreasing in size as the developing epicardium envelops the heart. 11,16 In the bird, epicardial cells have been shown to spread over the surface of the myocardium, in a rostral–dorsal direction, to form a continuous cell layer. 14,17 A process of epithelial-mesenchymal transformation (EMT) generates migratory cells from the epicardium, which penetrate heart tissues, providing a progenitor population that eventually seeds the entire coronary vascular bed. 13,17–24
Molecular Biology of Early Coronary Artery Development
Clues to our understanding of the molecular mechanisms that facilitate PEO cell migration, transformation, and differentiation into the coronary arteries are now emerging. The zinc finger protein FOG-2 is a GATA transcription factor repressor and likely to be a key player in the EMT process. 25 In mice, FOG-2 inactivation causes cardiac abnormalities, including the failure of coronary artery development. 26,27 Notably, this occurs despite the apparent normal development of the epicardium, which suggests that for EMT to occur, FOG-2 expression is required. Indeed the EMT process might be regulated by a complex interplay of inductive and repressive growth factors, consisting of FGF, VEGF, and EGF growth factor family members, and of bone morphogenetic proteins, the TGFβs. 28–32 Two molecules that may have roles in both migration and differentiation have recently been isolated: the transcription factor capsulin and the cell adhesion molecule Bves. Capsulin is a member of the basic helix-loop-helix (bHLH) transcription factor family and is associated with the EMT process in organogenesis. 33–38 This gene has alternative names, which include capsulin, epicardin, and Pod1. Capsulin is expressed in the PEO of the mouse at around embryonic day 9.5 (E9.5) and continues into the developing coronary vasculature. 33,38 This gene is required for epithelial cell differentiation in the lung, but a similar requirement in the development of the coronary vasculature has yet to be elucidated. 37 Blood vessel epicardial substance (Bves) is a prototype of a novel class of cell-adhesion molecules expressed in the chick and mouse hearts. 39–41 Mouse Bves also became known as popeye 1a, following its isolation, together with 2 other related peptides, by another laboratory. 42 In the chick, Bves is detected at HH10 in a subset of cells within the PEO. In the mouse Bves, positive cells are detected in the epicardium and subepicardium by E7. As development proceeds, Bves staining is associated with cells in the subendocardium and arterial vascular channels, where it localizes to smooth muscle cells of mature coronary arteries. 39 In accordance with the role played by Bves in cellular adhesion, Bader and colleagues have noted that the subcellular distribution of Bves shifts from a perinuclear localization to the cell membrane upon contact with other cells. 41 One further element of the mechanism that may be required for normal heart development and the processes of both EMT and PEO cell migration is the RhoA-RhoK GTPase signaling system. 43,44 These molecules participate in actin reorganization, and perturbation experiments prevent PEO cells from migrating into the myocardium. 44 In addition to these molecules, retinoic acid (RA) has recently been identified within the PEO. 45 Retinoids are morphogens implicated in the development of a number of different systems, 46 and recent investigations suggest that the heart is no exception. 47 By studying the expression pattern of the retinoic acid-synthetic enzyme (RALDH2), Xavier-Neto and colleagues 45 discovered a biphasic pattern, with heart development. The latter phase, pertinent here, begins at HH18 within the PEO and persists through the formation of the epicardium.
Cellular Phenotype and Ontogeny of Coronary Arteries
Mature coronary arteries, when viewed in cross section, are composed of 3 layers: an inner endothelial layer (tunica intima) enclosing the lumen, a central ring of smooth muscle cells (tunica media), and an outer layer of connective tissue and fibroblasts (tunica adventitia). The timing of coronary artery formation has been characterized in vertebrates by studying smooth muscle cell (SMC) differentiation. 16,17,48–51 In the rat, the SMC phenotype develops upon coronary ostia formation at E15.5 and continues in a proximal distal direction. 50,51 Smooth muscle cells begin to express alpha smooth muscle actin (aSMA) at E16, smooth muscle myosin heavy chain (SM-MHC) at E17, and finally smoothelin at E20. 51 Similarly, in avians, the accretion of SMCs to developing arteries occurs after the establishment of coronary blood flow at HH32. 16,17,48 Avian SMC differentiation is also well characterized by the sequential expression of alpha actin (SmaA), the 1E12 antigen, calponin, and SM-MHC. 16,48,49 Serum response factor (SRF) has been detected at the transcript and protein levels during SMC maturation. Loss-of-function experiments using SRF dominant negative approaches show that the SMC phenotypic expression pattern fails to develop, which indicates that SRF may play a role in the transcriptional regulation of SMC differentiation. 49
Lineage-tracing strategies in the chick show that the PEO contains the endothelial progenitor cells of the coronary vasculature and that these migrate into the myocardium at around HH16–20. 17,18 Consequent to expansion of these data, it is now known that cardiac fibroblasts, non-SMC perivascular cells, and SMCs of coronary arteries have a similar derivation from the PEO, distinct from aortic SMC populations. 19,20,22,52 Parallels exist between avian and mammalian systems, and observations made in mammalian models—in particular, the study of null mutant transgenic mice lacking either the vascular cell adhesion molecule-1 (VCAM-1) or its receptor integrin alpha4—have been revealing. 53,54 In both of these knockout mouse lines, the formation of epicardium is deficient, with the result that the coronary vasculature fails to form; this suggests that the mammalian PEO is key to the formation of cells of the mammalian coronary vasculature.
Coronary Arteriogenesis and Purkinje Fiber Differentiation
As discussed in the preceding section, the timing of PEO cell migration and subsequent differentiation to establish the coronary arterial bed is well defined in avians. 16,17,55–58 Proepicardial organ cells begin to migrate at HH18, while complete coronary blood flow circuits are established at HH32. Significantly, the formation of the coronary arteries predates the differentiation of a specific element of the chick heart conduction system: the periarterial Purkinje fibers. The primary function of this network of specialized cardiac cells is to transduce the pacemaker signal, thereby ensuring rapid and coordinated ventricular contractions. 59 More than 1 subclass of Purkinje fibers exists in the bird—periarterial Purkinje fibers (PPF) and subendocardial Purkinje fibers (SPF)—and together these form the distal parts of the peripheral conduction system (PCS) (Fig. 1).
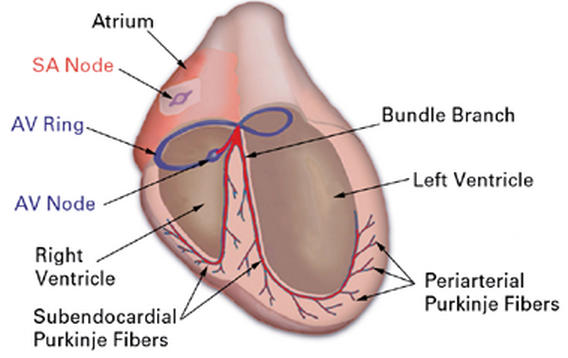
Fig. 1 Basic structural and functional organization of the embryonic pacemaking and conduction system. The organization of the conduction system in the avian heart is similar to its mammalian counterpart. Central conduction system elements include the sinoatrial node (SA node) or pacemaker, the atrioventricular ring (AV ring) and atrioventricular node (AV node), and bundle of His. The peripheral conduction system components are the left and right septal bundle branches, subendocardial Purkinje fibers, and periarterial Purkinje fibers. The description of periarterial Purkinje fibers in the chick is, to date, species specific, and no such fibers have been identified in the mammalian system thus far. In both mammals and avians, the subendocardial region contains subendocardial Purkinje fibers and is functionally distinct from the working myocardium.
Chick PPFs have been instructive insofar as they have shaped our ideas about the cellular and molecular factors involved in the differentiation of specialized cardiac lineages. It should nonetheless be noted that no such relationship between arteries and specialized myocardial cells has been found thus far in mammals, nor is it clear that lessons learned in the developing chick can be generalized to other species.
From around HH26, the developing chick PCS can be definitively visualized as a compartment separate from the working myocardium. 60–62 At this stage, conduction system cells begin to terminally differentiate, as shown by their lowered proliferative potential. 63 To date, only a single molecular marker of the PCS at these earliest stages has been described, the homeobox domain containing transcription factor Nkx2.5. In the chick, increased levels of Nkx2.5 expression, compared with surrounding myocardium, occur at HH35 and remain elevated until HH42. 64,65 Other markers of the PCS appear later in development and include (in the chick) the gap junction protein Connexin40 (Cx40) (from HH36), the neural-associated protein transitin (from HH40), and the slow tonic myosin heavy chain protein (sMHC), a structural protein expressed from HH42 forward. 66
Our earlier work demonstrated that Cx40, a thennovel gap junction protein, was expressed in the developing chick heart by both PEO-derived endothelial cells and adjacent myocardial cells 67 (Fig. 2).
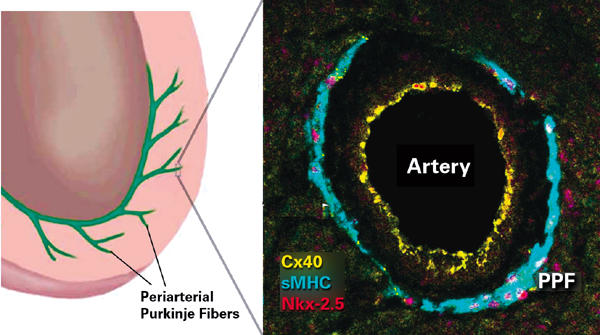
Fig. 2 Basic structural and functional organization of the embryonic pacemaking and conduction system. The model shown in the left-hand panel is based on the embryonic chick heart. In the chick, the terminal-most component of the conduction system penetrates into the ventricular muscle in intimate association with the coronary arteries. The periarterial Purkinje fiber (PPF) shown enlarged in the right-hand panel has been simultaneously labeled for 3 markers of conduction lineage: a gap junction protein connexin40 (Cx40, yellow); a myosin heavy chain (sMHC, green); and Nkx-2.5 (red), a transcription factor. The endothelial cells lining the artery also contain Cx40 gap junctions.
The myocardial populations could be shown to differentiate into definitive PPFs by the later expression of other markers, including transitin and sMHC. Using retrovirus lineage tracing, we were able to show that these PPFs differentiated from cardiomyogenic cells in the tubular heart. 61,68 Moreover, by analysis of the composition of clones of myocytes infected by retrovirus, working myocytes and PPFs were found to coexist within clonal domains (Fig. 3). Also, the frequency of heterogeneous clones was found to increase between stages HH40 and HH44.
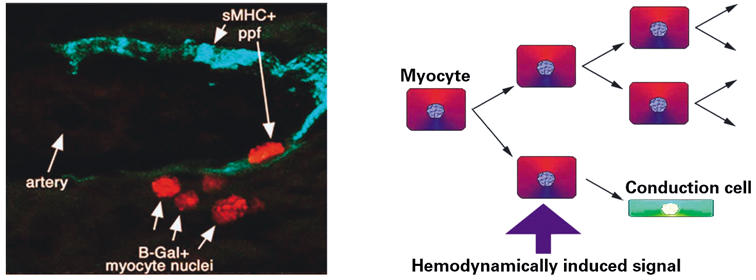
Fig. 3 Induction of periarterial Purkinje fiber (ppf) conduction cells. The left-hand panel shows a cluster of red nuclei delineating a clone of lacZ-expressing cells infected with a defective retrovirus. The clone contains both working myocytes and an sMHC+ (green) Purkinje fiber—a pattern consistent with the occurrence of localized recruitment of a multipotent progenitor cell to specialized myocardial lineages in the avian heart. The right-hand panel shows a model in which hemodynamically induced factors (for example, endothelin-1) from arterial tissues locally mediate this divergence into either working myocytes or Purkinje fiber conduction cells within a cardiomyogenic lineage.
On the basis of these data, a model was proposed in which conduction cells of myogenic origin were progressively recruited and signaled to differentiate in response to the ongoing process of coronary artery arborization. In essence, the developing and expanding arterial tree establishes a directional framework over which the peripheral conduction system is steadily recruited and patterned by intimate association.
Modulation of Coronary Artery Formation Directly Influences PPF Patterning
It is now widely accepted that a 2nd extracardiac population, the cardiac neural crest (CNC), has an important role in patterning the coronary vasculature. 48,69–71 In the chick, the CNC begins to migrate from the caudal hindbrain at around HH12 and reaches the base of the cardiac outflow tract at around HH18. 69 Experimental ablation of the CNC results in a number of abnormalities to the developing heart, including systemic rearrangements of the coronary vasculature 48,70 and reductions in the density of intramural arterial branches. 71 The CNC per se does not contribute to the cellular elements of the developing coronary arteries; however, parasympathetic ganglia (of CNC origin) are found in close association with them. 70 Recent work suggests that CNC cells undergo a high degree of apoptosis, but it is at present unknown what function this might serve. 72 Although a specific function has yet to be attributed to the CNC in coronary artery development, it is likely that a contribution to remodeling processes occurs. We have considered the PEO origin of coronary arteries and the CNC's contribution in initiating this patterning; but how does the vasculature undergo expansion as the developing heart increases in size? Growth factors are prime candidates for such a process, and FGFs have been shown to have a role in the arborization of coronary arteries in both avians and mammals. 71,73–79 Both FGF-1 and 2 have been identified in the chick heart; and incubation with, or overexpression of, FGF-2 results in ectopic vasculature in the chorioallantoic membrane and chick myocardium. 71,75,80 In mammals, members of the FGF family have been identified in cardiomyocytes and within the extracellular matrix. 73,74 In addition, expression has been localized to the endothelium and SMCs of coronary arteries. 74,76,77 Functional investigations in vitro and by the transgenic overexpression of FGF-1 in vivo have resulted in increased coronary artery density and branching, which suggests that FGFs regulate coronary artery differentiation. 78,81 In light of such knowledge, we undertook a detailed study in which we manipulated the coronary vasculature and observed effects on the patterning of peripheral conduction tissue. We found that intervention in normal coronary artery development in the chick, by CNC ablation, results in markedly decreased numbers of PPFs. 71 Conversely, the stimulation of ectopic vascularization by overexpression of FGF in vivo results in ectopic PPFs. These data provide strong evidence to suggest that the pattern of coronary artery ingrowth provides the inductive cues for the differentiation and organization of the periarterial Purkinje fibers of the peripheral conduction system.
Endothelin Induces Conduction Cell Phenotype in Embryonic Myocytes
Given the close spatial and temporal developmental relationship of PPFs with coronary arteries, we considered that paracrine signaling was a potential mechanism underlying such a recruitment process. In screening likely candidates, we found that the shear-stress-induced cytokine endothelin-1 (ET-1) could elicit just such an activity in myocytes in culture, increasing the levels of Cx40 and sMHC expression in vitro and in vivo. 65,82,83 This response appears to be specific to this molecule, because other candidates associated with the vasculature, such as VEGF, do not elicit up-regulation of conduction system markers. The effect of ET-1 on expression of conduction system markers is dose dependent and can be inhibited by using specific endothelin receptor antagonists. Endothelin-1 has been described in vascular endothelial cells and in the developing heart, where it is initially present as a precursor molecule, preproendothelin-1 (preproET-1). Preproendothelin-1 is subsequently cleaved to form big endothelin-1 (bigET-1), before active ET-1 is produced by 1 more round of cleavage by the endothelin-converting enzyme (ECE-1). 84–86 To gain insight into the effect of ET-1 on PCS development, an elegant experiment was conducted by means of viral-mediated co-overexpression of both preproET-1 and ECE-1 in the developing chick heart. 83 These experiments result in the precocious and ectopic induction of PPFs within zones of viral-mediated co-overexpression. This finding, together with in situ analyses of endogenous ECE-1 expression, firmly supports a theory wherein coronary-artery-derived ET-1 has a role to play in the terminal differentiation of PPFs (Fig. 4).
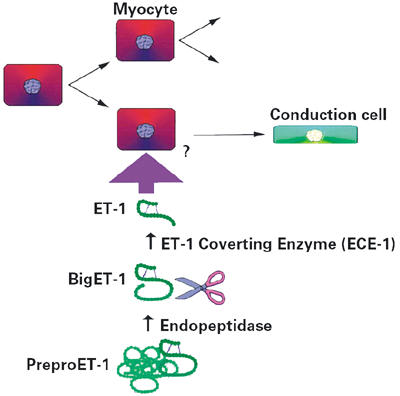
Fig. 4 A model for the molecular induction of Purkinje fiber conduction cells. The site of specific cleavage of bigET-1 by ECE-1 may be a mechanism by which localized induction of conduction cells occurs.
Are PEO Derivatives Involved in Patterning SPFs?
We have outlined experiments showing how the PEO gives rise to coronary arteries, which in turn locally recruit conduction system cells—PPFs—from a myocardial population. These experiments also show that the underlying mechanism of paracrine signaling may involve endothelin. However, PPFs are not the sole component of the chick peripheral conduction system; and how would a recruitment model apply to SPFs? Commonalities exist between PPFs and SPFs: for instance, the timing of formation in the developing heart; the expression of high relative levels of Cx40, transitin and sMHC; the proximity to endothelia; and the probable exposure to hemodynamic forces. With these points in mind, one might be surprised to find that diverse mechanisms underlie the formation of these 2 similar but separate subcompartments of the conduction system. Intriguingly, 3 independent studies that used similar chick-quail chimeras have identified quail-derived cells within the developing superficial myocardium of HH25–43 host chick hearts. 22,23,52 In specific regard to the conduction system, 1 study pointed to the accumulation of these cells in both subendocardial and periarterial locations. 52 This evidence shows that PEO-derived cells are also present in close association with SPFs, but it remains to be seen whether a direct relationship exists between these cells and the patterning of SPFs in the chick heart. Additionally, if such a mechanism was proved in the bird, it may be pertinent to consider whether a similar process occurs in the mammalian system.
Commonalities with Mammalian PCS Development
We have shown that parallels between avians and mammals exist in the normal development of the coronary arterial tree. Phylogenetic similarities also exist in the development of the conduction systems of different vertebrate species, and accumulating inferential evidence includes aspects of both lineage and protein expression. Until recently, the fate of mammalian CNC was difficult to investigate, but it is possible through the use of Cre/Lox mouse technology. 87,88 These studies show, as was concluded in the chick, that a neurogenic contribution to mammalian SPFs is unlikely. 61,68,72,87,88 Connexins are the subunits of gap junction channels that facilitate cell-to-cell coupling and propagation of electrical activation. 89,90 Interestingly, Cx40 has been shown to be the predominant isoform in the PCS in the majority of higher vertebrates. 67,89,91,92 In mice and human beings, as in the bird, the transcription factor Nkx-2.5 is expressed in the conduction system. 64 Investigators have identified a number of heterozygous Nkx-2.5 mutations that result in functional and structural defects to atrioventricular conduction in human beings. 93,94 Similar conduction system defects have been noted in transgenic mice that express one of the known human Nkx-2.5 mutants. 95 Such defects are consistent with Nkx-2.5 playing a role in the differentiation of the mammalian PCS. Recently, a lacZ-based marker of what Rentschler and colleagues 96 describe as the entire developing mouse cardiac conduction system was identified. The lacZ-expressing tissues were shown to correlate with the distribution of conduction fascicles. This transgenic mouse should provide an invaluable tool for the study of the murine conduction system.
We have presented data to outline a molecular and cellular mechanism that does indeed link coronary arterialization to the induction and patterning of specialized conduction cells in the chick heart. It remains to be seen whether this knowledge will give insights into the processes underlying conduction system development in other vertebrate species.
Acknowledgments
The authors would like to thank Jane Jourdan and Mary S. Rackley for their assistance in the preparation of this manuscript.
References
Key to Abbreviations
BigET-1 Big endothelin-1
Bves Blood vessel epicardial substance
CCS Central conduction system
CNC Cardiac neural crest
E7, E9, etc. Embryonic day as indicated by number
ECE-1 Endothelin converting enzyme-1
EGF Epidermal growth factor
EMT Epithelial-mesenchymal transformation
ET-1 Endothelin-1
FGF Fibroblast growth factor
FOG-2 Friend of GATA 2
HH Hamburger & Hamilton stage
PCS Peripheral conduction system
PEO Proepicardial organ
PPF Periarterial Purkinje fibers
PreproET-1 Preproendothelin-1
RA Retinoic acid
RALDH2 Retinoic acid-synthetic enzyme
SA node Sinoatrial node
SMC Smooth muscle cell
sMHC Slow tonic myosin heavy chain protein
SPF Subendocardial Purkinje fibers
SRF Serum response factor
TGFβ Transforming growth factor-beta
VEGF Vascular endothelial growth factor
Footnotes
Address for reprints: Robert G. Gourdie, PhD, MUSC/Cell Biology, Basic Science Bldg., Suite 641, 171 Ashley Ave., Charleston, SC 29425
This paper has its basis in a presentation made at the symposium Coronary Artery Anomalies: Morphogenesis, Morphology, Pathophysiology, and Clinical Correlations, held on 28 Feb.–1 March 2002, at the Texas Heart® Institute, Houston, Texas.
The authors have provided a short key to abbreviations at the end of the paper.
This work is supported by grants from the NIH (HL56728 to RGG), HD39446 to RGG and TXO), NSF (IBN 9734406 to RGG) and the office of Research and Development, Medical Research Service, Ralph H. Johnson Veterans Affairs Medical Center (TXO), Medical University of South Carolina, Charleston, SC.
References
- 1.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951;88:49–92. [PubMed]
- 2.Rosenquist GC, DeHaan RL. Migration of precardiac cells in the chick embryo: a radiographic study. 1966. Carnegie Inst. Washington Publ. 625 (Contrib. To Embryol). 38, 111–21.
- 3.Hatada Y, Stern CD. A fate map of the epiblast of the early chick embryo. Development 1994;120(10):2879–89. [DOI] [PubMed]
- 4.Garcia-Martinez V, Schoenwolf GC. Primitive-streak origin of the cardiovascular system in avian embryos. Dev Biol 1993;159(2):706–19. [DOI] [PubMed]
- 5.Rosenquist GC. A radioautographic study of labelled grafts in the chick blastoderm. 1966. Carnegie Inst. Washington Publ. 625 (Contrib. To Embryol). 38, 71–110.
- 6.Redkar A, Montgomery M, Litvin J. Fate map of early avian cardiac progenitor cells. Development 2001;128(12):2269–79. [DOI] [PubMed]
- 7.Manasek FJ. Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J Morphol 1968;125:329–65. [DOI] [PubMed]
- 8.Manner J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec 2000;259(3):248–62. [DOI] [PubMed]
- 9.Markwald RR, Wessels A. Overview of heart development. In: Tomanek RJ, Runyan RB, editors. Formation of the heart and its regulation. Boston: Birkhauser; 2001. p. 1–22.
- 10.Ho E, Shimada Y. Formation of the epicardium studied with the scanning electron microscope. Develop Biol 1978;66:579–85. [DOI] [PubMed]
- 11.Hiruma T, Hirakow R. Epicardial formation in embryonic chick heart: computer-aided reconstruction, scanning, and transmission electron microscopic studies. Am J Anat 1989;184:129–38. [DOI] [PubMed]
- 12.Manner J. The development of pericardial villi in the chick embryo. Anat Embryol (Berl) 1992;186:379–85. [DOI] [PubMed]
- 13.Viragh S, Gittenberger-de Groot AC, Poelmann RE, Kalman F. Early development of quail heart epicardium and associated vascular and glandular structures. Anat Embryol (Berl) 1993;188:381–93. [DOI] [PubMed]
- 14.Vrancken Peeters MP, Mentink MM, Poelmann RE, Gittenberger-de Groot AC. Cytokeratins as a marker for epicardial formation in the quail embryo. Anat Embryol (Berl) 1995;191(6):503–8. [DOI] [PubMed]
- 15.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail-chick chimera study. Dev Biol 1998;200(1):57–68. [DOI] [PubMed]
- 16.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Hungerford JE, Little CD, Poelmann RE. The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev Dyn 1997;208(3):338–48. [DOI] [PubMed]
- 17.Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bokenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res 1993;73:559–68. [DOI] [PubMed]
- 18.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A 1992;89:9504–8. [DOI] [PMC free article] [PubMed]
- 19.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 1996;174:221–32. [DOI] [PubMed]
- 20.Dettman RW, Denetclaw W Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol 1998;193:169–81. [DOI] [PubMed]
- 21.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. Contribution of the primitive epicardium to the subepicardial mesenchyme in hamster and chick embryos. Dev Dyn 1997;210(2):96–105. [DOI] [PubMed]
- 22.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res 1998;82:1043–52. [DOI] [PubMed]
- 23.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999;199(4):367–78. [DOI] [PubMed]
- 24.MacNeill C, French R, Evans T, Wessels A, Burch JB. Modular regulation of cGATA-5 gene expression in the developing heart and gut [published erratum appears in Dev Biol 2000;223(2):463]. Dev Biol 2000;217(1):62–76. [DOI] [PubMed]
- 25.Fossett N, Schulz RA. Conserved cardiogenic functions of the multitype zinc-finger proteins: U-shaped and FOG-2. Trends Cardiovasc Med 2001;11(5):185–90. [DOI] [PubMed]
- 26.Svensson EC, Huggins GS, Lin H, Clendenin C, Jiang F, Tufts R, et al. A syndrome of tricuspid atresia in mice with a targeted mutation of the gene encoding Fog-2. Nat Genet 2000;25(3):353–6. [DOI] [PubMed]
- 27.Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, et al. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell 2000;101(7):729–39. [DOI] [PubMed]
- 28.Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A 1991;88(4):1516–20. [DOI] [PMC free article] [PubMed]
- 29.Brown CB, Boyer AS, Runyan RB, Barnett JV. Antibodies to the Type II TGFbeta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol 1996;174(2):248–57. [DOI] [PubMed]
- 30.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science 1999;283:2080–2. [DOI] [PubMed]
- 31.Boyer AS, Runyan RB. TGFbeta Type III and TGFbeta Type II receptors have distinct activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Dyn 2001;221(4):454–9. [DOI] [PubMed]
- 32.Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev Biol 2001;234(1):204–15. [DOI] [PubMed]
- 33.Hidai H, Bardales R, Goodwin R, Quertermous T, Quertermous EE. Cloning of capsulin, a basic helix-loop-helix factor expressed in progenitor cells of the pericardium and the coronary arteries. Mech Dev 1998;73(1):33–43. [DOI] [PubMed]
- 34.Lu J, Richardson JA, Olson EN. Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mech Dev 1998;73(1):23–32. [DOI] [PubMed]
- 35.Lu J, Chang P, Richardson JA, Gan L, Weiler H, Olson EN. The basic helix-loop-helix transcription factor capsulin controls spleen organogenesis. Proc Natl Acad Sci U S A 2000;97(17):9525–30. [DOI] [PMC free article] [PubMed]
- 36.Quaggin SE, Vanden Heuvel GB, Igarashi P. Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech Dev 1998;71(1–2):37–48. [DOI] [PubMed]
- 37.Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, Rossant J. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development 1999;126(24):5771–83. [DOI] [PubMed]
- 38.Robb L, Mifsud L, Hartley L, Biben C, Copeland NG, Gilbert DJ, et al. epicardin: A novel basic helix-loop-helix transcription factor gene expressed in epicardium, branchial arch myoblasts, and mesenchyme of developing lung, gut, kidney, and gonads. Dev Dyn 1998;213(1):105–13. [DOI] [PubMed]
- 39.Reese DE, Zavaljevski M, Streiff NL, Bader D. Bves: A novel gene expressed during coronary blood vessel development. Dev Biol 1999;209(1):159–71. [DOI] [PubMed]
- 40.Reese DE, Bader DM. Cloning and expression of hbves, a novel and highly conserved mRNA expressed in the developing and adult heart and skeletal muscle in the human. Mamm Genome 1999;10(9):913–5. [DOI] [PubMed]
- 41.Wada AM, Reese DE, Bader DM. Bves: prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development 2001;128(11):2085–93. [DOI] [PubMed]
- 42.Andree B, Hillemann T, Kessler-Icekson G, Schmitt-John T, Jockusch H, Arnold HH, Brand T. Isolation and characterization of the novel popeye gene family expressed in skeletal muscle and heart. Dev Biol 2000;223(2):371–82. [DOI] [PubMed]
- 43.Wei L, Roberts W, Wang L, Yamada M, Zhang S, Zhao Z, et al. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development 2001;128(15):2953–62. [DOI] [PubMed]
- 44.Lu J, Landerholm TE, Wei JS, Dong XR, Wu SP, Liu X, et al. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Dev Biol 2001;240(2):404–18. [DOI] [PubMed]
- 45.Xavier-Neto J, Shapiro MD, Houghton L, Rosenthal N. Sequential programs of retinoic acid synthesis in the myocardial and epicardial layers of the developing avian heart. Dev Biol 2000;219(1):129–41. [DOI] [PubMed]
- 46.Means AL, Gudas LJ. The roles of retinoids in vertebrate development. Annu Rev Biochem 1995;64:201–33. [DOI] [PubMed]
- 47.Kubalak SW, Sucov HM. Retinoids in heart development. In: Harvey RP, Rosenthal N, editors. Heart development. San Diego: Academic Press; 1999. p. 209–19.
- 48.Hood LC, Rosenquist TH. Coronary artery development in the chick: origin and deployment of smooth muscle cells, and the effects of neural crest ablation. Anat Rec 1992;234:291–300. [DOI] [PubMed]
- 49.Landerholm TE, Dong XR, Lu J, Belaguli NS, Schwartz RJ, Majesky MW. A role for serum response factor in coronary smooth muscle differentiation from proepicardial cells. Development 1999;126(10):2053–62. [DOI] [PubMed]
- 50.Ratajska A, Fiejka E. Prenatal development of coronary arteries in the rat:morphologic patterns. Anat Embryol (Berl) 1999;200(5):533–40. [DOI] [PubMed]
- 51.Ratajska A, Zarska M, Quensel C, Kramer J. Differentiation of the smooth muscle cell phenotypes during embryonic development of coronary vessels in the rat. Histochem Cell Biol 2001;116(1):79–87. [DOI] [PubMed]
- 52.Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec 1999;255(2):212–26. [DOI] [PubMed]
- 53.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development 1995;121(2):549–60. [DOI] [PubMed]
- 54.Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development 1995;121(2):489–503. [DOI] [PubMed]
- 55.Rychter Z, Ostadal B. Mechanism of the development of coronary arteries in chick embryo. Folia Morphol (Praha) 1972;19:113–24. [PubMed]
- 56.Bogers AJ, Gittenberger-de Groot AC, Dubbeldam JA, Huysmans HA. The inadequacy of existing theories on development of the proximal coronary arteries and their connexions with the arterial trunks. Int J Cardiol 1988;20(1):117–23. [DOI] [PubMed]
- 57.Bogers AJ, Gittenberger-de Groot AC, Poelmann RE, Peault BM, Huysmans HA. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat Embryol (Berl) 1989;180:437–41. [DOI] [PubMed]
- 58.Waldo KL, Willner W, Kirby ML. Origin of the proximal coronary artery stems and a review of ventricular vascularization in the chick embryo. Am J Anat 1990;188:109–20. [DOI] [PubMed]
- 59.Janse MJ, Fast VG. Normal and abnormal conduction in the heart. In: Fozzard HA, Solaro RJ, editors. The Handbook of physiology: the cardiovascular system. Oxford: Oxford University Press; 2001. p. 455–527.
- 60.Sanders E, de Groot IJ, Geerts WJ, de Jong F, van Horssen AA, Los JA, Moorman AF. The local expression of adult chicken heart myosins during development. II. Ventricular conducting tissue. Anat Embryol (Berl) 1986;174(2):187–93. [DOI] [PubMed]
- 61.Gourdie RG, Mima T, Thompson RP, Mikawa T. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development 1995;121:1423–31. [DOI] [PubMed]
- 62.McCabe CF, Gourdie RG, Thompson RP, Cole GJ. Developmentally regulated neural protein eap-300 is expressed by myocardium and cardiac neural crest during chick embryogenesis. Dev Dyn 1995;203:51–60. [DOI] [PubMed]
- 63.Thompson RP, Kanai T, Germroth PG, Gourdie RG, Thomas PC, Barton PJ. Organization and function of early specialized myocardium. In: Clark EB, Markwald RR, Takao A, editors. Developmental mechanisms of heart disease. Armonk (NY): Futura Publishing Co., Inc.; 1995. p. 269–7.
- 64.Thomas PS, Kasahara H, Edmonson AM, Izumo S, Yacoub MH, Barton PJ, Gourdie RG. Elevated expression of Nkx-2.5 in developing myocardial conduction cells. Anat Rec 2001;263:307–13. [DOI] [PubMed]
- 65.Takebayashi-Suzuki K, Pauliks LB, Eltsefon Y, Mikawa T. Purkinje fibers of the avian heart express a myogenic transcription factor program distinct from cardiac and skeletal muscle. Dev Biol 2001;234:390–401. [DOI] [PubMed]
- 66.Gourdie RG, Kubalak S, Mikawa T. Conducting the embryonic heart: orchestrating development of specialized cardiac tissues. Trends Cardiovasc Med 1999;9:18–26. [DOI] [PubMed]
- 67.Gourdie RG, Severs NJ, Green CR, Rothery S, Germroth P, Thompson RP. The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of components of the cardiac atrioventricular conduction system. J Cell Sci 1993;105:985–91. [DOI] [PubMed]
- 68.Cheng G, Litchenberg WH, Cole GJ, Mikawa T, Thompson RP, Gourdie RG. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development 1999;126:5041–49. [DOI] [PubMed]
- 69.Kirby ML, Waldo KL. Role of neural crest in congenital heart disease. Circulation 1990;82(2):332–40. [DOI] [PubMed]
- 70.Waldo KL, Kumiski DH, Kirby ML. Association of the cardiac neural crest with development of the coronary arteries in the chick embryo. Anat Rec 1994;239(3):315–31. [DOI] [PubMed]
- 71.Hyer J, Johansen M, Prasad A, Wessels A, Kirby ML, Gourdie RG, Mikawa T. Induction of Purkinje fiber differentiation by coronary arterialization. Proc Natl Acad Sci U S A 1999;96(23):13214–8. [DOI] [PMC free article] [PubMed]
- 72.Poelmann RE, Gittenberger-de Groot. A subpopulation of apoptosis-prone cardiac neural crest cells targets to the venous pole: multiple functions in heart development? Dev Biol 1999;207:271–86. [DOI] [PubMed]
- 73.Casscells W, Speir E, Sasse J, Klagsbrun M, Allen P, Lee M, et al. Isolation, characterization, and localization of heparin-binding growth factors in the heart. J Clin Invest 1990;85(2):433–41. [DOI] [PMC free article] [PubMed]
- 74.Spirito P, Fu YM, Yu ZX, Epstein SE, Casscells W. Immunohistochemical localization of basic and acidic fibroblast growth factors in the developing rat heart. Circulation 1991;84(1):322–32. [DOI] [PubMed]
- 75.Wilting J, Christ B, Bokeloh M. A modified chorioallantoic membrane (CAM) assay for qualitative and quantitative study of growth factors. Studies on the effects of carriers, PBS, angiogenin, and bFGF. Anat Embryol (Berl) 1991;183(3):259–71. [DOI] [PubMed]
- 76.Engelmann GL, Dionne CA, Jaye MC. Acidic fibroblast growth factor and heart development. Role in myocyte proliferation and capillary angiogenesis. Circ Res 1993;72(1):7–19. [DOI] [PubMed]
- 77.Tomanek RJ, Haung L, Suvarna PR, O'Brien LC, Ratajska A, Sandra A. Coronary vascularization during development in the rat and its relationship to basic fibroblast growth factor. Cardiovasc Res 1996;31 Spec No:E116–26. [PubMed]
- 78.Fernandez B, Buehler A, Wolfran S, Kostin S, Espanion G, Franz WM, et al. Transgenic myocardial overexpression of fibroblast growth factor-1 increases coronary artery density and branching. Circ Res 2000;87:207–13. [DOI] [PubMed]
- 80.Tomanek RJ, Sandra A, Zheng W, Brock T, Bjercke RJ, Holifield JS. Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res 2001;88(11):1135–41. [DOI] [PubMed]
- 80.Patstone G, Pasquale EB, Maher PA. Different members of the fibroblast growth factor receptor family are specific to distinct cell types in the developing chicken embryo. Dev Biol 1993;155(1):107–23. [DOI] [PubMed]
- 81.Tomanek RJ, Zheng W, Peters KG, Lin P, Holifield JS, Suvarna PR. Multiple growth factors regulate coronary embryonic vasculogenesis. Dev Dyn 2001;221(3):265–73. [DOI] [PubMed]
- 82.Gourdie RG, Wei Y, Kim D, Klatt SC, Mikawa T. Endothelin-induced conversion of embryonic heart muscle cells into impulse-conducting Purkinje fibers. Proc Natl Acad Sci U S A 1998;95:6815–8. [DOI] [PMC free article] [PubMed]
- 83.Takebayashi-Suzuki K, Yanagisawa M, Gourdie RG, Kanzawa N, Mikawa T. In vivo induction of cardiac Purkinje fiber differentiation by coexpression of preproendothelin-1 and endothelin converting enzyme-1. Development 2000;127:3523–32. [DOI] [PubMed]
- 84.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411–5. [DOI] [PubMed]
- 85.Yoshizumi M, Kurihara H, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, Yazaki Y. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun 1989;161:859–64. [DOI] [PubMed]
- 86.Masaki T, Kimura S, Yanagisawa M, Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation 1991;84:1457–68. [DOI] [PubMed]
- 87.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development 2000;127(8):1607–16. [DOI] [PubMed]
- 88.Epstein JA, Li J, Lang D, Chen F, Brown CB, Jin F, et al. Migration of cardiac neural crest cells in Splotch embryos. Development 2000;127:1869–78. [DOI] [PubMed]
- 89.Gros DB, Jongsma HJ. Connexins in mammalian heart function. Bioessays 1996;18(9):719–30. [DOI] [PubMed]
- 90.Gourdie RG, Lo CW. Cx43 gap junctions in cardiac development and disease. In: Perrachia C, editor. Gap junctions. Molecular basis of cell communications in health and disease. Vol 49. Series: Current topics in membranes. San Diego: Academic Press; 2000. p. 581–602.
- 91.Gourdie RG, Green CR, Severs NJ, Anderson RH, Thompson RP. Evidence for a distinct gap-junctional phenotype in ventricular conduction tissues of the developing and mature avian heart. Circ Res 1993;72:278–89. [DOI] [PubMed]
- 92.Jalife J, Morley GE, Vaidya D. Connexins and impulse propagation in the mouse heart. J Cardiovasc Electrophysiol 1999;10:1649–63. [DOI] [PubMed]
- 93.Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 1998;281:108–11. [DOI] [PubMed]
- 94.Benson DW, Silberbach GM, Kavanaugh-McHugh A, Cottrill C, Zhang Y, Riggs S, et al. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J Clin Invest 1999;104:1567–73. [DOI] [PMC free article] [PubMed]
- 95.Kasahara H, Wakimoto H, Liu M, Maguire CT, Converso KL, Shioi T, et al. Progressive atrioventricular conduction defects and heart failure in mice expressing a mutant Csx/Nkx2.5 homeoprotein. J Clin Invest 2001;108:189–201. [DOI] [PMC free article] [PubMed]
- 96.Rentschler S, Vaidya DM, Tamaddon H, Degenhardt K, Sassoon D, Morley GE, et al. Visualization and functional characterization of the developing murine cardiac conduction system. Development 2001;128:1785–92. [DOI] [PMC free article] [PubMed]


